Abstract
AIMS
To assess the feasibility and acceptability of a community-based, culturally-specific, Diabetes Prevention Program (DPP)-adapted, group lifestyle intervention in Arab-Americans.
METHODS
Overweight (BMI≥27 kg/m2) Arab-Americans aged ≥30 years and without a history of diabetes were recruited to participate in a 24-week group lifestyle intervention. The DPP core-curriculum was culturally rewritten, translated into Arabic, and delivered in weekly sessions over a 12-week period. Follow-up was performed at week-24. The primary goals were to achieve ≥7% weight loss and ≥150 minutes/week of physical activity. An intent-to-treat analysis was performed.
RESULTS
Of the 71 participants (mean age±SD 47±10 years, 38% males), 44% achieved ≥7% weight loss, 59% achieved ≥5% reduction in weight, and 78% reached the physical activity goal of ≥150-minutes/week. The mean±SD weight loss was 5.2±4.4 kg at week-24 (p<0.0001), Marked reduction in body measurements, daily energy and fat intake were noted. Retention was high with 86% completing the intervention.
CONCLUSIONS
This trial demonstrates that a culturally-specific, DPP-adapted, group lifestyle intervention implemented in a community setting is feasible and effective in Arab-Americans.
Keywords: Diabetes, prevention, lifestyle intervention, Diabetes Prevention Program (DPP), Arab Americans
INTRODUCTION
The largest concentration of Arabs in the United States is in the Detroit Metropolitan area estimated at approximately 392,000 individuals according to the Arab American Institute Foundation[1]. The Arab-American community is primarily composed of recent immigrants[2]. A number of cultural elements distinguish this population including a deep religious orientation, reliance on the extended family, defined gender roles and strong gender taboos, use of the Arabic language, lack of acculturation, and adherence to traditional beliefs, and practices.
Diabetes is a growing clinical and public health problem in the Arab-American community. We have previously shown that the age- and sex- standardized prevalence rates of diabetes, impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) in Arab Americans aged 20–75 years are 18% and 23%, respectively[2]. Glucose intolerance is associated with obesity, physical inactivity, and lack of acculturation[3]. Several randomized controlled clinical trials have demonstrated that diabetes can be delayed or prevented with lifestyle and pharmacological interventions[4–10]. In the landmark Diabetes Prevention Program (DPP), modest lifestyle changes (≥7% reduction in body weight and ≥150-minutes/week of moderate physical activity) reduced the risk of progression from IGT to diabetes[4]. In the Finnish Diabetes Prevention Study, the risk reduction persisted after termination of the lifestyle intervention[11]. However, the implementation of lifestyle interventions in real-world settings has proven to be a challenge especially among minorities and in communities with limited resources[12–13]. Cultural differences may also influence the translatability of behaviorally-based interventions. Innovative population-based approaches that acknowledge cultural differences and enhance the individual and community’s adoption of lifestyle changes are needed if diabetes is to be prevented.
A few studies have examined the feasibility of programs that replicate the DPP lifestyle intervention[14–19]. The current prospective study was designed to primarily examine whether a community-based and culturally-specific group lifestyle intervention adapted from the DPP is feasible and acceptable to the Arab-American community. A secondary objective was to assess the effects of a structured educational intervention targeting knowledge gaps and health beliefs conducive to negative health behaviors on the willingness to engage in the lifestyle intervention.
MATERIALS AND METHODS
This was a community-based, prospective, non-randomized, feasibility demonstration trial of group lifestyle intervention for diabetes prevention in Arab Americans in Dearborn, MI. Participants were recruited from a previously constructed sampling list of housing units and from the general public through promotional materials. The study was approved by the Wayne State University and the University of Michigan Institutional Review Boards. All participants provided written informed consent. These consent forms were written in English and Arabic and pre-tested for content.
Participation was limited to self-identified Arab or Arab Americans ≥30 years of age and with a body mass index (BMI) ≥27 kg/m2 and without a history of diabetes. Eligible individuals were invited to participate in an orientation session at the Arab Community Center for Economic and Social Services (ACCESS) that provided detailed information about a DPP-modeled, group-conducted, culturally-specific, 24-week lifestyle intervention. During this informational session, the individual’s willingness to participate in the lifestyle intervention was assessed with standardized questions administered by a bilingual interviewer. Those who were willing to participate in the lifestyle intervention were directly enrolled. Those who declined the lifestyle intervention were asked to participate in a 4-week educational intervention. Following the educational intervention, their willingness to participate in the lifestyle intervention was reexamined. A subset of individuals who agreed to lifestyle intervention only after participating in the education intervention were enrolled in the lifestyle intervention.
All study-related procedures were carried out by trained bilingual personnel who were racially and ethnically identified with the community. These individuals were trained by the study investigators and consultants who had extensive experience in implementing behavioral interventions. To ensure fidelity of the intervention, the study nurse was primarily responsible for the delivery of the core-curriculum sessions of the lifestyle intervention. The nurse completed a structured training curriculum provided by the study investigators. Additional training was provided by the DPP lifestyle team at the University of Pittsburgh. Intervention fidelity accross study groups was further examined by direct observation and ongoing review of the formal core-curriculum sessions. All study procedures were conducted at ACCESS facilities located within the target community.
