Abstract
The cannabinoid type-1 (CB1) receptor is a G protein-coupled receptor (GPCR) that binds the main active ingredient of marijuana, Δ9-tetrahydrocannabinol, and has been implicated in several disease states, including drug addiction, anxiety, depression, obesity, and chronic pain. In the two decades since the discovery of CB1, studies at the molecular level have centered on the transmembrane core. This interest has now expanded as we discover that other regions of CB1, including the CB1 carboxyl-terminus, have critical structures that are important for CB1 activity and regulation. Following the recent description of the three dimensional structure of the full-length CB1 carboxyl-terminal tail (Ahn et al., Biopolymers (2009) 91: 565–573), several residues and structural motifs including two α-helices (termed H8 and H9) have been postulated to interact with common GPCR accessory proteins, such as G-proteins and β-arrestins. This discourse will focus on the CB1 carboxyl-terminus; our current understanding of the structural features of this region, evidence for its interaction with proteins, and the impact of structure on the binding and regulatory function of CB1 accessory proteins. The involvement of the carboxyl-terminus in the receptor life cycle including activation, desensitization, and internalization will be highlighted.
Keywords: cannabinoid receptor, G protein-coupled receptor, carboxyl-terminus, internalization, desensitization, helix 8
Introduction
The plant Cannabis sativa, in various forms including marijuana and hashish, has been utilized for its medicinal properties for centuries. The main psychoactive component of marijuana, Δ9-tetrahydrocannabinol (THC) and its derivatives, are classified as cannabinoids and affect a large number of physiological functions, including pain, body temperature, appetite control, motor coordination, learning and memory, sedation, anxiety, and fear (for review see Porter and Felder 2001). Endogenous cannabinoids, including anandamide and 2-arachidonyl glycerol, have been isolated from brain (Devane et al. 1992; Mechoulam et al. 1995; Felder et al. 1996; Sugiura et al. 1995), are highly lipophilic, and mediate cannabimimetic neurological effects (Fride and Mechoulam 1993; Crawley et al. 1993; Smith et al. 1994).
The cannabinoid receptors that bind THC with high affinity are found as two subtypes, the cannabinoid type-1 (CB1; Matsuda et al. 1990) and the cannabinoid type-2 (CB2; Munro et al. 1993) receptors. The CB1 is predominantly expressed in the central and peripheral nervous systems, and is among the most expressed receptors in the brain (Howlett et al. 2002). High-levels of CB1 expression have been reported in areas of the brain implicated in the actions of marijuana, including the cortex, amygdala, basal ganglia, cerebellum, and brainstem emetic centers (Tsou et al. 1998; Galiegue et al. 1995; Kumar et al. 2001). In contrast, the CB2 is largely restricted to cells associated with the immune system (Munro et al. 1993). Two splice variants of CB1 with shortened amino-termini have been identified, CB1a and CB1b (Rinaldi-Carmona et al. 1996; Shire et al. 1995; Ryberg et al. 2005), and recently, an orphan GPCR, GPR55, has been proposed as a third member of the cannabinoid receptor family. However, designation of GPR55 as a cannabinoid receptor is still pending (Godlewski et al. 2009; Brown and Robin 2009).
The CB1 is an interesting therapeutic target for a number of disorders, including treatment of anorexia in patients who suffer from AIDS wasting syndrome, reducing nausea and vomiting associated with chemotherapy treatment (Walsh et al. 2003), and relief of neuropathic pain in multiple sclerosis (Rahn and Hohmann 2009). To date, synthetic THC and analogues such as Marinol®, Cesamet®, and Sativex® are clinically available in a number of countries; however, the effort to improve efficacy, selectivity, and the therapeutic window of these drugs is ongoing (Walsh et al. 2003). CB1 antagonists/inverse agonists have also received much attention for their potential therapeutic applications such as for smoking cessation, weight loss, and drug addiction; however, CNS side effects have prevented their approval by the FDA (Le et al. 2008; Butler and Korbonits 2009; de Kloet and Woods 2009; Beardsley et al. 2009). Additional future avenues for CB1-specific pharmacotherapy may include use of orthosteric ligands and/or ligands that display bias towards the activation of selective cell signaling pathways (i.e. biased ligands); successful development of both will likely require a clear understanding of the CB1 carboxyl-terminus and its role in CB1 function.
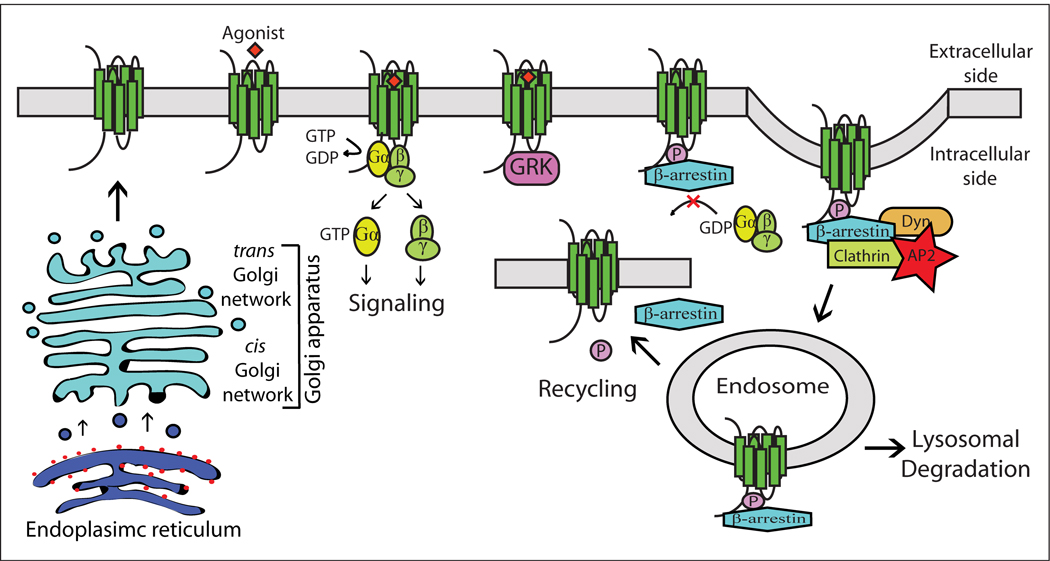
The CB1 receptor is a member of the rhodopsin-like class A G protein-coupled receptor (GPCR) superfamily, and like other GPCRs, contains an extracellular glycosylated amino-terminus, seven α-helical transmembrane domains (TMs), with intervening extracellular and intracellular loops, and an intracellular carboxyl-terminus. The cytoplasmic regions are involved in G protein-binding, desensitization, and cellular trafficking of the receptor. For prototypical GPCRs, as depicted in Fig. 1, binding of agonists induces heterotrimeric G-protein activation by exchanging GDP for GTP on the α subunit. The G-proteins then dissociate from the receptor and the α subunit dissociates from the β/γ subunits, allowing these to regulate downstream effectors. Upon prolonged agonist exposure, protein kinases (e.g. G-protein receptor kinases 2/3 (GRK2/3)) can become activated which in turn act to phosphorylate GPCRs, providing a scaffold for arrestins to bind. The G-proteins uncouple from the receptor (desensitization) and the receptors internalize. The carboxyl-terminus, a region found critical for these regulatory events, is the focus of this review. Sequence and structural motifs of the CB1 carboxyl-terminus will be described, as well as current findings regarding the function of this critical CB1 receptor domain.
