Abstract
Staphylococcus aureus continues to be a major health problem. This species' requirement for proline and proline transport from the extracellular environment is not well understood. Here, we identify a S. aureus low-affinity proline transport gene (opuD) with homology to the OpuD protein of Bacillus subtilis. Mutation of the opuD gene caused a significant decline in proline uptake under low affinity conditions as compared to wild-type, but the opuD mutant strain showed no significant attenuation in a murine abscess model of infection. The S. aureus opuD gene was transcriptionally activated during growth in moderate osmolarity media with high levels of proline or glycine betaine independent of SigB. In murine abscesses, the opuD gene was activated at a later time point, whereas opuD expression dropped over the course of an 18 h period within murine urinary tracts. Transcriptional regulation of opuD in S. aureus appears to be coordinated within this species when grown in moderate to high NaCl environments, but the level of extracellular proline had a marked effect on expression of this proline transport gene. The differential regulation of proline transport genes in S. aureus may be an adaptation for life in a variety of environments, including survival within the human body.
Keywords: proline uptake, murine abscess, gene regulation, osmotic stress, osmolytes, urinary tract
1. Introduction
Staphylococcus aureus is found as normal flora bacteria on humans, but the species can cause a variety of diseases, ranging from localized skin infections and soft-tissue infections to more serious infections of the respiratory tract, bone, joint, and endovascular systems (Lowy, 1998). Transport of proline and glycine betaine are believed to be part of the basic growth processes of S. aureus that significantly contributes to the accumulation of these osmolytes and subsequent growth in very high salt environments (1 to 2 M NaCl; Anderson & Witter, 1982; Bae & Miller, 1992; Graham & Wilkinson, 1992; Koujima et al., 1978; Miller et al., 1991; Pourkomailian & Booth, 1992; 1994; Townsend & Wilkinson, 1992; Vijarankul et al., 1997). Proline and glycine betaine have been shown to be the most effective osmoprotectants of S. aureus, since substantial impairments in S. aureus growth were observed when these solutes were excluded from defined high osmotic media (Amin et al., 1995; Graham & Wilkinson, 1992; Miller et al., 1991. A better understanding of proline and glycine betaine transport in S. aureus could not only increase our general knowledge of this organism, but may provide a possible target to control the growth of this organism.
In S. aureus, two proline transport systems have been identified: a low affinity system and a high affinity system (Bae & Miller, 1992; Bae et al., 1993; Pourkomailian & Booth, 1994; Townsend & Wilkinson, 1992; Wengender & Miller, 1995). A transposon mutation in the gene for the high affinity (putP) proline transport system of S. aureus rendered the bacteria less able to survive in several models of infection (Bayer et al., 1999; Schwan et al., 1998; 2004), suggesting that the proline transport systems may play an essential role in the in vivo survival of S. aureus. Moreover, initial observations suggest that the low-affinity proline transport system of S. aureus is activated under high osmotic conditions (Bae and Miller, 1992).
In this study, an opuD homolog gene was identified and characterized in S. aureus. The opuD mutant exhibited significantly less proline transport versus the wild-type strain. Moreover, we have demonstrated that an opuD-lux fusion was activated by high proline concentrations and relatively high osmotic conditions. These observations may help us understand how S. aureus grows in different environments with variations in proline levels and changes in the external osmolarity.
2. Materials and methods
2.1 Bacterial strains and plasmids
All strains and constructs are listed in Table 1. Strain O6464, a mariner transposon mutant in the Newman strain was obtained from Dr. Dominique Missiakas, University of Chicago (Bae et al. 2004). Escherichia coli DH5α-MCR (Gibco BRL, Rockville, MD) was used for the construction of the opuD∷lux fusion. Staphylococcus aureus strain RN4220 (Novick, 1990) was used for construction of an opuD mutation as well as for the opu-lux regulation studies. Strains RN6390 (Novick, 1990), Newman, 8325-4 (provided by Jean Lee, Channing Laboratory, Boston, MA), SH1000 and SH1003 (provided by Simon Foster, Sheffield University, Sheffield, UK, Horsburgh et al., 2002) were used to perform the regulation experiments. The pXen5 shuttle vector (Xenogen, Alameda, CA; Francis et al., 2001) was used as the backbone for the creation of the opuD∷lux fusion. This vector contains a promoterless luxABCDE operon, a kanamycin resistance gene located within a Tn4001 transposon, an erythromycin resistance gene, a gram-positive temperature-sensitive origin of replication and an E. coli origin of replication for use as a shuttle vector.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or Plasmid | Relevant Characteristics | Source or Reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | General cloning strain | Gibco/BBL |
| S. aureus | ||
| RN4220 | Transformation efficient strain | (Novick 1990) |
| RN6390 | SigB inactive, rsbU mutant | (Novick 1990) |
| Newman | Clinical isolate, SigB active | Jean Lee |
| 8325-4 | SigB inactive, rsbU mutant | Jean Lee |
| SH1000 | SigB active | (Horsburgh et al. 2002) |
| SH1003 | SigB inactive, sigB mutant | (Horsburgh et al. 2002) |
| DB3-11-37 | RN4220 opuD∷lux | This study |
| DAN2A | RN6390 opuD∷lux | This study |
| KJW4-4 | Newman opuD∷lux | This study |
| O6464 | Newman opuD∷mariner | (Bae et al. 2004) |
| SaBS 7-1 | RN4220 opuD∷Km | This study |
| SaBS11-A | SH1000 opuD∷lux | This study |
| SaBS12-1A | SH1003 opuD∷lux | This study |
| SaBS13-2 | 8325-4 opuD∷lux | This study |
| Plasmids | ||
| pXen5 | Ts origin, Tn4001, promoterless lux | (Francis et al. 2001) |
| pDB3-11 | opuD∷lux on pXen5 | This study |
| pKW8-5 | opuD on pAD123 | This study |
| pWS142-3 | Km cassette insertion into pKW8-5 | This study |
2.2 Media and growth conditions
Escherichia coli DH5α-MCR was maintained using Luria-Bertani agar (LA) and broth (LB); (Gibco BRL, Gaithersburg, MD) and S. aureus strains were propagated for routine use in brain heart infusion (BHI) agar and broth (Becton, Dickinson and Company, Sparks, MD; Difco) supplemented with antibiotics at the following concentrations: 5 μg/ml (gram-positive) or 300 μg/ml (gram-negative) of erythromycin (Sigma Chemical Co., St. Louis, MO) or 250 μg/ml of kanamycin (Sigma). A staphylococcal minimal media described by Rudin et al. (Rudin et al., 1974) that was subsequently modified (Schwan et al., 2006) was used to propagate the S. aureus strains under varying solute and osmotic conditions. To investigate the in vitro regulation of opuD in varying osmotic conditions, batches of standard defined staphylococcal medium (DSM; 1740 μM proline) were made containing 1.0 M NaCl, sucrose, or sorbitol (Sigma) and subsequently diluted with standard DSM to generate osmolyte concentrations ranging from 0 to 1000 mM. A modified version of DSM, containing only 1.74 μM proline, was also created and used for regulation experiments under varying concentrations of proline, betaine, choline, and L-carnitine which ranged from 1.74 μM to 1740 μM. Cultures were grown to mid-logarithmic phase and optical density readings were recorded at 600 nm. Regulation of opuD-lux was determined using a luminometer according to Schwan et al. (Schwan et al., 2006). At least three separate runs were performed for each culture condition and each strain.