Lifestyle intervention
The lifestyle intervention was modeled on the DPP but modified and adapted for Arab Americans based on our research experience and input from community members. Prior to fieldwork, a series of focus groups were convened to evaluate the cultural appropriateness of planned intervention, explore barriers and promoters to participation, and examine the preference for delivery of the intervention. In addition, a committee of study investigators and community members was convened to provide recommendations for cultural modification of the DPP core-curriculum. The following recommendations were provided: (1) The planned intervention must approach the issues of gender, family, religion, and community in a culturally-sensitive manner, (2) Gender-specific groups must be offered based on participant preference, (3) Physical activity sessions conducted at community centers should be segregated by sex, (4) The intervention should target women given that women are responsible for routine medical care and for promoting healthy lifestyle choices, (5) Family-based interventions should be incorporated to enhance male participation and promote communication regarding healthy food preparation, (6) the incorporation of wise old Arabic sayings, religious themes and imagery.
The DPP-adapted core-curriculum employed these culturally-specific strategies to promote weight loss, physical activity, and healthy lifestyle choices. Key features included a goal-oriented, rather than a process-oriented, focus aimed at achieving a ≥7% loss of initial body weight and ≥150-minutes/week of moderate physical activity and an Arab American focus. The entire 16 sessions of the DPP core-curriculum were revised to be culturally appropriate, reorganized into 12 sessions, and translated into Arabic. An additional session was developed to address issues related to fasting during Ramadan. Table 1 provides detailed information on session titles, content focus, and learning basis of the DPP-adapted curriculum[20]. “The DPP Lifestyle Balance Fat Counter” booklet was revised and reorganized to eliminate foods not commonly eaten and to incorporate Middle Eastern foods[21].
Table 1.
Session titles, content focus, and learning basis of the DPP-adapted curriculum
| Session Title (Arabic) | Session Title (English) | DPP Curriculum Sessions* | Basis* |
|---|---|---|---|
| Welcome to the AHLA Program | Welcome to the Lifestyle Balance Getting Started Being Active Getting Started Losing Weight | AHLA Sessions 1–7: | |
 |
Be a Fat Detective | Be a Fat Detective | 1. Presented the goals for the DPP-adapted lifestyle intervention |
| Three Ways to Eat Less Fat | Three Ways to Eat Less Fat | 2. Taught fundamental information about modifying energy intake and increasing energy output | |
 |
Healthy Eating | Healthy Eating | |
 |
Move Those Muscles | Move Those Muscles Being Active: A Way of Life | 3. Helped participants to self-monitor their intake and physical activity. |
| Tip the Calorie Balance | Tip the Calorie Balance | ||
 |
Take Charge of What’s Around You | Take Charge of What’s Around You | |
 |
Problem Solving | Problem Solving | AHLA Sessions 8–12: |
| Four Key Ways to Healthy Eating Out | Four Keys to Healthy Eating Out | 1. Focuses on psychological, social, and motivational challenges involved in maintaining these healthy lifestyle behaviors in the long term | |
| Slippery Slope of Lifestyle Change | Slippery Slope of Lifestyle Change Talk Back to Negative Thoughts | ||
| Make Social Cues Work for You | Make Social Cues Work for You You Can Manage Stress | ||
| Jump Start Your Activity Plan | Jump Start Your Activity Plan Ways to Stay Motivated | ||
| Be Ready for Ramadan | NA | Addressed issues related to fasting during Ramadan |
Diabetes Prevention Program content incorporated in the adapted lifestyle intervention[20].
Teaching formats consistent with the strong oral traditions of the Arab culture were used. These included the use of cultural themes and imagery. Wise old Arabic sayings related to attainment of goals were incorporated at the start of each session. Religious themes and imagery related to the medicine of nutrition served as visual aids during the core-curriculum sessions. Healthy snacks and menu planning demonstrations, modification of Arab cooking techniques, and grocery store trips were also incorporated. Many of these hands-on demonstrations were driven by participants who were encouraged to exchange experiences such as sharing of ethnic recipes within their respective groups.
Since social interactions are important to Arabs, study activities were conducted in Arabic and delivered in a group format. Same-sex groups were requested by about 20% of participants. Given the central role of the family, we actively sought family support by inviting family members to attend all sessions and activities.
Simple dietary modifications were emphasized. Weight loss was used to guide the dietary goals. Calorie and fat intake goals were derived by estimating the total calories needed to reduce the initial weight by 7% based on analysis of DPP data. Individual nutrition goals were established at session 1 by the study dietician. Four standard calorie levels were used: 1,200 kcal/day (33 g fat) for participants with an initial weight of 120–174 lbs, 1,500 kcal/day (42 g fat) for participants with a weight of 175–219 lbs, 1,800 kcal/day (50 g fat) for participants with a weight of 220–249 lbs and 2,000 kcal/day (55 g fat) for participants weighing 250 lbs or more[20]. The strategies to achieve these goals were modified by the participants as they gained knowledge regarding healthy food choices and portion control.