Fig. 1.
Model of the GPCR life cycle. GPCRs are synthesized, folded, and assembled associated with the endoplasmic reticulum (ER). Properly folded receptors are transported from the ER through the Golgi complex to the plasma membrane by passing a quality control process and undergoing post-translational modifications (e.g. glycosylation, methylation, and palmitoylation). Upon agonist stimulation, GPCRs activate their associated G-protein, which in turn dissociates to impact downstream signaling pathways. Prolonged exposure to agonist results in a rapid loss of responsiveness (desensitization) and removal of the receptors from the cell surface (internalization) by phosphorylation (e.g. via GRK) and subsequent arrestin recruitment. Internalized receptors are either targeted to lysosomes for degradation, or recycled back to the cell surface (resensitization).
Features of the CB1 carboxyl-terminus amino acid sequence
The CB1 carboxyl-terminus has 73 residues (i.e. human CB1 R400-L472; Bramblett et al. 1995; Xie and Chen 2005; Choi et al. 2005), a length similar to other GPCRs including the β2-adrenergic (84 residues; Kobilka et al. 1987) and the CB2 (59; Munro et al. 1993) receptors as shown in Fig. 2. Although the human CB1 and CB2 receptors only differ in their carboxyl-terminal length by 14 residues, share some ligands, and signal through similar pathways, there is no significant homology between the carboxyl-termini across these receptor subtypes.
Fig. 2.
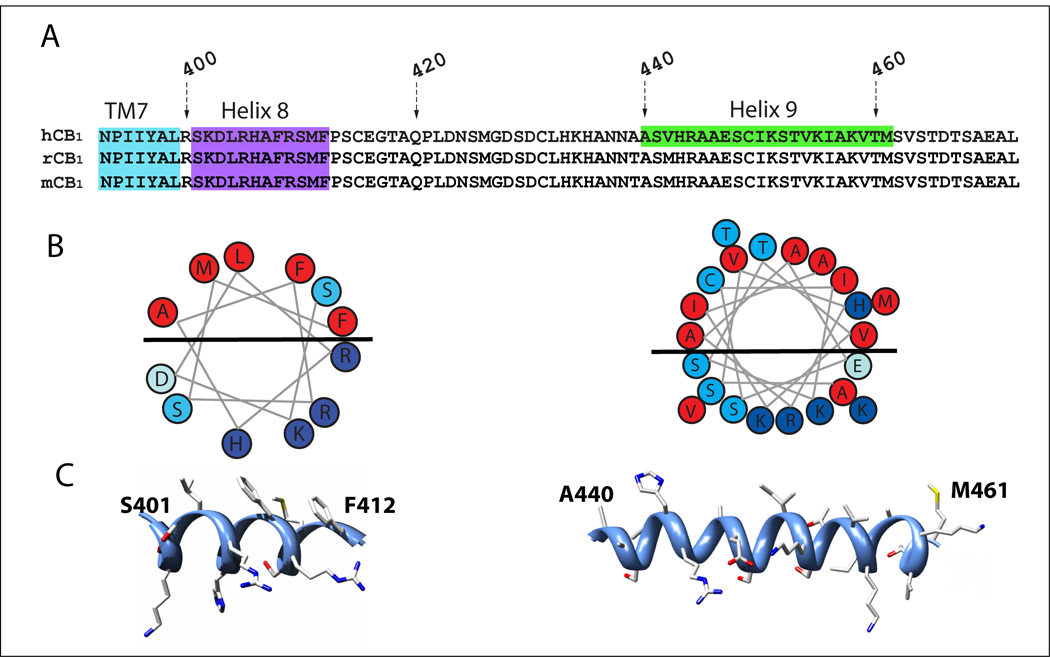
Sequence alignment of the carboxyl-termini of human CB1 and other selected GPCRs. The human CB1 carboxyl-terminus is presented with human CB2 and other rhodopsin-like family A GPCRs including hADRB2, human β2-adrenergic receptor (PDB code: 2RH1); bRho, bovine rhodopsin (PDB code: 1U19); sqRho, squide rhodopsin (PDB code: 2Z73); tADRB1, turkey β1-adrenergic receptor (PDB code: 2VT4); hADORA2, human adenosine 2A receptor (PDB code: 3EML). The carboxyl-terminal end of TM7, helix 8, and helix 9 are highlighted in cyan, purple, and green, respectively. The carboxyl-terminal sequences are aligned in two rows. Numbers correspond to the starting and ending residues in each line.
Potential functional roles for the CB1 carboxyl-terminus can be predicted from sequence analysis. The CB1 carboxyl-terminus contains three cysteine residues, which if palmitoylated may act as membrane anchors, as well as numerous serine and threonine residues (11 and 5 residues, respectively) that may become phosphorylated and play a role in associations with regulatory proteins and secondary signaling molecules (Gurevich and Gurevich 2006). The region also contains aspartate and glutamate residues, whose terminal carboxylic acid groups could mimic phosphorylated serine/threonine residues, as found in other receptors (Gurevich and Gurevich 2006). Nonetheless, defining the three dimensional structure of the CB1 carboxyl-terminus will help identify structural motifs involved in activities of the receptor. The amino acid numbering system of the human CB1 carboxyl-terminus has been utilized in this review.
The complexities of analyzing the structure of the CB1 carboxyl-terminus and strategies utilized to overcome them
Although riddled with their own tribulations, GPCR crystal structures have made significant contributions to the understanding of GPCR structure and function. However, in order to improve the crystal properties, the relatively unstructured and flexible carboxyl-terminus has been truncated in many of the GPCR crystal structures reported to date (Shimamura et al. 2008; Rasmussen et al. 2007; Murakami and Kouyama 2008). The exceptions include crystal structures of bovine rhodopsin, whose carboxyl-terminus is relatively short and is not appreciably resolved beyond the initial residues due to presumed inherent flexibility (Palczewski et al. 2000; Li et al. 2004). Therefore, what we know of the structure of the CB1 carboxyl-terminus has largely evolved from computer modeling studies, circular dichroism (CD) spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy.
Hydropathy plot analysis (Kyte and Doolittle, 1982) suggests that the CB1 carboxyl-terminal tail is not markedly hydrophobic, yet purification of the full-length carboxyl-terminus has been challenging and has lead several investigators to use small peptides corresponding to shorter regions. Only recently has a peptide corresponding to the full-length CB1 carboxyl-terminus been purified (Ahn et al. 2009) which has allowed for observations of additional structures. Because these studies do not examine the carboxyl-terminus within the context of the full-length receptor, interpretation of these studies must be performed with care. However, this approach has provided a good starting point for obtaining structural information.
