Abstract
Burkholderia pseudomallei is the causative agent of melioidosis, a fatal infectious disease endemic in tropical regions worldwide, and especially prevalent in southeast Asia and northern Australia. This intracellular pathogen can escape from phagosomes into the host cytoplasm, where it replicates and infects adjacent cells. We previously demonstrated that, in response to B. pseudomallei infection of macrophage cell line RAW 264.7, a subset of bacteria co-localized with the autophagy marker protein, microtubule-associated protein light chain 3 (LC3), implicating autophagy in host cell defence against infection. Recent reports have suggested that LC3 can be recruited to both phagosomes and autophagosomes, thereby raising questions regarding the identity of the LC3-positive compartments in which invading bacteria reside and the mechanism of the autophagic response to B. pseudomallei infection. Electron microscopy analysis of infected cells demonstrated that the invading bacteria were either free in the cytosol, or sequestered in single-membrane phagosomes rather than double-membrane autophagosomes, suggesting that LC3 is recruited to B. pseudomallei-containing phagosomes. Partial or complete loss of function of type III secretion system cluster 3 (TTSS3) in mutants lacking the BopA (effector) or BipD (translocator) proteins respectively, resulted in delayed or no escape from phagosomes. Consistent with these observations, bopA and bipD mutants both showed a higher level of co-localization with LC3 and the lysosomal marker LAMP1, and impaired survival in RAW264.7 cells, suggesting enhanced killing in phagolysosomes. We conclude that LC3 recruitment to phagosomes stimulates killing of B. pseudomallei trapped in phagosomes. Furthermore, BopA plays an important role in efficient escape of B. pseudomallei from phagosomes.
Introduction
Burkholderia pseudomallei is a Gram-negative, soil dwelling bacillus. It is the causative agent of melioidosis, a fatal infection of many animal species and humans and is endemic in tropical and subtropical areas of the world [1], [2]. Melioidosis generally presents as a febrile illness ranging from acute pneumonia or septicemia to chronic abscesses; prolonged periods of latency have also been documented [3]. The overall mortality associated with melioidosis remains high; at approximately 40% in northeast Thailand and 20% in northern Australia [2], [4]. While some B. pseudomallei virulence factors have been identified including capsule, flagella, lipopolysaccharide (LPS), pili, quorum sensing molecules and the type III secretion system cluster 3 (TTSS3), our current understanding of B. pseudomallei pathogenesis remains incomplete (reviewed in [1], [5], [6]).
B. pseudomallei is an intracellular pathogen that can invade both phagocytic [7] and non-phagocytic cells [8]. Following internalization, bacteria can escape from the phagosome into the host cytoplasm in a process that is dependent on a functional TTSS3 [9]. Once in the cytoplasm bacteria can replicate and induce actin polymerization at one pole of the bacterium, facilitating intracellular motility [10]. This actin-based motility is considered to facilitate bacterial spreading into adjacent cells leading to the formation of multinucleated giant cells (MNGC), which have been observed both in cultured cell lines and the tissues of patients [11].
Autophagy is a cellular degradation system that eliminates unwanted molecules, damaged proteins and organelles from within the cell and it plays an important role in many physiological and pathological processes, including the cellular response to starvation, cell development and tumor suppression (reviewed in [12], [13]). Autophagy is also a component of innate immune defence, as it is involved in the clearance of a variety of pathogenic bacteria [14], [15], [16]. Autophagy is critical for the elimination of cytoplasmic Group A Streptococcus [17] and inhibits the intracellular survival of Mycobacterium tuberculosis [18]. However, some host-adapted intracellular pathogens including Shigella flexneri, Listeria monocytogenes and Salmonella enterica serovar Typhimurium, have developed means to evade killing by autophagy. The molecular strategies used by some pathogens to evade autophagy have been reported [19], [20], [21], [22]. Other pathogens can divert phagosome maturation towards the autophagy pathway, taking control of this host defence pathway to the benefit of the pathogen [23], [24]. Legionella pneumophia, Coxiella burnetii, Brucella abortus, Porphyromonas gingivalis, Staphylococcus aureus, and Anaplasma phagocytophilum each exploit modified autophagosomes as their intracellular niche [25], [26], [27], [28], [29].
We have shown that, in response to B. pseudomallei infection of macrophage cell line RAW 264.7, only a subset of bacteria co-localized with the autophagy marker protein LC3 [30]. When cells were treated with rapamycin, an inducer of autophagy, bacterial co-localization with LC3 was significantly increased and bacterial survival reduced. Thus, autophagy was implicated as part of the host defence system against B. pseudomallei infection, although the strategy by which most invading bacteria avoided host autophagic attack remained unclear. Moreover, we showed the involvement of the bacterial TTSS3-effector protein BopA in modulating the host cell response, as bopA mutant bacteria showed increased co-localization with LC3 and reduced intracellular survival [30].
A recent report showed that LC3 can be recruited directly to bacteria-containing phagosomes [31]; a process designated LC3-associated phagocytosis (LAP) [32]. In RAW 264.7 cells infected with Escherichia coli, LAP was induced by lipopolysaccharide (LPS) via toll-like receptors (TLR), and involved the rapid translocation of the autophagic proteins Beclin1 and LC3 to the bacteria-containing phagosomes, leading to an increased level of phagocytosis and bacterial killing [31]. Such reports led us to assess the nature of the compartment in which intracellular B. pseudomallei is sequestered. Here we demonstrate, through analysis of electron microscopic (EM) images of infected cells, that intracellular B. pseudomallei bacteria are free in the cytosol or sequestered in single-membrane phagosomes, but rarely in double-membrane autophagosomes, suggesting that LC3 is recruited to B. pseudomallei-containing phagosomes. Furthermore analysis of a mutant lacking a functional TTSS3 showed that when bacteria are unable to escape from the phagosome they become strongly co-localised with LC3, confirming that LC3 is recruited to B. pseudomallei-containing phagosomes. A high percentage of LC3 positive phagosomes become LAMP1 positive, implicating LC3 recruitment in efficient phagosome maturation. Importantly, we also show that efficient escape of B. pseudomallei from phagosomes requires the presence of the predicted TTSS3 effector protein BopA. Finally, as we were unable to observe more than a single bacterium within a double membrane vesicle, we conclude that B. pseudomallei can efficiently avoid engulfment by canonical autophagosomes. Taken together these data show that LC3 recruitment to B. pseudomallei-containing phagosomes plays a role in destruction of bacteria trapped in phagosomes, but that most bacteria can escape from the phagosome and once free in the cytoplasm are rarely targeted by autophagosomes.