Walking is an activity that is preferred by this community and brisk walking was promoted to achieve the physical activity goal. A weekly supervised physical activity session in the form of neighborhood group walks, or aerobic classes was regularly offered. Individuals were required to have a medical clearance letter completed by their primary care physicians (PCP) attesting to absence of contraindications to physical activity.
In terms of self-monitoring, emphasis was placed on recording body weights, physical activity minutes, food types and portion sizes and in identifying high-fat foods. Demonstrations using food replicas were used to estimate portion sizes.
Participants who agreed to the lifestyle intervention were allocated to groups of their choice; either mixed gender or all female. These groups consisted of 10–12 persons. At the first session, group members chose a group leader responsible for organizing unsupervised group activities. Participants were required to attend the 12 weekly core-curriculum sessions, each lasting approximately 60–90 minutes. For participants intending to fast during Ramadan, an additional session was scheduled. Thereafter, group members met on a monthly basis for another 12 weeks to assess achievement of goals and to discuss barriers and strategies to overcome them. Although these meetings were optional, most group members attended. Visits on an individual basis were offered but were not requested at any point. The trained study nurse facilitated all core-curriculum sessions. A part-time dietitian supported the study coordinator by conducting hands-on demonstrations during nutrition intensive sessions 3 and 4 (Table 1).
Educational intervention
The educational intervention used the Health Belief Model as its theoretical framework[22]. Table 2 provides detailed information on session titles, content focus, and Health Belief Model constructs[23]. The educational intervention sought to identify and modify health beliefs that negate willingness to engage in diabetes prevention activities, to improve knowledge regarding diabetes, and to correct misconceptions. Four 60–90 minute weekly group sessions were conducted. Same topic sessions were offered twice and scheduled at different times to accommodate participant availability over the 4-week period.
Table 2.
Session titles, content focus, and Health Belief Model constructs for the Educational Intervention
| Session Title | Content Focus | Health Belief Model Construct* |
|---|---|---|
| Diabetes is a Serious Disease | Myths regarding diabetes Epidemiology of diabetes Types of diabetes Risk factors for diabetes Associated complications |
Perceived susceptibility Perceived severity |
| The Science: Diabetes Prevention | Pre-diabetes, its risk factors and complications Evidence for diabetes prevention |
Perceived susceptibility Perceived severity Perceived benefits |
| Small Steps. Big Rewards. Your GAME PLAN for Preventing Type 2 Diabetes toolkit of the National Diabetes Education Program (NDEP) | Content of toolkit reviewed | Perceived barriers Perceived benefits Self-efficacy |
| Question and Answer | Correction of misconceptions Question and Answer session |
Perceived susceptibility Perceived severity Perceived benefits Perceived barriers |
Health belief model constructs addressed in educational intervention[23]
During the individual sessions, the content areas of the Small Steps. Big Rewards. Your GAME PLAN for Preventing Type 2 Diabetes toolkit of the National Diabetes Education Program (NIH Publication, 2003) were addressed. Content areas of individual sessions included: 1) diabetes, its complications, and the associated disorders and risks; 2) prediabetes, its risk factors and consequences; 3) the evidence for diabetes prevention with lifestyle modification or medications; and 4) correction of misconceptions and addressing questions and concerns.
Measures
After providing written informed consent, all individuals consenting to either the lifestyle intervention or the education intervention completed verbally-administered, previously validated and standardized questionnaires assessing baseline sociodemographic information, health beliefs, risk perception, and selected psychosocial variables[2,3,24–26]. These questionnaires were translated into Arabic, back-translated, and pilot tested prior to use. The degree of acculturation was assessed with a four-item survey that was previously validated in the target population[3]. Risk perception was measured with the Risk Perception Survey for Developing Diabetes (RPS-DD) which evaluates multiple dimensions of perceived risk[24]. Health-related quality of life was measured with the EQ-5D, a standardized instrument applicable to a wide range of health conditions and outcome[25]. Depressive symptom severity was assessed with the 9-item PRIME-MD Patient Health Questionnaire (PHQ-9)[26]. In addition, we developed and pre-tested the Health Beliefs Barriers survey for content validity in 25 randomly selected Arab-Americans to evaluate redundant responses and socially unacceptable scale items.
For individuals participating in the lifestyle intervention, the following additional data were collected: 1) Anthropometric determinations including measurements of height, weight (in light clothing and without shoes), and waist and hip circumferences at baseline, week-12 (conclusion of core-curriculum), and week-24 (conclusion of the study). Participants were weighed weekly during the core-curriculum and monthly thereafter using the same scale and time of day. 2) Nutrient intake was assessed with a 24-hour dietary recall at baseline, 12 and 24 weeks. Total daily calorie intake including calories from fat was assessed. 3) Self-reported physical activity was assessed with the DPP Modifiable Activity questionnaire at baseline, 12 and 24 weeks[27]. Physical activity assessment included capturing the duration and frequency of each type of activity in minutes/week. This was summed for all activities performed. Attendance at scheduled sessions was documented and adherence was defined as attendance of a minimum of 80% of scheduled sessions.