The cytoplasmic end of TM7 and the H8 helical segment
In all reported crystal structures TM7 includes an α-helical structure followed by a kink and either a second helix (e.g. squid and bovine rhodopsin and α2-adrenergic receptors; Murakami and Kouyama 2008; Jaakola et al. 2008; Palczewski et al. 2000) or an unstructured region (e.g. turkey β1 and human β2-adrenergic receptors; Warne et al. 2008; Rasmussen et al. 2007). In all cases, the highly conserved NPXXY motif, a region at the carboxyl-terminal end of TM7 thought to be critical for receptor activation (Fritze et al. 2003), is membrane imbedded. Beyond that, the TM7 carboxyl-terminal end is not universal and data from crystal structures cannot be easily applied to other GPCRs.
Due to the combination of its proximity to TM7 and its defined structure, the most commonly studied region within the carboxyl-terminus of all rhodopsin-like class A GPCRs is the region that encompasses helix 8 (H8). Although the amino acid sequence homology in this region is low, the H8 structure has been identified in all GPCR crystal structures and its existence suggested by numerous receptor modeling and NMR studies, covering a wide-range of GPCR subtypes (e.g. Palczewski et al. 2000; Murakami and Kouyama 2008; Jaakola et al. 2008; Warne et al. 2008; Rasmussen et al. 2007; Li et al. 2004; Piserchio et al. 2005).
One of the first analyses that predicted the CB1 H8 employed Fourier transform methods, with the nPRIFT hydrophobicity scale and with a variability profile to calculate the α-helical periodicity in the primary amino acid sequence of the human full-length CB1 receptor (Bramblett et al. 1995). A discrete α-helical segment was identified extending from TM7 and spanning the membrane-cytoplasm interface. The relative orientations of the hydrophobic and variability moment vectors of this TM7 extension suggested that the helix has two faces, one polar and one nonpolar, 180° apart, and indicated that this helical segment is not fully embedded in the plasma membrane (Bramblett et al. 1995).
CD spectroscopy has been a useful tool to study the H8 in isolated peptide fragments of the CB1 carboxyl-terminus. Results indicate that the helicity of this region is dependent on the solvent/detergent utilized. CD studies in phosphate buffer and water have found that low concentrations of a peptide inclusive of the CB1 H8 region exhibit random coil (Mukhopadhyay et al. 1999; Choi et al. 2005). However, evidence for a limited number of low energy conformations was provided from molecular dynamics (MD) simulations (Cowsik et al. 1997), and taken together with findings from Fourier transform methods (Bramblett et al. 1995), suggests that greater helical structure within the carboxyl-terminus is likely. More recently, utilizing a peptide corresponding to the full-length CB1 carboxyl-terminus, helicity was found to be concentration-dependent in aqueous solution leading the authors to conclude that at higher peptide concentrations amphiphilic helices form by self-association to sequester hydrophobic regions of the peptide (Ahn et al. 2009). In contrast, in zwitterionic (DPC) and anionic (SDS and DOC) detergents the peptide exhibits high helical content (36–38% and 48–51%, respectively), at all concentrations of peptide studied. Thus, a mimetic of the membrane leaflet can induce helical formation (Ahn et al. 2009). Similar solvent-dependent effects on H8 helicity have been reported by Mukhopadhyay et al (1999) and for other GPCR H8 regions (Choi et al. 2002; Mukhopadhyay et al. 2000; Bechinger et al. 1991; Jung et al. 1996; Johnson and Cornell, 1999).
NMR studies utilizing short peptide fragments found little to no evidence for H8 secondary structure in aqueous media (Grace et al. 2007; Choi et al. 2005); yet using nuclear Overhauser effect spectroscopy, spectra typical of a high degree of α-helix formation in hydrophobic environments were found (Grace et al. 2007; Xie and Chen 2005; Tyukhtenko et al. 2009; Choi et al. 2005; Ahn et al. 2009), consistent with CD data. Secondary structural analyses of such small peptides are typically prone to difficulties including underestimates of secondary structure due to the high proportion of peptide constituted by the floppy ends. Consequently, in the intact receptor, H8 is likely to have a very high propensity for adopting a helical domain, comprised of residues S401-F412, in amphipathic environments (Xie and Chen 2005; Ahn et al. 2009).
In the GPCR crystal structures reported thus far, the TM7-H8 interface is comprised of 1–2 residues that allow for flexibility of the amino acid backbone, with the TM7 and H8 helices lying perpendicular to each other in three dimensional space and the H8 almost parallel to the membrane surface (Palczewski et al. 2000; Cherezov et al. 2007; Jaakola et al. 2008; Murakami and Kouyama 2008; Rasmussen et al. 2007; Shimamura et al. 2008; Warne et al. 2008). Results from both computer modeling and NMR studies of the CB1 receptor leave a single residue, R400, as the pivot point in the potential “L-shaped” linker, or flexible hinge, formed between the TM7 and H8 domains (Bramblett et al. 1995; Xie and Chen 2005; Ahn et al. 2009). Following activation of bovine rhodopsin, it has been suggested that the cytoplasmic end of TM7 undergoes conformational changes exposing the carboxyl-terminus of TM7, as well as the H8 domain via the flexible hinge, to an aqueous environment. Such a conformational change is believed to unmask critical G protein-binding domains (Abdulaev and Ridge, 1998). Furthermore, a critical interaction between TM7 and H8 (i.e. the NPXXY(X)4,5F motif), which has been found to stabilize rhodopsin in the inactive state, is displaced upon activation (Li et al. 2004; Scheerer et al. 2008). The location of H8 and its movement upon rhodopsin activation may be assisted by palmitoylation/depalmitoylation of neighboring cysteine residues (Shimamura et al. 2008). Consistent with this possibility, NMR-based computations of CB1 suggest that a cysteine residue just carboxyl-terminal to the H8 (position 415) faces the membrane surface, and if palmitoylated, could stabilize the location of H8 at the membrane surface (Xie and Chen 2005). Thus, the structure of this region seems to be well-suited to be constrained and membrane interactive when the receptor is inactive; yet has the potential for significant movement and exposure of possible regulatory protein binding sites when activated.
The amphipathic nature, membrane association, and potential functional relevance of H8
The amino acid sequence of the CB1 H8 is interspersed with residues containing polar side chains, yet NMR analyses of H8 in detergent indicate that the nonconsecutive positively-charged residues, including K402, R405, and R409, orient on the same side of the helix (Xie and Chen 2005; Choi et al. 2005; Ahn et al. 2009; Tyukhtenko et al. 2009; Grace et al. 2007) while the hydrophobic residues, including L404, F408, and F412, reside on the opposite face of the helix, as depicted in Figs. 3B and 3C. NOE interactions between D403 and H406 are indicative of a salt bridge (2.6 Å long), whose relative distance and orientation supports the helical nature of this region (Tyukhtenko et al. 2009; Xie and Chen 2005). MD simulations (at 150-ps) establish that the energetic stability favors helix association with the membrane surface (Ahn et al. 2009) and NMR studies find the F408 side chain and L404 amide protons interact with DPC acyl chains and head groups (Choi et al. 2005), together supporting a model in which the hydrophobic face of the helix is oriented toward the membrane and sits in proximity to the surface. Moreover, the H8 in all reported GPCR crystal structures is similarly amphipathic and has a similar orientation suggesting this requirement for correct folding, membrane association, or both.