Materials and Methods
Bacterial strains and cell culture
B. pseudomallei wild-type strain K96243 [33] and mutants were cultured in Luria–Bertani (LB) broth at 37°C. E. coli strain SM10λpir was used as a conjugative donor of the λpir-dependent suicide replicon pDM4 (ori R6K, mob RP4, sacBR, cat) and its derivatives [34]. The RAW 264.7 cell line stably expressing GFP-LC3 was constructed as described [30]. Cells were maintained at 37°C in 5% CO2 without antibiotics in RPMI 1640 medium (Gibco Laboratories), supplemented with 10% (v/v) heat-inactivated fetal bovine serum (JRH Biosciences). Bacteria were heat-killed at 98°C for 30 min. The rat anti-LAMP1 antibody was obtained from the Development Studies Hybridoma Bank developed by J.T. August under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA. All other chemicals were purchased from Sigma unless otherwise indicated.
Mutagenesis of TTSS3 genes
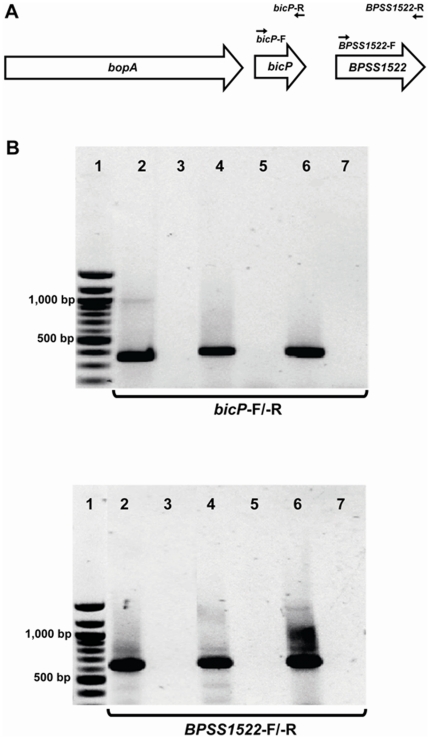
The B. pseudomallei bopA deletion mutant has been described previously [30]. This mutant is unmarked and contains an in-frame deletion within bopA. Although we were unable to complement this mutant, we have subsequently used RT-PCR to analyse the transcription of the bicP and bpss1522 genes, which are downstream of bopA. Primer pairs (bicP-F, 5′-AACGTGTCGATCAGGCTTTC-3′ and bicP-R, 5′-ACGCACACCGAATGGTTGAA-3′) and (BPSS1522-F, 5′-GGCGCGCACGCGTTCGCATA-3′ and BPSS1522-R, 5′- GGGTGCTCGTCGTCGACAGC-3′) were used to amplify bicP and bpss1522 RT-PCR products respectively. RT-PCR products of the expected size, 368 bp (bicP) and 637 bp (bpss1522) were generated from cDNA derived from both the wild-type and bopA mutant strains (Figure 1), indicating that the deletion mutation within bopA does not disrupt the transcription of these genes.
Figure 1. Reverse transcription (RT)-PCR of bicP and bpss1522 in wild-type and bopA mutant B. pseudomallei.
RNA extracted from the wild-type K96243 or bopA deletion mutant strain was reverse-transcribed to obtain cDNA. Each cDNA preparation was used as a template for RT-PCR. (A) Schematic diagram showing the position of primers used for RT-PCR. (B) Electrophoretic separation of RT-PCR products amplified with primers for bicP (top panel) and bpss1522 (lower panel). These products were generated from reactions: (2) bopA mutant cDNA; (3) bopA mutant cDNA, no RT control; (4) wild-type cDNA, (5) wild-type cDNA, no RT control; (6) wild-type genomic DNA control; and (7) No DNA control. DNA size markers (bp) are shown in lane 1.
A B. pseudomallei bapA mutant was constructed by double-crossover allelic exchange using the λpir-dependent vector pDM4 which carries the negative-selectable marker sacB [34]. PCR primer pairs (5′-GGGCCCACTAGTCCGATCCGAAGCAACCGACAAGA-3′ and 5′-GGGCCCAGATCTACCATGTCGACGAGATTCGTC-3′; 5′-GGGCCCAGATCTCTTTATCCGCTCGTCGACGATGCTT-3′ and 5′-GGGCCCTCTAGATTGGCGTATTGGCGTATTGGCGTA-3′) were used to amplify upstream and downstream sequences flanking the bapA open reading frame, respectively. A 918-bp fragment spanning the 5′ section of bapA and upstream DNA was cloned into SpeI/BglII digested pBluescript (Stratagene) then a 1,241 bp downstream fragment spanning the 3′ section of bapA and downstream DNA was cloned into the BglII/XbaI sites. The tetracycline resistance cassette tetA(C) was digested from the plasmid pUTminiTn5 [35] and ligated into the BglII site between the two cloned bapA fragments, generating a plasmid containing a 1,565 bp internal deletion in bapA. The mutagenesis construct was then transferred to pDM4. This pDM4 derivative was introduced into B. pseudomallei by conjugation and transconjugants selected on plates containing tetracycline (25 µg/ml). Colonies were then screened for chloramphenicol sensitivity in the presence of sucrose.
A bipD mutant was constructed by single crossover insertional mutagenesis. PCR primers (5′-GGGCCCACTAGTAACCTGCTCGAGCGCCTGGAAA-3′ and 5′-GGGCCCTCTAGAGCCGCCGTCGATCTTCATGT-3′) were used to amplify a 261 bp internal fragment from within the target open reading frame. This fragment was digested with the appropriate enzymes, ligated to SpeI/XbaI-digested pDM4 and introduced into B. pseudomallei as above. Single crossover mutants were selected on LB plates containing chloramphenicol (50 µg/ml). Each of the mutants was verified by PCR and sequence analysis.
Bacterial replication assays
To determine the ability of B. pseudomallei strains to replicate intracellularly, RAW 264.7 cells stably expressing green fluorescent protein-LC3 (GFP-LC3) were infected with B. pseudomallei K96243 as previously described [30]. Briefly, RAW 264.7 cells (seeded at 1.0×105 cells/well) in 24-well trays (BD Biosciences) were infected with B. pseudomallei at a multiplicity of infection (MOI) of 10∶1, and incubated at 37°C for 1 h to allow bacterial invasion. Infected cells were washed 3 times with phosphate buffered saline pH 7.2 (PBS) and then inoculated into fresh medium supplemented with kanamycin (800 µg/ml) and ceftazadime (800 µg/ml) to kill extracellular bacteria. At 2, 4 and 6 h after addition of bacteria, cells were washed 4 times with PBS and intracellular bacteria were released by addition of 0.1% Triton X-100. For each time point, cell lysates were prepared from cells grown in 3 separate wells. Serial dilutions of each lysate were plated onto LB agar and the numbers of intracellular bacteria (expressed as colony forming units (CFU)), were enumerated by direct colony counts after 48 h. Bacterial survival was normalised to counts obtained at 2 h post-infection (p.i.), the time point at which extracellular bacteria were killed with antibiotics, and data presented as relative survival (%).