2.3 Bioinformatics
The nucleotide sequence of the putative Staphylococcus aureus opuD was identified by BLASTN query using the opuD nucleotide sequence from Bacillus subtilis (Kunst et al., 1997) against the S. aureus NTCT 8325 genomic sequence (Iandolo et al., 2002; located on the Oklahoma Genomic Research Center web site, www.genome.ou.edu/Staph.html). This putative S. aureus opuD nucleotide sequence was then translated into an amino acid sequence and was used to perform a second BLASTP query against all available protein sequences. The results of this secondary query showed a high degree of homology to known OpuD glycine betaine transporters and was present in all sequenced S. aureus strains.
2.4 Construction of an opuD mutant
All primers used in construction were synthesized commercially by Integrated DNA Technology (Coralville, IA). PCR reactions were cycled in a GeneAmp PCR System 2400 machine (Perkin-Elmer, Norwalk, CT). Each reaction was set up as previously described (Schwan et al., 2006) using S. aureus strain RN4220 genomic DNA as the template. Genomic DNA of S. aureus strain RN4220 was isolated using a commercial kit (EdgeBioSystems, Gaitherberg, MD) with the addition of lysostaphin (100 μg/ml; Sigma) and incubated for 20 min at 37°C during the first step of the isolation procedure. Relative DNA quality and quantity was measured spectrophotometrically at 260 nm and 280 nm with a BioSpec-1601 DNA/Protein/Enzyme analyzer (Shimadzu Scientific Instruments, Inc., Columbia, MD).
Initially, the pXen5 shuttle vector plasmid was digested with BamHI and religated, eliminating the Tn4001 transposon as well as the kanamycin resistance gene. The plasmid was then digested with XbaI, to remove the lux operon, and religated to create plasmid pWSXen5. A full length opuD gene (including it's native staphylococcal promoter and upstream region & the stop and a downstream region) was PCR amplified using low-fidelity GoTaq polymerase (Promega, Madison, WI) and the primers: ProP1B (5' ACTGGTACCAATTGTACAGCAGCAATCAGGA 3') and ProP2B (5' GCTGGATCCATACCGTCAAACTTCACTTTAG 3') which contained a KpnI site on the ProP1B end and a BamHI site on the ProP2B end. These sites were used to directionally clone the ProP1B/2B PCR fragment into the pWSXen5 backbone, resulting in the plasmid pKW8-5. To create the opuD mutation in S. aureus RN4420, a kanamycin cassette was PCR amplified using pXen5 DNA as the template with the X5Kan1 (5' CCAGCTCAGAATGCAGCCTAAACATG 3') and X5Kan2 (5' CCAGTCTAGATCAAACATCAGCCAAT 3') primer pair. Both the pKW8-5 DNA and X5Kan1/2 PCR product were cut with XbaI and then ligated together, creating the pWS142-3 plasmid. Plasmid DNA from pWS142-2 was electroporated into RN4220 cells (Iandolo and Kraemer, 1990) and homologous recombination occurred which resulted in strain SaBS7-1. To confirm the mutation, PCR analysis was performed with opuD specific primers. No PCR product was generated using these primers (data not shown), but a product was generated with wild-type staphylococcal DNA as a positive control.
2.5 Radioactive proline uptake
All proline uptake assays were done as described before (Bae and Miller, 1992) using 2.5 μM or 400 μM 3H-proline (Perkin Elmer, Waltham, MA).
2.6 Construction of a opuD∷lux reporter system
PCR primers were designed to amplify a 403 bp DNA fragment containing the putative opuD promoter region included ProP3B (5'-TCG CGA ATT CAC CAA TTA ATG GCA TGA TGA-3') and ProP4 (5'-GAT AGA GCT CAT TGC ACT ATA GAT GAA GAC-3'). The resulting 403 bp product was processed as noted before (Schwan et al., 2006) and verified by restriction digest.
To create the opuD∷lux reporter fusion, pXen5 plasmid DNA was extracted from using a commercial kit (Qiagen), digested using the restriction endonuclease EcoRI (NEB), cleaned using a QIAquick kit (Qiagen), blunted using the Klenow fragment of E. coli (NEB), and then digested again using SstI (Invitrogen, Carlsbad, CA). The blunted/SstI pXen5 DNA was ligated to the 403 bp ProP3B/4 PCR product DNA that was cut with SstI and used to transform E. coli DH5α-MCR cells (Gibco BRL) (Sambrook et al., 1989). Transformants were selected for on LA containing erythromycin. Several transformants were selected for plasmid DNA extraction and were screened by electrophoresis for a 403 bp increase in overall size compared to pXen5. Further screening was performed by PCR amplification of a 322bp internal region of the opuD promoter, using the following primers: 5'-CAT CAC AAC AGC AAT TGC TA-3' (ProP3C) and 5'-ACT GGT GAA TAC TTC TTT CC-3' (ProP4B), to confirm the creation of the opuD∷lux reporter fusion, resulting in pDB3-11.