Statistical analysis
Data management and analysis were performed by the Biostatistics and Economic Modeling Core of the Michigan Diabetes Research and Training Center. The primary endpoints were the percent who attained the target weight loss, the percent who converted (agreed to participate in the lifestyle intervention), and the percent who completed the lifestyle intervention. In addition, the outcome measures (weight, body fat measurements, nutritional intake) were tested for change from pre- to post-intervention. Differences between those who initially accepted the lifestyle intervention and those who completed the lifestyle intervention after the education program were also examined. An intent-to-treat analysis (defined as all individuals who attended at least one session) was utilized. Participants who dropped out of the intervention were assigned a change of zero (no improvement) for the purpose of testing for a difference. A per protocol analysis (individuals who provided data through week 24) was also performed.
Prior to analysis, data were examined for errors, outliers, and asymmetry. Errors and outliers were queried. When appropriate, transformations were used to reduce asymmetry. Baseline measures were compared between the two groups; since no imbalance was noted, these measures were not included in the analysis below.
Success rates and their 95% confidence intervals were estimated based on an intent-to-treat approach (all withdrawals treated as failures). Changes due to intervention were tested by a two-tailed paired t-test. Differences between groups were tested by Fisher’s exact test (two-tailed) and a two-sample t-test, respectively. Analyses were performed using SAS, version 9.1 (SAS Institute, Cary, NC).
RESULTS
A total of 178 Arab Americans were screened. Of these, 14 individuals were excluded because they did not meet the age and/or BMI criteria (n=10) or they had diabetes (n=4). Of the remaining 164 eligible individuals, 48 (23%) declined participation in any intervention. The reported reasons for refusing to participate were lack of time (31%), work (29%), family/health related issues (23%), and plans to travel abroad (8%).
Of the remaining 116 individuals, 53 initially agreed to the lifestyle intervention and were subsequently enrolled. The remaining 63 individuals initially declined the lifestyle intervention but agreed to participate in the educational program and were subsequently enrolled.
At the conclusion of the educational intervention, 49 of the 63 enrolled individuals (78%) converted and were willing to participate in the lifestyle intervention. Due to a higher than expected response rate and the available study resources, we were only able to enroll in the lifestyle intervention the first 18 participants who accepted the lifestyle after the educational intervention.
As a result, 71 individuals (53 initially accepting lifestyle and 18 accepting following educational program) participated in the lifestyle intervention. Their baseline demographic characteristics are presented in Table 3. The mean (±SD) age was 47.0±9.4 years. Most participants were from Lebanon (73%) followed by Iraq (18%); 38% were male. All had a BMI≥27 kg/m2 and most were obese (BMI≥30). Participants consumed mostly traditional Middle Eastern foods. The majority (70%) did not engage in regular physical activity.
Table 3.
Baseline Demographic Characteristics of study population in the lifestyle intervention
| Characteristic | Overall | Males | Females |
|---|---|---|---|
| N (%) | 71 (100.0) | 27 (38.0) | 44 (62.0) |
| Age, years ± SD | 47±10 | 48±10 | 46±9 |
| Country of origin, n (%) | |||
| Lebanon | 52 (73.2) | 18 (66.7) | 34 (77.3) |
| Iraq | 13 (18.3) | 5 (18.5) | 8 (18.2) |
| Other | 6 (8.5) | 4 (14.8) | 2 (4.5) |
| Weight, kg ± SD | 91 ± 16 | 94 ± 14 | 89 ± 17 |
| BMI, kg/m2 ± SD | 34.3 ± 6.1 | 32.5 ± 3.9 | 35.5 ± 7.0 |
| Waist circumference, cm ± SD | 105 ± 12 | 107 ± 9 | 103 ± 14 |
| Hip circumference, cm ± SD | 118 ± 13 | 113 ± 10 | 122 ± 13 |
| Caloric intake, kcal ± SD | 2770 ± 1028 | 3104 ± 1305 | 2551 ± 735 |
| Fat intake, grams ± SD | 128 ± 62 | 144 ± 82 | 117 ± 43 |
| Saturated fat intake, grams ± SD | 37 ± 20 | 41 ± 24 | 34 ± 16 |
| Physical activity ≥ 150 minutes, n (%) | 21 (29.6) | 8 (29.6) | 13 (29.5) |
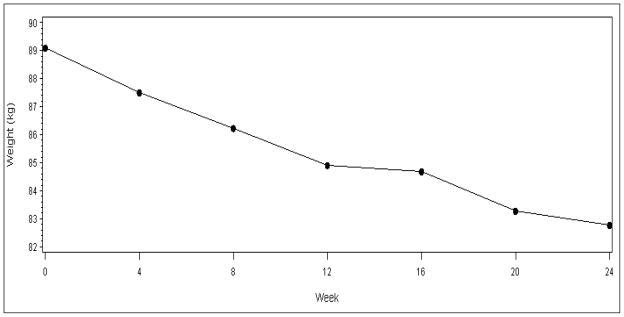
Mean weight over time is depicted in Figure 1 for the subjects who participated for at least 19 weeks. To avoid variation due to missed measurements, the mean weight loss at each week was calculated and subtracted from the mean weight at baseline. We observed an approximately linear reduction in weight over time. The mean weight loss was 3.5±3.4 and 5.2±4.4 kg at 12 and 24 weeks, respectively (p<0.0001). The body weight loss goal of ≥7% was achieved by 44% of participants at week 24; 59% lost at least 5% of their baseline weight.