Immunofluorescence and confocal microscopy
To determine co-localization with GFP-LC3, cells were cultured in 24-well trays (BD Biosciences) containing 13-mm-diameter glass coverslips (ProScitech). At the indicated time points p.i., the cells were fixed with methanol for 10 min and blocked for 1 h in PBS containing 1% (w/v) bovine serum albumin (BSA) and 0.1% (v/v) Triton X-100. Coverslips were incubated with rabbit anti-B. pseudomallei outer membrane serum [30] at a 1∶100 dilution. The fluorescently labeled secondary antibody Alexa Fluor 405-conjugated goat anti-rabbit IgG serum (Molecular Probes) was used at a 1∶250 dilution. LAMP1 was labeled with rat anti-LAMP1 antibody (Development Studies Hybridoma Bank, University of Iowa, IA) at a 1∶100 dilution followed by Alexa Fluor 568-conjugated goat anti-rat IgG serum (Molecular Probes) at a 1∶250 dilution. Stained cells were washed with PBS, coverslips mounted in Permafluor aqueous mounting medium (Immunotech) and visualised using a confocal laser scanning microscope (CLSM; Olympus FV500) equipped with a 1.2 NA water immersion lens (Olympus 60X UPlanapo). Image analysis and processing was performed using Olympus FluoView TIEMPO software version 4.3 and the public domain software Image-J version 1.41a (http://rsb.info.nih.gov/ij). The intracellular location of bacteria was assessed using Z-stack CLSM analysis. For actin staining, RAW 264.7 cells, cultured on coverslips at the indicated time points p.i., were fixed in 3.5% (w/v) para-formaldehyde (PFA) and 0.1% (v/v) Triton X-100 consecutively for 10 min prior to staining as described above. Stained cells were counter stained with Alexa-Flour 488 conjugated phalloidin (Molecular Probes) at a 1∶500 dilution for 50 min to visualize cellular actin filaments.
Transmission electron microscopy
For standard transmission electron microscopy, infected (at a MOI of 10 or 100) and uninfected RAW 264.7 cells were fixed for 2 h at room temperature with 2.5% (w/v) glutaraldehyde (EM Sciences) in 0.1 M cacodylate buffer, pH 7.2 at the indicated time points. Cells were collected, washed twice and postfixed for 1 h at room temperature with 1% (w/v) osmium tetroxide and then subsequently incubated with 2% (w/v) uranyl acetate for 1 h. After dehydration and embedding in Epon resin (EM Sciences), ultra-thin 70 nm sections were cut and stained with lead citrate and uranyl acetate. Sections were viewed at 80 kV using a Hitachi H-7500 transmission electron microscope fitted with a Gatan Multiscan 791 CCD camera.
Statistical analyses
For quantification studies, at least 100 bacteria were counted for each condition in each experiment, unless otherwise indicated. Values were expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using GraphPad Prism software version 5.00 (http://www.graphpad.com). Differences between groups were analyzed by 2-tailed, 2-sample, unequal variance Student's t test or ANOVA analysis with Dunn post-hoc test where appropriate. A p value of <0.05 was considered to be statistically significant.
Results
Intracellular B. pseudomallei are localized free in the cytosol or in phagosomes
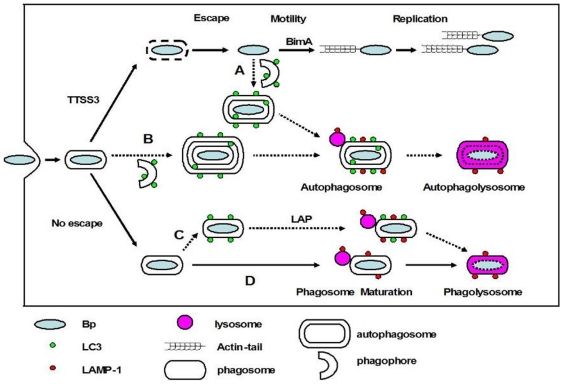
Only a small subset of intracellular B. pseudomallei co-localizes with the autophagy marker protein LC3 in infected RAW 264.7 macrophage cells, although this co-localization increases when cells are treated with the autophagy inducer rapamycin [30]. However, intracellular pathogens may be subject to LC3-dependent host autophagic processes by three distinct pathways as depicted in Figure 2: A) autophagosomes may directly sequester bacteria free in the cytosol [21], [36]; B) autophagosomes may engulf bacteria-containing phagosomes [18]; C) LC3 may be recruited directly to bacteria-containing phagosomes thus stimulating phagosome maturation [31]. The operation of each of these pathways could give rise to co-localization of bacteria with LC3. To investigate which of these pathways may be responsible for co-localization of B. pseudomallei with LC3 in infected RAW 264.7 cells, we used transmission electron microscopy (TEM) to view sections prepared from cells infected with wild-type bacteria (at MOI of 100) (Figure 3).
Figure 2. Possible fates of B. pseudomallei in infected macrophages.
Following phagocytic uptake by macrophages, bacteria are first located within phagosomes. The majority of wild-type B. pseudomallei (Bp) can escape from the phagosome into the cytosol in a process which is largely uncharacterized but involves the TTSS3. Once free in the cytosol bacteria activate BimA-mediated actin-based motility, replicate and invade adjacent cells via membrane protrusions. Potentially some cytosolic bacteria may be sequestered in canonical autophagosomes (pathway A). Some bacteria which remain in phagosomes might be sequestered by double-membrane autophagosomes (pathway B). The autophagy marker protein LC3 can be recruited to bacteria-containing phagosomes, a process designated LC3-associated phagocytosis (LAP) which stimulates further phagosomal maturation via recruitment of other proteins including LAMP1, a late endosome/lysosome marker, and the subsequent fusion of phagosomes with lysosomes, leading to bacterial killing (pathway C). Finally phagosomes may mature to phagolysosomes without LC3 recruitment (pathway D).
