Abstract
Binding of antigen to B–cell antigen receptor (BCR) leads to antigen internalization and presentation to T cells, a critical process in the initiation of the humoral immune response. However, antigen internalization has been demonstrated for soluble antigen, in vivo antigen is often encountered in insoluble form or tethered to a cell surface. Here, we show that not only can B cells internalize and present large particulate antigen (requiring a signalling-competent BCR to drive antigen uptake), but they can also extract antigen that is tethered tightly to a non-internalizable surface. The form in which the antigen is displayed affects the B cell's ability to discriminate antigen–BCR affinity. Thus, arraying an antigen on a particle or surface allows efficient presentation of low affinity antigens. However, the presentation efficiency of antigen arrayed on an internalizable particle plateaus at low affinity values. In contrast, extraction and presentation of antigen from a non-internalizable surface depends on antigen–BCR affinity over a wide affinity range. The results have implications for understanding both the initiation and affinity maturation of the immune response.
Keywords: affinity/antigen presentation/B cell/extraction
Introduction
Activation of B cells is triggered by interaction of antigen with the B–cell antigen receptor (BCR). BCR fulfils this function through two distinct processes: transmembrane signalling and antigen internalization/presentation. Transmembrane signalling through the BCR (reviewed in Reth and Wienands, 1997) initiates a cascade of protein tyrosine phosphorylation and drives the B cell into cycle as well as up-regulating the expression of cell surface molecules involved in B cell–T cell collaboration. Internalization of antigen through the BCR leads to proteolytic processing of the antigen and loading of antigen-derived peptides onto major histocompatibility complex (MHC) class II molecules for presentation to T cells and the recruitment of T–cell help (Rock et al., 1984; Lanzavecchia, 1985).
In many ligand–receptor interactions, the receptor need only discriminate the high affinity ligand from low affinity, irrelevant molecules. However, with lymphocytes, the receptor needs to give a graded response dependent on ligand affinity. To initiate the humoral response, B cells should respond to antigens of low affinity since a high affinity receptor often may not be available in the primary repertoire. In contrast, during development of the immune response, the B–cell response depends upon antigen–BCR affinity over a wide affinity range to allow affinity maturation.
In recent years, we and others have performed studies to determine how the B–cell response varies according to antigen affinity (Lanzavecchia, 1985; Batista and Neuberger, 1998; Guermonprez et al., 1998; Kouskoff et al., 1998). Working with a soluble, monomeric antigen, we found that for specific BCR-mediated antigen presentation to cognate T cells (that rises above the background attributable to fluid phase pinocytosis), the antigen needed to have an affinity greater than ∼7 × 105 M–1. As the antigen–BCR affinity increased, there was a corresponding diminution in the amount of antigen needed to trigger a response, until the ability to discriminate further affinity increases disappeared at affinities greater than ∼1010 M–1. Thus, affinity discrimination in this situation occurred over a range of ∼106–1010 M–1.
However, whereas our (Batista and Neuberger, 1998) and most other previous in vitro studies of BCR-mediated presentation have focused on soluble antigen, it is likely that the majority of antigens encountered in vivo are in an insoluble form. Not only may the antigen itself be particulate or cellular in nature (e.g. a microbe or virus), but it is probable that during maturation (and possibly initiation) of the response even to soluble antigens the antigen is encountered tethered to a cell surface as part of an immune complex (reviewed in Möller, 1980). In this work, we have compared the ability of B cells to present an antigen that has been encountered in soluble, particulate or immobilized forms. We find that not only can B cells internalize antigen encountered in either soluble or particulate form, but they can also extract antigen that is tightly bound to a non-internalizable surface. However, the relationship between presentation and antigen–BCR affinity differs depending upon the form in which the antigen is encountered, a finding that is probably of importance to our understanding of both the initiation and affinity maturation of the humoral immune response.
Results
The experimental system we have used is the presentation of hen egg lysozyme (HEL) by HEL-specific B–cell transfectants to T cell-specific hybridomas that recognize various HEL peptides in the context of MHC class II. The B–cell transfectants express one of two BCRs (D1.3 and HyHEL10; reviewed in Davies and Padlan, 1990) that bind distinct sites on HEL and exhibit a >100-fold difference in affinity (Table I). The HEL antigen itself was provided in three formats: as soluble monomer, displayed on beads or tethered to a plastic plate.
Table I. Affinities of mutant lysozymes.
| Lysozyme | Ka × 108 M–1 |
|---|---|
| Affinities for D1.3 | |
| HEL | 3 |
| V120 | 0.08 |
| Q121 | 0.03 |
| TEL | ∼0.007 |
| Affinities for HyHEL10 | |
| HEL | 500 |
| R21, D101 | 42 |
| R21, D101, G102, N103 | 5 |
The derivation of the HEL mutants and references for the affinity determinations for wild-type lysozymes are provided in Batista and Neuberger (1998). The mutations described that diminish D1.3 binding have little effect on the affinity for HyHEL10, HyHEL5 or F10; a similar result applies to the mutations designed to diminish HyHEL10 binding, except for the R21→A substitution, which causes a small reduction in affinity for D1.3 (Batista and Neuberger, 1998).
Presentation of particulate antigen
As a form of particulate antigen, HEL was bound onto 2.8 μm streptavidin-coated beads by use of a biotinylated anti-HEL monoclonal antibody (mAb) bridge. Incubation of these HEL-coated beads with transfectants of the LK35.2 B–cell lymphoma that expressed either the D1.3 or HyHEL10 HEL-specific BCR led to efficient antigen presentation as judged by interleukin 2 (IL-2) production from a co-cultured T–cell hybridoma (Figure 1A). It is likely that the bulk of the presentation is due to internalization of the beads by the B cells. Such uptake of particulate antigen by B cells can be observed under the microscope and has been described previously (Lombardi et al., 1987; Vidard et al., 1996). A major role for scavenging of spontaneously dissociated antigen is unlikely since presentation is diminished substantially if the mAb bridge and BCR recognize the same epitope on HEL (Figure 1A). Furthermore, presentation can be achieved readily even if the antigen is covalently conjugated to the bead (Figure 1B) and presentation correlates with proteolytic degradation of the HEL antigen and mAb bridge (Figure 2). A major role for extracellular protein degradation is also unlikely in view of the fact that the efficiency of presentation of HEL that is covalently linked to beads diminishes radically if the bead size is increased to 25 μm (data not shown), agreeing well with the results of Vidard et al. (1996) who observed a similar upper size limit to particle internalization by B–cell lines.