Fig. 3.
CB1 carboxyl-terminal sequences and structural representations of two helical motifs within the carboxyl-terminus. (A) Amino acid sequence alignment of the carboxyl-terminus of CB1 from different species; hCB1, human CB1 receptor, rCB1, rat CB1 receptor, mCB1, mouse CB1 receptor. Two helical motifs within the CB1 carboxyl-terminus were defined based on the NMR structure (Ahn et al. 2009). Helix 8 and helix 9 are highlighted in purple and green, respectively. Residues are numbered according to their gene-specific position in the protein sequence. (B) Helical wheel projections of CB1 helix 8 (left) and helix 9 (right). Hydrophobic and positively charged residues are colored red and blue, respectively. Negatively charged residues and serines/threonines are colored light blue and turquoise, respectively. The black bar through each helical wheel highlights the amphipathic nature of the helix and the nonpolar and polar faces. (C) Illustration of the amphipathic nature of the two α-helices observed for the carboxyl-terminus of the human CB1 receptor. The helical domains CB1 (401–412) (left) and CB1 (440–461) (right) identified in Ahn et al. (2009) are shown as ribbons.
Recently, the impact of specific H8 residues on helicity, ligand binding, and subcellular localization was examined by mutational studies of both a peptide corresponding to the CB1 carboxyl-terminus and the full-length receptor. In the presence of DPC both a wild type and a K402Q/R405Q/R409Q mutant CB1 carboxyl-terminal peptide displayed high helical content, whereas a L404A/F408A/F412A mutant peptide displayed substantially reduced helicity (Ahn et al. 2010). This suggests that the highly hydrophobic residues are critical for helix formation, likely due to their direct interactions with the membrane mimetic. Moreover, the full-length CB1 receptor L404A/F408A/F412A mutant, relative to the wild-type CB1, exhibited aberrant localization in cells and markedly lower Bmax values indicating that helix formation is needed for proper CB1 trafficking (Ahn et al. 2010). Similar dependence on the hydrophobic H8 residues for proper receptor trafficking was found for the leukotriene BLT2 receptor (Yasuda et al. 2009).
Although H8 is found in all GPCR crystal structures, and based on sequence alignment, a similar amphipathic motif can be found in a large variety of class A GPCRs (Han et al. 2001), it is not yet clear if a common functional role for H8 exists. Numerous studies, including the analysis of crystal structures, peptide crosslinking and peptide competition studies, implicate H8 in productive G-protein coupling (Konig et al. 1989; Ernst et al. 2000; Cai et al. 1999; Swift et al. 2006). These studies find that H8 interacts with cytoplasmic loops and TM extensions of the receptor (e.g. Wess et al. 2008; Konig et al. 1989; Shimamura et al. 2008; Murakami and Kouyama 2008), and these interactions become disrupted upon activation (Sheerer et al. 2008; Li et al. 2004). However, whether these alterations are due to direct binding of G protein to H8 or are a consequence of indirect binding of G protein remains to be elucidated. Other groups find the carboxyl-terminus necessary for GPCR exit from the endoplasmic reticulum (e.g. Tai et al. 1999; Bermak et al. 2001; Robert et al. 2005; Duvernay et al. 2004), with a defective H8 leading to impaired receptor localization and β-arrestin translocation to the plasma membrane (e.g. Ahn et al. 2010; Suvorova et al. 2009; Yasuda et al. 2009). While deletion of H8 of the leukotriene BLT2 receptor led to ER trapping, cell surface expression could be recovered by treatment with ligands that act like pharmacological chaperones (Yasuda et al. 2009). These finding suggests that H8 impacts the proper folding of the receptor and, in its absence, ligands can induce receptor assembly into a transport-competent form. Recently, results from a CB1 model generated by MD simulation suggested that once the helix forms, the hydrophobic residues of H8, TM1, IC1, and TM7 form a strong hydrophobic pocket (Shim 2009). This pocket may directly interact with the hydrophobic tails of membrane lipids, contributing to receptor stabilization during biosynthesis and trafficking. Alternatively, one face of H8 may interact with its counterpart on another CB1 receptor or other GPCR to promote interactions in a receptor dimer that may be crucial for ER assembly. Indeed, in the crystal structures of photoactivated rhodopsin (Salom et al. 2008; PDB code: 2137), the β1-adrenergic receptor (Warne et al. 2008; PDB code: 2vt4), and the β2-adrenergic receptor (Cherezov et al. 2007; PDB code: 2rh1) H8 along with various TM domains (e.g. TM1 and TM2) are found at the dimer interface. Interestingly, the crystal structure of the β2-adrenergic receptor also reveals a cholesterol-binding pocket in the receptor dimer interface. This is consistent with the observed interactions of CB1 H8 with lipids (Choi et al. 2005; Ahn et al. 2009). Regardless of the site of H8 interaction, its importance to receptor assembly is emphasized by the finding that ER-resident chaperones such as calnexin (Free et al. 2007) and calreticulin (Duvernay et al. 2009) have been found to interact with some GPCRs, including those with substantial H8 mutations, to ensure that only properly folded proteins leave the ER. It is also possible the H8 may directly interact with chaperones or cargo carriers such as Drip 78 (Bermak et al. 2001), BiP (Siffroi-Fernandez et al. 2002), calmodulin (Labasque et al. 2008; Navarro et al. 2009), and COPII vesicles (Dong et al. 2008), and without a functional H8, receptor maturation and trafficking are unachievable.
H9 and the remainder of the CB1 carboxyl-terminus
Little structural information regarding GPCR carboxyl-termini beyond the H8 is available. Recently, NMR studies examining the structure of the entire CB1 carboxyl-terminal tail confirmed the presence and location of the CB1 H8 and identified an additional helix, termed H9, located towards the end of the CB1 carboxyl-terminus and encompassing residues A440-M461 (Fig. 3B and 3C; Ahn et al. 2009). Unfortunately, the lack of a tertiary fold in the structure prevents mapping of the relative and topological orientation of the H9. However, NMR line-broadening indicates that H9 interacts with DPC micelles, suggesting that the amphiphilic H9, like H8, lies on the inner-membrane surface and is perpendicular to the TM7 bundle. Similar to results from NMR studies, findings from a 150-ps MD simulation of the L374–L472 peptide (TM7 and the full-length CB1 carboxyl-terminus) fully solvated in a lipid bilayer indicate that the CB1 H9 (residues A440-M461) energetically favors lying on the membrane surface, with the 28 intervening residues (P413-A439) flexible and fluctuating (Ahn et al. 2009). Yet, the distance between residue F412 (H8) and A440 (H9) remains at 26 Å, indicating that despite movement of the intervening regions, the location of the helices remains fixed. Residues carboxyl-terminal to H9 were also found to be unstructured (Ahn et al. 2009).