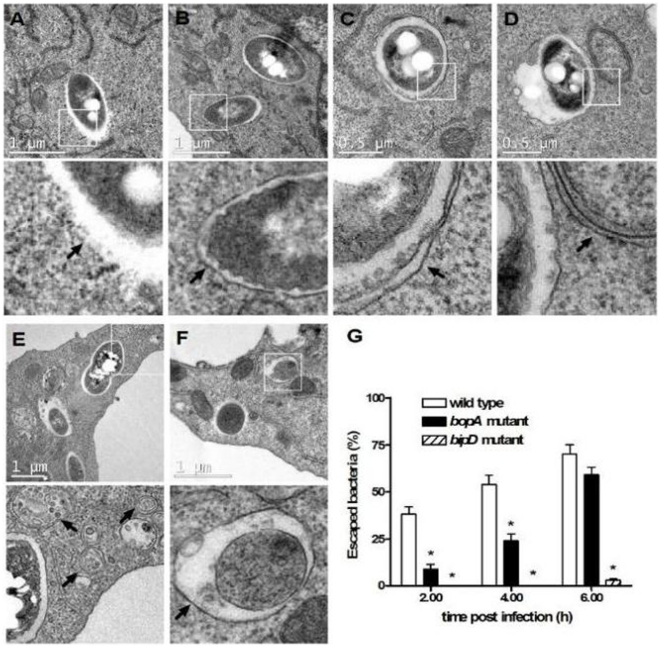
Figure 3. B. pseudomallei-containing vacuoles are bound by single membranes and the TTSS3 and the effector BopA are required for bacterial escape.
(A–F) Transmission electron micrographs show the intracellular location of B. pseudomallei in RAW 264.7 cells at 2–6 h post infection (p.i.). The scale bar is indicated. Boxed areas are shown as magnified images below each panel. Intracellular bacteria were observed either free in the cytosol and not membrane bound (panel A), or within single-membrane phagosomal compartments (panel B). Only one bacterium was found in a double-membrane compartment (panel C), which could be an autophagosome. Canonical autophagosomes having a double-membrane were observed in infected and uninfected RAW 264.7 cells (panels D–F). Arrows indicate detailed membrane ultrastructure. (G) The percentage of bacteria free in the cytosol of RAW 264.7 cells following infection with B. pseudomallei wild-type, bopA mutant and the bipD mutant at 2, 4, and 6 h p.i. Data represent the mean ± SEM of three separate experiments (n = 100 bacteria). Where shown * indicates p<0.05 relative to the wild-type strain at each time point.
Cell sections were scored for the presence of bacteria and whether the bacteria were within single-membrane compartments (phagosomes), double- or multi- membrane compartments (autophagosomes) or unbounded by any detectable membranes (free in the cytosol). These analyses showed that B. pseudomallei was primarily present either free in the cytoplasm (Figure 3A), or within single-membrane phagosomes (Figure 3B). For wild-type B. pseudomallei the proportion of bacteria free in the cytosol was 39%, 54% and 71% at 2, 4 and 6 h p.i. respectively (Figure 3G), while the proportion of bacteria within phagosomes was 61%, 46% and 29% at 2, 4 and 6 h p.i. respectively. Of the 500 bacteria identified and scored in TEM sections, only a single bacterium was observed within a double-membrane compartment that could be considered to be an autophagosome (Figure 3C). Importantly, the number of canonical double-membrane structures representing different stages of autophagosome maturation (phagophore, autophagosome, amphisome) increased in infected RAW 264.7 cells (Figure 3D–F), confirming our previous observation that autophagy is induced in response to B. pseudomallei infection [30]. However, these autophagosome structures did not contain bacteria. Therefore, B. pseudomallei is not efficiently targeted by canonical double-membrane autophagosomes via the pathways A or B as shown in Figure 2. However, as we have previously shown that approximately 5–10% of wild-type B. pseudomallei are associated with LC3 at 2 h p.i. [30], these results suggest that LC3 may be recruited to B. pseudomallei-containing phagosomes (as depicted in pathway C of Figure 2). Qualitatively identical data were obtained by TEM analysis of RAW 264.7 cells infected with wild-type B. pseudomallei at MOI of 10.
The putative TTSS3 effector BopA is required for efficient escape of B. pseudomallei from phagosomes
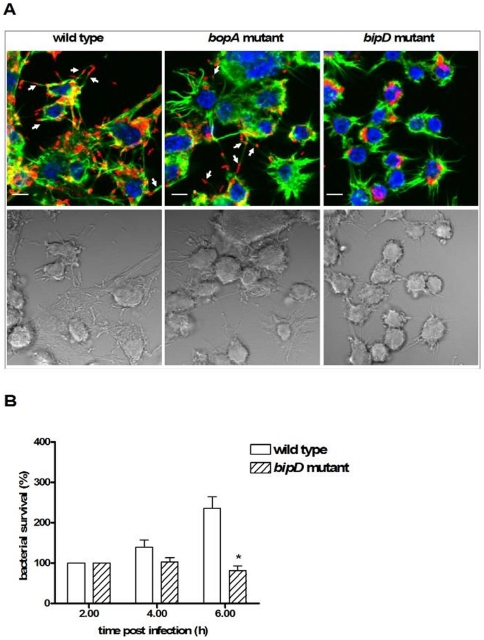
We next used TEM to analyse the intracellular location of the B. pseudomallei TTSS3 bipD and bopA mutants in RAW 264.7 macrophage cells. BipD is a component of the needle tip complex of the B. pseudomallei TTSS3 apparatus [37]; a bipD mutant is unable to escape from the phagosomes of macrophage cells up to 6 h p.i. [9]. BopA is a predicted secreted TTSS3 effector protein [38]. As expected the bipD mutant was observed only in single membrane phagosomes at 2 and 4 h p.i. and even at 6 h p.i. 98% of bacteria were still localised in phagosomes while the remaining 2% of bacteria were observed free in the cytoplasm. Furthermore, fluorescence microscopy of infected cells labelled with Alexa Fluor 488-conjugated phalloidin showed that the bipD mutant did not form actin tails or MNGC (Figure 4A), consistent with the earlier observations of Stevens et al. [9], These data confirm a critical role of the TTSS3 in phagosome escape. The bopA mutant also showed significantly reduced phagosome escape compared to the wild-type strain with 9% of the bopA mutant cells observed free in the cytosol at 2 h p.i., compared to 39% for the wild-type strain. Furthermore, the bopA mutant also showed reduced escape at 4 h p.i. (24% compared to 54% for the wild-type strain) and 6 h p.i. (59% compared to 71% for the wild-type strain). Fluorescence microscopy analysis indicated that the bopA mutant could form actin tails when free in the cytoplasm and caused MNGC formation in RAW 264.7 cells (Figure 4A), but at later time points compared to wild-type cells (data not shown). Therefore, the predicted TTSS3 effector protein BopA is important for efficient escape from phagosomes.
