Abstract
OBJECTIVE
To better understand the natural history of weight change with androgen-deprivation therapy (ADT), we investigated the effect of ADT on body weight among men from the Shared Equal Access Regional Cancer Hospital (SEARCH) database.
Men undergoing ADT lose lean muscle but gain fat mass, contributing to an overall gain in weight.
PATIENTS AND METHODS
We identified 132 men in SEARCH who received ADT after radical prostatectomy.
‘Weight change’ was defined as the difference in weight before starting ADT (6 months before ADT) and the on-ADT weight (between 6 and 18 months after starting ADT).
In a subanalysis, baseline characteristics of weight-gainers and -losers were analysed using univariate and multivariate analysis to test association with weight change.
RESULTS
In all, 92 men (70%) gained weight, and 40 (30%) either lost or maintained a stable weight.
On average, weight on ADT was 2.2 kg higher than the weight before ADT, with the mean change for weight-gainers and -losers being +4.2 kg and −2.4 kg, respectively.
This compared with no significant weight change in the year before starting ADT (paired t-test, change −0.7 kg, P = 0.19) or in the second year on ADT (paired t-test, change −0.5 kg, P = 0.46) for 84 men in whom these additional weight values were recorded.
There was no significant association between any of the features examined and weight change on univariate and multivariate analysis.
CONCLUSIONS
In this longitudinal study, ADT was accompanied by significant weight gain (+2.2 kg). This change occurred primarily in the first year of therapy, with men neither losing nor gaining additional weight thereafter.
Keywords: prostate cancer, androgen deprivation, side-effects, weight
INTRODUCTION
Prostate cancer is the most commonly diagnosed malignancy in American men, with a lifetime risk of one in six. In 2009 alone, it has been estimated that prostate cancer accounted for 25% of cancer incidences in men and 9% of cancer deaths [1]. In men diagnosed with metastatic prostate cancer, or those with recurrent disease, androgen-deprivation therapy (ADT) is commonly administered with the intent of reducing morbidity and improving survival [2]. However, this benefit does not come without cost, as the hypogonadal state produced by androgen deprivation has been linked to hyperglycaemia and insulin resistance [3,4], cardiovascular disease [5–7], sexual dysfunction, and decreased quality of life [8].
These adverse side-effects typically go hand-in-hand with changes in body mass composition. Previous studies have shown that men with prostate cancer undergoing ADT for a duration of 12 months exhibit a significant decrease in muscle and bone mass and an increase in fat mass, contributing to an overall gain in weight [9,10]. Galvao et al. [11] recently reported that after 9 months of ADT, both whole-body lean mass and bone mineral density decreased by a mean of 2.4%, while fat mass increased by a mean of 13.8%. In addition, men on ADT exhibited a decrease in physical activity and increase in fatigue levels. Smith et al. [10] showed that in men with localized prostate cancer, 48 weeks of ADT increased both body weight and body mass index (BMI) by 2.4%.
While these studies show an important trend of overall weight gain, there is limited information about the natural history of weight change on ADT. Likewise, the features, e.g. demographic, clinical, or pathological, which can be used to identify the men at greatest risk for body weight change, are unknown. Therefore, we sought to investigate trends and predictors of weight change in men undergoing ADT using data from the Shared Equal Access Regional Cancer Hospital (SEARCH) database.
PATIENTS AND METHODS
After obtaining Institutional Review Board approval, data were collected from 2123 men who underwent radical prostatectomy (RP) between 1988 and 2009 at four Veterans Affairs Medical Centres (West Los Angeles and Palo Alto, CA; Augusta, GA; Durham, NC, USA) and compiled into the SEARCH database. This database excludes men who received preoperative ADT or radiation therapy. Within this population, we identified a population of 277 men who received ADT, defined as bilateral orchidectomy or GnRH agonist, at some point in their postoperative course. Of the 277 men treated with ADT after RP, 143 had recorded weights that could be abstracted within 6 months before starting ADT and between 6 to 18 months after starting ADT. Values for patient weight were taken from prospectively recorded medical records and analysed retrospectively. Outliers, defined as a change in body weight between these two measurements greater than two standard deviations (sds) from the mean, were excluded from analysis (11 patients). Thus, the final study population was 132 men.