The functional relevance of the CB1 H9 has yet to be determined and for only a few GPCRs has structure in the carboxyl-terminus beyond the H8 been reported. Although limited, what we have learned from other H9 regions may provide insight into the significance of the CB1 H9. For example, the squid rhodopsin H9 indirectly associates with the membrane though interactions with the membrane-anchored H8 and the carboxyl-terminal end of TM6 (Shimamura et al. 2008; Murakami and Kouyama 2008; Schertler 2008). Together with the IC2, IC3, and the intracellular end of TM5, these domains tightly fold together, suppressing H9 rotational freedom. The negatively charged H9 residues add an electrostatic potential to the otherwise predominately positively-charged intracellular protein surface. This complex has been postulated to comprise a Gαq-binding site. NMR studies find that the bradykinin receptor, which also signals through Gαq, also has an H9 (Piserchio et al. 2005). This helix contains a number of hydroxylated (and possibly phosphorylated) residues that were found imperative for Gαq signaling. Although the bradykinin H9 is amphipathic, likely membrane-associated, and its interactions with other intracellular structures are unknown, these findings may indicate that like squid rhodopsin, H9 negative charges are important for Gαq binding. The CB1 H9, which is also amphipathic, likely membrane-associated, and contains a number of polar residues on its hydrophilic face (Ahn et al. 2010; Fig. 3), could fold similarly with other intracellular components to interact with signaling molecules and regulatory proteins. This possibility is currently under investigation.
It is important to note that what we have learned from peptides corresponding to regions of GPCRs is extensive and informative; however, in all cases the necessary extrapolation of data to the full-length receptor must be weighed carefully. At present we can’t predict how the presence of the full-length receptor may alter the structures, orientations, and membrane-interactions identified in the peptides corresponding to regions of the carboxyl-terminal tail. Furthermore, none of these structural strategies utilize biological membranes and thus we can only infer biological information from each model system.
Functional roles of the CB1 carboxyl-terminus
The carboxyl-termini of GPCRs have been implicated in both the binding of numerous proteins and regulation of receptor activation, signaling, and subcellular localization. The remainder of this review will focus on the role of the CB1 carboxyl-terminus in these processes and highlight the proteins that are thought to interact with this region of the receptor as summarized in Fig. 4.
Fig. 4.
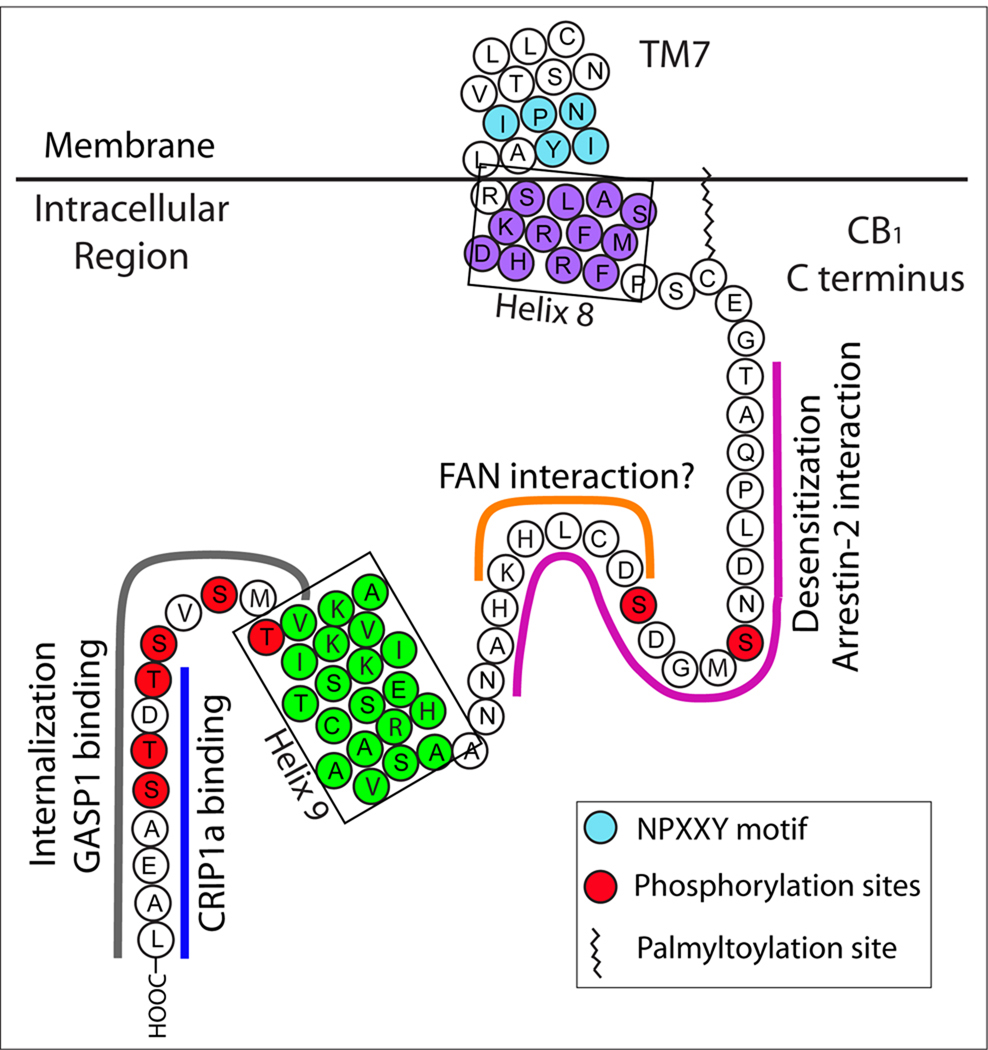
Schematic diagram of the carboxyl-terminus of the human CB1 showing the proposed structural and functional domains. The relevant portion of TM7 is shown. The NPXXY motif, helix 8, and helix 9 are highlighted in cyan, purple, and green, respectively, as described in Fig. 2. Fuschia, orange, grey, and blue lines indicate potential interaction domains with β-arrestin 1, FAN, GASP1, and CRIP1a, respectively. A putative palmitoylated cysteine at position 415 is depicted and potential phosphorylation sites are indicated by red filled circles. Two domains implicated in desensitization (Jin et al. 1999; Bakshi et al. 2007) and internalization (Heieh et al. 1999) are indicated.
CB1 G-protein coupling
Agonist-induced activation of the CB1 receptor results in the inhibition of pertussis toxin (PTX)-sensitive inhibition of adenylate cyclase activity and leads to the inhibition of cAMP accumulation, inhibition of N- and P/Q-type calcium channels, and decreases in Ca2+ conductance (for review see Howlett 2005). Activation of CB1 receptors also leads to an increase in G protein-gated inward-rectifying K+ channel (GIRK) activity and activation of mitogen-activated protein kinases (MAPKs). These effects result from coupling to one of the three subtypes of Gαi or either of the two subtypes of Gαo proteins (i.e. Gαi1,2,3 and Gαo1,2, respectively; Howlett et al. 1986). Cannabinoids have also been shown to stimulate cAMP (Bonhaus et al. 1998; Maneuf and Brotchie, 1997; Glass and Felder, 1997) as well as activate Ca2+ signalling (Lauckner et al. 2005; DePetrocellis et al. 2007), suggesting the receptors can also couple to Gαs and Gαq proteins, however the significance of these interactions is unknown.