Figure 4. B. pseudomallei-associated actin-tails, host cell membrane protrusions and MNGC formation require TTSS3 translocator BipD, but not effector BopA.
(A) Representative confocal micrographs with DIC images of RAW 264.7 cells infected with the wild-type strain, the bopA mutant, or the bipD mutant at 6 h p.i. Bacteria were stained red, filamentous actin was stained green and nuclei were stained blue. Bacteria with associated actin-tails are marked with arrows. Scale bar = 5 µm. (B) Intracellular survival of B. pseudomallei wild-type and bipD mutant in RAW 264.7 cells at 2, 4, and 6 h p.i. Bacterial survival was normalized to CFU counts obtained at 2 h p.i. and presented as relative survival (%). Data represent the mean ± SEM of three separate experiments, each carried out in triplicate. Where shown * indicates p<0.05 relative to the wild-type strain at each time point.
B. pseudomallei mutants defective in phagosome escape show increased LC3 co-localization
A B. pseudomallei bopA mutant displays increased co-localization with LC3 (Figure 5C and [30]). Our TEM data presented here indicate that wild-type B. pseudomallei and the bopA and bipD mutants are very rarely found within double membrane autophagosomes and that the bopA mutant displays reduced phagosome escape. Taken together these observations support the proposal that B. pseudomallei co-localization with LC3 occurs as a result of recruitment of LC3 to phagosomes that contain bacteria. To further explore this proposal we analysed the co-localization of the B. pseudomallei bipD mutant with LC3. Fluorescence microscopy analysis showed that the bipD mutant displayed a high level of co-localization with LC3 at 2 h, 4 h and 6 h p.i. (Figure 5C). As shown above, the bipD mutant displayed no escape from phagosomes at 2 h and 4 h p.i (Figure 3G). Therefore, this association with LC3 at these time points must result from LC3 recruitment to phagosomes. To investigate whether LC3 recruitment to phagosomes was affected by other TTSS3 effectors, we utilised a mutant defective in another putative effector, BapA, constructed for another study. The bapA deletion mutant did not show increased co-localization with LC3 and its ability to escape from phagosomes was indistinguishable from that of wild-type bacteria (data not shown). Collectively these data strongly suggest that all B. pseudomallei co-localization with LC3 results from LC3 recruitment to B. pseudomallei-containing phagosomes and not to engulfment of cytosolic bacteria by autophagosomes. Thus, the previously reported increased co-localization of a B. pseudomallei bopA mutant with LC3 [30] results from the reduced ability of this mutant to escape from phagosomes.
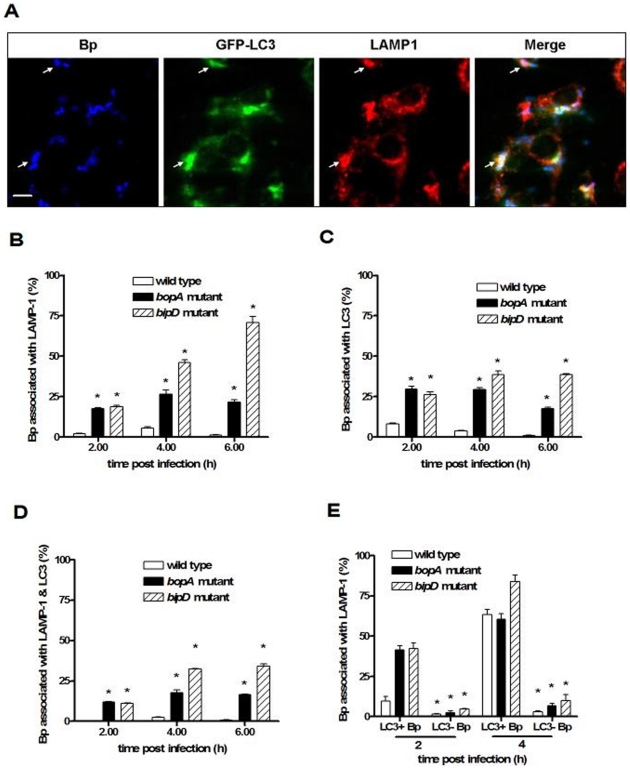
Figure 5. B. pseudomallei with defective TTSS3 show enhanced co-localization with autophagy marker protein LC3 and the lysosome marker LAMP1.
(A) Confocal images of RAW 264.7 cells expressing GFP-LC3 (green) and infected with B. pseudomallei (Bp). Cells were fixed at 2 h p.i., permeabilized, and stained for B. pseudomallei (blue) and LAMP1 (red). Arrows indicate bacteria associated with LC3 that are also within LAMP1-positive vacuoles. Bacterial co-localization with LC3 or LAMP1 was defined by the presence of labelled B. pseudomallei (blue) which were fully overlaid by intense green/red or fully contained within a green/red ring. Scale bar = 5 µm. (B–D) Quantitative analysis of bacterial co-localization with LC3 (B), LAMP1 (C) or both LC3 and LAMP1 (D) in RAW 264.7 cells infected with B. pseudomallei wild-type, bopA mutant or bipD mutant at 2, 4, and 6 h p.i. (E) The percentage of LC3-positive (LC3+ Bp) or LC3-negative bacteria (LC3− Bp) of the wild-type, bopA mutant or bipD mutant at 2 or 4 h p.i. that co-localized with LAMP1. Data represent the mean ± SEM of three separate experiments (n = 100 bacteria). Where shown * indicates p<0.05 relative to wild-type strain at each time point.