The pDB3-11 plasmid was electroporated into S. aureus strain RN4220 cells, as stated previously. Transformants resistant to both erythromycin and kanamycin (Sigma) were checked for luciferase activity using a Femtomaster FB 12 luminometer (Zylux Corp., Maryville, TN). One of the resulting clones labeled S. aureus RN4220/pDB3-11 #2 was incubated in BHI broth containing no antibiotics at 43°C overnight and then plated onto BHI agar containing kanamycin to select for Tn4001 inserts in the S. aureus chromosome. Clones resistant to kanamycin and sensitive to erythromycin were tested for luciferase activity to confirm the presence of the opuD∷lux fusion within the S. aureus cells. One of the resulting strains, showing a high level of luminescence, was labeled S. aureus RN4220 Tn pDB3-11 #37.
2.7 Transduction of the opuD∷lux fusion into clinically relevant strains of S. aureus
To investigate regulation in clinically relevant strains of S. aureus, the opuD∷lux reporter fusion was transduced into S. aureus strains RN6390 (SigB inactive), Newman (SigB active), 8325-4 (SigB inactive), SH1000 (SigB active), and SH1003 (SigB inactive) via transduction using the bacterial phage ϕ80α (Pattee & Neveln, 1975). Transductants were selected on BHI agar containing kanamycin and luciferase activity was measured. One transductant from strain RN6390, labeled DAN2A; one transductant from strain Newman, labeled KJW4-4; as well as transductants in the 8325-4 (SaBS13-2), SH1000 (SaBS11-A), and SH1003 (SaBS12-1A) backgrounds were used for additional in vitro analysis.
2.8 Southern blot hybridization
To confirm that the opuD∷lux fusion was successfully inserted only once into the S. aureus strains, Southern blot hybridization was performed. Genomic DNAs extracted from the transduced S. aureus strains listed previously were isolated using a commercial kit as previously described, digested with HindIII, separated on a 0.8% agarose gel, and transferred to a positively charged nylon membrane (Amersham, Piscataway, NJ) and prehybridized for 4 h at 65°C (Sambrook et al., 1989). The Tn4001 containing DNA fragment from pXen5, separated by BamHI digestion and gel purification, was radiolabeled with 32P-dCTP (PerkinElmer, Waltham, MA) using a Prime-It© random-primed nick translation kit (Stratagene, La Jolla, CA) and used to probe the nylon membrane overnight at 65°C as previously described (Schwan et al., 1998). Southern blots were exposed overnight on a phosphorimager screen cassette and developed using a model 475 Phosphorimager (Amersham).
2.9 Murine models of infection
A murine urinary tract infection model was used to assess regulation of the opuD gene in S. aureus cells infecting murine urinary tract tissues (Schaeffer et al., 1987). Fifty microliters of mid-logarithmetic phase grown bacteria, diluted to 108 CFU/ml in PBS, were injected into the bladder and time points at 0, 4, and 18 h post-inoculation were analyzed. Five mice per time point per strain of bacteria were used. Organs were homogenized in 1 ml of phosphate buffered saline (PBS), and the entire homogenate from each organ was placed into a 1.5 ml microfuge tube and read in a luminometer for the relative luminescence units (RLU). Aliquots (100 μl) of the organ homogenates were plated onto BHI agar with kanamycin to obtain viable counts. The RLU per bacterial cell was calculated by taking the RLU number, subtracting out the background and then dividing by the viable count.
The murine thigh abscess model of infection was also used to test regulation of the opuD gene as well as to compare the opuD mutant to wild-type bacteria using the Newman background (Beonton et al., 2004). Bacteria grown in BHI broth to midlogarithmetic phase were diluted to 106 CFU/ml and mixed 1:1 with Cytodex beads (Sigma). Five female Swiss Webster mice per time point (0, 6, 18 and 72 h) were injected intramuscularly in the thigh with 100 μl of the inocula. Mice were sacrificed and organs processed as described above.
2.10 Statistics
Student's t-test was used for statistical analyses of the in vitro growth conditions. P values of ≤0.05 were considered significant. A repeated measures analysis of variance (ANOVA) with a Bonferroni correction was used for assessing the significance of strain KJW4-4 in the animal studies.
3. Results
Confirmation of proline transport by OpuD from Staphylococcus aureus NCTC 8235
Introduction of the S. aureus opuD homolog into E. coli strain WG389 (Wengender & Miller, 1995), which is devoid of all proline transport genes, was successful at restoring the growth of the WG389 strain in M9 minimal media containing 25 μM of proline, confirming that the opuD homolog was indeed a proline transporter. Additionally, when strain WG389/pKW8-5 was grown in M9 minimal media with increasing concentrations of NaCl (0–800 mM), growth increased as NaCl levels increased up to 400 mM and then decreased as the concentration became higher (data not shown).
Next, proline uptake by the opuD mutant strain SaBS7-1, in a RN4220 background, was measured using radiolabeled L-proline under high affinity (2.5 μM) and low affinity (400 μM) conditions. No significant difference in proline uptake was observed between the opuD mutant (28.2 nmol/mg protein) and wild-type (32.1 nmol/mg protein) strains using 2.5 μM proline (TABLE 2). However, proline transport decreased by about half in the opuD mutant strain (489 nmol/mg protein) versus wild-type (802 nmol/mg protein) when proline was at 400 μM. Complementation of the opuD mutation using pKW8-5 restored proline uptake to a wild-type level (856 nmol/mg protein). Furthermore, proline uptake by the O6464 (opuD∷mariner) strain versus the wild-type Newman strain showed results similar to those observed using the RN4220 background (data not shown). These results demonstrate that OpuD is indeed a low-affinity proline transporter.
TABLE 2.