Figure 1.
Mean body weight over time.
Changes in body fat measurements, nutritional intake, and physical activity are shown in Table 4. BMI, and waist and hip circumferences were significantly reduced at 12 and 24 weeks (p<0.0001). Women achieved a greater reduction at 24 weeks than men in BMI (2.6±2.0 vs. 1.8±1.0 kg/m2; p=0.032), waist (13.4±7.0 vs. 6.6±6.6 cm; p=0.0003) and hip (13.4±8.0 vs. 7.5±6.9 cm; p=0.0034) circumferences. Nutritional intake goals were achieved at 12 weeks with no further improvement at 24 weeks. Daily energy, and total and saturated fat intakes were significantly reduced at 12 and 24 weeks (p<0.0001). The 150-minute physical activity goal was achieved by 69% and 78% of participants at 12 and 24 weeks, respectively.
Table 4.
Change from baseline in body fat measures, nutritional intake, and physical activity following lifestyle intervention
| All Participants (n=71)*** | Initially Accepting Lifestyle (n=53) | Lifestyle Following Education (n=18) | |||||
|---|---|---|---|---|---|---|---|
| 12 Weeks | 24 Weeks | 12 Weeks | 24 Weeks | 12 Weeks | 24 Weeks | ||
| Body fat measurements** | |||||||
| Weight, kg ± SD | |||||||
| Intent to treat | −3.5 ± 3.4* (−4.3, −2.7) | −5.2 ± 4.4* (−6.3, −4.2) | −3.5 ± 3.5 (−4.5, −2.5) | −5.3 ± 4.6 (−6.5, −3.9) | −3.6 ± 2.9 (−5.1, −2.2) | −5.0 ± 3.8 (−6.9, −3.2) | |
| Per protocol | −4.1 ± 3.3* (−5.0, −3.3) | −6.0 ± 4.2* (−7.0, −4.9) | −4.1 ± 3.5 (−5.1, −3.0) | −5.9 ± 4.5 (−7.3, −4.6) | −4.3 ± 2.7 (−5.8, −2.9) | −6.0 ± 3.3 (−7.8, −4.2) | |
| BMI, kg/m2 ± SD | |||||||
| Intent to treat | −1.3 ± 1.3* (−1.6, −1.0) | −1.9 ± 1.7* (−2.4, −1.5) | −1.3 ± 1.4 (−1.7, −0.9) | −2.0 ± 1.8 (−2.5, −1.5) | −1.3 ± 1.3 (−2.0, −0.7) | −1.8 ± 1.6 (−2.6, −1.0) | |
| Per protocol | −1.6 ± 1.3* (−1.9, −1.2) | −2.3 ± 1.7* (−2.7, −1.8) | −1.5 ± 1.3 (−1.9, −1.1) | −2.2 ± 1.8 (−2.8, −1.7) | −1.7 ± 1.2 (−2.4, −1.1) | −2.3 ± 1.4 (−3.1, −1.5) | |
| Waist circumference, cm ± SD | |||||||
| Intent to treat | −6.6 ± 7.7* (−8.4, −4.8) | −9.2 ± 7.9* (−11.1, −7.4) | −6.9 ± 7.9 (−9.0, −4.7) | −9.7 ± 8.4 (−12.0, −7.4) | −5.7 ± 7.0 (−9.2, −2.2) | −7.9 ± 6.2 (−11.0, −4.8) | |
| Per protocol | −7.7 ± 7.7* (−9.6, −5.7) | −10.6 ± 7.6* (−12.5, −8.7) | −7.9 ± 8.0 (−10.3, −5.6) | −10.9 ± 8.1 (−13.3, −8.6) | −6.9 ± 7.2 (−10.8, −2.9) | −9.5 ± 5.6 (−12.6, −6.4) | |
| Hip circumference, cm ± SD | |||||||
| Intent to treat | −6.6 ± 7.8* (−8.4, −4.7) | −9.5 ± 8.4* (−11.5, −7.5) | −6.8 ± 8.2 (−9.1, −4.6) | −10.3 ± 8.9 (−12.8, −7.9) | −5.9 ± 6.6 (−9.1, −2.6) | −7.1 ± 6.4 (−10.3, −3.9) | |
| Per protocol | −7.7 ± 7.9* (−9.7, −5.6) | −10.9 ± 8.1* (−13.0, −8.9) | −7.9 ± 8.3 (−10.3, −5.4) | −11.7 ± 8.6 (−14.2, −9.1) | −7.0 ± 6.7 (−10.7, −3.3) | −8.6 ± 6.1 (−11.9, −5.2) | |
| Nutritional Intake** | |||||||
| Proportion achieving caloric intake goal, n (%) | |||||||
| Intent to treat | 24 (33.8) (22.8, 44.8) | 27 (38.0) (26.7, 49.3) | 18 (34.0) (21.2, 46.7) | 22 (41.5) (28.2, 54.8) | 6 (33.3) (11.6, 55.1) | 5 (27.8) (7.1, 48.5) | |
| Change in daily caloric intake, kcal ± SD | |||||||
| Per protocol | −898±1088* (−1179, −617) | −989±1259* (−1311,−617) | −1016±1160 (−1365, 668) | −1109±1342 (−1508,−711) | −542±762 (−964,−120) | −620±899 (−1118,−122) | |
| Proportion achieving fat intake goal, n (%) | |||||||
| Intent to treat | 10 (14.1) (6.0, 22.2) | 10 (14.1) (6.0, 22.2) | 9 (17.0) (6.9, 27.1) | 8 (15.1) (5.5, 24.7) | 1 (5.6) (0, 16.1) | 2 (11.1) (4, 25.6) | |
| Change in daily fat intake, grams ± SD | |||||||
| Per protocol | −48 ± 70* (−66, −30) | −56 ± 80* (−77, −36) | −54±73 (−76,−33) | −62±86 (−88,−37) | −30±57 (−61, 2) | −38±56 (−69, −7) | |