LC3 recruitment stimulates phagosome maturation
In the process of LC3-associated phagocytosis (LAP), recruitment of LC3 to the phagosome was found to lead to rapid acidification of the compartment and enhanced killing of ingested E. coli in RAW 264.7 cells [31], [32]. In an earlier study we demonstrated decreased intracellular survival of the bopA mutant [30]. In this study the bipD mutant also showed reduced intracellular survival at 6 h p.i. (Figure 4B). Furthermore, B. pseudomallei mutants lacking either BipD or BopA display reduced virulence in a mouse melioidosis model [38]. To test the hypothesis that decreased intracellular survival of these mutant bacteria is associated with LAP, we investigated the co-localization of B. pseudomallei with LC3 and the lysosome marker lysosomal-associated membrane glycoprotein-1 (LAMP1) in RAW 264.7 cells (Figure 5). LAMP1 is an abundant lysosomal membrane protein that is both delivered to phagosomes during their maturation and required for fusion of lysosomes with phagosomes [39] and autophagosomes [40]. Both the bopA and bipD mutants showed increased levels of co-localization with LAMP1 (Figure 5B) and LC3 (Figure 5C) compared to the wild-type strain at all time points. At 4 h and 6 h p.i. the bipD mutant showed higher levels of co-localization with each marker protein than did the bopA mutant. We also determined dual co-localization of bacteria with LC3 and LAMP1 (Figure 5D), as a measure of the maturation of bacteria-containing LC3 phagosomes by fusion with lysosomes. The percentage of bacteria co-localized with both LC3 and LAMP1 was also significantly higher for both the bopA and bipD mutants (Figure 5D). Importantly, for the wild-type and mutant strains a high percentage of LC3-positive bacteria-containing phagosomes was also LAMP1 positive (Figure 5E), suggesting that LC3 recruitment is associated with enhanced levels of phagolysosome maturation. Consistent with this proposal is the observation that only a very low percentage of LC3-negative bacteria-containing phagosomes are LAMP1 positive but more than 60% of LC3-positive bacteria-containing phagosomes at 4 h p.i. were LAMP1 positive. These data are consistent with the hypothesis that LC3 recruitment to B. pseudomallei-containing phagosomes stimulates fusion of phagosomes with lysosomes, leading to enhanced killing of the bacterial contents.
The recruitment of LC3 to phagosomes is diminished in RAW 264.7 cells which have taken up heat-killed B. pseudomallei
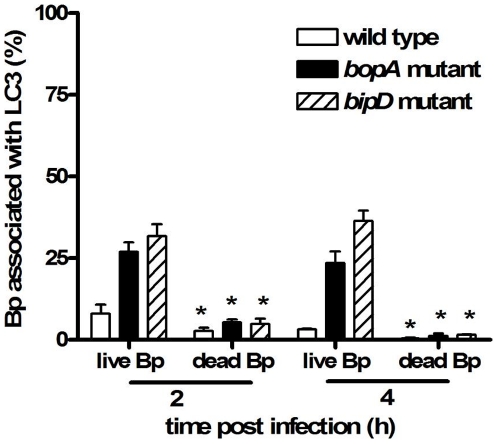
In order to determine whether LC3 recruitment to phagosomes always occurs when B. pseudomallei is present within phagosomes, we analysed the co-localization of LC3 with heat-killed bacteria taken up by RAW 264.7 cells. In these experiments only a very small number of internalised bacteria were observed to co-localize with LC3 (Figure 6). Therefore, retention of bacteria within phagosomes is not sufficient for LAP to occur. A low level of co-localization with LC3 was observed for all three heat-killed strains tested: wild-type, bopA mutant and bipD mutant. Similar results were observed in cells infected with B. pseudomallei which had been killed by treatment with PFA or methanol (data not shown). These data suggest that LAP requires the presence of specific bacterial factor(s) produced by viable B. pseudomallei.
Figure 6. The recruitment of LC3 to bacteria-containing phagosomes is diminished in RAW 264.7 cells infected with heat-killed B. pseudomallei.
Quantitative analysis of bacterial co-localization with LC3 in RAW 264.7 cells infected with live B. pseudomallei (wild-type strain, the bopA mutant or the bipD mutant) or heat-killed strains at 2 and 4 h p.i. The data represent the mean ± SEM of three separate experiments (n = 100 bacteria). Where shown * indicates p<0.05 relative to the wild-type strain at each time point.
Discussion
LC3-associated phagocytosis (LAP)
TEM analysis of infected RAW 264.7 macrophage cells revealed that invading B. pseudomallei bacteria are found either free in the cytosol or sequestered in single-membrane phagosomes (Figure 3). Of 500 bacteria identified and scored in TEM sections only one bacterium was observed within a double-membrane structure likely to be a canonical autophagosome. As we have shown in a previous study that B. pseudomallei can be observed co-localized with the autophagosome marker LC3 these data suggest that LC3 is recruited to B. pseudomallei-containing phagosomes and that cytoplasmic bacteria are very rarely engulfed by autophagosomes. Moreover, B. pseudomallei-containing phagosomes are apparently not targeted by autophagy (Figure 2, pathway B) as no triple membrane structures were observed. These data were supported by analysis of the B. pseudomallei bipD mutant which is unable to escape from the phagosome but which showed high levels of co-localization with LC3. Although conventional autophagosomes appear to be rarely involved in clearance of B. pseudomallei, LC3-associated phagocytosis (LAP) can be considered to be an autophagy-related mechanism as it requires autophagic proteins Beclin1, Atg5 and Atg7, and is inhibited by the PI3K inhibitors wortmannin or LY294002 [31], [32]. Indeed, treatment of RAW 264.7 macrophage cells with wortmannin reduced the co-localization of the bopA mutant with LC3 and increased bacterial survival compared to untreated cells [30].
The LPS of B. pseudomallei stimulates both TLR2 and TLR4 while only TLR2 contributes to host defense [41]. We have observed that the treatment of RAW 264.7 cells with B. pseudomallei LPS induces a significant increase in the formation of GFP-LC3 puncta [30]. Therefore, in light of the data presented here we propose that LAP in B. pseudomallei-infected macrophages is induced in response to LPS, probably via a TLR2- or TLR4-dependent mechanism. Consistent with this proposal TLRs are known to activate the NOX2 NADPH oxidase, and recently it was shown that NOX2-generated reactive oxygen species are necessary for LC3 recruitment to phagosomes [42]. Interestingly we observed that retention of bacteria within phagosomes is insufficient for LAP to occur, as heat-killed B. pseudomallei showed dramatically reduced co-localization with LC3 suggesting that LAP requires other bacterial factor(s) produced by, or present on, live bacteria.
Role of T3SS3 and BopA in the evasion of LAP
The TEM data presented here show the importance of the TTSS3 for escape from phagosomes and identify the putative effector protein BopA as having an important role in phagosome escape. Importantly, bacterial escape from the phagosome results in reduced bacterial killing through avoidance of sequestration within phagolysosomes. The bipD mutant showed almost no escape from phagosomes within RAW 264.7 cells up to 6 h p.i.; similar results have been observed for a B. pseudomallei bipD mutant within J774.2 murine macrophage-like cells [9]. BipD is predicted to be a component of the secretion needle tip [37], [43] and thus a bipD mutant would be unable to secrete any effector proteins. The mechanism by which TTSS3 facilitates phagosome escape is not currently known. Several other putative effector proteins are specified by the B. pseudomallei TTSS3 gene cluster [9]. Inactivation of single TTSS3 effector proteins can lead to different outcomes with respect to co-localization with LC3 which presumably reflects the ability of single gene mutants to escape from the phagosome. Thus, the bopA mutant exhibited increased co-localization with LC3 and delayed escape from phagosomes, but a mutant defective in another putative effector, BapA, showed a similar level of co-localization with LC3 as the wild-type strain. Furthermore, the observation of a small number of free bipD mutant bacteria at 6 h p.i. suggests that the bipD mutant can escape the phagosome at late time points, a phenotype observed in another TTSS3-defective strain, a bsaZ mutant [44]. Consistent with our observations regarding localization of bacteria in single-membrane compartments is that the presence of actin tails and MNGC formation could be observed in RAW 264.7 cells infected with wild-type or bopA mutant bacteria as expected because many bacteria are free in the cytosol. Notably, these phenotypes are not observed for bipD mutant bacteria as we, and others [9], have shown that bacteria are almost completely sequestered in phagosomes at 6 h p.i..
