Abstract
FtsY, the Escherichia coli homologue of the eukaryotic signal recognition particle (SRP) receptor α-subunit, is located in both the cytoplasm and inner membrane. It has been proposed that FtsY has a direct targeting function, but the mechanism of its association with the membrane is unclear. FtsY is composed of two hydrophilic domains: a highly charged N–terminal domain (the A–domain) and a C–terminal GTP-binding domain (the NG–domain). FtsY does not contain any hydrophobic sequence that might explain its affinity for the inner membrane, and a membrane-anchoring protein has not been detected. In this study, we provide evidence that FtsY interacts directly with E.coli phospholipids, with a preference for anionic phospholipids. The interaction involves at least two lipid-binding sites, one of which is present in the NG–domain. Lipid association induced a conformational change in FtsY and greatly enhanced its GTPase activity. We propose that lipid binding of FtsY is important for the regulation of SRP-mediated protein targeting.
Keywords: E.coli/FtsY/protein targeting/signal recognition particle
Introduction
In eukaryotic cells, co-translational targeting and insertion of proteins into the membrane of the endoplasmic reticulum (ER) is mediated by the signal recognition particle (SRP) and its receptor (SR) (reviewed in Rapoport et al., 1996). SRP binds via its 54 kDa subunit (SRP54) to hydrophobic targeting signals in short nascent polypeptides, thereby inhibiting further elongation. Interaction of the complex with the α-subunit of the SR, which is anchored to the membrane by SRβ, relieves the arrest in elongation and allows insertion of the nascent protein into the translocation pore of the ER membrane. SRP54, SRα and SRβ are GTPases, and hydrolysis of GTP by SRP54 and SRα is required to dissociate the SRP–SRα complex and recycle these targeting factors.
The more recently discovered SRP pathway in Escherichia coli involves cytosolic factors that strongly resemble components involved in protein targeting to the eukaryotic ER membrane (reviewed in Luirink and Dobberstein, 1994; Wolin, 1994). The E.coli SRP consists of 4.5S RNA and a 48 kDa GTPase designated P48 (or Ffh for fifty four homologue) that show homology to the eukaryotic 7S RNA and SRP54, respectively. Recent in vitro and in vivo data indicate that proteins bearing strongly hydrophobic targeting signals (e.g. integral inner membrane proteins) are particularly dependent on the SRP for efficient membrane targeting and insertion (MacFarlane and Müller, 1995; de Gier et al., 1996; Ulbrandt et al., 1997; Valent et al., 1997, 1998).
The E.coli GTPase FtsY displays significant sequence similarity to SRα and is considered a true SRP receptor based on the following observations. First, depletion of FtsY affects the synthesis and secretion of proteins that depend on SRP for proper membrane targeting (Luirink et al., 1994; Seluanov and Bibi, 1997). Second, FtsY and SRP form a complex in vitro and regulate each others' GTPase activity (Miller et al., 1994; Kusters et al., 1995; Powers and Walter, 1995). Third, FtsY is essential for the release of SRP from a nascent substrate and its subsequent association with the E.coli translocase (Valent et al., 1998).
FtsY and SRα contain two distinct domains: a highly charged N–terminal domain (the A–domain) and a C–terminal domain (the NG–domain), the crystal structure of which has been determined recently (Montoya et al., 1997a). In mammalian cells, SRα is targeted co- translationally to the membrane and is anchored via its A–domain to the integral membrane subunit SRβ (Young et al., 1995; Young and Andrews, 1996). In contrast, FtsY is located in part in the cytosol and in part at the cytoplasmic membrane (Luirink et al., 1994). The mechanism of its association with the membrane is unclear. No hydrophobic helix, which would suggest a transmembrane location of FtsY, has been detected from the sequence data (Gill et al., 1986) and no obvious SRβ homologues have been identified in the E.coli genome sequence.
Based on in vivo localization studies, it has been suggested that the association of FtsY with the membrane involves two distinct types of association (de Leeuw et al., 1997). First, FtsY may associate with the membrane via a saturatable protein–protein interaction, in which the A–domain would be primarily involved. Second, FtsY may associate via a direct protein–lipid interaction.
In this study, we investigated the possibility of a direct FtsY–lipid interaction. Both full-length FtsY and the FtsY–NG domain were shown to associate directly with liposomes and insert into monolayers composed of E.coli phospholipids, with a preference for anionic phospholipids. Liposomes induced a conformational change in FtsY and greatly enhanced its GTPase activity. It is proposed that the dynamic interaction of FtsY with membrane lipids plays a crucial role in the regulation of SRP-mediated protein targeting in E.coli.
Results
FtsY and FtsY–NG associate with liposomes
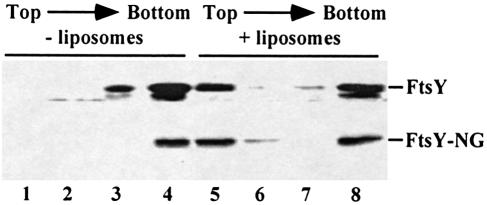
FtsY is needed for an efficient release of SRP from the nascent chain at the membrane (Valent et al., 1998). The nature of the association of FtsY with the membrane is unclear. FtsY has been suggested to interact directly with phospholipids, possibly through the FtsY–NG domain (de Leeuw et al., 1997). To investigate the interaction of FtsY with phospholipids, purified FtsY and FtsY–NG were subjected to floatation gradient centrifugation in the presence or absence of liposomes. Both FtsY and FtsY–NG co-localized in part with the liposomes in the top fraction (Figure 1). To examine whether association depends on a specific nucleotide-bound conformation, liposome association was determined in the presence of guanine nucleotides. Neither GTP, GDP nor GMP-PNP present at 2 mM in the incubation buffer influenced liposome association. Both FtsY and FtsY–NG associate independently of the presence of guanine nucleotides (data not shown).
Fig. 1. FtsY and FtsY–NG bind to liposomes composed of E.coli phospholipids. Purified FtsY or FtsY–NG (250 ng) was incubated for 10 min at 37°C in the absence (lanes 1–4) or presence (lanes 5–8) of 50 μg of liposomes prepared from E.coli lipids in 50 mM HEPES pH 7.6, 0.5 M KOAc, 5 mM Mg(OAc)2 in a final volume of 25 μl. Samples were subjected to floatation gradient centrifugation as described in Materials and methods. The gradient was collected in four fractions from the top. Fractions were TCA precipitated and analysed by immunoblotting using affinity-purified αFtsY serum. The lower band of the FtsY doublet represents a degradation product that lacks the 14 N–terminal amino acids (Luirink et al., 1994).
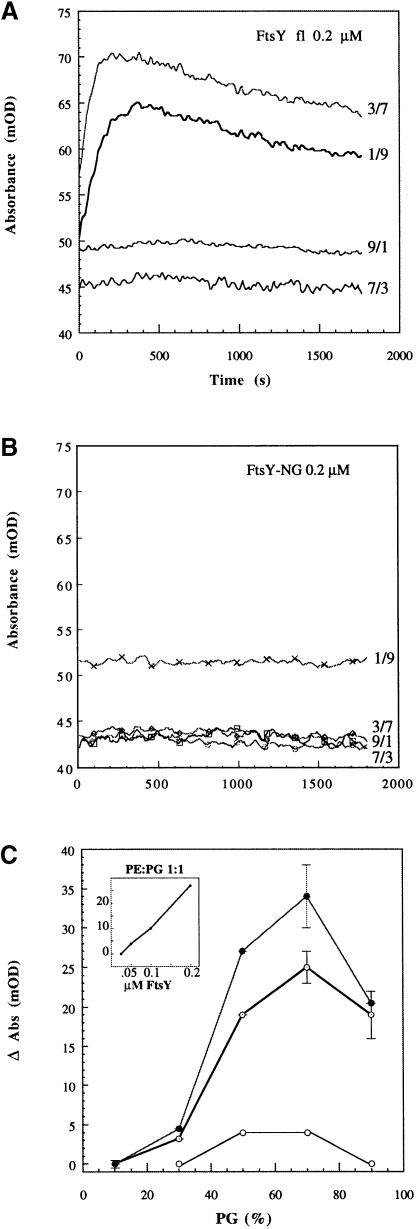
To examine the valence of the lipid association, the ability of FtsY to induce membrane aggregation was determined by measuring the turbidity of a large unilamellar vesicle (LUV) suspension upon addition of FtsY. FtsY (but not FtsY–NG) caused a fast absorbance increase followed by a slower decreasing phase (Figure 2A and B). This indicates the formation of aggregates of increasing size. In the first phase, the turbidity increases with the molecular size of the scattering particles, whereas in the second phase the ensuing reduction in the number of scattering centres prevails. This aggregation occurs only with phosphatidylglycerol (PG):phosphatidylethanolamine (PE) LUVs where the anionic lipid PG is present at >30% of the molar fraction and is a concentration-dependent reaction (Figure 2C). Similar aggregation was observed upon addition of FtsY to phosphatidic acid (PA):palmitoyl-oleoyl-phosphatidylcholine (POPC) LUVs when the anionic lipid PA was >30% (not shown). No aggregation occurs with vesicles comprised only of the zwitterionic lipid POPC.