Radioactive proline uptake in strains RN4220, SaBS7-1, and SaBS7-1/pKW8-5 over a 30 sec time course
| Proline uptake (nmol/mg protein)a | |||
|---|---|---|---|
| Strain | Genotype | 2.5 μM | 400 μM |
| RN4220 | Wild-type | 32.1+8.4b | 802+136 |
| SaBS7-1 | opuD∷Km | 28.2+4.7 | 489+158 |
| SaBS7-1/pKW8-5 | opuD∷Km, opuD+ | 35.3+2.6 | 856+42 |
Proline uptake measured after 30 sec.
The data are the mean ± standard deviation for two or three separate reactions.
OpuD alone does not contribute significantly to S. aureus survival in murine abscesses
Since proline uptake was affected in vitro by the opuD mutation, the O6464 and wild type Newman strains were inoculated into murine abscesses. Bacterial numbers from abscess homogenates were compared by viable counts. After three days post-inoculation, the mean viable count numbers were lower for the O6464 (4.61 × 106 CFU/g) compared to the Newman (8.81 × 106 CFU/g) strain, but the difference was not significant (P < 0.42).
Effect of compatible solute concentration on opuD regulation
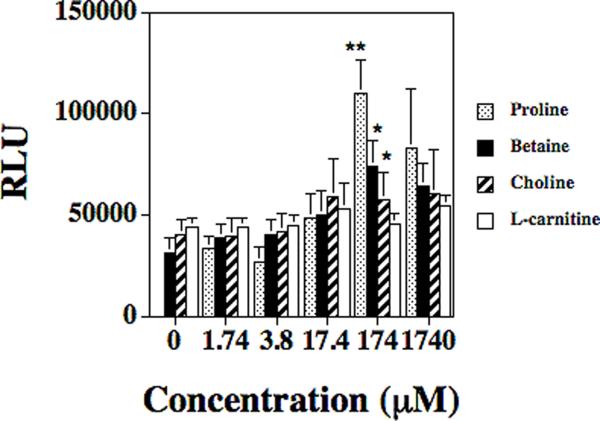
Since nothing is known about the environmental factors that regulate the opuD gene in S. aureus, several substrates that have previously been shown to activate homologous osmoregulatory genes in other bacteria (e.g. B. subtilis and E. coli) were tested using the S. aureus strain DAN2A. The substrates that were tested included: betaine, L-carnitine, choline, and proline. Six substrate concentrations were tested: 1740 μM, 174 μM, 17.4 μM, 3.8 μM, 1.74 μM, and base assay media (modified DSM which contains a 1.74 μM concentration of proline) with no additives. In base assay media as well as modified DSM containing 1.74 μM substrate, the RLUs from the midlogarithmetic phase cultures ranged from 32,446 to 45,362 depending on the substrate that was analyzed (FIG. 1) showing no effect on the regulation of opuD. Given that the S. aureus strain will not grow in media without proline, no RLU values were determined for 0 μM proline. As the substrate concentrations increased to 17.4 μM and greater, both proline and betaine were shown to activate the expression of the opuD gene. Indeed, maximum expression of opuD was observed when the betaine (77,160 RLUs) and proline (107,714 RLUs) concentrations were at 174 μM (FIG. 1), representing a significant two and a half to three fold increase when compared to the base assay media (P < 0.021 and P < 0.0002, respectively).
FIG. 1.
Effects of proline (open column), betaine (closed column), choline (striped column), and carnitine (gray column) concentration on S. aureus strains DAN2A (opuD∷luxABCDE) as determined with an luxABCDE transcriptional fusion. The RLU/bacterial cell were measured with a luminometer and then divided by viable counts; means ± standard deviations are indicated from at least three separate runs. The * denotes significance of P < 0.05 and ** denotes significance of P < 0.0002.
Choline concentrations below 17.4 μM did not appear to regulate opuD expression (P > 0.06). However, choline concentrations of 174 μM and 1740 μM showed a weak induction of opuD expression (58,548 and 65,201 RLUs, respectively) as compared to the 0 μM level (P < 0.026 for 174 μM and P < 0.028 for 1740 μM). On the other hand, L-carnitine levels did not affect opuD transcription at any substrate concentration tested. This data shows that opuD can be transcriptionaly regulated by the presence of the compatible solutes betaine, proline and choline.
To rule out that the observed regulation results were not strain specific, the opuD∷lux reporter fusion was transferred into the Newman strain background, which created strain KJW4-4. Both the DAN2A and KJW4-4 S. aureus strains were compared in media containing different concentrations of betaine. Although betaine concentrations affected both strains in a comparable manner, there were 15–20 fold higher RLU readings obtained for strain KJW4-4 (667,330 to 1,169,782 RLUs) compared to strain DAN2A (32,446 to 77,160 RLUs) (data not shown).
Effect of osmolyte concentration on opuD regulation
To examine whether osmolyte concentrations may be regulating the opuD gene, S. aureus strain DAN2A was grown in standard DSM with varying NaCl conditions that ranged from 0 mM to 1000 mM. The opuD expression rose approximately two-fold in strain DAN2A from 58,215 RLUs at 0 mM NaCl to 106,798 RLUs in 400 mM NaCl (FIG. 2A; P < 0.003). As NaCl concentrations were further increased up to 1000 mM, opuD expression declined by about half as compared to growth in 400 mM NaCl (P < 0.005). When sucrose or sorbitol replaced NaCl as the osmolyte, opuD expression increased three- to four-fold going from 0 mM (24,612 RLUs for sucrose and 26,6738 RLUs for sorbitol) to 1000 mM [71,684 RLUs for sucrose (P < 0.016) and 109,647 RLUs for sorbitol (P < 0.015)]. Thresholds were not reached for either sucrose or sorbitol when testing strain DAN2A.
FIG. 2.


Effects of osmolarity on S. aureus strains A) DAN2A and B) KJW4-4 as determined with a opuD∷luxABCDE transcriptional fusion. The RLU/bacterial cell were measured with a luminometer and then divided by viable counts; means ± standard deviations are indicated from at least three separate runs. Osmolarity effects were tested by using sucrose (closed column), sorbitol (striped column), or NaCl (open column) as the osmolyte. The * denotes significance of P < 0.05.