What then is the specific role of BopA in escape of bacteria from phagosomes? Given that the bopA mutant displays a reduced ability to escape the phagosome, it is likely that BopA plays a direct role in disruption of the phagosome membrane, facilitating escape of sequestered bacteria into the host cytosol. Interestingly, it was recently reported that BopA contains a Rho GTPase inactivation domain at its carboxy terminus which may function as a protease or acyltransferase acting on host molecules [45]. Another recent report showed that BopA also contains a cholesterol binding domain [46]. Binding of cholesterol by BopA might lead to the accumulation of cholesterol on phagosome membranes which could limit lysosomal recognition and/or fusion. Such a view is supported by the finding that cholesterol depletion in macrophages infected with Mycobacterium avium triggered phagolysosomal or autophagolysosomal formation with consequent bacterial degradation [47]. A detailed investigation of the cellular location and biochemical function of BopA is warranted in order to provide further insight into its role in phagosome escape and intracellular survival. Notably the bopA mutant shows reduced virulence in mouse melioidosis models, suggesting that its role in intracellular survival is critical for the pathogensis of B. pseudomallei.
Cytosolic B. pseudomallei is resistant to canonical autophagic attack
Wild-type B. pseudomallei is highly resistant to LAP and lysosomal killing, as the majority of the intracellular population escapes from phagosomes. Once free in the cytosol, bacteria activate BimA-mediated actin-based motility, replicate, invade adjacent cells via membrane protrusion and form MNGCs (Figure 4). As demonstrated by TEM analysis cytosolic bacteria are highly resistant to uptake by canonical autophagosomes. Indeed, while we were able to observe many autophagosomes in infected RAW 264.7 cells, we identified only one which contained a bacterium. How then do these free bacteria avoid attack by canonical autophagy? It has recently been suggested that it is unlikely that BopA acts in a manner analogous to IcsB of S. flexneri which acts to inhibit sequestration of bacteria in autophagosomes by preventing Atg5 binding to BimA [46]. This proposal fits with the results we present here showing that a bopA mutant is more susceptible to LAP, but not to cytoplasmic engulfment by autophagosomes.
Autophagosomes engulf several species of cytoplasmic bacteria such as Group A Streptococcus and S. enterica serovar Typhimurium [17], [48]. Although the signals inducing this autophagic attack are largely unknown, ubiquitination of cytoplasmic bacteria and the binding of the autophagy adaptor protein p62/SQSTM1 play a role in this process (reviewed in [49]). Some bacteria have developed mechanisms to evade or exploit the processes activated by ubiquitination, producing both ubiquitin ligases and deubiquitinases that modulate host defence responses. Recently, the B. pseudomallei TssM protein was identified as exhibiting deubiquitinase activity [50], and this activity may represent a mechanism by which B. pseudomallei avoids recognition by the machinery of canonical autophagy. Clearly a major challenge that remains is to understand how bacteria free in the cytosol are resistant to attack by canonical autophagy.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by grants from the Australian Research Council and the National Health and Medical Research Council, Australia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lazar Adler NR, Govan B, Cullinane M, Harper M, Adler B, et al. The molecular and cellular basis of pathogenesis in melioidosis: how does Burkholderia pseudomallei cause disease? FEMS Microbiol Rev. 2009;33:1079–1099. doi: 10.1111/j.1574-6976.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 2.White NJ. Melioidosis. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng AC, Hanna JN, Norton R, Hills SL, Davis J, et al. Melioidosis in northern Australia, 2001-02. Commun Dis Intell. 2003;27:272–277. [PubMed] [Google Scholar]
- 5.Galyov EE, Brett PJ, DeShazer D. Molecular insights into Burkholderia pseudomallei and Burkholderia mallei pathogenesis. Annu Rev Microbiol. 2010;64:495–517. doi: 10.1146/annurev.micro.112408.134030. [DOI] [PubMed] [Google Scholar]
- 6.Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2006;4:272–282. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 7.Pruksachartvuthi S, Aswapokee N, Thankerngpol K. Survival of Pseudomonas pseudomallei in human phagocytes. J Med Microbiol. 1990;31:109–114. doi: 10.1099/00222615-31-2-109. [DOI] [PubMed] [Google Scholar]
- 8.Jones AL, Beveridge TJ, Woods DE. Intracellular survival of Burkholderia pseudomallei. Infect Immun. 1996;64:782–790. doi: 10.1128/iai.64.3.782-790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens MP, Wood MW, Taylor LA, Monaghan P, Hawes P, et al. An Inv/Mxi-Spa-like type III protein secretion system in Burkholderia pseudomallei modulates intracellular behaviour of the pathogen. Mol Microbiol. 2002;46:649–659. doi: 10.1046/j.1365-2958.2002.03190.x. [DOI] [PubMed] [Google Scholar]
- 10.Kespichayawattana W, Rattanachetkul S, Wanun T, Utaisincharoen P, Sirisinha S. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect Immun. 2000;68:5377–5384. doi: 10.1128/iai.68.9.5377-5384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harley VS, Dance DA, Drasar BS, Tovey G. Effects of Burkholderia pseudomallei and other Burkholderia species on eukaryotic cells in tissue culture. Microbios. 1998;96:71–93. [PubMed] [Google Scholar]
- 12.Mehrpour M, Esclatine A, Beau I, Codogno P. Overview of macroautophagy regulation in mammalian cells. Cell Res. 2010;20:748–762. doi: 10.1038/cr.2010.82. [DOI] [PubMed] [Google Scholar]
- 13.Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol. 2009;335:71–84. doi: 10.1007/978-3-642-00302-8_3. [DOI] [PubMed] [Google Scholar]