Fig. 2. FtsY–induced aggregation of lipid vesicles suggests two binding sites. (A) Absorbance changes (at 405 nm) induced by the addition of 0.2 μM FtsY to lipid vesicles composed of PE:PG at the indicated molar fractions. The lipid:protein molar ratio was 500. (B) The same as (A), but with 0.2 μM FtsY–NG. (C) Absorbance increase versus molar fraction of the anionic lipid (PG) for three different concentrations of FtsY (open circles, 0.05 μM; dotted circles, 0.1 μM; filled circles, 0.2 μM). The lipid concentration was always 100 μM. Inset: dose dependence of FtsY–induced aggregation of PG:PE 1:1 LUV.
Taken together, these data indicate that both FtsY and the FtsY–NG domain are able to contact E.coli phospholipids directly, independently of the nucleotide. The difference in efficiency of lipid association between FtsY and FtsY–NG and the ability of FtsY to cause LUV aggregation suggest the presence of two lipid attachment sites in FtsY: one in the A–domain and one in the NG–domain. At least one of them has a preference for anionic phospholipids.
FtsY and FtsY–NG insert into lipid monolayers with a preference for anionic phospholipids
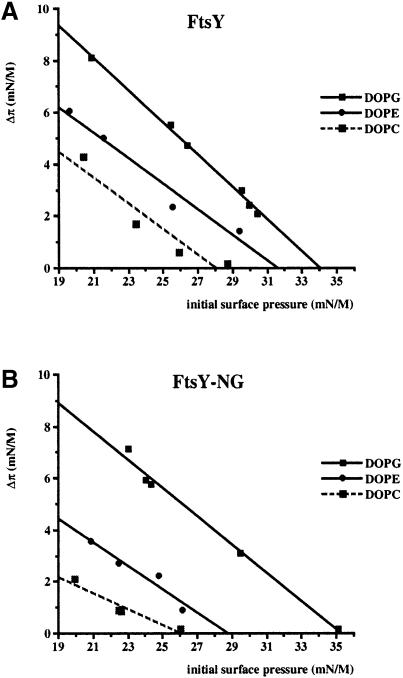
The nature and extent of the FtsY–lipid interaction were characterized further using the lipid monolayer technique. In this approach, the insertion of a protein into the lipid phase causes an increase in surface pressure of the monolayer (Demel, 1994). Both FtsY and FtsY–NG were tested for insertion into dioleoyl-PG (DOPG; anionic), DOPE (zwitterionic) or DOPC (zwitterionic) monolayers as a function of varying initial surface pressure. It should be noted that FtsY and FtsY–NG show surface activity by themselves, as in the absence of a phospholipid monolayer they give rise to a surface pressure increase of 18.5 and 19.5 mN/m, respectively. At an initial surface pressure above the surface pressure of the protein in the absence of a monolayer, the increase in surface pressure can only be interpreted as insertion of the protein into the monolayer (reviewed in Demel, 1994). Therefore, the initial surface pressure was kept at or above 20 mN/m. Both FtsY and FtsY–NG showed significant insertion into monolayers of the three lipid types. The insertion was more efficient when DOPG was used as compared with DOPE and DOPC, independently of the initial surface pressure (Figure 3A and B). In addition, the maximal surface pressure allowing insertion was significantly higher when DOPG monolayers were used as compared with DOPC or DOPE. Insertion into DOPE was slightly more efficient than into DOPC, although both are zwitterionic lipids. This is possibly related to the fact that DOPC is not a natural E.coli lipid and has a relatively large headgroup. For both zwitterionic phospholipids, the maximal surface pressure allowing insertion is higher for FtsY than for FtsY–NG. Again, this may suggest the presence of two membrane attachment sites.
Fig. 3. FtsY (A) and FtsY–NG (B) insert into synthetic lipid monolayers. The increase in surface pressure upon injection of the protein into the subphase was measured as a function of the initial surface pressure. Monolayers of DOPG, DOPE and DOPC were used.
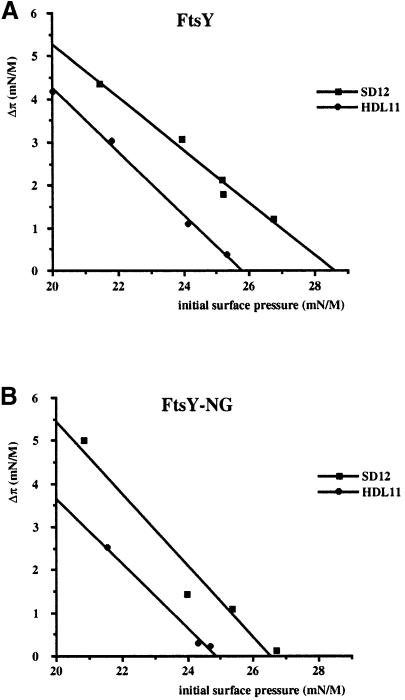
To examine further the role of negatively charged phospholipids in the insertion of FtsY, phospholipid extracts were prepared from inner membrane vesicles derived from E.coli strain SD12 (wild type) and HDL11 (a conditional strain in which PG synthesis was down-regulated). Both FtsY and FtsY–NG showed a reduced insertion into PG–depleted phospholipid monolayers as compared with monolayers based on wild-type phospholipid extract, irrespective of the initial surface pressure (Figure 4). These results are consistent with a preference of FtsY to aggregate LUVs containing negatively charged phospholipids (Figure 2).
Fig. 4. Insertion of FtsY (A) and FtsY–NG (B) into monolayers is stimulated by phosphatidylglycerol. The increase in surface pressure upon injection of the protein into the subphase was measured as a function of the initial surface pressure. Monolayers were prepared from phospholipid extracts of wild-type inner membrane vesicles of E.coli strain SD12, and of PG–depleted inner membrane vesicles of E.coli strain HDL11.
FtsY–lipid interaction is dominated by electrostatic interactions
FtsY does not contain any predicted hydrophobic membrane–spanning sequence (Gill et al., 1986). It is a strongly negatively charged protein at physiological pH with ∼50 net negative charges, so that an electrostatic repulsion between the negative membrane surface and the protein, especially the acidic A–domain, is anticipated. On the other hand, we observed an enhanced interaction of FtsY and FtsY–NG with negatively charged lipids as compared with zwitterionic lipids in monolayer and aggregation studies, suggesting an interaction based on electrostatic attraction.