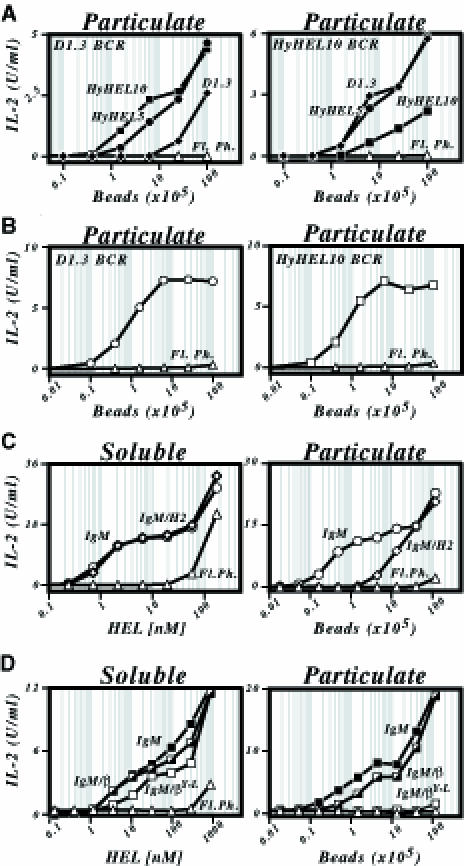
Fig. 1. Presentation of particulate antigen by B cells. (A) Presentation of HEL25–43/I-Ak by HEL-specific transfectants of the LK35.2 B–cell lymphoma that were incubated with HEL-coated beads was monitored by measuring IL-2 production from the co-cultured 2B6.3 T–cell hybridoma. The LK35.2 transfectants expressed either a D1.3 or a HyHEL10 BCR as indicated. HEL was bound onto streptavidin-coated beads via a biotinylated HyHEL10 (▪), HyHEL5 (•) or D1.3 (♦) mAb bridge. Untransfected LK35.2 B cells provided a control for presentation through fluid phase uptake (Fl. Ph., ▵) and give the same result as presentation by HEL-specific B–cell transfectants incubated in the absence of a bridging mAb. (B) Presentation of HEL108–116/I-Ad to the 1E5 T–cell hybridoma by A20[D1.3] (left-hand panel, ○) or A20[HyHEL10] (right-hand panel, □) B–cell transfectants that had been co-cultured with HEL covalently conjugated to tosyl-activated beads. Fluid phase presentation (▵). (C) Presentation of HEL1–18/I-Ek to the 2G7 T–cell hybridoma by transfectants of the LK35.2 B–cell lymphoma that express either the canonical D1.3 IgM HEL-specific BCR (○) or a D1.3 IgM/H2 chimera (⋄). Fluid phase presentation by untransfected LK35.2 cells (▵). Presentation was monitored using either soluble HEL (Soluble; left-hand panel) or biotinylated HEL bound to streptavidin-coated beads (Particulate; right-hand panel). (D) Presentation of HEL108–116/I-Ad to the 1E5 T–cell hybridoma by transfectants of the A20 B–cell lymphoma that express the canonical D1.3 IgM HEL-specific BCR (IgM; ▪), an IgM–β chimera (▪) or an IgM–β chimera carrying a Y→L mutation of the membrane-proximal cytoplasmic tyrosine (□). Fluid phase presentation by untransfected A20 cells (▵). Antigen was displayed in either soluble or particulate form as in (C).
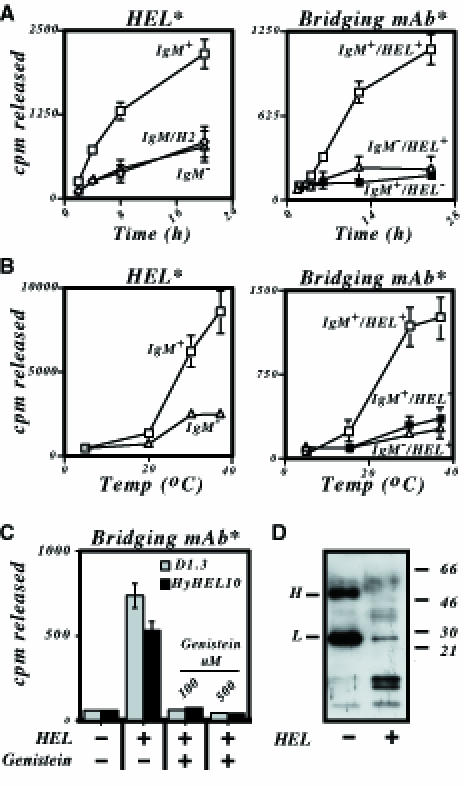
Fig. 2. Degradation of particulate antigen by B cells. (A) Degradation of antigen or bridging mAb as a function of time. Transfectants of LK35.2 expressing either a canonical HEL-specific IgM BCR (IgM+; □), a HEL-specific IgM–H2 chimeric receptor (IgM/H2, ⋄) or no transfected BCR (IgM–, ▵) were incubated at 37°C either with beads displaying [125I]HEL (left-hand panel) or with beads displaying non-radioactive HEL bound by an 125I-labelled bridging mAb (right-hand panel). Degradation of HEL or bridging mAb is presented as radioactivity released into the culture supernatant. In the right-hand panel, degradation of the bridging mAb was monitored in situations where it had (IgM+ HEL+; □ and ▵) or had not (IgM+ HEL–; ▪) been pre-loaded with soluble HEL. Both the [125I]HEL in the right-hand panel and the 125I-labelled bridging mAb (HyHEL5) in the right-hand panel were covalently conjugated to beads. (B) Degradation of antigen or bridging antibody as a function of temperature. The experiment is as described in (A) except that the incubation was performed for 24 h at various temperatures. (C) Sensitivity of degradation to genistein. LK[HyHEL10] or LK[D1.3] transfectants were incubated for 6 h at 37°C with tosyl-activated beads containing 35S-labelled covalently conjugated HEL-specific bridging mAb (HyHEL5) which had or had not been pre-loaded with soluble HEL. The incubation was performed in the absence or presence of genistein and the results are presented as radioactivity released into the culture supernatant. (D) Degradation of bridging antibody detected by a SDS–PAGE on a 20% polyacrylamide gel. LK[HyHEL10] transfectants were incubated for 24 h at 37°C with a biotinylated HEL-specific bridging mAb (F10) immobilized on the surface of streptavidin-coated beads that had or had not been pre-loaded with soluble HEL. At the end of the incubation, the anti-HEL mAb was boiled off the beads, subjected to SDS–PAGE and detected by Western blotting with peroxidase-conjugated streptavidin.