To determine if strain variability affected the fusion, S. aureus strain KJW4-4 was tested under the same osmotic conditions. Again, overall luminescence in strain KJW4-4 was 5 to 24 times greater than in strain DAN2A (FIG. 2B) at all osmotic conditions examined. For strain KJW4-4, optimal opuD expression occurred at a 200 mM NaCl concentration (496,145 RLUs; FIG. 2B). As the NaCl concentration increased to 1000 mM NaCl a gradual decrease in opuD expression was observed (211,900 RLUs; P < 0.02 as compared to 0 mM NaCl).
Within E. coli, opuD regulation was affected the same way by the osmolarity as shown in S. aureus DAN2A. Strain DH5α/pDB3-11grown to stationary phase in LB cultures with 0–800 mM added NaCl were affected by the NaCl concentration. The culture grown in LB with no added NaCl had luminescence counts of 96,818 (FIG. 3), whereas the cultures grown in LB with 400 mM NaCl or 800 mM NaCl had RLUs of 806,397 and 264,118, respectively.
FIG. 3.
Effects of osmolarity on E. coli DH5α determined with an opuD∷luxABCDE transcriptional fusion. The RLU/bacterial cell were measured with a luminometer and then divided by viable counts; means ± standard deviations are indicated from at least three separate runs. Osmolarity effects were tested by using NaCl as the osmolyte. The * denotes significance of P < 0.05 and ** denotes significance of P < 0.001.
Moreover, the use of non-ionic osmolytes (sucrose and sorbitol) in the assay also resulted in increased activation of the opuD∷lux fusion, but showed different trends than what was observed for the DAN2A strain. As the concentration of sorbitol increased from 0 mM to 1000 mM, luminescence increased proportionally from 518,223 to 854,847 RLUs. Although this is a similar to what was seen for the DAN2A strain, the amount of increase between sorbitol concentrations was not as dramatic as was observed for the DAN2A strain resulting in an overall 1.6-fold increase in opuD expression as sorbitol concentrations were increased from 0 mM to 1000 mM (P < 0.04; FIG. 2B). As sucrose concentrations increased, opuD expression also increased until a plateau was reached at a concentration of 600 mM (916,309; P < 0.014 as compared to 0 mM sucrose). Again, as with the sorbitol trial, overall activation of opuD increased only 1.5-fold (P < 0.003) over the entire 0 mM to 1000 mM concentration range assayed. Thus, non-ionic osmolytes appear to have a different effect on opuD regulation than the ionic osmolyte NaCl in strain KJW4-4.
To verify that SigB was not affecting opuD expression, several strains that we have used before (Schwan et al., 2006) were tested (8325-4, SH1000, and SH1003). No difference in opuD expression was observed among the strains when they were grown in media with 0 mM, 400 mM, or 1 M NaCl (data not shown).
Regulation of the opuD gene tied to the early stage of the growth cycle
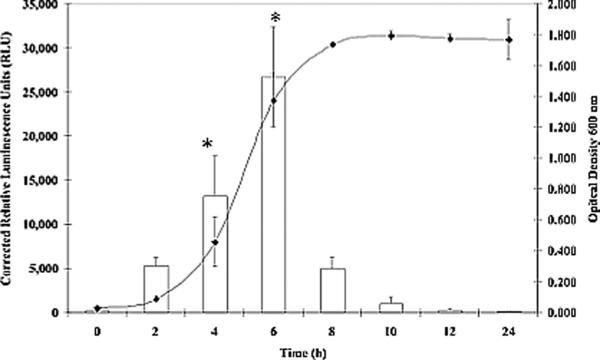
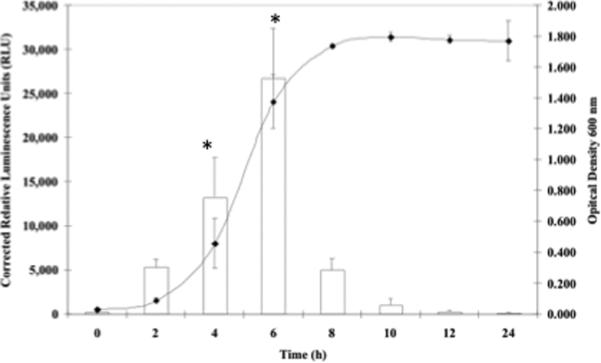
Since previous studies have shown that osmotically shocked S. aureus cells rapidly transport glycine betaine and proline intracellularly to counter the effects of switching to a high osmotic environment (Amin et al., 1995; Graham & Wilkinson, 1992; Miller et al., 1991), growth curves were performed to plot opuD transcriptional activation as measured by luminescence versus time. Regulation of opuD transcription in S. aureus strains DAN2A and KJW4-4 was monitored in standard DSM containing three concentrations of NaCl conditions over a 24 h growth period. In standard DSM with no added NaCl, strain DAN2A (FIG. 4A) exhibited maximal expression of opuD after 4 h (16,630 RLUs, P < 0.05). However, growth of strain DAN2A in standard DSM with either 400 mM (FIG. 4B) or 800 mM NaCl (FIG. 4C) led to optimal opuD expression after 6 h instead, representing a 2 h shift (26,708 and 20,821 RLUs, P < 0.05). This observed 2 h delay following growth in higher NaCl concentration media coincided with a delay in overall growth of the strain. Strain KJW4-4 exhibited an activation curve similar to the DAN2A curve, although the total luminescence was much higher (data not show).
FIG. 4.
Growth stage and osmolarity effects on S. aureus strain DAN2A as determined with a opuD∷luxABCDE, transcriptional fusion. The conditions tested were A) DSM broth plus 0 mM NaCl, B) DSM plus 400 mM NaCl, and C) DSM broth plus 800 mM NaCl. Both RLUs (open column) and O.D.600 (closed triangle) readings were measured. Means ± standard deviations are indicated from at least three separate runs.