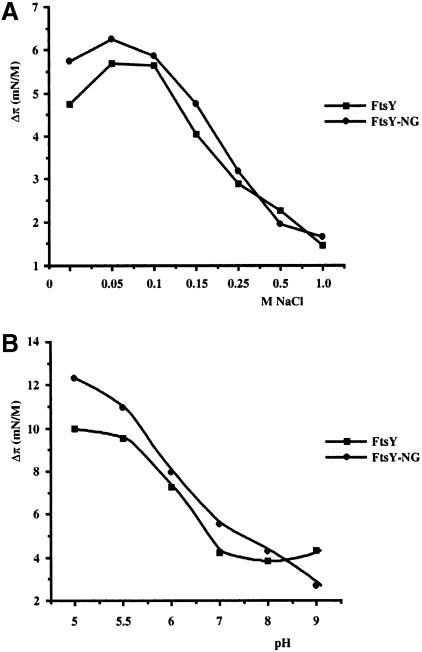
To investigate the nature of the FtsY–lipid interaction in more detail, the effect of the salt concentration in the subphase buffer on the insertion of FtsY(-NG) into DOPG monolayers was measured (Figure 5A). Optimal insertion occurred at ∼100 mM NaCl for both FtsY and FtsY–NG. Insertion decreased gradually when the NaCl concentration was raised, suggesting that the insertion indeed depends on electrostatic interactions. To change the charge profile of the protein, the pH of the subphase phosphate buffer was varied from 5 to 9. Lowering the pH from 7 to 5.5 resulted in an almost linear increase of insertion for both FtsY and FtsY–NG into a DOPG monolayer (Figure 5B). It is likely that a relatively low pH counteracts the negative surface charge of the FtsY protein, thus reducing electrostatic repulsion at the DOPG monolayer.
Fig. 5. Insertion of FtsY and FtsY–NG into a DOPG monolayer (initial surface pressure of 25 mN/m) is influenced by the salt concentration (A) and pH (B) of the subphase buffer. FtsY and FtsY–NG were injected into a subphase of 50 mM Tris–HCl pH 7.5 containing the indicated concentrations of NaCl (A) or 50 mM Na phosphate, 100 mM NaCl at the indicated pH (B).
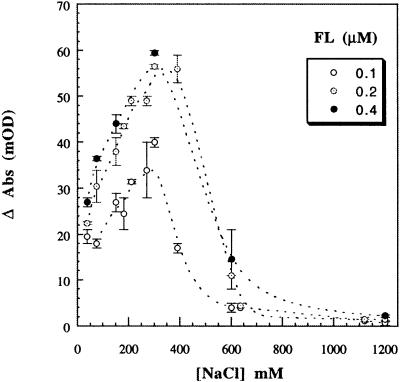
To confirm the predominantly electrostatic interaction of FtsY with the membrane, the aggregation of the vesicles was monitored as a function of the ionic strength of the incubation buffer (Figure 6). Above 500 mM NaCl, the aggregation of LUVs induced by FtsY was abolished, indicating disruption of electrostatic interactions involving at least one of the two suggested binding sites. Maximal aggregation occurred at ∼250 mM NaCl, and aggregation decreased with decreasing ionic strength. The insertion as well as the aggregation may be optimal at intermediate (100–250 mM) NaCl concentrations by shielding of the negatively charged A–domain, thus minimizing initial electrostatic repulsion at low (<100 mM) NaCl concentrations. In conclusion, these results indicate that the insertion of FtsY into a DOPG monolayer and its association with LUVs is dominated by electrostatic interactions.
Fig. 6. Effect of salt concentration on FtsY–induced LUV aggregation (measured as in Figure 2) for different FtsY (FL) concentrations (open circles, 0.1 μM; dotted circles, 0.2 μM; filled circles, 0.4 μM) and constant lipid concentration (100 μM).
Insertion of FtsY–NG into lipid monolayers is inhibited by GTP and GDP
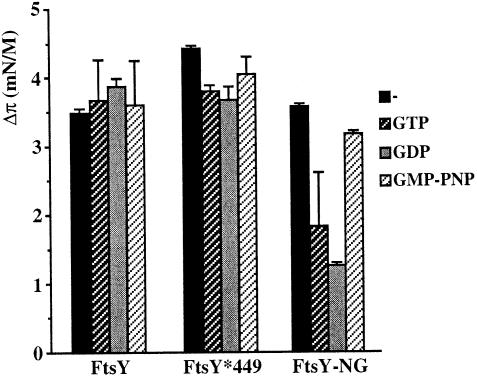
The effects of GTP and GDP on the insertion of FtsY and FtsY–NG into a DOPG monolayer were examined. In the absence of nucleotide, both FtsY and FtsY–NG show a comparable increase of initial surface pressure (Figure 7). Insertion of FtsY–NG was impaired upon addition of GDP and, to a lesser extent, GTP to the subphase buffer, whereas the non-hydrolysable analogue GMP-PNP did not affect insertion. Considering the equilibrium and kinetic constants of guanine nucleotide binding to the NG–domain and its rate of GTP hydrolysis, the majority of the NG–domain will be in the GTP-bound state in the presence of 2 mM GTP (Moser et al., 1997). The difference in insertion between the GTP- and GMP-PNP-bound states of the NG–domain may reflect subtle conformational changes upon binding of these different nucleotides (see below).
Fig. 7. Effect of nucleotides on the insertion of FtsY, FtsY*449 and FtsY–NG into a DOPG monolayer (initial surface pressure of 25 mN/m). Protein was injected into a subphase buffer of 50 mM Tris–HCl, 100 mM NaCl pH 7.5 containing 2 mM MgCl2. GTP, GDP or GMP-PNP were added to a final concentration of 2 mM prior to injection of protein.
In contrast, insertion of full-length FtsY appeared unaffected by the presence of GTP, GMP-PNP or GDP. Mutant FtsYA*449, which displays strongly impaired GTP binding (Kusters et al., 1995), showed a comparable insertion. These data indicate that insertion of full-length FtsY occurs independently of its nucleotide occupancy. It is possible that the A–domain influences the conformation of the NG–domain, facilitating insertion in the GTP- or GDP-bound form. Alternatively, the A–domain itself may interact with the DOPG monolayer without being influenced by nucleotide binding to the NG–domain.
Lipid association induces a conformational change in FtsY
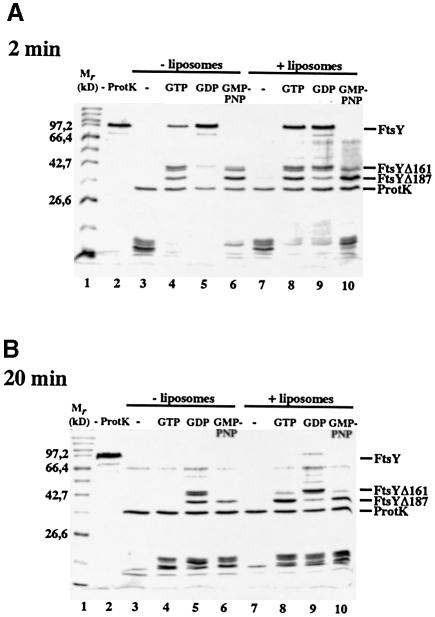
Limited proteolysis has been shown to detect conformational changes in FtsY that are due to its nucleotide occupancy (Kusters et al., 1995). The influence of guanine nucleotides and liposomes on the conformation of FtsY was studied by monitoring its resistance to proteolysis by proteinase K with time (Figure 8). In the absence of liposomes, FtsY was degraded readily by proteinase K, already resulting in degradation products of low molecular weight after 2 min incubation (Figure 8A, lane 3, bottom). In the presence of GTP, FtsY was stabilized temporarily, resulting in a doublet band of 40 kDa and a product of 37 kDa (Figure 8A, lane 4). N–terminal sequencing revealed that the 40 kDa product lacks the N–terminal 161 amino acids (FtsYΔ161; data not shown). In time, this fragment is converted further into a 37 kDa product corresponding to the previously described FtsYΔ187 (Kusters et al., 1995). In the presence of GMP-PNP, FtsY was converted more rapidly to FtsYΔ187, which was still present after 20 min (Figure 8A and B, lane 6), indicative of a difference in conformation when GTP or GMP-PNP is bound. GDP stabilized full-length FtsY initially but after 20 min mainly FtsYΔ161 was detected (Figure 8A and B, lane 5).
Fig. 8. FtsY is relatively resistant to protease treatment in the presence of liposomes and GTP. A 4 μg aliquot of FtsY was incubated in the absence (lanes 3–6) or presence (lanes 7–10) of liposomes and 2 mM of nucleotide where indicated. Samples were treated with proteinase K (lanes 3–10) or left untreated (lane 2) at 37°C for 2 min (A) or 20 min (B). Samples were subjected to TCA precipitation, analysed by SDS–PAGE and visualized by Coomassie Blue staining.