- 14.Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120:159–162. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Deretic V. Autophagy in infection. Curr Opin Cell Biol. 2010;22:252–262. doi: 10.1016/j.ceb.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, et al. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa Y, Ogawa M, Hain T, Yoshida M, Fukumatsu M, et al. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat Cell Biol. 2009;11:1233–1240. doi: 10.1038/ncb1967. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa M, Sasakawa C. Bacterial evasion of the autophagic defense system. Curr Opin Microbiol. 2006;9:62–68. doi: 10.1016/j.mib.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, et al. Escape of intracellular Shigella from autophagy. Science. 2005;307:727–731. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- 22.Birmingham CL, Canadien V, Gouin E, Troy EB, Yoshimori T, et al. Listeria monocytogenes evades killing by autophagy during colonization of host cells. Autophagy. 2007;3:442–451. doi: 10.4161/auto.4450. [DOI] [PubMed] [Google Scholar]
- 23.Dorn BR, Dunn WA, Progulske-Fox A. Bacterial interactions with the autophagic pathway. Cell Microbiol. 2002;4:1–10. doi: 10.1046/j.1462-5822.2002.00164.x. [DOI] [PubMed] [Google Scholar]
- 24.Lerena MC, Vazquez CL, Colombo MI. Bacterial pathogens and the autophagic response. Cell Microbiol. 2010;12:10–18. doi: 10.1111/j.1462-5822.2009.01403.x. [DOI] [PubMed] [Google Scholar]
- 25.Amer AO, Swanson MS. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell Microbiol. 2005;7:765–778. doi: 10.1111/j.1462-5822.2005.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, et al. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med. 2003;198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez MG, Vazquez CL, Munafo DB, Zoppino FC, Beron W, et al. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell Microbiol. 2005;7:981–993. doi: 10.1111/j.1462-5822.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues PH, Belanger M, Dunn W, Progulske-Fox A. Porphyromonas gingivalis and the autophagic pathway: an innate immune interaction? Front Biosci. 2008;13:178–187. doi: 10.2741/2668. [DOI] [PubMed] [Google Scholar]
- 29.Schnaith A, Kashkar H, Leggio SA, Addicks K, Kronke M, et al. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J Biol Chem. 2007;282:2695–2706. doi: 10.1074/jbc.M609784200. [DOI] [PubMed] [Google Scholar]
- 30.Cullinane M, Gong L, Li X, Lazar-Adler N, Tra T, et al. Stimulation of autophagy suppresses the intracellular survival of Burkholderia pseudomallei in mammalian cell lines. Autophagy. 2008;4:744–753. doi: 10.4161/auto.6246. [DOI] [PubMed] [Google Scholar]
- 31.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 32.Sanjuan MA, Milasta S, Green DR. Toll-like receptor signaling in the lysosomal pathways. Immunol Rev. 2009;227:203–220. doi: 10.1111/j.1600-065X.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 33.Holden MT, Titball RW, Peacock SJ, Cerdeno-Tarraga AM, Atkins T, et al. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc Natl Acad Sci U S A. 2004;101:14240–14245. doi: 10.1073/pnas.0403302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milton DL, O'Toole R, Horstedt P, Wolf-Watz H. Flagellin A is essential for the virulence of Vibrio anguillarum. J Bacteriol. 1996;178:1310–1319. doi: 10.1128/jb.178.5.1310-1319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Lorenzo V, Herrero M, Jakubzik U, Timmis KN. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson S, Roversi P, Espina M, Olive A, Deane JE, et al. Self-chaperoning of the type III secretion system needle tip proteins IpaD and BipD. J Biol Chem. 2007;282:4035–4044. doi: 10.1074/jbc.M607945200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens MP, Haque A, Atkins T, Hill J, Wood MW, et al. Attenuated virulence and protective efficacy of a Burkholderia pseudomallei bsa type III secretion mutant in murine models of melioidosis. Microbiology. 2004;150:2669–2676. doi: 10.1099/mic.0.27146-0. [DOI] [PubMed] [Google Scholar]
- 39.Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, et al. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007;26:313–324. doi: 10.1038/sj.emboj.7601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eskelinen EL. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med. 2006;27:495–502. doi: 10.1016/j.mam.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Wiersinga WJ, Wieland CW, Dessing MC, Chantratita N, Cheng AC, et al. Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (Melioidosis). PLoS Med. 2007;4:e248. doi: 10.1371/journal.pmed.0040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, et al. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci U S A. 2009;106:6226–6231. doi: 10.1073/pnas.0811045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Espina M, Ausar SF, Middaugh CR, Baxter MA, Picking WD, et al. Conformational stability and differential structural analysis of LcrV, PcrV, BipD, and SipD from type III secretion systems. Protein Sci. 2007;16:704–714. doi: 10.1110/ps.062645007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burtnick MN, Brett PJ, Nair V, Warawa JM, Woods DE, et al. Burkholderia pseudomallei type III secretion system mutants exhibit delayed vacuolar escape phenotypes in RAW 264.7 murine macrophages. Infect Immun. 2008;76:2991–3000. doi: 10.1128/IAI.00263-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pei J, Grishin NV. The Rho GTPase inactivation domain in Vibrio cholerae MARTX toxin has a circularly permuted papain-like thiol protease fold. Proteins. 2009;77:413–419. doi: 10.1002/prot.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kayath CA, Hussey S, El hajjami N, Nagra K, Philpott D, et al. Escape of intracellular Shigella from autophagy requires binding to cholesterol through the type III effector, IcsB. Microbes Infect. 2010;12:956–966. doi: 10.1016/j.micinf.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 47.de Chastellier C, Thilo L. Cholesterol depletion in Mycobacterium avium-infected macrophages overcomes the block in phagosome maturation and leads to the reversible sequestration of viable mycobacteria in phagolysosome-derived autophagic vacuoles. Cell Microbiol. 2006;8:242–256. doi: 10.1111/j.1462-5822.2005.00617.x. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimori T, Amano A. Group a Streptococcus: a loser in the battle with autophagy. Curr Top Microbiol Immunol. 2009;335:217–226. doi: 10.1007/978-3-642-00302-8_10. [DOI] [PubMed] [Google Scholar]
- 49.Collins CA, Brown EJ. Cytosol as battleground: ubiquitin as a weapon for both host and pathogen. Trends Cell Biol. 2010;20:205–213. doi: 10.1016/j.tcb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Tan KS, Chen Y, Lim YC, Tan GY, Liu Y, et al. Suppression of host innate immune response by Burkholderia pseudomallei through the virulence factor TssM. J Immunol. 2010;184:5160–5171. doi: 10.4049/jimmunol.0902663. [DOI] [PubMed] [Google Scholar]