Testing the opuD∷lux fusion in S. aureus cells injected into mice
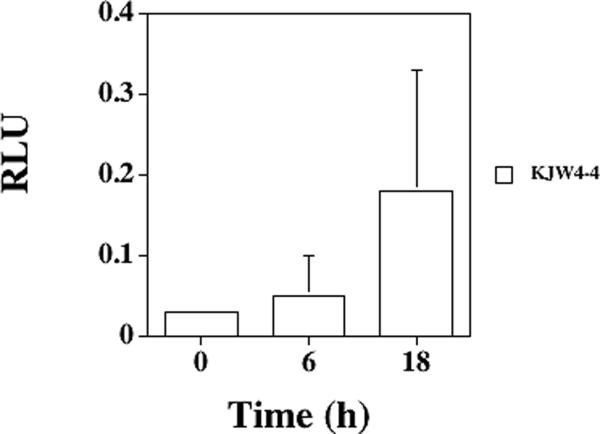
From the growth media experiments with various proline or osmolyte concentrations, it appeared that high proline levels and a moderate osmolarity environment transcriptionally activated opuD expression. Previously, we examined regulation of the putP gene in S. aureus cells found in vivo in murine tissues (Schwan et al., 2006), so two animal models of infection were used to decipher the regulation of the opuD gene in S. aureus cells growing in murine tissues. With the murine urinary tract infection model, opuD expression was examined in S. aureus cells infecting bladders and kidneys. In murine bladders, RLUs increased in strain KJW4-4 from 0.039 at 0 h to 0.187 after 4 h, representing a nearly five-fold increase (P < 0.05, FIG. 5A). After 18 h, the RLUs dropped for strain KJW4-4 (0.068), representing less than a two-fold increase compared to 0 h (P > 0.05). Strain KJW4-4 bacterial counts changed from 1.84 × 104 at 4 h to 1.29 × 104 after 18 h, representing a slight decline in viable counts over the time course tested. In murine kidneys, the opuD expression in strain KJW4-4 decreased fourfold going from the 0 h to the 4 h time point (P < 0.05, FIG. 5B), and the expression continued to be two-fold lower after 18 h (P > 0.05). Viable bacterial counts in the kidneys dropped from 1.13 × 105 at 0 h to 1.44 × 104 by 18 h. Strain DAN2A that contained an opuD∷lux fusion in a strain RN6390 background also displayed similar kinetics compared to strain KJW4-4 (data not shown).
FIG. 5.


Expression of opuD in murine tissues after infection with S. aureus strain KJW4-4 as determined with a opuD∷luxABCDE transcriptional fusion after 0, 4 or 6, and 18 h post-inoculation. Murine tissues included A) bladder, B) kidney, and C) thigh abscess. The RLU were measured with a luminometer and then divided by viable counts; means ± standard deviations are indicated. Five animals per time were examined per tissue. The * denotes significance of P < 0.05.
In a thigh abscess model of infection of mice, S. aureus strain KJW4-4 bacterial counts dropped slightly from 1.75 × 105 at 4 h to 1.02 × 105 after 18 h post-inoculation. The S. aureus opuD expression increased 1.5-fold after 4 h (0.052; P > 0.05) and exhibited a two-fold decrease after 18 h (0.0023) compared to the initial inoculum (0.039; FIG. 5C).
4. Discussion
The transport of compatible solutes from the extracellular environment is a major strategy employed by bacteria to rapidly adapt to changing osmotic environments (Wood, 1988). In S. aureus, a disruption of the high-affinity proline transport gene (putP) in S. aureus caused diminished growth in high osmotic environments as well as decreased survival in several animal models of infection (Bayer et al., 1999; Schwan et al., 1998; 2004; 2006), but proline transport was not completely abolished. In this study, we report on the identification and regulation of a low-affinity proline transport system encoded by the opuD gene within S. aureus.
Bioinformatic analysis identified the putative low-affinity system in S. aureus as a homolog of the opuD gene of B. subtilis (Kappes et al., 1996). An opuD mutation resulted in lower proline uptake by the bacteria in an environment rich in proline, but no change in proline uptake occurred when the proline concentration was very low, confirming that OpuD was indeed a low-affinity proline transport system. However, proline transport was not completely abolished and the mutant was not significantly attenuated compared to wild-type bacteria in a murine abscess model of infection.
An opuD∷lux reporter system was created to monitor the transcriptional regulation of the opuD gene in several S. aureus strains under different environmental conditions. Although DNA sequence upstream of the opuD gene was used for the construct, the DNA sequence was part of the mscL gene, which would be transcribed in the opposite direction and encodes for a large-conductive mechanosensitive channel (Liu et al., 2009) that should not affect regulation of the opuD gene. For the S. aureus strain RN6390, proline and glycine betaine activated opuD at concentrations ≥ 174 μM. The proline uptake assays showed OpuD was a low-affinity transporter and the gene activation affinities are identical to those observed for the proP gene of E. coli (Culham et al., 1993; Kempf & Bremer, 1998) but differ from the high-affinity for glycine betaine shown for OpuD from B. subtilis (Kappes et al., 1996). Of the remaining compatible solutes tested, no significant statistical activation was observed for L-carnitine or choline, which was in line with observed affinities of the B. subtilis OpuD (Kappes et al., 1996). Several studies have indicated that high osmotic conditions activate the proline transport systems in S. aureus (Anderson & Witter, 1982; Graham & Wilkinson, 1992; Koujima et al., 1978; Miller et al., 1991; Pourkmailian & Booth, 1992; 1994; Townsend & Wilkinson, 1992; Vijarankul et al., 1997). An immediate transcriptional upregulation of the opuD gene occurred as the NaCl molarity increased from 0 to 400 mM, followed by a rapid decrease in opuD transcription as the NaCl concentration increased further that coincided with a previous study (Townsend & Wilkinson, 1992). Maximal activation of opuD in E. coli also occurred when grown in medium containing 400 mM NaCl. Nonionic osmolytes (sucrose and sorbitol) also activated opuD expression, corresponding with a previous study that showed sucrose stimulates proline transport through the activation of the proP low-affinity proline transport gene in E. coli and Salmonella (Grothe et al., 1986).