| Proportion achieving saturated fat intake goal, n (%) | |||||||
| Intent to treat | 35 (49.3) (37.7, 60.9) | 33 (46.5) (34.9, 58.1) | 26 (49.1) (35.6, 62.5) | 24 (45.3) (31.9, 58.7) | 9 (50.0) (26.9, 73.1) | 9 (50.0) (26.9, 73.1) | |
| Change in daily saturated fat intake, grams ± SD | |||||||
| Per protocol | −16 ± 29* (−23, −9) | −17 ± 26* (−24, −10) | −18±32 (−27, −8) | −19±29 (−27,−10) | −11±14 (−19, −4) | −12±16 (−21, −3) | |
| Physical Activity | |||||||
| Proportion achieving 150-minute activity goal, n (%) | |||||||
| Intent to treat | 49 (69.0) (58.3, 79.8) | 55 (77.5) (67.7, 87.2) | 39 (73.6) (61.7, 85.5) | 41 (77.4) (66.1, 88.6) | 10 (55.6) (32.6, 78.5) | 14 (77.8) (58.6, 97.0) | |
P-value vs. baseline <0.0001. Only p-values for the entire cohort are presented.
Mean±standard deviation or n (%), (95% confidence interval) are included.
Changes in outcomes due to intervention were tested by the two-tailed paired t-test.
Intent-to-treat calculation included all participants (n=71) where drop-outs were assigned a change of zero; Per protocol calculation included only participants (n=61) who provided the week 24 assessment.
Differences between individuals initially accepting lifestyle intervention and those accepting intervention following the educational program were tested by the Fisher’s exact test (two-tailed) or the two-sample t-test; no differences were noted.
Adherence to scheduled sessions was consistently high with 92% of participants attending at least 80% of weekly sessions. Participants attended an average of 10±3 sessions and 65% attended all 12 sessions. In contrast, adherence to self-monitoring was uniformly low with <15% of participants completing 80% of dietary records. Nevertheless, the majority of participants recorded the types and quantities of foods consumed.
Retention was high with 61 participants completing the entire 24-week lifestyle intervention yielding a completion rate of 86%; 2 additional individuals completed 12 and 18 weeks before traveling abroad. The remaining 8 individuals dropped-out before session 5. The reasons for ending participation included: time constraints (n=6) and failure to obtain clearance from PCP (n=2). No adverse events were attributed to the study intervention.
We compared the retention and performance between the two cohorts: those that initially accepted the lifestyle intervention (n=53) and those that initially declined then accepted the lifestyle intervention after the educational intervention (n=18). Subgroup analysis did not reveal statistically-significant differences in any of the measures tested (Table 4).
DISCUSSION
Despite compelling evidence that diabetes can be prevented or delayed, the translation of effective diabetes prevention strategies in diverse communities has been limited. The most effective strategy, namely lifestyle modification, entails substantial time, resources, and costs and its effectiveness depends on numerous cultural, social, and economic factors[12–13]. Culture shapes health promotion behaviors and marginalization of culture is a barrier to care. The lifestyle intervention involves culturally embedded behaviors such as eating and physical activity. This is the first study demonstrating the feasibility of recruitment and retention in a community-based, group-conducted, DPP-adapted lifestyle intervention in the culturally-unique, medically-underserved, largely immigrant Arab-American community. The study provides evidence that incorporating cultural and social preferences enhances implementation while maintaining the fidelity of the DPP lifestyle intervention.