In the presence of liposomes, the degradation characteristics of FtsY remained relatively unaffected in the absence of nucleotide or in the presence of GDP or GMP-PNP. However, in the presence of GTP, FtsY was partly stabilized by the presence of liposomes at 2 min incubation (Figure 8A, cf. lanes 4 and 8) whereas FtsYΔ187 was remarkably stable even after 20 min incubation (Figure 8B, cf. lanes 4 and 8). These data suggest that liposomes stabilize FtsY in the GTP-bound conformation.
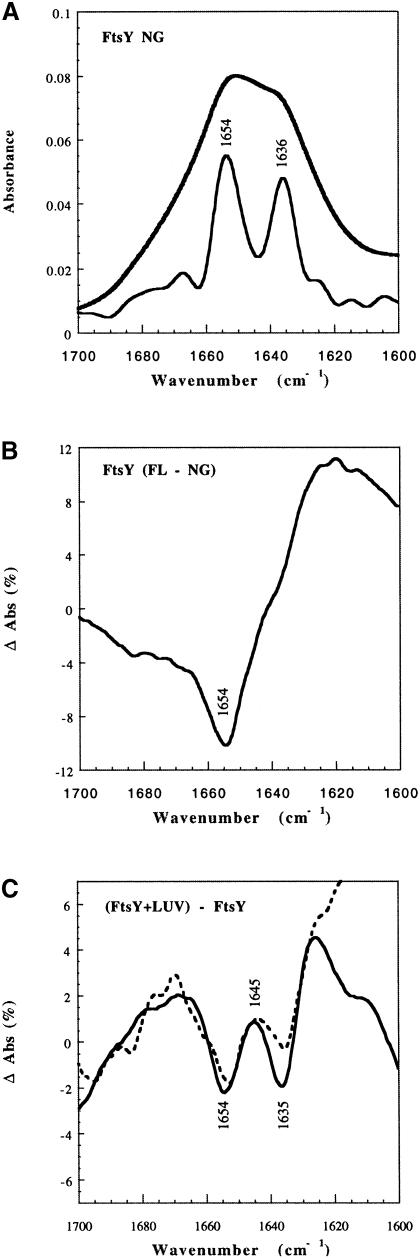
In order to determine whether lipids affect the conformation of FtsY in the absence of nucleotides, in a way that is not detected by the above-described proteolysis experiment, we used Fourier transform infrared (FT-IR) spectroscopy (Figure 9), a technique that can report conformational changes undergone by proteins upon binding to lipid membranes (Tamm and Tatulian, 1997), as shown for influenza haemagglutinin (Tatulian et al., 1995) and toxins such as aerolysin (Cabiaux et al., 1997). FT-IR spectra of deuterated films of FtsY or FtsY–NG were obtained with or without LUVs. The amide I′ region (1600–1700 cm–1) was analysed as described (Menestrina et al., 1999). FtsY–NG was found to contain ∼50% α-helix and 40% β-sheet (Figure 9A). A differential spectrum with FtsY indicated that the whole protein contained on average slightly less α-helix and more β-sheet than the NG fragment (Figure 9B). Upon addition of LUVs, both FtsY and FtsY–NG show a decrease in either α-helical or β-sheet content (indicated, in the differential spectrum, by two negative minima at 1654 and 1635 cm–1, respectively, Figure 9C). A positive peak at 1645 cm–1 suggests an increase in random coil structure, whereas the increase at 1625 cm–1 may indicate the formation of intermolecular hydrogen bonds in extended β-strands (Tamm and Tatulian, 1997). These changes, albeit clear, amount to only ∼5% (as a comparison, influenza haemagglutinin increases its α-helical content ∼10% upon binding to lipid vesicles at low pH; Tatulian et al., 1995). They are consistent with a localized, limited, unfolding and oligomerization of both FtsY and FtsY–NG upon binding to lipids.
Fig. 9. Effect of LUVs on the conformation of FtsY derived from infrared-ATR spectra. (A) Amide I′ spectrum of deuterated films of FtsY–NG (upper solid line) and its deconvolution (lower solid line) obtained with 1.6 resolution enhancement factor and Bessel smoothing. Two major components at 1654 ± 1 cm–1 (α-helix) and 1636 ± 1 cm–1 (β-sheet), six minor components at 1682 cm–1 (β-turn), 1675 cm–1 (antiparallel β-sheet), 1666 cm–1 (β-turn), 1625 cm–1 (β-sheet), 1614 cm–1 and 1600 cm–1 (side chains), and a shoulder at 1648 cm–1 (random coil) are present, assigned according to Byler and Susi (1986) and Tatulian et al. (1995). An initial set of nine Lorentzian components with these positions was used to generate a least-squares fit of the original data. The resulting average secondary structure was 46% α-helix, 39% β-sheet, 10% β-turn and 5% random coil. Errors are typically ± 5% of the determined values. (B) Differential spectrum obtained by subtracting the FtsY–NG spectrum from the normalized FtsY spectrum. (C) Differential spectra obtained by subtracting the soluble form from the normalized lipid-bound form, for either full-length (FL) FtsY (dashed line) or FtsY–NG fragment (solid line).
In conclusion, the data from both experiments suggest that liposomes induce conformational changes in FtsY and that FtsY–NG is stabilized in a GTP-bound conformation. The marked influence of GTP, GDP and GMP-PNP on the degradation characteristics suggests the existence of various conformations of the C–terminal region (FtsYΔ187 and FtsYΔ161) that contain the NG–domain.
The GTPase activity of the FtsY–NG domain is regulated by the A–domain and lipids
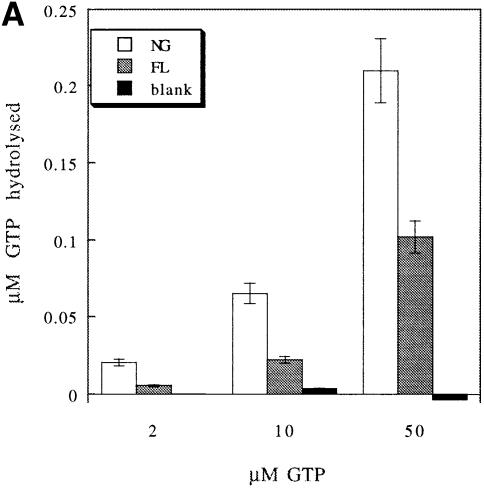
FtsY has been reported to display very low intrinsic GTPase activity (Miller et al., 1994; Kusters et al., 1995; Powers and Walter, 1995). Only when labelled GTP of high specific activity was used could low but significant GTP hydrolysis by FtsY and FtsY–NG be detected (Figure 10A). FtsY–NG hydrolysed twice as much GTP as full-length FtsY when used in equimolar amounts. This suggests that in the absence of liposomes, the A–domain acts as a repressor of GTP hydrolysis.
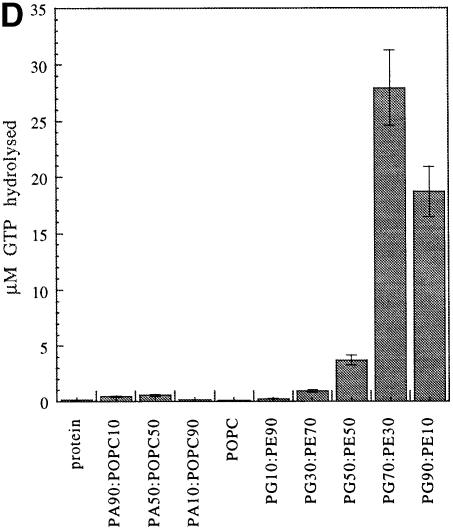
fig. 10. Effect of LUVs on GTP hydrolysis by FtsY and FtsY–NG. (A) The A–domain acts as a repressor of GTP hydrolysis in FtsY in the absence of lipids. FtsY or FtsY–NG (1 μM) was incubated in hydrolysis buffer (see Materials and methods) at the indicated final concentration of GTP for 22 min at 37°C. (B) FtsY, but not FtsY–NG, is stimulated upon lipid interaction. Initial reaction rates for FtsY and for FtsY–NG are shown as a function of the protein:lipid ratio (the lipid is PG). FtsY (100 nM) and FtsY–NG (1 μM) were incubated at 37°C with 10 μM GTP. Time points were taken at 0, 2, 4 and 10 min. The lipid is PG. (C) Lipid interaction of FtsY seems to affect mainly the vmax. Initial reaction rates have been determined as a function of substrate and lipid concentration and are fitted with the Michaelis–Menten equation (the error of the fit is given in parentheses). FtsY (100 nM) was incubated at 37°C with PG and GTP at the indicated concentrations. The Km and vmax are given. (D) Stimulation of GTP hydrolysis in FtsY depends on the lipid composition of the LUVs. Conditions are: 1 μM protein, 50 μM GTP, 160 μM LUVs; time is 11 min. The key for (A), (B) and (D) is as indicated in the inset in (A).