Rapid activation of proline transporters in high osmotic environments is essential for survival of several bacteria (Csonka & Hanson, 1991; Wood, 1988), and our results showed maximum activation of the opuD gene at 4 h post-inoculation in the absence of NaCl and at 6 h post-inoculation in the presence of 800 mM NaCl (FIG. 4) that corresponded to the mid-logarithmic growth phase of the assay culture. Furthermore, SigB, an alternative sigma factor triggered by osmotic stress in S. aureus (Chan et al., 1998) did not affect opuD expression in S. aureus, although SigB at least partially regulates the opuD gene in Bacillus subtilis (Ziegler et al., 2010). It is possible that the MtrAB regulatory system that regulates opuD in Corynebacterium glutamicum (Moker et al., 2004) may also regulate the S. aureus opuD gene in an osmotically stressed environment.
Based on our previous putP study (Schwan et al., 2006), opuD expression was examined in bacteria inoculated into mice. We found opuD expression increased significantly in the bacteria found within murine bladders four hours post-inoculation, but then waned after 18 h, kinetics that were the exact opposite of those observed for putP expression. In the murine kidneys where the osmolarity has been shown to be as high 3 mol/kg (Loeb & Quimby, 1989), the opuD gene was down-regulated, which correlated well with the in vitro analysis done with different osmolytes that demonstrated down-regulation of the opuD gene under high osmotic conditions. In S. aureus infected murine abscesses, opuD expression increased, although not significantly, with time. Again, this contrasted with the putP expression kinetics where putP was maximally activated very early and then expression levels plummeted. Early abscess formation likely occurs under a paucity of available proline, so greater putP expression would be advantageous to get more PutP for uptake of that limited concentration of proline. However, virulence factors expressed by S. aureus will damage tissue as the abscess matures (Lowy, 1998), releasing proline from the damaged tissue that would now activate the opuD gene encoding a low-affinity proline transporter.
The low-affinity proline transport OpuD and the high-affinity proline transporter PutP may be working in unison to provide sufficient levels of proline and osmoprotection to S. aureus cells growing in many different murine or human tissues. Early in the growth phase of S. aureus in environments where there is a low concentration of proline or high osmolalities (e.g., the kidneys or an early abscess), the high-affinity putP gene is turned on (maximum expression at 2 h) followed by a decrease in expression (Schwan et al., 2006). As the level of putP expression wanes, increased expression of the opuD gene occurs. During early growth, cells are trying to accumulate nutrients and building blocks, which makes a transporter with high-affinity more advantageous and thus up-regulation of the putP gene warranted. Moreover, this would create an osmotic stabilizing condition within the cell. However, later in the growth cycle (e.g, an older abscess), long term osmoprotection is needed, thus up-regulation of both the opuD gene and presumably the proP gene would lead to more gene products from both of these genes that would protect the S. aureus cells. S. aureus cells adapt to a given environment by up-regulating or down-regulating the proline transport genes to allow for their survival in many niches within a mammalian host.
Acknowledgements
We wish to thank Xenogen Corporation for the pXen5 plasmid, Ambrose Cheung for the phage, Jean Lee for the Newman and 8325-4 strains, Dominique Missiakas for strain 06464, and Simon Foster for strains SH1000 and SH1003. This work was supported by an UW-L Undergraduate Research Grant to D. B., a UW-L Graduate Research Grant to K. J. W., as well as a grant from the NIH (AREA Grant 1R15AI47801-01A2) to W. R. S.
References
- Amin US, Lash TD, Wilkinson BJ. Proline betaine is a highly effective osmoprotectant for Staphylococcus aureus. Arch Microbiol. 1995;163:138–142. doi: 10.1007/BF00381788. [DOI] [PubMed] [Google Scholar]
- Anderson CB, Witter LD. Glutamine and proline accumulation by Staphylococcus aureus with reduction in water activity. Appl Environ Microbiol. 1982;43:1501–1503. doi: 10.1128/aem.43.6.1501-1503.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J-H, Miller KJ. Identification of two proline transport systems in Staphylococcus aureus and their roles in osmoregulation. Appl Environ Microbiol. 1992;58:471–475. doi: 10.1128/aem.58.2.471-475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J-H, Anderson SH, Miller KJ. Identification of a high-affinity glycine betaine transport system in Staphylococcus aureus. Appl Environ Microbiol. 1993;59:2734–2736. doi: 10.1128/aem.59.8.2734-2736.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae T, Banger AK, Wallace A, Glass EM, Aslund F, Schneewind O, Missiakas DM. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci U S A. 2004;101:12312–12317. doi: 10.1073/pnas.0404728101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AS, Coulter SN, Stover CK, Schwan WR. Impact of the high-affinity proline permease gene (putP) on the virulence of Staphylococcus aureus in experimental endocarditis. Infect Immun. 1999;67:740–744. doi: 10.1128/iai.67.2.740-744.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beonton BM, Zhang JP, Pope C, Christian T, Lee L, Winterberg KM, Schmid MB, Buysse JM. Large-scale identification of genes required for full virulence of Staphylococcus aureus. J Bacteriol. 2004;186:8478–8489. doi: 10.1128/JB.186.24.8478-8489.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PF, Foster SJ, Ingham E, Clements MO. The Staphylococcus aureus alternative sigma factor sigmaB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J Bacteriol. 1998;180:6082–6089. doi: 10.1128/jb.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka LN, Hanson AD. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- Culham DE, Lasby B, Marangoni AG, Milner JL, Steer BA, van Nues RW, Wood JM. Isolation and sequencing of Escherichia coli gene proP reveals unusual structural features of the osmoregulatory proline/betaine transporter, ProP. J Mol Biol. 1993;229:268–276. doi: 10.1006/jmbi.1993.1030. [DOI] [PubMed] [Google Scholar]