A direct interaction between G-proteins and the CB1 receptor has been shown following co-immunoprecipitation experiments and utilizing toxins that inhibit G protein-binding (Mukhopadhyay et al. 2000; Mukhopadhyay and Howlett 2001). Several studies indicate that the interactions of select G-protein subtypes occur with CB1 intracellular loops (e.g. Ulfers et al. 2002; Abadji et al. 1999), however, discussion of G protein-CB1 interactions in this review focuses on those associated with the CB1 carboxyl-terminus.
Evidence for direct G-protein interactions with the CB1 carboxyl-terminus
Findings from G-protein activation and co-immunoprecipitation studies, utilizing a peptide corresponding to residues R400-E416 of the CB1 carboxyl-terminus, implicate this region in Gαi/o protein binding and activation. In the absence of CB1 ligands, the R400-E416 peptide stimulated GTPγS binding to rat brain membrane fractions and inhibited adenylate cyclase activity in membrane homogenates from N18TG2 cells (Howlett et al. 1998). Peptide activity was also measured in CHO cells lacking CB1 receptors and was not reversed by co-incubation with a CB1 inverse agonist (Mukhopadhyay et al. 1999), indicating that the R400-E416 peptide can autonomously activate G-proteins. In co-immunoprecipitation studies, co-incubation of high concentrations (0.2–0.5 mM) of the R400-E416 peptide with CB1-expressing cell membrane homogenates prevents co-immunoprecipitation of the full-length CB1 receptor with Gαi3 and Gαo, but not Gαi1,2 (Mukhopadhyay et al. 2000; Mukhopadhyay and Howlett 2001), suggesting that the peptide can specifically disrupt CB1-Gαi3 and CB1-Gαo immunoprecipitable complexes. In contrast, three different peptides of similar length encompassing distinct regions of the IC3 loop (Mukhopadhyay et al. 2000), a peptide containing the putative H8 region of CB2 (Mukhopadhyay and Howlett 2001), and mastoparan (Mukhopadhyay and Howlett 2001), a cationic bee venom peptide that can form an amphipathic α-helix and that can autonomously activate G-proteins (Higashijima et al. 1990), were unable to individually compete with the full-length CB1 receptor for Gαi3 or Gαo subunits. These findings suggest that the R400-E416 region of the CB1 carboxyl-terminal tail is involved in Gαi3/o recognition, binding, and activation (Mukhopadhyay et al. 2000).
Structural features of the CB1 H8 region potentially involved in G-protein recognition, binding and activation
The CB1 R400-E416 peptide consists of residues corresponding to the “TM7 linker region” (R400), the residues encompassing H8 (S401-F412), and four additional residues carboxyl-terminal to the H8, one of which is a C415S substitution (Howlett et al. 1998). Since the bulk of this peptide includes residues corresponding to the H8, it is tempting to speculate that this domain is necessary for the observed interactions of the peptide with G-proteins. As shown in Fig. 3, the H8 contains many potentially bioactive residues, including a cationic patch. Although, charge neutralization of residue R400 with norleucine (which contains similar bulk, yet lacks a charge) and shortening of the peptide by removal of R400 (i.e. peptide S401-E416) results in a significant decrease in affinity and efficacy (5-fold and ~25% loss, respectively), further peptide shortening (i.e. peptides D403-E416, R405-E416, A407-E416) results in no additional losses in peptide activity, underscoring the importance of the R400 residue, rather than the charge or length of the peptide in G-protein activation (Mukhopadhyay et al. 1999). Furthermore, charge neutralization of the peptide (i.e. due to acetylation of K402) does not drastically affect R400-E416 peptide activity in adenylate cyclase activity assays (Mukhopadhyay et al. 1999) and more recently Ahn et al. (2010), reported that substitution of the K402, R405, and R409 residues with glutamine on the full length receptor has wild type-like CB1 agonist and GTPγS binding, further supporting the conclusion that the particular hydrophilic residues of the H8 do not directly contribute to G-protein activation. Although the above studies indicate that residue R400 is the key residue for G-protein activation, it is still possible that the adjacent H8 region may be involved in G-protein recognition and/or binding as well.
Regulatory roles of the CB1 carboxyl-terminus in G-protein binding and/or activation
Some evidence suggests that residues carboxyl-terminal to the H8 may play a modulatory role in G-protein binding and/or activity of the full-length receptor. Nie & Lewis (2001a; 2001b) have measured G-protein activation following truncation after the TM7 (i.e. residues following R400) and separately after the H8 (i.e. following G417) domains of the full-length CB1 receptor. Truncation of the entire carboxyl-terminus was found to eliminate 90% of measured Gαi/o activation, whereas truncation following the H8 resulted in only a ~50% loss (Nie and Lewis 2001a; Nie and Lewis 2001b). It is not entirely clear if the observed reduction in G-protein activity is due to a direct loss in G-protein binding sites or to an indirect effect due to structural changes in the receptor that impact G-protein binding (e.g. expression or localization).
The CB1 receptor has been found to be constitutively active when expressed heterologously in non-neuronal cells (Bouaboula et al. 1997; Nie and Lewis 2001a). In CB1-endogenously expressing neurons, constitutive activity has been measured by some (Pan et al. 1998; Hillard et al. 1999; Bouaboula et al. 1997; Vasquez and Lewis, 1999), but not all (Savinainen et al. 2003; Breivogel et al. 2004; Shi et al. 2003) laboratories. Results from studies performed in the Lewis laboratory utilizing truncated CB1 receptor mutants suggest that residues carboxyl-terminal to H8 may be involved in regulating CB1 constitutive activity. For example, when truncated 6 residues carboxyl-terminal to H8 (M1-G417) this CB1 mutant displays similar surface expression, but exhibits greater reversal of tonic inhibition of voltage-dependent Ca2+ current in superior cervical ganglion (SCG) neurons following N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A) treatment (Nie and Lewis 2001a; Nie and Lewis 2001b), suggesting that truncation resulted in higher constitutive activity. Furthermore, co-expression of the α2-adrenergic receptor with the wild type CB1 receptor leads to a reduction in UK14304-mediated inhibition of Ca2+ currents (Vasquez and Lewis, 1999;Nie and Lewis 2001a), while co-expression with the CB1 M1-G417 mutant receptor completely abolished these effects (Nie and Lewis 2001a). These studies suggest that the CB1 M1-G417 mutant constitutively associates with G-proteins to a greater extent than the full-length receptor, confirm that the CB1 M1-G417 mutant has an enhanced ability to sequester activated G-proteins as compared to the wild-type receptor, and support the hypothesis that residues distal to G417 are involved in attenuating CB1 constitutive activity. Similar dependence on the carboxyl-terminus to mediate constitutive activity has been found for the dopamine D5 (Demchyshyn et al. 2000), the serotonin 5-HT4 (Claeysen et al. 1999), and the β2-adrenergic (Parker and Ross, 1991) receptors. However, the extent to which the observations by Nie and Lewis (2001a; 2001b) represent basal activity mediated by endogenous ligands, rather than constitutive activity, requires clarification.