DEMOGRAPHIC, CLINICAL, AND PATHOLOGICAL VARIABLES
‘Weight change’ was defined as the difference in weight before starting ADT (within 6 months before the start of ADT) and the on-ADT weight (between 6 and 18 months after starting ADT). In men with multiple weight values in these intervals, we selected the weight closest to the start of ADT and closest to 1 year on ADT, respectively. In a subanalysis, we sought to examine weight change in the year before ADT and in the second year after ADT. To accomplish this, we abstracted weight values in the year before ADT (between 18 and 6 months before the start of ADT) and in the second year after starting ADT (between 18 and 30 months after the start of ADT) and calculated differences in weight change over time. In men with multiple weight values in these intervals, we selected the weight closest to 12 months before and 24 months after the start of ADT, respectively.
‘Weight-gainers’ were defined as men who gained weight during the ‘pre-ADT’ and ‘post-ADT’ time interval, and ‘weight-losers’ were defined as men losing weight or those maintaining the exact same weight. Patient age and year were defined at the start of ADT and evaluated as continuous variables. Centre and race were defined as categorical variables, with race including White, Black, and Other. Pre-ADT BMI was calculated by dividing the weight closest to, but preceding, the start of ADT, over the square of height (kg/m2) and was examined as a continuous logarithmically transformed variable. Pre-ADT PSA values (ng/mL) were obtained from the measurement closest to the date of, but preceding, the start of ADT. Extracapsular extension, surgical margin status, seminal vesicle invasion, and lymph node involvement were determined at the time of RP. Gleason score was obtained from the surgical specimen and placed into one of three categories: ≤6, 3 + 4, and ≥4 + 3.
Weight change was assessed via paired t-test between the intervals of 1 year pre-ADT, pre-ADT, post-ADT, and 2 years post-ADT. The distribution of demographic, clinical, and pathological characteristics of weight-gainers vs weight-losers was compared via t-test, rank-sum, and chi-squared test as appropriate. Normally distributed variables were described using means with sds, and non-normally distributed variables were described using medians with interquartile (IQR) ranges. All P-values were two-sided with a threshold of P < 0.05 for statistical significance. Multivariate analysis of the characteristics predictive of weight change, defined as a continuous variable, was performed using linear regression. In all, 106 men had complete data for all of the aforementioned variables and were included in the full multivariate model.
RESULTS
Baseline characteristics of the 132 men included in this study are shown in Table 1. All patients received GnRH agonist, excepting one subject who underwent bilateral orchidectomy. The mean (sd) age at the time of ADT for the study cohort was 66 (8) years. In all, 50% of the men were White, 42% were Black, and 8% were Other. The mean (sd) BMI before starting ADT was 29.0 (4.8) kg/m2. The median (IQR) time from the measurement of pre-ADT weight to the start of ADT was 33 (11–76) days, and the median time from the start of ADT to the measurement of post-ADT weight was 363 (310–381) days.
TABLE 1.
Clinical and pathological characteristics of men in the SEARCH database who started ADT after RP stratified by whether they gained weight or lost weight/had no change in the weight in the first year of ADT
| Variable | Total | Weight gainers | Weight losers | P* |
|---|---|---|---|---|
| No. of patients (%) | 132 | 92 (70) | 40 (30) | |
| Mean (sd): | ||||
| weight change, kg | 2.2 (4.1) | 4.2 (2.9) | −2.4 (2.4) | |
| age at ADT, years | 66 (8) | 66 (8) | 66 (8) | 0.81† |
| Median (IQR): | ||||
| year of ADT | 2003 (‘01–‘05) | 2003 (‘01–‘05) | 2003 (‘01–‘05) | 0.75‡ |
| N (%): | ||||
| Race: | 0.25 | |||
| White | 66 (50) | 47 (51) | 19 (48) | |
| Black | 56 (42) | 36 (39) | 20 (50) | |
| Other | 10 (8) | 9 (10) | 1 (2) | |
| Centre: | 0.61 | |||
| 1 | 38 (29) | 27 (29) | 11 (27) | |
| 2 | 26 (20) | 20 (22) | 6 (15) | |
| 3 | 16 (12) | 12 (13) | 4 (10) | |
| 4 | 52 (39) | 33 (36) | 19 (48) | |
| Mean (sd): | ||||
| pre-ADT BMI, kg/m2 | 29.0 (4.8) | 28.4 (4.4) | 30.0 (5.5) | 0.10† |
| Median (IQR): | ||||
| pre-ADT PSA level, ng/mL | 1.0 (0.2–4.8) | 1.0 (0.2–5.6) | 1.2 (0.4–2.8) | 0.86‡ |
| N (%): | ||||
| Pathological Gleason: | 0.74 | |||
| ≤6 | 22 (17) | 15 (16) | 7 (18) | |
| 3 + 4 | 46 (35) | 34 (37) | 12 (30) | |
| ≥4 + 3 | 64 (48) | 43 (47) | 21 (53) | |
| Extracapsular extension: | 0.33 | |||
| positive | 57 (44) | 42 (47) | 15 (38) | |
| negative | 73 (56) | 48 (53) | 25 (63) | |
| Surgical margins: | 0.56 | |||
| positive | 91 (31) | 62 (67) | 29 (73) | |
| negative | 41 (69) | 30 (33) | 11 (27) | |
| Seminal vesicle invasion: | 0.99 | |||
| positive | 46 (35) | 32 (35) | 14 (35) | |
| negative | 85 (65) | 59 (65) | 26 (65) | |
| Lymph node status: | 0.68 | |||
| 0 | 100 (76) | 71 (77) | 29 (73) | |
| 1 | 8 (6) | 6 (7) | 2 (5) | |
| 2 | 24 (18) | 15 (16) | 9 (22) |
P-value from chi-squared except where noted;
P-value from t-test;
rank sum.