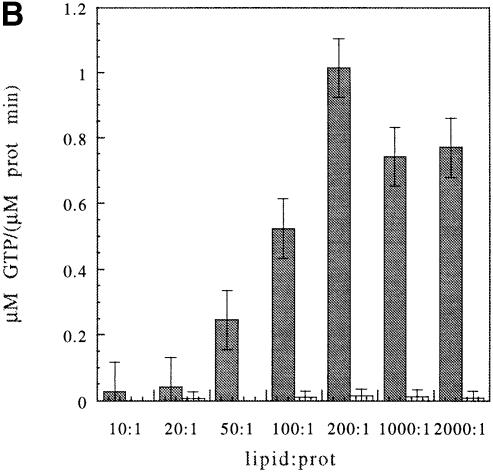
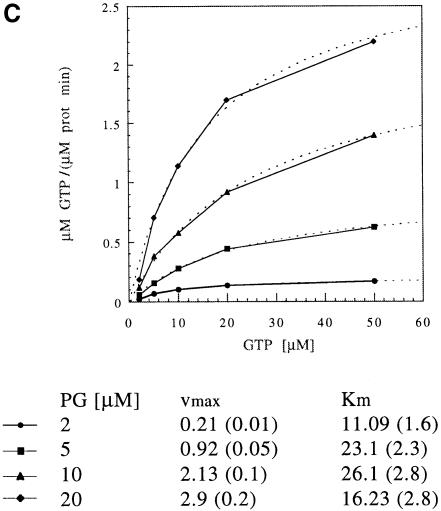
FtsY and FtsY–NG were shown to associate with liposomes, which is accompanied by conformational changes. To examine the effect of liposome association on the GTPase activity of FtsY and FtsY–NG, LUVs were used in the hydrolysis assay and found to stimulate GTP hydrolysis strongly in FtsY, whereas the effect on FtsY–NG was negligible (Figure 10B). This reflects the importance of the A–domain for GTPase stimulation upon lipid association. These data also indicate that the A–domain is not only crucial for sensing the membrane, but also for releasing the block in GTP hydrolysis and for stimulating the hydrolysis over the level of membrane-bound FtsY–NG. To exclude a possible effect of different affinities for the membrane, FtsY and FtsY–NG were titrated with PG LUVs. Both proteins show a maximum at a molar ratio of ∼1:200 (Figure 10B). Lipid interaction of FtsY appears to have a clear effect on the vmax but only a minor effect on the Km (Figure 10C). This suggests that there is no major change in the affinity for the nucleotide.
To investigate the dependence of GTPase stimulation on the lipid composition of the LUVs, the GTPase activity of FtsY was tested in the presence of PA:phosphatidylcholine (PC) or PG:PE LUVs. The content of negatively charged lipids was varied from 10 to 90%. Stimulation was low for all the ratios of PA:PC tested, but varied substantially with the amount of PG present, being optimal at 70% PG (Figure 10D). This indicates that not only the charge, but also the nature of the lipid headgroup is important to promote GTP hydrolysis. PE and PA are both known to be HII-phase promoting lipids (Cullis and de Kruijff, 1979). However, the phase transition temperature for 100% egg PE vesicles is 45°C (Ellens et al., 1986) and therefore it is well above the assay temperature. We presume that the effects observed here are due predominantly to the charge distribution and headgroup nature, and not to lipid polymorphism effects. Stimulation can also be achieved with E.coli cardiolipin (CL) liposomes (not shown). Taken together, the results show that natural E.coli anionic phospholipids can stimulate the hydrolysis of FtsY.
Discussion
In this study, we show that the E.coli SRP receptor FtsY interacts directly with membrane lipids. Previously, we have demonstrated that FtsY is located in part in the cytoplasm and in part in the inner membrane (Luirink et al., 1994). Cytoplasmic FtsY was shown to associate with SRP bound to ribosome–nascent chain complexes in the absence of membranes (Valent et al., 1998). The affinity of FtsY for lipids may contribute to the targeting of nascent chains.
FtsY binds in its nucleotide-free form to liposomes derived from E.coli total lipid extracts. Interestingly, these liposomes also induced significant FtsY–mediated release of the SRP from the nascent chains, indicating that the interaction of FtsY with lipids is functional and sufficient to induce a conformational change in the SRP that lowers its affinity for the nascent chains (Scotti et al., 1999).
In addition, FtsY was shown to insert into monolayers of all lipid species analysed. The most efficient insertion is observed with anionic lipids at moderate salt concentration. Hence, insertion into the monolayer appears to involve electrostatic interactions. It should be noted that the increase in surface pressure measured in the monolayer experiment reflects insertion of a significant portion of the protein between the headgroups in between the acyl chains and is not merely a peripheral attachment to the charged surface of the monolayer (Demel, 1994). The insertion might explain the relative resistance of membrane-associated FtsY to salt and alkali extraction in vivo (Luirink et al., 1994). Interaction with anionic phospholipids was unexpected considering that FtsY itself is strongly negatively charged, carrying ∼50 net negative charges at physiological pH (Gill et al., 1986). On the other hand, despite its overall negative charge, positive clusters of R/K residues are found in the A–domain as well as in the NG–domain that could contribute to interaction with anionic phospholipids.
FtsY is shown to aggregate liposomes, indicating the presence of at least two lipid attachment sites. Analytical ultracentrifugation experiments (K.te Kaat, unpublished observation) show FtsY as being monomeric, despite its different migration behaviour in gel filtration (Luirink et al., 1994). Extensive aggregation is observed when LUVs are composed of phospholipids normally present in E.coli membranes. However, optimal results are obtained at a molar ratio of 7:3 PG:PE, whereas the physiological molar ratio of PG:PE described in wild-type E.coli membranes is 3:7 (Morein et al., 1996). It is unclear whether local areas with a high density of negatively charged phospholipids exist in the inner membrane. If so, they might contribute to the targeting specificity of FtsY. It should be noted that the interaction with PE is also important since aggregation is not optimal with pure PG vesicles.
The aggregation data presented here suggest that a second lipid-binding site is present in the A–domain, which shows no clear nucleotide dependence. Unfortunately, this could not be demonstrated directly since purified A–domain proved to be unstable (data not shown). The acidic domain of FtsY is poorly conserved and varies dramatically in size, being basically non-existent in a predicted FtsY from the genome of Helicobacter pylori to ∼200 residues in the E.coli FtsY (Samuelsson and Zwieb, 1999). Interestingly, almost all bacterial FtsY homologues possess an N–terminus rich in positively charged residues. Frequently, the clusters of positive residues are flanked by aromatic residues often found in membrane-interacting proteins. It has been reported that fusion of the FtsY–NG domain to an unrelated transmembrane protein partly complements FtsY depletion (Seluanov and Bibi, 1997). These data suggest that the A–domain is important for the membrane targeting of bacterial FtsY. The α-subunit of the eukaryotic SRP receptor (SRα) was shown to interact at its very N–terminus with the β-subunit, an integral membrane protein (Young et al., 1995; Young and Andrews, 1996). Accordingly, eukaryotic SRα sequences do not show clustering of positive residues at the N–terminus suggesting a different mechanism by which FtsY and SRα species are targeted to the membrane.
It has been demonstrated that E.coli FtsY complements the loss of SRα in co-translational targeting of pre-prolactin to microsomal membranes (Powers and Walter, 1997). When the N–terminal 46 amino acids (encompassing the cluster of positive charges) were deleted from FtsY, a higher concentration of FtsYΔ46 was necessary to support both targeting of pre-prolactin and localization of FtsY to the microsomal membranes. Presumably the affinity of both the acidic domain (especially its extreme N–terminus) and the NG–domain for lipids explains the ability of FtsY to function in heterologous protein targeting.