Particulate antigen requires a signalling-competent BCR
In previous work (Aluvihare et al., 1997), we noted that a signalling-incompetent BCR was well able to mediate the internalization and presentation of soluble monomeric HEL in a 24 h co-culture assay. A very different picture emerges with the particulate form of HEL. Here we see that an IgM–H2 chimeric receptor (which does not associate with the Ig-α/β sheath since the transmembrane and cytoplasmic domains of membrane IgM have been substituted by corresponding portions of MHC class I) is highly compromised in its ability to mediate presentation of HEL-coated beads whilst well able to mediate presentation of soluble HEL (Figure 1C). This reflects a need for functional immunoreceptor tyrosine-based activation motifs (ITAMs), since an IgM–β chimera (which is a derivative of IgM–H2 but with the cytoplasmic domain substituted by that of Ig-β) is active whereas an IgM–β with a mutated ITAM is ineffective (Figure 1D).
The fact that functional ITAMs are required for presentation of particulate but not soluble HEL is interpreted most readily by proposing that whereas constitutive endocytosis appears sufficient to deliver monomeric HEL for presentation (Aluvihare et al., 1997), the uptake of HEL-coated beads is essentially a phagocytic effect and depends upon functional ITAMs in the same way as, for example, uptake of immune complexes through FcγRIII (Daeron, 1997). If this interpretation is correct, one would expect that degradation of the particulate antigen should also be dependent on the BCR having functional ITAMs and that the process would be sensitive to tyrosine kinase inhibitors. This is indeed the case. Degradation of both HEL itself and of the antibody used to tether it to the bead is a time- and temperature-sensitive process that requires a functional BCR and which can be blocked by genistein (Figure 2).
Affinity-dependence of the presentation of particulate antigen
It is notable that the HyHEL10 BCR presents soluble HEL much more effectively than does the D1.3 BCR, whilst little discrimination between the two BCRs is evident when they are provided with HEL coupled to beads (Figure 3A). This probably reflects that presentation of soluble and particulate antigens has a differential dependence on antigen affinity. To confirm this, we compared presentation through the HyHEL10 BCR of wild-type HEL with that of a mutated HEL ([R21, D101, G102, N103]), which exhibits a 100-fold reduction in affinity for HyHEL10 (Table I). Wild-type HEL was far better presented than the lower affinity mutant when they were both encountered as soluble monomers, but no discrimination was evident when they were arrayed on the surface of a bead (Figure 3B).
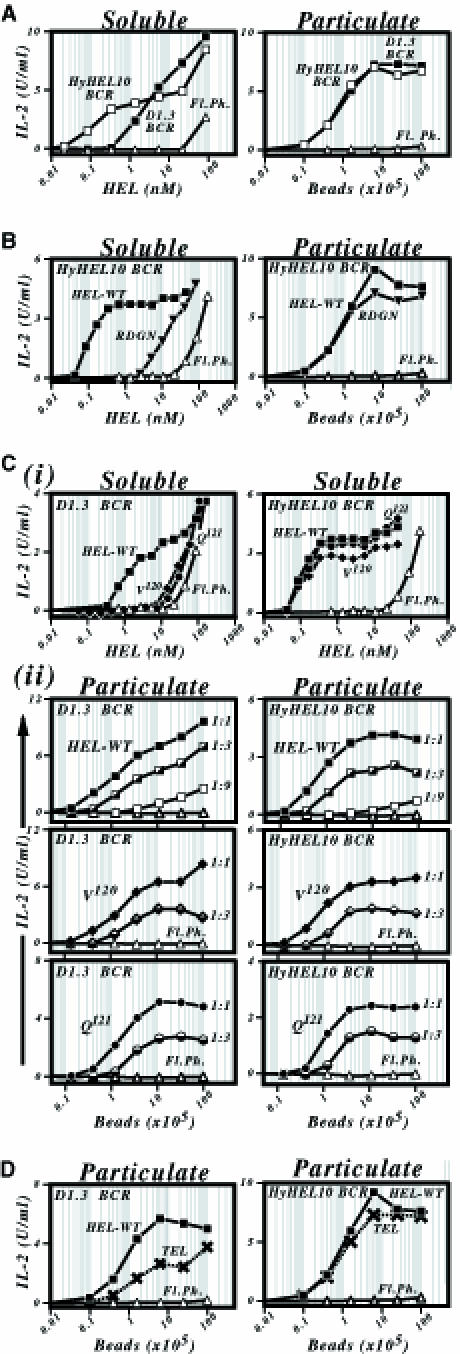
Fig. 3. Dependence of the presentation of particulate antigen on antigen–BCR affinity. (A) Comparison of presentation of HEL108–116/I-Ad by LK35.2 B–cell transfectants carrying a HyHEL10 (□) or D1.3 (▪) BCR that have been co-cultured with HEL in either soluble (left panel) or particulate forms (right panel; covalently conjugated to tosyl-activated beads). (B) Comparison of presentation of wild-type HEL (▪) and of a mutant (HEL[R21, D101, G102, N103] designated RDGN, ▾) that shows reduced affinity for the HyHEL10 BCR by LK[HyHEL10] transfectants when the antigen is encountered either in soluble form (left-hand panel) or covalently conjugated to tosyl-activated beads (right-hand panel). Presentation of wild-type HEL by untransfected LK35.2 cells is shown as a control (▵). (C) Comparison of presentation of wild-type HEL (▪) and of two mutants displaying reduced affinity for the D1.3 BCR (HEL[V120], ♦; HEL[Q121], •) by LK[D1.3] transfectants. (i) The top pair of panels shows that presentation of these mutants through the D1.3 BCR (but not through the HyHEL10 BCR) is much reduced when they are encountered in soluble form. (ii) The lower panels show presentation of these mutant lysozymes through the D1.3 BCR (left-hand panels) as well as through the HyHEL10 BCR (control, right-hand panels) when encountered arrayed on a bead. The lysozymes were arrayed at various densities on streptavidin-coated beads by use of a biotinylated HEL-specific mAb bridge that was established by incubating 107 streptavidin-coated beads in 1 ml of PBS/BSA/Tween with biotinylated F10 mAb at concentrations of 5 (filled symbols), 1.67 (half-filled symbols) or 0.56 (open symbols) μg/ml prior to loading with saturating amounts of HEL. Presentation in (i) was monitored using 2B6 T cells, and in (ii) using 1E5 cells. HEL[Q121] gives a slightly reduced amplitude of IL-2 production from 1E5 T cells with both D1.3 and HyHEL10 transfectants, possibly reflecting the proximity of the Q121 mutation to the T–cell epitope recognized; this same reduction is not evident when the presentation of several other T–cell epitopes is monitored. (D) Presentation of HEL108–116/I-Ad by LK35.2 B–cell transfectants incubated with turkey egg lysozyme (TEL; ×) or HEL (▪) that have been covalently conjugated directly onto tosyl-activated beads. Presentation by LK[D1.3], left-hand panel; by LK[HyHEL10], right-hand panel. TEL, when encountered in solution, does not yield a level of presentation through the D1.3 BCR above that attributable to fluid phase uptake (see Figure 5D).