Another finding of this study was the demonstration that culturally-based impediments to lifestyle intervention are modifiable. We have shown the effectiveness of an educational intervention targeting knowledge gaps and misconceptions in modifying the health beliefs conducive to negative health behaviors and consequently improving the adoption and participation in diabetes prevention activities. Seventy-eight percent of individuals initially unwilling to engage in the lifestyle intervention agreed to participate following the educational intervention.
The effectiveness of the lifestyle intervention was evaluated on the basis of the short-term outcomes identified by DPP as the benchmarks for the effectiveness of lifestyle modification in preventing diabetes, namely weight loss and physical activity. Weight loss was the main determinant of diabetes risk reduction[28]. By week 24, 44% of participants achieved at least a 7% weight loss and 78% reached the physical activity goal of at least 150 minutes/week. These findings are comparable to those reported in the DPP where 50% of participants met the weight loss goal and 74% met the physical activity goal at week 24[4]. The weight loss observed in our study was associated with a significant reduction in BMI and in waist and hip circumferences. The proportion of obese participants (BMI≥30 kg/m2) was 51% at baseline and 34% at week 24. Similarly, central obesity (defined as waist circumference >102 cm in men and >88 cm in women) was present in 83% of participants at baseline, but only in 47% by week 24. Nutritional changes at 24-weeks were comparable to dietary changes reported at one-year by the DPP. The mean fat intake at baseline was high in our participants (41% of total calories) compared to DPP (34%). Fat intake decreased by 5.6% in our participants compared to a 6.6% in DPP. Adherence (92%) and retention (86%) observed in our study were excellent.
Our study differed from the DPP in several key ways. Unlike the DPP, the presence of glucose intolerance was not an inclusion criterion. The selection of participants using age (≥30 years) and BMI (≥27 kg/m2) was based on our previous finding that prediabetes was likely to be detected in obese individuals aged 30 or older[2,29]. Older age and adiposity were also shown to be useful screening tools to identify individuals at risk for diabetes in the DPP[30]. Despite these differences in eligibility criteria, our participants were generally comparable to the DPP population in terms of age, sex distribution, and anthropometric measures including weight, BMI, and waist circumference. The recruitment approach utilized in our study may be an efficient, practical, and cost-effective strategy to identify individuals at-risk for diabetes who merit intervention.
Unlike the DPP where the lifestyle intervention required tailored and individualized counseling by specialized staff who had undergone extensive training, our intervention employed a group format and was delivered by one person who required only a moderate amount of training. The group format and the involvement of family members likely contributed to improved adherence and performance of study participants (especially men) and, although unmeasured, appeared to lead to additional benefits for the entire family.
Another departure from the DPP was in the self-monitoring requirement. Initially, participants were asked to record daily calorie intake including grams of fat, minutes of physical activity, and weight. The majority had difficulty in estimating calories and fat content. Subsequently, emphasis was placed on recording weight, physical activity minutes, types of food eaten including portion sizes, and on identifying high-fat foods.
There are several potential limitations to our study. First, this investigation was a feasibility study examining group lifestyle intervention for diabetes prevention in Arab Americans and lacked a control group. Second, nutritional intake was assessed with only a 24-hour dietary recall. Finally, there were differences in exposure to and experience with study related procedures between those individuals accepting lifestyle intervention initially and those enrolled following the educational program. We have examined and presented outcome data between these cohorts to overcome this potential limitation.
In summary, we describe the development and implementation of a community-based, culturally-specific, group lifestyle intervention. The study demonstrates the feasibility and effectiveness of this DPP adapted lifestyle intervention in overweight Arab Americans and provides compelling evidence that education targeting gaps in knowledge and misconceptions is effective in promoting participation. Future trials focusing on partnerships with established community organizations are likely to demonstrate the broad dissemination of effective and sustainable diabetes prevention measures in various populations at increased risk of developing diabetes.
Acknowledgments
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institute of Health (Grant R34 DK076663). Support for the Biostatistics and Economic Modeling Core and the Behavioral, Clinical, and Health Systems Intervention Research Core of the Michigan Diabetes Research and Training Center from the National Institute of Health (Grant P60 DK20572).
The funding organization had no role in the design and conduct of the study; collection, management, analysis or interpretation of the data; preparation, review, or approval of the manuscript.
We acknowledge the dedication of the study coordinator, Ms Hiam Hamade, and Mayssoun Hamade for their valuable contributions to the success of the project. We are also grateful for the commitment of the study participants.
Footnotes
Abstract Presentation: An abstract describing parts of this study was presented at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, June 8, 2009. An abstract was also presented at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, June 26, 2010.
Conflict of interest: No potential conflicts of interest relevant to this study were reported.