Does FtsY also interact with membrane proteins? In contrast to the salt-sensitive interaction with liposomes observed here, FtsY cannot be extracted from crude inner membranes by high salt treatment (Luirink et al., 1994). In addition, it has been observed that, upon overexpression, the acidic domain of E.coli FtsY competes endogenous FtsY from the membrane, whereas the NG–domain does not (de Leeuw et al., 1997). These results suggest a titratable membrane receptor site in native inner membranes, presumably proteinaceous in nature, to which the acidic domain binds. On the other hand, we have not been able to identify any protein receptor using a variety of techniques to detect protein interactions (J.Luirink, unpublished results).
The protease protection assay and the FT-IR spectra both suggest a conformational change of the protein upon interaction with the membrane. FT-IR reveals a local unfolding of the full-length protein and the NG fragment to similar extents. Protease protection in the presence of GTP and liposomes conferred resistance against digestion to a fragment that encompasses the NG–domain, also indicating a conformational change involving mainly the NG–domain. Conceivably, liposome association stimulates GTP binding to FtsY that has been shown to stabilize the NG–domain.
FtsY and SRP have been described to act as GTPase-activating proteins for each other in an in vitro assay based on purified components. The reciprocal stimulation of GTP hydrolysis triggers dissociation of the SRP–FtsY complex, which was proposed to allow the cycling of these components in vivo (Miller et al., 1994; Powers and Walter, 1995). The membrane so far has escaped attention as a component in the SRP–GTPase cycle. We demonstrate here a strong increase in the GTPase activity of FtsY upon binding to liposomes. The presence of liposomes containing either PG or CL greatly stimulated the hydrolysis, and only a very small stimulation is achieved by PA. The stimulation seems therefore to be more dependent upon the nature of the lipid headgroup than are binding and aggregation. The GTPase activity of FtsY–NG is stimulated much less by liposomes than is the full-length protein, whereas in the absence of liposomes the hydrolytic activity of FtsY–NG exceeds that of FtsY. Apparently, the acidic domain modulates the GTPase activity of FtsY–NG in two ways: repressing it in solution and highly stimulating it at the membrane.
FtsY and SecA function in different targeting routes (Scotti et al., 1999) but are remarkably similar, at least at a phenomenological level. Both serve as a receptor of a precursor–chaperone complex: SecA binds SecB–precursor complexes (Hartl et al., 1990) and FtsY binds SRP–nascent chain complexes (Valent et al., 1998). Both are nucleotide-binding proteins (Mitchell and Oliver, 1993; Kusters et al., 1995; Powers and Walter, 1995) showing a low basal hydrolysis activity in vitro that is stimulated by membranes and precursor complexes (for SecA, reviewed in Driessen, 1996; Powers and Walter, 1995; this study). Both show an unusual subcellular distribution in vivo: partly soluble and partly firmly associated with the inner membrane (Cabelli et al., 1991; Luirink et al., 1994). They interact directly with phospholipids, with a preference for anionic phospholipids despite their overall acidic nature (pI SecA = 5.50, pI FtsY = 4.49) (Breukink et al., 1992; this study). Lipid attachment of SecA (Ulbrandt et al., 1992) and FtsY (this study) is also accompanied by a partial unfolding of the protein and insertion into monolayers. The lipid binding of SecA has, in contrast to FtsY, additionally a strong hydrophobic component (Breukink et al., 1992). Finally, both SecA and FtsY are required to connect the targeting and membrane insertion at the SecYEG translocon (reviewed in Driessen et al., 1998; Valent et al., 1998).
Conclusions
Although the SRP-mediated targeting pathways in prokaryotes and eukaryotes show many similarities, it seems that membrane targeting of FtsY is fundamentally different from that of SRα. Despite different approaches, no functional interaction between FtsY and a membrane-embedded protein could be detected (J.Luirink, unpublished observations). Our data suggest a general affinity of FtsY for the membrane, and a strong increase in GTP hydrolysis in FtsY upon membrane binding. Therefore, an additional membrane-embedded receptor may not be required. At this point, also in the light of the strong similarities to SecA, it is tempting to speculate that after targeting, FtsY interacts transiently with the translocon to ensure proper delivery of the nascent chain. A more detailed understanding of the events between targeting and insertion of nascent chains into the translocon will require full reconstitution of the SRP/FtsY–mediated targeting reaction.
Materials and methods
Chemicals
POPC, DOPC, PA, egg PE, DOPE, egg PG, DOPG and E.coli phospholipids were all purchased from Avanti Polar Lipids (Pelham, AL).
Strains and constructs
The E.coli strain BL21 F– hsdS gal (DE3) harbouring pLysS was used for expression of FtsY or FtsY–NG cloned in pET vectors as described (Studier et al., 1990). The E.coli TOP10F strain (Stratagene) was used for general cloning. Escherichia coli HDL11, a mutant with inducible phosphatidylglycerolphosphate synthase activity, and its isogenic wild-type SD12 were used to prepare phospholipid extracts for monolayer experiments (Kusters et al., 1991).
Expression and purification of FtsY and FtsY–NG
FtsY and FtsY–NG were expressed and purified as described (Montoya et al., 1997b). Proteins were purified in their apoform, as no GTP or GDP could be detected in the preparations by reversed-phase HPLC (Moser, 1998). Protein for the hydrolysis assays was quantified using Bradford reagent (Bio-Rad) and by measuring the absorption at 280 nm in 6 M guanidine–HCl using a molar extinction coefficient of 19 630 for FtsY and of 8250 for FtsY–NG.
Preparation of liposomes
Liposomes were prepared from E.coli phospholipids (Avanti Polar Lipids) by incubating 20 mg/ml phospholipids in 50 mM Tris–HCl pH 8.0 and 40% glycerol for 5 min on ice, followed by rapid 50-fold dilution in 50 mM Tris–HCl pH 7.5 and 50 mM KCl. After 30 min incubation on ice, liposomes were collected by centrifugation for 20 min at 120 000 g. Liposomes were resuspended to 4 mg/ml, sonicated three times for 10 min and stored at –80°C until use.
Preparation of large unilamellar vesicles
Lipid LUVs were prepared by the extrusion technique (Hope et al., 1985). Multilamellar liposomes prepared in 10 mM Tris–HCl, 150 mM NaCl pH 7.6 (buffer B) were freeze–thawed five or six times and then passed 21 times through two stacked 100 nm pore polycarbonate filters (Nuclepore) in a two-syringe extruder (LiposoFast from Avestin Inc., Ottawa, Canada). Either single lipids or different mixtures (as specified) were used at a concentration of 2–5 mg/ml.
Floatation gradient centrifugation
Purified FtsY or FtsY–NG (250 ng) was incubated for 10 min at 37°C in the absence or presence of 50 μg of liposomes prepared from E.coli phospholipids in 50 mM HEPES pH 7.6, 0.5 M KOAc, 5 mM Mg(OAc)2 (buffer A) in a final volume of 25 μl and subjected to floatation gradient analysis as described (Valent et al., 1998). Fractions were trichloroacetic acid (TCA) precipitated and analysed by immunoblotting using affinity-purified αFtsY serum.
Light scattering experiments
LUV and FtsY or FtsY–NG were mixed at a typical concentration of 100 μM (lipid) and 0.1 μM (protein) in buffer B. The turbidity of the solution was monitored continuously at 405 nm in 96-well flat-bottom microplates using a reader (UV-Max, Molecular Devices, USA). Between each reading, the plate was mixed for 3 s to avoid precipitation. For the salt dependence, solutions containing the reported amount of NaCl were buffered with 10 mM Tris–HCl pH 7.6.