Presumably this finding means that whereas when encountered in solution there is a ceiling to affinity discrimination at ∼1010 M–1 (Batista and Neuberger, 1998), the avidity increase effected by displaying the antigen arrayed on a bead has resulted in the ceiling being achieved at lower affinity values. At what point, then, is the affinity ceiling reached for particulate antigen? We used mutant HELs that have diminished affinities for the D1.3 BCR to ask whether affinity discrimination occurs in the low affinity range. Reducing the antigen–BCR affinity from 3 × 108 to 3 × 106 M–1 resulted in a substantial drop in presentation when the lysozymes were encountered in solution, but there was no clear discrimination between these antigens when they were displayed on a bead (Figure 3C). However, an experiment with turkey egg lysozyme (Ka in the order of 7 × 105 M–1) reveals that a fall off in presentation is seen when antigen affinity is reduced further (Figure 3D).
Although effective affinity discrimination in the >106 M–1 range was not even observed if the total amount of antigen was diminished by decreasing the number of HEL-coated beads in the culture, we wondered whether affinity discrimination could be obtained by diminishing the concentration of antigen on each bead. This does not appear to be the case. Antigen presentation is very dependent on the concentration of antigen on the bead and falls off rapidly at low antigen densities. However, affinity discimination in this situation is not enhanced evidently by decreasing antigen density (Figure 3C). The likely explanation for this observation is that a minimum density of antigen on the bead is needed to give the degree of BCR clustering required to trigger the phagocytosis; this density is high enough such that even a low affinity antigen will yield sufficient avidity when arrayed on the bead to give specific B–cell binding.
Thus, arraying the antigen on a bead allows efficient presentation of very low affinity antigens that bind BCR too weakly for specific presentation (above the background of fluid phase pinocytosis) to be achieved when encountered as a soluble monomer. However, arraying the antigen on the bead also means that there is little affinity discrimination at affinity values much greater than 106 M–1.
Presentation of immobilized antigen
The role of B–cell-mediated antigen presentation in affinity maturation is not fully defined. It is possible that affinity discrimination is effected solely by differential transmembrane signalling through the BCR, with presentation merely serving to ensure that the high affinity B cell selected in this way still displays a peptide epitope in its MHC that can recruit T–cell help. Alternatively, the increased ability of a high affinity B cell preferentially to scavenge and internalize the antigen for loading onto MHC class II could form part of the competitive process driving affinity maturation. If the latter proposal is correct, then the results with the HEL-conjugated beads suggest that particulate antigen or, for example, iccosomes (Szakal et al., 1988) are unlikely to be the form of antigen that drives affinity maturation. Since antigen is retained in the germinal centre bound to the surface of follicular dendritic cells via complement or Fc receptors (reviewed in Möller, 1980), we were interested in asking whether B cells were able to extract antigen immobilized on a surface.
Extraction of tightly tethered antigen
To investigate whether such extraction was possible and, if so, study the parameters governing it, we devised an assay in which the HEL antigen was displayed tethered to a non-internalizable surface, i.e. a plastic plate. The high affinity HyHEL10 BCR was well able to extract HEL antigen that had been tethered to the plate via the medium affinity D1.3 anti-HEL mAb (Figure 4A). This extraction could also occur if the extracting BCR was of relatively weak affinity and the tethering was strong. Thus, the D1.3 BCR could extract HEL tethered by HyHEL5 or HyHEL10 mAb, and both the HEL-specific BCRs could extract biotinylated lysozymes that had been tethered by the biotin–steptavidin interaction (Figure 4B and C), which has an affinity >1013 M–1 (Green, 1990).