Of the 132 men in our study, 92 (70%) gained weight, 35 (26%) lost weight and five (4%) maintained a stable weight. The mean weight change for all men was +2.2 kg (P < 0.001). The mean weight change for the groups of weight-gainers and -losers were +4.2 (2.9) kg and −2.4 (2.4) kg, respectively. The mean age for both groups was 66 (8) years. The mean BMI before the start of ADT was 28.4 (4.4) kg/m2 and 30.0 (5.5) kg/m2 (P = 0.10), respectively. Weight-gainers and -losers did not differ in any clinical or pathological features (all P ≥ 0.10; Table 1). Similarly, on multivariate analysis among men with complete data (106 men), we found no demographic, clinical, or pathological features to be significantly predictive of weight change.
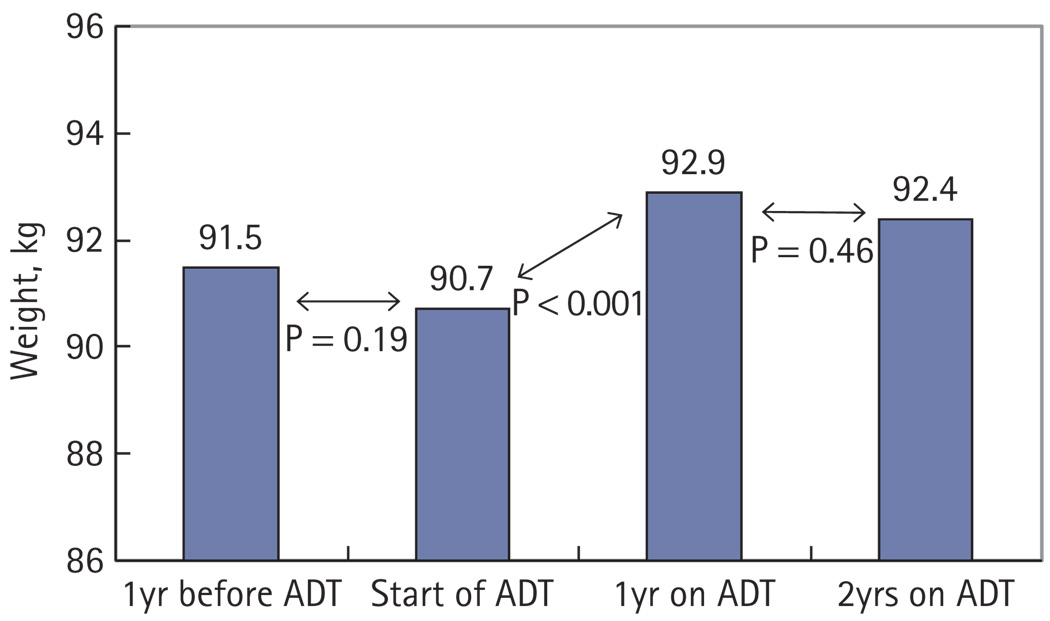
Given that the men in the present study had significant weight gain in their first year on ADT, we sought to characterize weight change in the intervals before and after this period. To accomplish this, we examined whether the men in the present cohort had weight change in the year preceding ADT and in the second year of ADT use, and noted that 84 (64%) men had recorded weights during these time intervals. Among these 84 men, the average weight on ADT was 2.1 kg higher than the pre-ADT weight (P < 0.001), paralleling the findings from the entire group. There was no significant weight change in the year before starting ADT (weight change −0.7 kg; P = 0.19) or in the second year of ADT (weight change −0.5 kg; P = 0.46; Fig. 1). The median (IQR) time intervals from these measurements were 403 (378–460) days before the start of ADT and 728 (683–757) days after the start of ADT, respectively.