The importance of the “L-shaped” arm and the NPXXY motif in CB1-G protein interactions
The NPXXY motif at the end of TM7 likely participates through aromatic stacking interactions with a relatively conserved phenylalanine in the H8 domain of many GPCRs, playing a key role in switching between the inactive and active states (Prioleau et al. 2002; Fritze et al. 2003; Ernst et al. 2000). The interaction between the NPXXY tyrosine and the H8 phenylalanine in rhodopsin has been proposed to provide structural constraints on the H8 region, driving H8 rearrangement in response to photoactivation and altering the affinity for G-proteins (Fritze et al. 2003). In the serotonin 5-HT2C receptor, the tyrosine-phenylalanine interaction has been reported to be important for conformational switching of the receptor between inactive and active states (Prioleau et al. 2002). Substitution of this H8 phenylalanine residue in the 5-HT2C serotonin receptor and in rhodopsin with amino acids lacking aromatic side chains yields results that suggest disruption of the aromatic stacking is detrimental to the receptors' ability to both activate and dock specific G-proteins (e.g. Prioleau et al. 2002; Fritze et al. 2003). Although H8 sequence alignment (Okuno et al. 2005) finds that the phenylalanine residue is relatively conserved across various GPCRs (135/180 GPCRs), the CB1 receptor contains a leucine (L404, see Fig. 2) at this position, the second most common residue (18/180). Mutation of the full-length CB1 receptor to restore the more conserved residue (i.e. L404F) results in wild type-like ligand binding affinities and expression profiles; however, CB1 agonist-induced GTPγS maximal stimulation (i.e. Emax values) is reduced (Anavi-Goffer et al. 2007). Furthermore, unlike the wild-type receptor (Anavi-Goffer et al. 2007; Mukhopadhyay et al. 2000), the L404F mutant is not able to co-immunoprecipitate Gαi3 subunits (Anavi-Goffer et al. 2007). MD studies, examining possible CB1 TM7-H8 interactions in both the wild type and L404F mutant found evidence for π interactions between the tyrosine-phenylalanine residues in the later, but not the former receptor (Anavi-Goffer et al. 2007). However, the elbow region, specifically the R400 residue which is thought to play a critical role in CB1 affinity for G-proteins (Mukhopadhyay et al. 1999), is encompassed within a helical region in the L404F mutant, while in the wild-type receptor, it remained uncoiled (Anavi-Goffer et al. 2007). Collectively, these data suggest that CB1 has evolved a somewhat different structural scheme that does not employ aromatic stacking of the tyrosine in NPXXY and a phenylalanine in H8; yet it does retain the elbow motif that appears needed for Gαi3 binding and activation.
Overall, the structure of the CB1 carboxyl-terminal tail with its two amphiphathic helices is well-suited for residing at the membrane-cytoplasm interface. This provides a means for association and tight folding of the carboxyl-terminus with the intracellular loops and TM extensions of the receptor. In addition to adding stability to the inactive state of the receptor, this form provides a mechanism by which distinct regions of the receptor could be sequestered, making them accessible to protein binding partners only after critical conformational changes in the receptor occur due, for example, to agonist binding. This could include access to and exposure of specific sites for G protein interactions, whether or not these are directly on the CB1 carboxyl-terminal tail.
CB1 internalization, recycling, and desensitization
Prolonged exposure of CB1 agonists results in rapid attenuation of behavioral responsiveness, also termed tolerance, in human and animal models (Abood and Martin, 1992; Martin et al. 1994; Pertwee et al. 1993; Martin et al. 2004) that has been attributed to both a decrease in the ability of the receptor to activate effector pathways (i.e. desensitization) and in the number of cell surface-expressed receptors (i.e. internalization; Sim et al. 1996; Gainetdinov et al. 2004; Claing et al. 2002; Ferguson et al. 1998; Perry and Lefkowitz 2002). In the classical model of desensitization, the agonist-bound GPCR becomes a substrate for GRKs; these kinases phosphorylate serine and/or threonine residues on GPCR cytoplasmic domains, which then become a high affinity target for arrestins. Binding of arrestins uncouples G-proteins (Sim et al. 1996) and inhibits additional G-protein associations, as well as stimulates the internalization of the receptor. Following internalization, GPCRs either recycle back to the cell surface or are degraded in lysosomes. This classical model was first described for the β2-adrenergic receptor and has been found applicable to many other GPCRs (e.g. Inglese et al. 1993; Freedman and Lefkowitz, 1996; Kovoor et al. 1997; Krupnick and Benovic, 1998); however, the identified protein interactions that mediate these processes vary among GPCRs (Jala et al. 2005; Hsieh et al. 1999), and likely differ for the CB1 receptor.
CB1 internalization
CB1 localization and the mechanisms for internalization and recycling remain largely unknown. Elucidation of CB1 trafficking patterns has been complicated by findings that basal CB1 activity leads to some constitutive internalization and endosomal localization. In model cell lines such as naïve HEK293 cells (Leterrier et al. 2004; Bakshi et al. 2007; Rozenfeld and Devi 2008; Ahn et al. 2009; D'Antona et al. 2006), epithelial LLC-PK1 cells, and SHSY-5Y neuroblastoma cells (Leterrier et al. 2004), high levels of CB1 are associated with the endosomal compartment. In cultured hippocampal neurons (Coutts et al. 2001; Leterrier et al. 2006; McDonald et al. 2007) and cortical neurons (Mikasova et al. 2008), defining subcellular localization is more complicated; CB1 receptors are intracellular in the somatodendritic regions, consistent with constitutive internalization, yet the receptors accumulate on the cell surface of axons. One explanation could be that at synapses, but not so much at the cell soma, receptor functions and differences in expression of regulatory proteins inhibit CB1 internalization and/or promote its rapid recycling.
The carboxyl-terminus has been found to be important for the internalization of some (e.g. Trapaidze et al. 1996), but not all (e.g. Liggett et al. 1992; Pals-Rylaarsdam and Hosey 1997; Tsuga et al. 1998) GPCRs. Studies utilizing CB1 receptor mutants have identified residues that govern endocytosis at the carboxyl-terminal end of H9. When expressed in AtT20 cells, truncations removing residues carboxyl-terminal to V459 (M1-V459) and V464 (M1-V464) do not alter surface expression levels. However, the M1-V459, but not the M1-V464, mutant receptor fails to internalize following agonist treatment, suggesting that in AtT20 cells residues between V459 and V464 are required for CB1 internalization (Hsieh et al. 1999; Daigle et al. 2008b). The CB1 M1-V459 truncation removed six potential phosphorylation sites. In HEK293 cells, mutation to alanine of two of these residues in the full-length receptor (i.e. T460A/S462A, S464A/T465A, or T467/S468) has no effect, yet mutation of four (T460A-T465A) or all six (T460A-S468A) of these putative phosphorylation sites drastically reduces the extent of agonist-induced internalization of CB1 receptors (Daigle et al. 2008b). In contrast, residues S425 and S429 (which are required for CB1 desensitization), are not required for endocytosis, as a CB1 S425A/S429A receptor mutant displays similar agonist-induced internalization as wild-type CB1 in AtT20 cells. These data implicate the last 14 residues of the carboxyl-terminus in the regulation of CB1 internalization. Still, the analyses are confounded by a variety of different mutational studies that make clear comparisons difficult. Also, mutations can affect multiple parameters in unknown ways making the analyses more complicated than sometimes are assumed. More in-depth studies of the phosphorylation states and structural requirements for CB1 endocytosis will be valuable for clarifying these issues.