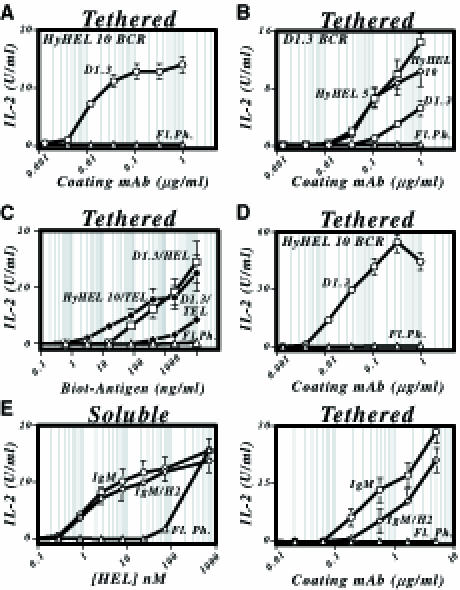
Fig. 4. Presentation by B cells of antigen immobilized on a plate. (A) Presentation of HEL108–116/I-Ad by LK[HyHEL10] B cells (○) that have been incubated on plates onto the surface of which HEL has been tethered through a D1.3 mAb bridge coated at various concentrations. Presentation by untransfected LK35.2 cells, ▵. (B) Presentation of HEL108–116/I-Ad by LK[D1.3] B cells that have been incubated on plates onto the surface of which HEL has been tethered through a HyHEL5 (□), HyHEL10 (⋄) or D1.3 (○) mAb bridge. (C) Presentation of HEL108–116/I-Ad by B–cell transfectants incubated on streptavidin-coated plates displaying biotinylated lysozyme. Presentation by LK[HyHEL10] transfectants incubated on plates displaying biotinylated HEL (•). Presentation by LK[D1.3] transfectants incubated on plates displaying biotinylated HEL (□) or TEL (♦). Plates were coated with streptavidin (20 μg/ml) in PBS and, after washing and blocking, incubated with biotinylated HEL/TEL at the indicated concentration. (D) Presentation of HEL108–116/I-Ad by LK[HyHEL10] B cells (○) that have been incubated on Reacti-Bind™ plates which display HEL tethered by covalently linked D1.3 mAb. (E) Presentation of HEL1–18/I-Ek by HEL-specific transfectants of LK35.2 that have been incubated with either soluble HEL (left-hand panel) or HEL tethered onto HyHEL5-coated plastic plates (right-hand panel). Presentation by LK[D1.3 canonical BCR], ○; by LK[D1.3 IgM–H2 chimeric BCR], ⋄; untransfected LK35.2 cells, ▵.
The extraction appears to take the antigen from the tethering antibody rather than simply pulling both the tethering antibody and antigen off the plastic plate, since the extraction occurs well even if the tethering antibody is covalently linked to the plate (Figure 4D), although little presentation is achieved if HEL is covalently conjugated to the plate directly (not shown). The extraction probably does not simply reflect scavenging of HEL that has dissociated spontaneously from the tethering moiety, since not only does extraction occur even when the half-life of the antigen–tether interaction is several days, but it is also notable that extraction is diminished substantially when the BCR and tethering antibody compete for the same epitope on the antigen (Figure 4B). We therefore envisage that extraction occurs when the tethered HEL is bound simultaneously by the BCR and the tethering antibody. Experiments with B–cell transfectants carrying chimeric BCRs reveal that, unlike when the antigen was in particulate form, a signalling-competent receptor is not essential for this extraction, although it does appear to confer some advantage (Figure 4E).
Affinity-dependence of antigen extraction
The extraction also shows great differences from the presentation of particulate antigen with respect to its dependence on antigen affinity. Analysis of the presentation of HEL mutants through the HyHEL10 BCR revealed that the extraction of tethered antigen was sensitive to antigen affinity even in the high (5 × 108 M–1–5 × 1010 M–1) affinity range (Figure 5A; Table I). Experiments using the D1.3 BCR revealed that this affinity discrimination also extends through to the low affinity range (Figure 5B). Furthermore, tethering the antigen on the plate allows specific presentation through the D1.3 BCR of an antigen (TEL) whose affinity (<106/M) is too low for specific presentation when encountered as soluble monomer (Figure 5B; Table I). Thus, tethering the antigen on the plate has lowered the threshold for specific antigen presentation whilst maintaining a wide window of affinity discrimination. It is also notable that the degree of affinity discrimination is often greater at lower concentrations of tethering antibody.
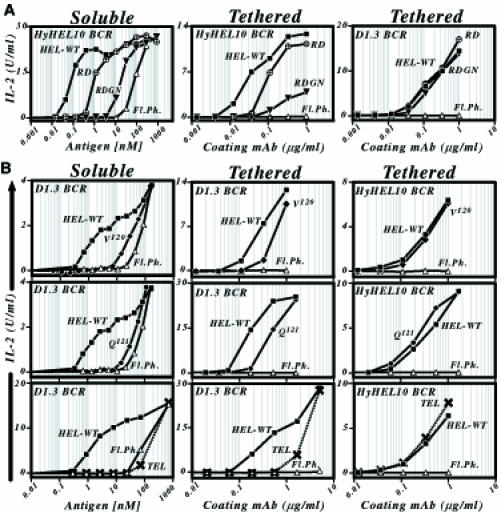
Fig. 5. Dependence of the presentation of plate-immobilized antigen on antigen–BCR affinity. (A) Presentation of lysozyme mutants showing a diminished affinity for HyHEL10. The left-hand panel shows presentation by LK[HyHEL10] B–cell transfectants of the antigens when encountered in solution; the middle and right-hand panels show presentation by LK[HyHEL10] or LK[D1.3] transfectants of the same antigens when displayed immobilized on a plate tethered by an anti-HEL mAb (D1.3 tether, middle panel; HyHEL5 tether, right-hand panel). Wild-type HEL, ▪; HEL[R21, D101], ○× (RD); HEL[R21, D101, G102, N103], ▾ (RDGN); untransfected LK35.2 control, ▵. (B) Presentation of lysozyme variants showing a diminished affinity for D1.3. The left-hand panel shows presentation by LK[D1.3] B–cell transfectants of the antigens when encountered in solution; the middle and right-hand panels show presentation by LK[D1.3] or LK[HyHEL10] B–cell transfectants of the same antigens when displayed tethered to the plate via HyHEL5 mAb. Wild-type HEL, ▪; HEL[V120], ♦; HEL[Q121], •; TEL, ×; untransfected LK35.2 control, ▵. Presentation was monitored using 2G7 T cells, except in the experiment investigating presentation of soluble HEL[V120], where 2B6 was used.