FIG. 1.
The mean weight of 84 men receiving ADT over 3 years.
DISCUSSION
Androgen deprivation is a well-established therapy for men with metastatic prostate cancer. Moreover, ADT is commonly administered to men whose only indication is a rising PSA level after RP or radiation therapy [12]. Despite its clinical efficacy in advanced disease, ADT is associated with an array of adverse side-effects, such as insulin resistance and increasing risk for cardiovascular disease [3–7,13–15]. Additionally, a growing number of studies show that men receiving ADT undergo a shift in body mass composition favouring weight gain, with a net decrease in bone density and lean muscle mass and an increase in fat mass [9–11,16,17]. However, previous studies have been limited in that they have focused primarily on a short time-frame immediately after initiation of ADT. In the present study, we sought to characterize weight change on ADT during a 3-year interval, from 1 year before the start of ADT to 2 years afterwards.
Initially, the results of the present study validated previous reports that men gain weight while on ADT. Among the present 132 men, there was an average increase in weight of 2.2 kg, or 2.4%, a proportion identical to the results published by Smith et al. [10] in a prospective study of men with prostate cancer receiving ADT. The authors of that study remarked that their cohort was comprised mostly of White men and required validation in a more racially diverse population. The present study addressed this concern, with a fair representation of Black men (42%) and men of other races (8%) in the cohort. Notably, our finding of an average weight gain of 2.2 kg represents a composite of increasing fat and lean muscle mass. In this context, the increase in fat mass could be interpreted as exceeding 2.2 kg, though actual data on fat mass per se were not available. However, it should be noted that this and other studies have not adequately addressed the contribution of fluid changes to increased body weight.
Importantly, in the present study ADT-associated weight gain occurred primarily in the first year of therapy. Among 84 men with recorded weights for three consecutive years, they did not change their weight in the year before starting therapy, nor did they gain additional weight during the second year of therapy. We conclude that most of the weight gain occured in the first year of ADT, with men neither losing nor gaining additional weight thereafter. This is consistent with previous studies on the adverse side-effects of ADT. For example, Lee et al. [16] investigated the effects of long-term ADT, finding that fat mass increases and lean body mass decreases mainly during initial therapy (12 months). In men receiving long-term ADT (up to 30 months), only bone mineral density steadily declined beyond the initial 12 months. Additionally, Smith et al. [13] reported that the trend of weight gain in the first 12 months of ADT is approximately linear, with two-thirds of the weight gain occurring at 6 months of therapy. Finally, this is also consistent with data showing that insulin resistance can develop within 12 weeks after ADT, implying that even a few months of ADT can have deleterious effects [4].
The overall health implications of ADT are now known to include weight gain [4,9,10], decreased lean muscle and bone mass [11,16], insulin resistance [3,4], and increased risk of diabetes [5,6,18] among other effects. The degree to which these factors lead to increased coronary artery disease (CAD) remains unclear. While some studies have suggested increased risk of CAD among men undergoing ADT [6,14,19], other studies have found no increased risk [18,20]. Among the studies that have linked ADT to CAD, Keating et al. [6] reported that as little as 1–4 months of ADT increased the risk of CAD, with this risk remaining equally elevated among men who continued treatment for a longer period. Additionally, D’Amico et al. [14] showed that ADT increases the incidence of fatal myocardial infarction, with the greatest elevation of risk occurring within the first year of therapy. While the risk of myocardial infarction in that study did not return to baseline after this initial year, it was not further increased by continued ADT. However, these studies must be tempered by other data showing no impact of ADT on CAD [18,20]. While the link between ADT and CAD remains unclear, the present findings lend further support that the adverse metabolic effects of ADT occur early during therapy and persist over time.