Monolayer experiments
Monolayer surface pressure was measured essentially as described by the platinum Wilhelmy plate method in a thermostatically controlled box, using a Cahn 2000 microbalance (Demel, 1994). Escherichia coli phospholipids from strains SD12 and HDL11 were prepared according to Breukink et al. (1992). Unless stated otherwise, monomolecular lipid layers were spread from a chloroform solution on a subphase buffer of 50 mM Tris–HCl pH 7.6 and 100 mM NaCl, to give an initial surface pressure of 25 mN/m. The Teflon dish had a volume of 5 ml and a surface area of 8.81 cm2. In each measurement, 10 μg of purified protein (either FtsY or FtsY–NG) were used since this amount of protein was shown to yield near to maximum insertion levels in pilot experiments. All buffers were filtered and degassed prior to use.
Protease protection assay
Protease sensitivity of FtsY was studied essentially as described (Kusters et al., 1995) by incubating 4 μg of FtsY and 200 μg/ml liposomes in 100 μl of Tris–acetate pH 7.5, 10 mM Mg2+-acetate for 10 min at 37°C, followed by incubation with 8 ng/μl proteinase K (Roche Diagnostics) at 37°C. Nucleotides (Roche Diagnostics) were included at a concentration of 2 mM where indicated. The reaction was stopped at the indicated time points by addition of TCA to a final concentration of 10%. Samples were analysed by SDS–PAGE, and Coomassie-stained protein bands were visualized using the FluorS imager.
Hydrolysis experiments
Hydrolysis experiments were carried out as described (Samuelsson and Olsson, 1993), with slight modifications. The protein was incubated in hydrolysis buffer (20 mM Tris–HCl pH 7.5, 65 mM NaCl, 2 mM MgCl2, 1% glycerol). The reaction was started by adding GTP to the indicated final concentrations (as measured by UV absorption using a molar extinction coefficient of 13 700 for GTP) along with 10 μCi of [γ-32P]GTP (Amersham). Immediately, a time point at 0 min was taken. The reactions were stopped on ice in a 12% charcoal slurry containing 10 mM KH2PO4 and 0.1 M HCl. The absorption mix was vortexed briefly and centrifuged for 10 min at 15 000 g. A 150 μl aliquot of supernatant was transferred to a scintillation cocktail and counted by Cerenkov counting.
Preparation of membrane-bound protein for FT-IR experiments
Aliquots of PE:PG LUV (molar ratio 3:7) were diluted to 250 μg/ml in 200 μl of buffer B and incubated with 20 μg/ml of FtsY or FtsY–NG (lipid to protein molar ratio 833:516). After 1 h at 25°C, the LUVs were separated from unbound protein by ultracentrifugation (in a Beckman Optima TL 100 with swing-out rotor TLS 55) at 190 000 g and 5°C for 10 h. The amount of free protein remaining in the supernatant was estimated by SDS–PAGE. The pellet, consisting of vesicles and bound protein, was resuspended in 90 μl of water and used for FT-IR analysis. Controls with the same amount of protein centrifuged in the absence of the vesicles indicated that the protein alone does not precipitate in these conditions.
Fourier transform infrared spectroscopy
FT-IR spectra were collected in the attenuated total reflection (ATR) configuration (Fringeli and Günthard, 1981) on a Bio-Rad FTS 185 spectrometer with linearized MCT detector and KBr beamsplitter, constantly purged with CO2-free dry air. Ten-reflection Ge crystals (45° cut) were used in a vertical liquid cell. Typically, 50 interferograms were co-added at a resolution of 0.5 cm–1. For the soluble form, either 25 μg of FtsY or 40 μg FtsY–NG in low salt buffer were deposited and gently dried under nitrogen on one side of the crystal (Arkin et al., 1995). For the lipid-bound protein, 90 μl of protein–LUV pellet (see above) were spread similarly. Deuterated spectra were collected after flushing for 20 min with D2O-rich nitrogen, ensuring equilibration of the fast-exchangeable amide protons. The relative content of secondary structure was estimated as described (Menestrina et al., 1999) in accordance with Byler and Susi (1986) and Goormaghtigh et al. (1990). In the case of membrane-bound protein, the lipid contribution was removed by subtracting the spectra of lipid alone weighted to minimize the residual signal of the phospholipid carbonyl group at 1738 cm–1. Differential spectra were obtained by subtracting the spectrum of the protein in soluble form from that of the lipid-bound form, with a weight that minimizes the remaining integral in the amide I′ region.
Acknowledgments
Acknowledgements
We would like to thank Audrey Kuhn (EMBL), Cristina Potrich (Trento), Corinne Hagen-Jongman (Amsterdam) and Marien de Jonge (Amsterdam) for excellent technical assistance, Anton Rietveld for stimulating discussions and advice concerning lipids, and Hans van der Zandt for help with analytical ultracentrifugation. The work was supported by a TMR-Network grant to I.S. and J.L. (ERBFMRXCT-960035), a grant from the FCI to K.t.K., a grant from The Netherlands Organization for Scientific Research to E.deL. and an EMBO short-term fellowship to C.M.; G.M. was supported financially by the Italian Consiglio Nazionale delle Ricerche (CNR) and by the Instituto Trentino di Cultura (ITC).
References
- Arkin I.T., Rothman, M., Ludlam, C.F.C., Aimoto, S., Engelman, D.M., Rothschild, K.J. and Smith, S.O. (1995) Structural model of the phospholamban ion channel complex in phospholipid membranes. J. Mol. Biol., 248, 824–834. [DOI] [PubMed] [Google Scholar]
- Breukink E., Demel, R.A., de Korte-Kool, G. and de Kruijff, B. (1992) SecA insertion into phospholipids is stimulated by negatively charged lipids and inhibited by ATP: a monolayer study. Biochemistry, 31, 1119–1124. [DOI] [PubMed] [Google Scholar]
- Byler D.M. and Susi, H. (1986) Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers, 25, 469–487. [DOI] [PubMed] [Google Scholar]
- Cabelli R.J., Dolan, K.M., Qian, L. and Oliver, D.B. (1991) Characterization of membrane-associated and soluble states of SecA protein from wild-type and SecA51 (TS) mutant strains of Escherichia coli.J. Biol. Chem., 266, 24420–24427. [PubMed] [Google Scholar]
- Cabiaux V., Buckley, J.T., Wattiez, R., Ruysschaert, J.M., Parker, M.W. and van der Goot, F.G. (1997) Conformational changes in aerolysin during the transition from the water-soluble protoxin to the membrane channel. Biochemistry, 36, 15224–15232. [DOI] [PubMed] [Google Scholar]
- Cullis P.R. and de Kruijff, B. (1979) Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta, 559, 399–420. [DOI] [PubMed] [Google Scholar]
- De Gier J.W.L., Mansournia, P., Valent, Q.A., Phillips, G.J., Luirink, J. and von Heijne, G. (1996) Assembly of a cytoplasmic membrane protein in Escherichia coli is dependent on the signal recognition particle. FEBS Lett., 399, 307–309. [DOI] [PubMed] [Google Scholar]
- De Leeuw E., Poland, D., Mol, O., Sinning, I., ten Hagen-Jongman, C.M., Oudega, B. and Luirink, J. (1997) Membrane association of FtsY, the E.coli SRP receptor. FEBS Lett., 416, 225–229. [DOI] [PubMed] [Google Scholar]
- Demel R.A. (1994) Monomolecular layers in the study of biomembranes. Subcell. Biochem., 23, 83–120. [DOI] [PubMed] [Google Scholar]
- Driessen A.J.M. (1996) Translocation of proteins across the bacterial cytoplasmic membrane. In Konings,W.N., Kaback,H.R. and Lolkema,J.S. (eds), Handbook of Biophysics, 2. Transport Processes in Membranes. Elsevier, Amsterdam, The Netherlands, pp. 759–790. [Google Scholar]
- Driessen A.J.M., Fekkes, P. and van der Wolk, J.P.W. (1998) The Sec system. Curr. Opin. Microbiol., 2, 216–222. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz, J. and Szoka, F.C. (1986) Fusion of phosphatidyl- ethanolamine-containing liposomes and mechanism of the l alpha–HII phase transition. Biochemistry, 25, 285–294. [DOI] [PubMed] [Google Scholar]