Discussion
B cells can internalize and present antigen that has been encountered in soluble form, as particles or when tethered to a non-internalizable surface. The dependence of the efficiency of presentation on antigen–BCR affinity differs for these three forms of antigen.
The ability of B cells to present particulate antigens has been noted by several groups, and convincing evidence has been put forward demonstrating that this presentation occurs by way of particle internalization (Malynn et al., 1985; Lombardi et al., 1987; Zhang et al., 1988; Vidard et al., 1996). However, uptake of particulate antigen has not been noted in all studies (Galelli et al., 1993) and its efficiency probably depends on the nature and size of the particle as well as on the nature and differentiation stage of the B cell analysed. In addition, it is clear that the presence of a signalling-competent antigen-specific BCR is needed to drive the efficiency of the process. Furthermore, our results show that the presentation of particulate antigen by B cells depends critically upon the density of antigenic epitopes on the particle, a feature that appears more important than the individual affinity of these epitopes.
The ability of B cells to extract and present antigen that has been tethered to a non-internalizable surface is, however, a novel finding. Whilst, as discussed in Results, we cannot exclude the possibility that extracellular proteolysis of the antigen by the B cell or the scavenging of antigen that has dissociated spontaneously from the tether contribute to antigen extraction, it is unlikely that these processes play a dominant role. Rather, the evidence points to the major role being played by BCR-mediated wrenching of the antigen from its tether. It is notable that a weak BCR apparently can wrench a tightly tethered antigen from the plate. Clearly the effect is cumulative: any antigen that is wrenched from its tether, even for a short time, can be internalized and processed. It may at first sight seem surprising that a low affinity BCR can extract an antigen that is tethered tightly to the plate in cases where the affinity difference is of several orders of magnitude (Figures 4 and 5). However, the BCR–antigen–tether interaction is not a static one. The BCR is part of the B–cell surface, and the large, motile nature of the cell may cause distortion of the antigen (and diminution of antigen–tether affinity) as a consequence of BCR binding. Indeed, recent experiments using dynamic force microscopy have revealed that the dissociation half-life of the biotin–streptavidin interaction can be reduced readily from several days to ∼1 ms if it is subjected to a force of 5 pN at a slow loading rate (Merkel et al., 1999). A simplified analysis ignoring buoyancy and other confounding effects suggests that a B cell restrained on a steep antigen-coated incline by a dozen BCR molecules will exert a force of this order on each of the antigen–BCR pairs simply by virtue of the cell's weight.
With regard to the affinity threshold, soluble monomeric antigen in the assay systems described here needs an affinity of greater than ∼7 × 105 M–1 if the BCR is to mediate presentation at a concentration of antigen lower than that needed for presentation by non-specific fluid phase pinocytosis (Batista and Neuberger, 1998). The results reveal that this threshold can be lowered substantially by arraying the antigen on the surface of a particle or of a plate. This presumably is due to the increased avidity of the antigen–BCR interaction, a similar effect being achievable by oligomerizing the antigen in solution by use of specific antibody (Batista and Neuberger, 1998). All these forms of antigen array will allow B cells to recognize low affinity antigens that would otherwise be below the detection threshold.
When the antigen is arrayed on a bead, the efficiency of presentation is critically dependent on the surface density of the antigen array, a similar density being required for both low- and high-affinity antigen. This observation is interpreted most reasonably by proposing that a minimum degree of BCR clustering is needed to trigger phagocytosis of the bead. As discussed above, the avidity increase effected by antigen array probably lowers the affinity needed for antigen uptake. A relatively low affinity might give a sufficient avidity and stability of bead–cell association to allow bead internalization and subsequent presentation. Presumably, once such a stability of interaction is achieved, little is to be gained from further reduction in the antigen–BCR dissociation rate. This presumably accounts for the low ceiling to affinity discrimination that we have observed for the presentation of antigen densely coated on a bead. A distinction between different beads, however, may well be effected if they differ in the density of antigen coating.
Arraying antigen on a non-internalizable surface, as with the beads, facilitates specific presentation of low affinity antigen. Presumably, the avidity of the arrayed antigen–BCR interaction drives close apposition of the surface of the B cell to the plate, thereby effecting a high local density of antigen. However, in contrast to what is observed with beads, the efficiency of extraction and presentation of antigen arrayed on a non-internalizable surface is sensitive to antigen–BCR affinity over a wide affinity range, plateauing at affinities >1010 M–1, similar to what is seen with monomeric soluble antigen. The reason for this distinction is probably that with a bead it is sufficient for the antigen–BCR interaction to bind the bead to the B cell: internalization and presentation will then result. However, interaction between the BCR and antigen tethered to a plate, whilst sufficient to form an intimate contact between the B cell and the plate, probably does not lead automatically to antigen extraction. The efficiency of the extraction will probably still depend on the quality of individual BCR–antigen interactions.