The principal investigator (Linda A. Jaber) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zogby J. Arab Americans. [Accessed October 8, 2010];Demographics. http://www.aaiusa.org/arab-americans/22/demographics.
- 2.Jaber LA, Brown MB, Hammad A, et al. Epidemiology of diabetes among Arab Americans. Diabetes Care. 2003;26:308–313. doi: 10.2337/diacare.26.2.308. [DOI] [PubMed] [Google Scholar]
- 3.Jaber LA, Brown MB, Hammad A, Zhu Q, Herman WH. Lack of acculturation is a risk factor for diabetes in Arab immigrants in the US. Diabetes Care. 2003;26:2010–2014. doi: 10.2337/diacare.26.7.2010. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 6.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and diabetes study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 8.Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes: the STOP-NIDDM randomized trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 9.Gerstein HC, Yusuf S, Boxch J, Pogue J, Holman RR, et al. DREAM (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication) Trial Investigators: effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomized controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijav V. Indian Diabetes Prevention Programme (IDPP): the Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-I) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 11.Lindstrom J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: the follow-up results of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 12.Glasgow RE, Bull SS, Gillette C, Klesges LM, Dzewaltowski DA. Behavior change intervention researchin health care settings: a review of recent reports with emphasis on external validity. Am J prev Med. 2002;23:62–69. doi: 10.1016/s0749-3797(02)00437-3. [DOI] [PubMed] [Google Scholar]
- 13.Garfield SA, Malozowski S, Chin MH, et al. The Diabetes Mellitus Interagency Coordinating Committee (DMICC) Translation Conference Working Group. Considerations for diabetes translational research in real-world settings. Diabetes Care. 2003;26:2670–2674. doi: 10.2337/diacare.26.9.2670. [DOI] [PubMed] [Google Scholar]
- 14.Davis-Smith YM, Boltri JM, Seale JP, Shellenberger S, Blalock T, Tobin B. Implementing a diabetes prevention program in a rural African-American church. J Natl Med Assoc. 2007;99:440–446. [PMC free article] [PubMed] [Google Scholar]
- 15.Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the diabetes prevention program into an urban medically underserved community: a nonrandomized prospective intervention study. Diabetes Care. 2008;31:684–689. doi: 10.2337/dc07-1869. [DOI] [PubMed] [Google Scholar]
- 16.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community: the DEPLOY Study. Am J Prev Med. 2008;35:357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Absetz P, Valve R, Pldenburg B, et al. Type 2 diabetes prevention in the “real world”. One-year results of the GOAL implementation trial. Diabetes Care. 2007;30:2465–2470. doi: 10.2337/dc07-0171. [DOI] [PubMed] [Google Scholar]
- 18.McTigue KM, Conroy MB, Bigi L, Murphy C, McNeil M. Weight loss through living well: Translating an effective lifestyle intervention into clinical practice. The Diabetes Educator. 2009;35:199–208. doi: 10.1177/0145721709332815. [DOI] [PubMed] [Google Scholar]
- 19.Amundson HA, Butcher MK, Gohdes D, et al. Translating the Diabetes Prevention Program into practice in the general community: Findings from the Montana Cardiovascular disease and Diabetes Prevention Program. The Diabetes Educator. 2009;35:209–223. doi: 10.1177/0145721709333269. [DOI] [PubMed] [Google Scholar]
- 20.The Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP) – Description of the Lifestyle Intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. [Accessed October 8, 2010]; http://www.bsc.gwu.edu/dpp/lifestyle/dpp_part.html.
- 22.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. 1988;15(2):175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 23.Mensing C. The Art and Science of Diabetes Self-Management Education. American Association of Diabetes Educators; Chicago, IL: 2006. [Google Scholar]
- 24.Walker EA, Mertz CK, Kalten MR, Flynn J. Risk perception for developing diabetes: comparative risk judgments of physicians. Diabetes Care. 2003;26:2543–2548. doi: 10.2337/diacare.26.9.2543. [DOI] [PubMed] [Google Scholar]
- 25. [Accessed 2010 July 21];EQ-5D. http://www.euroqol.org/home.html.
- 26.Spitzer RL, Kroenke K, Williams JBW the Patient Health Questionnaire Primary Care Study Group. Validation and utility of a self-report version of PRIME-MD. The PHQ Primary Care Study. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 27.Kriska AM, Caspersen CJ. Introduction to a collection of physical activity questionnaires. Med Sci Sports Exerc. 1997;29(suppl):5–9. [PubMed] [Google Scholar]
- 28.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaber LA, Brown MB, Hammad A, Zhu Q, Herman WH. The prevalence of the metabolic syndrome among Arab Americans. Diabetes Care. 2004;27:234–238. doi: 10.2337/diacare.27.1.234. [DOI] [PubMed] [Google Scholar]
- 30.The Diabetes Prevention Program Research Group. Strategies to identify adults at high risk for type 2 diabetes: The Diabetes Prevention Program. Diabetes Care. 2005;28:150–156. doi: 10.2337/diacare.28.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]