- Fringeli U.P. and Günthard, H.H. (1981) Infrared membrane spectroscopy. Mol. Biol. Biochem. Biophys., 31, 270–332. [DOI] [PubMed] [Google Scholar]
- Gill D.R., Hatfull, G.F. and Salmond, G.P.C. (1986) A new cell division operon in Escherichia coli.Mol. Gen. Genet., 205, 134–145. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., Cabiaux, V. and Ruysschaert, J.-M. (1990) Secondary structure and dosage of soluble and membrane proteins by attenuated total reflection Fourier-transform infrared spectroscopy on hydrated films. Eur. J. Biochem., 193, 409–420. [DOI] [PubMed] [Google Scholar]
- Hartl F.-U., Lecker, S., Schiebel, E., Hendrick, J.P. and Wickner, W. (1990) The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E.coli plasma membrane. Cell, 63, 269–279. [DOI] [PubMed] [Google Scholar]
- Hope M.J., Bally, M.B., Webb, G. and Cullis, P.R. (1985) Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta, 812, 55–65. [DOI] [PubMed] [Google Scholar]
- Kusters R., Dowhan, W. and de Kruijff, B. (1991) Negatively charged phospholipids restore prePhoE translocation across phosphatidyl– glycerol-depleted Escherichia coli inner membrane vesicles. J. Biol. Chem., 266, 8659–8662. [PubMed] [Google Scholar]
- Kusters R., Lentzen, G., Eppens, E., van Geel, A., van der Weijden, C.C., Wintermeyer, W. and Luirink, J. (1995) The functioning of the SRP receptor FtsY in protein targeting in E.coli is correlated with its ability to bind and hydrolyse GTP. FEBS Lett., 372, 253–258. [DOI] [PubMed] [Google Scholar]
- Luirink J. and Dobberstein, B. (1994) Mammalian and Escherichia coli signal recognition particles. Mol. Microbiol., 11, 9–13. [DOI] [PubMed] [Google Scholar]
- Luirink J., ten Hagen-Jongman, C.M., van der Weijden, C.C., Oudega, B., High, S., Dobberstein, B. and Kusters, R. (1994) An alternative protein targeting pathway in Escherichia coli: studies on the role of FtsY. EMBO J., 13, 2289–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane J. and Müller, M. (1995) Functional integration of a polytopic membrane protein of E.coli requires the bacterial signal recognition particle. Eur. J. Biochem., 223, 766–771. [DOI] [PubMed] [Google Scholar]
- Menestrina G., Cabiaux, V. and Tejuca, M. (1999) Secondary structure of sea anemone cytolysins in soluble and membrane bound form by infrared spectroscopy. Biochem. Biophys. Res. Commun., 254, 174–180. [DOI] [PubMed] [Google Scholar]
- Miller J.D., Bernstein, H.D. and Walter, P. (1994) Interaction of E.coli Ffh/4.5S ribonucleoprotein and FtsY mimics that of mammalian signal recognition particle and its receptor. Nature, 367, 657–659. [DOI] [PubMed] [Google Scholar]
- Mitchell C. and Oliver, D. (1993) Two distinct ATP-binding domains are needed to promote protein export by Escherichia coli SecA ATPase. Mol. Micobiol., 10, 483–497. [DOI] [PubMed] [Google Scholar]
- Montoya G., Svensson, C., Luirink, J. and Sinning, I. (1997a) Crystal structure of the NG domain from the signal recognition particle receptor FtsY. Nature, 385, 365–368. [DOI] [PubMed] [Google Scholar]
- Montoya G., Svensson, C., Luirink, J. and Sinning, I. (1997b) Expression, crystallization and preliminary X-ray diffraction study of FtsY, the docking protein of the signal recognition particle of E.coli.Proteins, 28, 285–288. [DOI] [PubMed] [Google Scholar]
- Morein S., Andersson, A., Rilfors, L. and Lindblom, G. (1996) Wild-type Escherichia coli cells regulate the membrane lipid composition in a ‘window’ between gel and non-lamellar structures. J. Biol. Chem., 271, 6801–6809. [DOI] [PubMed] [Google Scholar]
- Moser C. (1998) Functional studies on the receptor of the signal recognition particle. PhD thesis, University of Heidelberg. [Google Scholar]
- Moser C., Mol, O., Goody, R.S. and Sinning, I. (1997) The signal recognition particle receptor of Escherichia coli (FtsY) has a nucleotide exchange factor built into the GTPase domain. Proc. Natl Acad. Sci. USA, 94, 11339–11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T. and Walter, P. (1995) Reciprocal stimulation of GTP hydrolysis by two directly interacting GTPases. Science, 269, 1422–1424. [DOI] [PubMed] [Google Scholar]
- Powers T. and Walter, P. (1997) Co-translational protein targeting catalyzed by the Escherichia coli signal recognition particle and its receptor. EMBO J., 16, 4880–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport T.A., Jungnickel, B. and Kutay, U. (1996) Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu. Rev. Biochem., 65, 271–303. [DOI] [PubMed] [Google Scholar]
- Samuelsson T. and Olsson, M. (1993) GTPase activity of a bacterial SRP-like complex. Nucleic Acids Res., 21, 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T. and Zwieb, C. (1999) The signal recognition particle database (SRPDB). Nucleic Acids Res., 27, 169–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti P.A., Valent, Q.A., Manting, E.H., Urbanus, M.L., Driessen, A.J.M., Oudega, B. and Luirink, J. (1999) SecA is not required for SRP-mediated targeting and initial membrane insertion of a nascent inner membrane protein. J. Biol. Chem., 274, 29883–29888. [DOI] [PubMed] [Google Scholar]
- Seluanov A. and Bibi, E. (1997) FtsY, the prokaryotic signal recognition particle receptor homologue, is essential for biogenesis of membrane proteins. J. Biol. Chem., 272, 2053–2055. [DOI] [PubMed] [Google Scholar]
- Studier F.W., Rosenberg, A.H., Dunn, J.J. and Dubendorff, J.W. (1990) Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol., 185, 60–89. [DOI] [PubMed] [Google Scholar]
- Tamm L.K. and Tatulian, S.A. (1997) Infrared spectroscopy of proteins and peptides in lipid bilayers. Rev. Biophys., 30, 365–429. [DOI] [PubMed] [Google Scholar]
- Tatulian S.A., Hinterdorfer, P., Baber, G. and Tamm, L.K. (1995) Influenza hemagglutinin assumes a tilted conformation during membrane fusion as determined by attenuated total reflection FTIR spectroscopy. EMBO J., 14, 5514–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrandt N.D., London, E. and Oliver, D.B. (1992) Deep penetration of a portion of Escherichia coli SecA protein into model membranes is promoted by anionic phospholipids and by partial unfolding. J. Biol. Chem., 267, 15184–15192. [PubMed] [Google Scholar]
- Ulbrandt N.D., Newitt, J.A. and Bernstein, H.D. (1997) The E.coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell, 88, 187–196. [DOI] [PubMed] [Google Scholar]
- Valent Q.A., de Gier, J.-W.L., van Heijne, G., Kendall, D.A., ten Hagen-Jongman, C.M., Oudega, B. and Luirink, J. (1997) Nascent membrane and presecretory proteins synthesised in Escherichia coli associate with signal recognition particle and trigger factor. Mol. Microbiol., 25, 53–64. [DOI] [PubMed] [Google Scholar]
- Valent Q.A., Scotti, P.A., High, S., de Gier, J.-W.L., von Heijne, G., Lentzen, G., Wintermeyer, W., Oudega, B. and Luirink, J. (1998) The E.coli SRP and SecB targeting pathways converge at the translocon. EMBO J., 17, 2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin S.L. (1994) From the elephant to E.coli: SRP-dependent protein targeting. Cell, 77, 787–790. [DOI] [PubMed] [Google Scholar]
- Young J.C. and Andrews, D.W. (1996) The signal recognition particle receptor α subunit assembles co-translationally on the endoplasmic reticulum membrane during an mRNA-encoded translation pause in vitro.EMBO J., 15, 172–181. [PMC free article] [PubMed] [Google Scholar]
- Young J.C., Ursini, J., Legate, K.R., Miller, J.D., Walter, P. and Andrews, D.W. (1995) An amino-terminal domain containing hydrophobic and hydrophilic sequences binds the signal recognition particle receptor α subunit to the β subunit on the endoplasmic reticulum membrane. J. Biol. Chem., 270, 15650–15657. [DOI] [PubMed] [Google Scholar]