Incidentally, we identified a moderately sized group of men (30%) that did not gain weight, and in fact lost or maintained a stable weight while on ADT. When stratified into their respective groups, weight-gainers and -losers had a mean weight change of +4.2 kg and −2.4 kg, respectively. Given this heterogeneous response in body weight to ADT, we sought to analyse various demographic, clinical, and pathological factors to identify men at most risk for weight gain. However, in both univariate and multivariate analysis, we were unable to find any statistically significant relationship predicting the direction of weight change. Of note, patient age, pre-ADT BMI, disease aggressiveness, race, or centre were not predictive of weight change. It is unclear at this time whether this lack of a statistically significant association is due to limited statistical power or a true lack of association. Thus, future efforts using more sophisticated metabolic measures are needed to identify men at the greatest risk of metabolic abnormalities from ADT.
The present study is not without limitations. First, because of the SEARCH database’s retrospective nature, we were limited to analysis of vital sign statistics, namely weight. These data are inherently subject to human error and therefore variation in reporting. Moreover, the SEARCH database does not contain specific information on body composition, such as fat mass, abdominal vs limb obesity, muscle mass or bone mineral density. We thus measured weight change as a proxy of metabolic change, as these detailed data on body composition were not available. Further characterization of weight change due to ADT should closely examine individual components of body mass, such as lean mass, fat mass, and bone density.
Second, to qualify for inclusion into the SEARCH database, men must have undergone RP at one of four Veterans Affairs hospitals. This may be important in that men with biochemical recurrence after RP are but one subset of the total population of men with prostate cancer receiving ADT. Additionally, the men in the SEARCH database may have been younger and healthier than the typical patient with prostate cancer, and many patients lacking recorded weight values for the time interval of interest were excluded from study. Indeed, it might have been interesting to analyse weight change in a population of men who are generally older and less fit, as these patients might respond differently to ADT. Unfortunately, the SEARCH database only includes men treated with RP and thus, the conclusions from this study may not be applicable beyond the RP population. Finally, we were unable to control for various co-interventions not detailed in the SEARCH database, such as dietary habits or exercise frequency. Nevertheless, the results from the present study warrant prospective analysis of the natural history of weight change in men receiving ADT.
In conclusion, while ADT has been a mainstay treatment for recurrent or metastatic prostate cancer, physicians are increasing the use of ADT to treat localized disease [21]. This rising utilization of ADT warrants a closer examination of the side-effects of therapy, which include metabolic changes that potentially contribute to an increased incidence of diabetes and perhaps other comorbidities. In the present study, men on ADT tended to gain weight. Our work, in conjunction with previous studies, suggest that these changes take place within the first year of therapy and persist thereafter, but do not necessarily continue to worsen over time.
ACKNOWLEDGEMENTS
Supported by the Department of Veterans Affairs, National Institute of Health R01CA100938 (W.J.A.), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (W.J.A.), the Georgia Cancer Coalition (M.K.T.), the Department of Defense, Prostate Cancer Research Program (L.L.B./S.J.F.), the American Urological Association Foundation/Astellas Rising Star in Urology Award (S.J.F.) and the Howard Hughes Medical Institute Medical Fellows Program (H.S.K.). Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
Abbreviations
- ADT
androgen-deprivation therapy
- BMI
body mass index
- CAD
coronary artery disease
- IQR
interquartile range
- RP
radical prostatectomy
- SEARCH
the Shared Equal Access Regional Cancer Hospital (database)
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–1788. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 3.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–588. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 4.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 5.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 7.Tsai HK, D’Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 8.Basaria S, Lieb J, 2nd, Tang AM, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 2002;56:779–786. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63:742–745. doi: 10.1016/j.urology.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 10.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 11.Galvao DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102:44–47. doi: 10.1111/j.1464-410X.2008.07539.x. [DOI] [PubMed] [Google Scholar]
- 12.Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol. 2000;163:1632–1642. [PubMed] [Google Scholar]
- 13.Smith MR, Lee H, McGovern F, et al. Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: differences from the classic metabolic syndrome. Cancer. 2008;112:2188–2194. doi: 10.1002/cncr.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 15.Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, McGovern K, Finkelstein JS, Smith MR. Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer. 2005;104:1633–1637. doi: 10.1002/cncr.21381. [DOI] [PubMed] [Google Scholar]
- 17.Smith MR. Osteoporosis and other adverse body composition changes during androgen deprivation therapy for prostate cancer. Cancer Metastasis Rev. 2002;21:159–166. doi: 10.1023/a:1020840311573. [DOI] [PubMed] [Google Scholar]
- 18.Alibhai SM, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–3458. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 20.Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol. 2009;27:92–99. doi: 10.1200/JCO.2007.12.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]