- Francis KP, Yu J, Bellinger-Kawahara C, Joh D, Hawkinson MJ, Xiao G, Purchio TF, Caparon MG, Lipitsch M, Contag PR. Visualizing pneumonococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect Immun. 2001;69:3350–3358. doi: 10.1128/IAI.69.5.3350-3358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JE, Wilkinson BJ. Staphylococcus aureus osmoregulation: roles of choline, glycine betaine, proline, and taurine. J Bacteriol. 1992;174:2711–2716. doi: 10.1128/jb.174.8.2711-2716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe S, Krogsrud RL, McClellan DJ, Milner JL, Wood JM. Proline transport and osmotic stress response in Escherichia coli K12. J Bacteriol. 1986;166:253–259. doi: 10.1128/jb.166.1.253-259.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. σB modulates virulence determinants expression and stress resistance: characterization of a functional rsbU strains derived from Staphylococcus aureus 8325-4. J Bacteriol. 2002;184:5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iandolo JJ, Kraemer GR. High frequency transformation of Staphylococcus aureus by electroporation. Curr Microbiol. 1990;21:373–376. [Google Scholar]
- Iandolo JJ, Worrell V, Groicher KH, et al. Comparative analysis of the genomes of the temperate bacteriophages phi 11, phi 12, and pi 13 of Staphylococcus aureus 8325. Gene. 2002;289:109–118. doi: 10.1016/s0378-1119(02)00481-x. [DOI] [PubMed] [Google Scholar]
- Kappes RM, Kempf B, Bremer E. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J Bacteriol. 1996;178:5071–5079. doi: 10.1128/jb.178.17.5071-5079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf B, Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol. 1998;170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- Koujima I, Hayashi H, Tomochika K, Okabe A, Kanemasa Y. Adaptional change in proline and water content of Staphylococcus aureus after alteration of environmental salt concentration. Appl Environ Microbiol. 1978;35:467–470. doi: 10.1128/aem.35.3.467-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst F, Ogasawara N, Moszer I, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gandhi CS, Rees DC. Structure of a tetrameric MscL in an expanded intermediate state. Nature. 2009;461:120–124. doi: 10.1038/nature08277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb WF, Quimby FW. The Clinical Chemistry of Laboratory Animals. Pergamon Press; New York, NY: 1989. [Google Scholar]
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Zelt SC, Bae J-H. Glycine betaine and proline are the principle compatible solutes of Staphylococcus aureus. Curr Micorobiol. 1991;23:131–137. [Google Scholar]
- Moker N, Brocker M, Schaffer S, Kramer R, Morbach S, Bott M. Deletion of the genes encoding the MtrA-MtrB two-component system of Corynebacterium glutamicum has a strong influence on cell morphology, antibiotic susceptibility and expressiuon of genes involved in osmoregulation. Mol Microbiol. 2004;54:420–438. doi: 10.1111/j.1365-2958.2004.04249.x. [DOI] [PubMed] [Google Scholar]
- Novick RP. The Staphylococcus as a Genetic System. In: Novick RP, editor. Molecular Biology of the Staphylococci. VCH Publishing; New York, NY: 1990. pp. 1–40. [Google Scholar]
- Pattee PA, Neveln DS. Transformation analysis of three linkage groups in Staphylococcus aureus. J Bacteriol. 1975;124:210–211. doi: 10.1128/jb.124.1.201-211.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourkomailian B, Booth IR. Glycine betaine transport by Staphylococcus aureus: evidence for two transport systems and for their possible roles in osmoregulation. J Gen Microbiol. 1992;138:2515–2518. doi: 10.1099/00221287-138-12-2515. [DOI] [PubMed] [Google Scholar]
- Pourkomailian B, Booth IR. Glycine betaine transport by Staphylococcus aureus: evidence for feedback regulation of the activity of the two transport systems. Microbiology. 1994;140:3131–3138. doi: 10.1099/13500872-140-11-3131. [DOI] [PubMed] [Google Scholar]
- Rudin L, Sjostrom JE, Lindberg M, Philipson L. Factors affecting competence for transformation in Staphylococcus aureus. J Bacteriol. 1974;118:155–164. doi: 10.1128/jb.118.1.155-164.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. 2nd ed. Cold Springs Harbor Laboratory Press; Plainview, NY: 1989. [Google Scholar]
- Schaeffer AJ, Schwan WR, Hultgren SJ, Duncan JL. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect Immun. 1987;55:373–380. doi: 10.1128/iai.55.2.373-380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan WR, Lehmann L, McCormick J. Transcriptional activation of the Staphylococcus aureus putP gene by low-proline-high osmotic conditions and during infection of murine and human tissues. Infect Immun. 2006;74:399–409. doi: 10.1128/IAI.74.1.399-409.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan WR, Wetzel KJ, Gomez TS, Stiles MA, Beitlich BD, Grunwald S. Low-proline environments impair growth, proline transport and in vivo survival of Staphylococcus aureus strain-specific putP mutants. Microbiology. 2004;150:1055–1061. doi: 10.1099/mic.0.26710-0. [DOI] [PubMed] [Google Scholar]
- Schwan WR, Coulter SN, Ng EYW, Langhorne MH, Ritchie HD, Brody LL, Westbrock-Wadman S, Bayer AS, Folger KR, Stover CK. Identification and characterization of the PutP proline permease that contributes to in vivo survival of Staphylococcus aureus in animal models. Infect Immun. 1998;66:567–572. doi: 10.1128/iai.66.2.567-572.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DE, Wilkinson BJ. Proline transport in Staphylococcus aureus: a high-affinity system and a low-affinity system involved in osmoregulation. J Bacteriol. 1992;174:2702–2710. doi: 10.1128/jb.174.8.2702-2710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijarankul U, Nadakavukaren MJ, Bayles DO, Wilkinson BJ, Jayaswal RK. Characterization of a NaCl-sensitive Staphylococcus aureus mutant and rescue of the NaCl-sensitive phenotype by glycine betaine but not by other compatible solutes. Appl Environ Microbiol. 1997;63:1889–1897. doi: 10.1128/aem.63.5.1889-1897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengender PA, Miller KJ. Identification of a PutP proline permease gene homolog from Staphylococcus aureus by expression cloning of the high-affinity proline transport system in Escherichia coli. Appl Environ Microbiol. 1995;61:252–259. doi: 10.1128/aem.61.1.252-259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JM. Proline porters effect the utilization of proline as nutrient or osmoprotectant for bacteria. J Membr Biol. 1988;106:183–202. doi: 10.1007/BF01872157. [DOI] [PubMed] [Google Scholar]
- Ziegler C, Bremer E, Kramer R. The BCCT family of carriers: from physiology to crystal structure. Mol Microbiol. 2010;78:13–34. doi: 10.1111/j.1365-2958.2010.07332.x. [DOI] [PubMed] [Google Scholar]