Whilst these studies on the affinity dependence of different forms of antigen presentation necessarily were performed in vitro, they have likely implications for our understanding of in vivo processes. For example, the analysis of presentation of HEL-conjugated beads suggests that, in vivo, even B cells bearing low affinity BCRs may be able to internalize and present viruses or microbes providing they have a sufficient density of epitopes on their surface. A high affinity B cell would show little competitive advantage over one with a medium affinity BCR in this regard. Affinity maturation is therefore unlikely to be driven by competitive BCR-mediated internalization of free virus/microbe (or vesicularized cell fragments such as iccosomes). Rather, our experiments with immobilized antigen raise the possibility that BCR-mediated extraction of antigen tethered to a cell surface via complement or Fc receptors could well play a role in the maturation of the response: affinity discrimination with tethered antigen is still evident in the high affinity range. Indeed, such discrimination was most evident when the density of tethered antigen was sparse, a situation that will probabaly pertain during the later stages of the immune response. Finally, whilst a role for presentation (as opposed to BCR-mediated signalling) in driving affinity maturation remains to be established, it is interesting to note that if such presentation works by way of the extraction of tethered antigen, then the process will be likely to select for linkage between B- and T–cell epitopes (a situation that would not obviously pertain when particulate or vesicularized antigen is phagocytosed). This could prove of benefit for the avoidance of autoimmunity.
Materials and methods
Cell lines
Mouse B–cell lymphomas A20 (IgG2a, κ; H2d) and LK35.2 (IgG2a, κ; H2kxd) are described in Kim et al. (1979) and Kappler et al. (1982), respectively. Transfectants of these lymphomas that express HEL-specific IgM BCRs or IgM–H2 chimeras with the VH and VL regions deriving from the D1.3 or HyHEL10 hybridomas have been described previously (Aluvihare et al., 1997; Batista and Neuberger, 1998). The HEL-specific IgM–β chimeras (with or without a Y→L mutation in the membrane-proximal cytoplasmic tyrosine) were assembled by replacing the NP-specific VH domains of the chimeras described in Patel and Neuberger (1993) with VH of D1.3, and were the gift of Petra Budde. Transfectants were established by electroporation, cloned by limiting dilution with expression of the transfected genes analysed by flow cytometry and cultured as described previously (Batista and Neuberger, 1998).
The HEL-specific mAbs D1.3, F10, HyHEL5 and HyHEL10 were obtained from hybridomas kindly provided by R.Poljak and S.J.Smith-Gill. The T–cell hybridomas 1E5.111, 2B6.3 and 2G7 (specific for HEL108–116/I-Ad, HEL25–43/I-Ak and HEL1–18/I-Ek, respectively; Adorini et al., 1993) were kindly provided by L.Adorini.
Antigens
HEL and TEL were purchased from Sigma and, if required, biotinylated using sulfo-NHS-LC-LC-biotin (Pierce). Mutant lysozymes were prepared using a plasmacytoma expression system as described previously (Batista and Neuberger, 1998). Lysozymes were bound onto streptavidin-coated beads by mixing 7 × 107 streptavidin Dynabeads™ (2.8 μm diameter; Dynal) in 1 ml of phosphate-buffered saline (PBS)/2% bovine serum albumin (BSA)/0.01% Tween with either saturating amounts of biotinylated HEL (50 μg) or with various concentrations of biotinylated anti-HEL mAb (in the range of 0.1–5 μg) followed by saturating lysozyme (>10 μg/ml) prior to extensive washing. For direct conjugation of HEL to beads, saturating amounts of HEL were covalently conjugated to tosyl-activated Dynabeads™ (4.5 μm diameter) according to the manufacturer's instructions.
For provision of antigen tethered to the surface of plastic plates, anti-HEL mAbs (1 μg in 100 μl of PBS for each well) or streptavidin (20 μg/ml) were either bound to MaxiSorp™ plates (Nunc) by overnight incubation at 4°C or the anti-HEL mAbs were covalently conjugated (1 h incubation at 37°C) to Reacti-Bind™ maleic anhydride-activated polystyrene plates (Pierce). Following blocking with PBS/2% BSA/Tween and extensive washing with PBS/Tween, the mAb or streptavidin tethers were loaded using saturating concentrations (0.5 μg/ml) of the desired lysozyme/biotinylated lysozyme.
Presentation assays
For analysis of antigen presentation, triplicate 24 h co-cultures were performed comprising 8 × 104 cells each of the relevant B–cell transfectant and T–cell hybridoma together with the designated amount of antigen in 300 μl of medium [RPMI/10% fetal bovine serum (FBS)/10 mM HEPES pH 7.4/50 μM 2-mercaptoethanol]. Antigen consisted of a wild-type or mutant lysozyme provided either in soluble form, coated on a bead or tethered to the surface of the plate in which the presentation assay was performed. In all cases, fluid phase presentation was monitored by performing parallel experiments using untransfected B cells. Presentation was assessed by measuring IL-2 production in the culture supernatant as described previously (Batista and Neuberger, 1998).
Monitoring proteolysis
[125I]HEL [generated by use of Iodobeads (Pierce)] was covalently conjugated to tosyl-activated beads (Dynal; 4.5 μm). [35S]HyHEL5 (prepared by biosynthetic labelling in medium containing l-[35S]methionine and subsequent immunoprecipitation) was covalently cross-linked by use of dimethyl pimelimidate dihydrochloride to rat anti-mouse IgG1-coated beads (Dynal; 4.5 μm) as described in the manufacturer's instructions and then loaded (or not) with saturating amounts of soluble HEL. The radioactive beads (105 beads; 10 000–50 000 c.p.m.) in duplicate samples were incubated in medium (1 ml of RPMI/10% FBS) with a 10-fold excess of B cells for the times indicated prior to centrifigation and measuring the radioactivity in the pellet and supernatants. Where desired, genistein (Sigma) was included in the culture as well as during a 1 h pre-incubation of the cells.
For SDS–PAGE analysis of proteolysis of the bridging mAb, biotinylated F10 anti-HEL mAb (0.1 μg) was bound onto 106 streptavidin-coated beads and loaded (or not) with soluble HEL. A total of 105 beads were incubated for 24 h with a 10-fold excess of B cells. After purification by use of a magnet, biotinylated mAb fragments were boiled off the beads using SDS loading buffer and detected using peroxidase-conjugated streptavidin and ECL after SDS–PAGE through a 20% gel and transfer onto a PVDF membrane.
Acknowledgments
Acknowledgements
We thank Petra Budde for generating one of the B–cell transfectants used in this work. This work was supported in part by an EMBO fellowship (to F.D.B.), an HHMI International Research Scholar's award (to M.S.N.) and a grant (B0637) from the Arthritis Research Campaign.
References
- Adorini L., Guery, J.C., Fuchs, S., Ortiz, N.V., Hammerling, G.J. and Momburg, F. (1993) Processing of endogenously synthesized hen egg-white lysozyme retained in the endoplasmic reticulum or in secretory form gives rise to a similar but not identical set of epitopes recognized by class II-restricted T cells. J. Immunol., 151, 3576–3586. [PubMed] [Google Scholar]
- Aluvihare V.R., Khamlichi, A.A., Williams, G.T., Adorini, L. and Neuberger, M.S. (1997) Acceleration of intracellular targeting of antigen by the B–cell antigen receptor: importance depends on the nature of the antigen–antibody interaction. EMBO J., 16, 3553–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista F.D and Neuberger, M.S. (1998) Affinity dependence of the B cell response to antigen: a threshold, a ceiling and the importance of off-rate. Immunity, 8, 751–759. [DOI] [PubMed] [Google Scholar]
- Daeron M. (1997) Fc receptor biology. Annu. Rev. Immunol., 15, 203–234. [DOI] [PubMed] [Google Scholar]
- Davies D.R. and Padlan, E.A. (1990) Antibody–antigen complexes. Annu. Rev. Biochem., 59, 439–473. [DOI] [PubMed] [Google Scholar]
- Galelli A., Charlot, B., Deriaud, E. and Leclerc, C. (1993) B cells do not present antigen covalently linked to microspheres. Immunology, 79, 69–76. [PMC free article] [PubMed] [Google Scholar]
- Green N.M. (1990) Avidin and streptavidin. Methods Enzymol., 184, 51–67. [DOI] [PubMed] [Google Scholar]
- Guermonprez P., England, P., Bedouelle, H. and Leclerc, C. (1998) The rate of dissociation between antibody and antigen determines the efficiency of antibody-mediated antigen presentation to T cells. J. Immunol., 161, 4542–4548. [PubMed] [Google Scholar]
- Kappler J., White, J., Wegmann, D., Mustain, E. and Marrack, P. (1982) Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc. Natl Acad. Sci. USA, 79, 3604–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.J., Kanellopoulos, L.C., Merwin, R.M., Sachs, D.H. and Asofsky, R. (1979) Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol., 122, 549–554. [PubMed] [Google Scholar]
- Kouskoff V., Famiglietti, S., Lacaud, G., Lang, P., Rider, J.E., Kay, B.K., Cambier, J.C. and Nemazee, D. (1998) Antigens varying in affinity for the B cell receptor induce differential B lymphocyte responses. J. Exp. Med., 188, 1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. (1985) Antigen-specific interaction between T and B cells. Nature, 314, 537–539. [DOI] [PubMed] [Google Scholar]
- Lombardi G., del Gallo, F., Vismara, D., Piccolella, E., de Martino, C., Garzelli, C., Puglisi, C. and Colizzi, V. (1987) Epstein–Barr virus-transformed B cells process and present Mycobacterium tuberculosis particulate antigens to T–cell clones. Cell. Immunol., 107, 281–292. [DOI] [PubMed] [Google Scholar]
- Malynn B.A., Romeo, D.T. and Wortis, H.H. (1985) Antigen-specific B cells efficiently present low doses of antigen for induction of T cell proliferation. J. Immunol., 135, 980–988. [PubMed] [Google Scholar]
- Merkel R., Nassoy, P., Leung, A., Ritchie, K. and Evans, E. (1999) Energy landscapes of receptor–ligand bonds explored with dynamic force spectroscopy. Nature, 397, 50–53. [DOI] [PubMed] [Google Scholar]
- Möller G. (1980) Accessory cells in the immune response. Immunol. Rev.. 53. [Google Scholar]
- Patel K.J. and Neuberger, M.S. (1993) Antigen presentation by the B cell antigen receptor is driven by the α/β sheath and occurs independently of its cytoplasmic tyrosines. Cell, 74, 939–946. [DOI] [PubMed] [Google Scholar]
- Reth M. and Wienands, J. (1997) Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol., 15, 453–479. [DOI] [PubMed] [Google Scholar]
- Rock K.L., Benacerraf, B. and Abbas, A.K. (1984) Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J. Exp. Med., 160, 1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakal A.K., Kosco, M.H. and Tew, J.G. (1988) A novel in vivo follicular dendritic–cell dependent iccosome-mediated mechanism for delivery of antigen to antigen-processing cells. J. Immunol., 140, 341–353. [PubMed] [Google Scholar]
- Vidard L., Kovacsovics-Bankowski, M., Kraeft, S.K., Chen, L.B., Benacerraf, B. and Rock, K.L. (1996) Analysis of MHC class II presentation of particulate antigens of B lymphocytes. J. Immunol., 156, 2809–2818. [PubMed] [Google Scholar]
- Zhang Y.P., Tzartos, S.J. and Wekerle, H. (1988) B–T lymphocyte interactions in experimental autoimmune myasthenia gravis: antigen presentation by rat/mouse hybridoma lines secreting monoclonal antibodies against the nicotinic acetylcholine receptor. Eur. J. Immunol., 18, 211–218. [DOI] [PubMed] [Google Scholar]