CB1 trafficking
Recent evidence suggests that the H8 domain can also play a significant role in CB1 receptor trafficking. The full-length CB1 receptor with a L404F substitution, a mutation found to affect CB1 receptor-mediated G-protein activation, displays a faster rate of agonist-induced internalization as compared to wild type (Anavi-Goffer et al. 2007). Furthermore, molecular modeling studies of the L404F mutant found that the phenylalanine places structural constraints on the H8 domain, possibly limiting its mobility (Anavi-Goffer et al. 2007). Thus H8 flexibility may be crucial for endocytosis. More recently, disruptions of the hydrophobic face of the CB1 H8 (i.e. L404A/F412A and L404A/F408A/F412A) as well as lengthening the distance between the CB1 TM7 and H8 regions by the successive addition of the neutral amino acid, glutamine, have been found to decrease maximal agonist binding (i.e. Bmax values), with no effect on agonist affinity (Ahn et al. 2010). Effects on binding were attributed to CB1 receptor trafficking defects observed via confocal microscopy (Ahn et al. 2010). Unlike the wild-type receptor, which co-localizes with the late endosome/lysosome marker, LAMP-1, these mutants displayed a more diffuse pattern of CB1 expression, with significant co-localization with an ER marker (Ahn et al. 2010). As the H8 hydrophobic residues are critical for maintenance of helicity (Ahn et al. 2010), these data suggest that H8 helical conformation and location are critical for proper CB1 trafficking. This emphasizes the likelihood of H8 interactions with the membrane and/or other intracellularly-oriented regions of the receptor and that these interactions are key for CB1 assembly.
CB1 desensitization
Results from mutational studies, performed to determine residues involved in CB1 desensitization, find that truncation at residue 417, but not at 438 and 459 causes a dramatic attenuation of desensitization, without affecting agonist activation (Jin et al. 1999). This led researchers to further examine the residues between H8 and H9 helices as potential residues critical for GRK3/β-arrestin 2-mediated desensitization. Like the receptor truncated at position 417, a deletion mutant with residues 417–438 removed fails to exhibit agonist-induced desensitization in oocytes (Jin et al. 1999). Two putative GRK3 phosphorylation sites exist within this region, at residues S425 and S429, of the CB1 carboxyl-terminus. Point mutations that remove these potential phosphorylation sites (i.e. S425A/S429A), yield CB1 receptors with reduced levels of agonist-induced desensitization as measured through activation of GIRK channels (Jin et al. 1999) and ERK1/2 phosphorylation (Daigle et al. 2008a), yet have levels of agonist-induced internalization (Daigle et al. 2008a; Jin et al. 1999) and recruitment of β-arrestin to the plasma membrane comparable to wild type (Daigle et al. 2008a). These later studies highlight the role of phosphorylation in desensitization and distinguish separate mechanisms for CB1 receptor desensitization and internalization. Therefore, these specific serine residues could be involved in GRK and/or β-arrestin binding, or act as a regulatory region mediating the binding and/or activities of these proteins.
Interactions of the CB1 carboxyl-terminus with other accessory proteins
Arrestin interactions
Of the four known arrestin subtypes, only β-arrestin 1 and 2 (also known as arrestin 2 and 3, respectively) have been shown to interact with non-visual GPCRs under physiological conditions. β-arrestins play a key role in modulating the duration and amplitude of signal transduction by promoting desensitization and/or internalization of the receptors. Unlike most effector proteins, β-arrestins do not recognize a unique well-defined consensus sequence across GPCRs and thus mapping these binding sites has been difficult. In general, arrestin binding sites contain at least two phosphorylated residues (or mimicks of phosphorylated residues; e.g. aspartic or glutamic acid) within close proximity to each other (see Gurevich and Gurevich 2006). However, it has been proposed that arrestin binding sites are more-likely dependent on topological structure (Chen et al. 1993), and although GPCRs are predominately phosphorylated prior to β-arrestin interactions, for some GPCRs phosphorylation is not a prerequisite (Gurevich and Gurevich 2006). Class A GPCRs have been proposed to have a higher affinity for β-arrestin 2 as compared to β-arrestin 1, while class B GPCRs do not distinguish a preference for either β-arrestin subtype (Oakley et al. 2000), inferring that the CB1 receptor is more likely regulated by β-arrestin 2.
The CB1 carboxyl-terminus contains residues that regulate β arrestin-mediated desensitization and internalization. Potential phosphorylation sites at S425 and S429 have been proposed to be critical for β arrestin-mediated desensitization in AtT20 cells (Jin et al. 1999), but not internalization (Jin et al. 1999; Daigle et al. 2008a), differentiating β-arrestin’s role in these processes. In contrast to desensitization studies, the last 14 residues of the CB1 receptor have been implicated in CB1 internalization, yet effects are dependent on the cellular expression system studied. Truncation at V459 in AtT20 but not HEK293 cells results in attenuated agonist-induced β-arrestin translocation to the plasma membrane (Daigle et al. 2008a). However in HEK293 cells, mutation of more than 2 serine/threonine residues carboxyl-terminal to V459 results in a receptor unable to alter β-arrestin 2 subcellular distribution. This discrepancy in the required residues for β arrestin 2-mediated internalization is intriguing and once clarified, may help explain CB1 receptor trafficking inconsistencies not only across cell lines, but also between neuronal soma and axons.
Few studies have examined direct binding of arrestin to the CB1 receptor and the sequence and structural features involved. Recently, alternative cell-based screening assays for Gαi/o protein-coupled GPCRs have been examined, utilizing CB1 as the prototype (van der Lee et al. 2009; Vrecl et al. 2009), and have provided some findings that suggest direct binding of β-arrestins to the CB1 receptor. In one set of studies, the imaging-based Redistribution assay (Thermo) and two non-imaging based assays, Tango (Invitrogen) and PathHunter (DicoverRX), were utilized to examine CB1 agonist-induced binding of β-arrestin 2 to CB1 receptors labeled on their carboxyl-termini (van der Lee et al. 2009). In these studies, β-arrestin 2 was found to redistribute and co-localize with CB1 in an agonist-dependent manner (van der Lee et al. 2009), suggesting that CB1 and β-arrestin 2 directly interact. However, care must be taken when interpreting these data, as the CB1 carboxyl-terminus was altered by the addition of a tag in all three assays, possibly impacting the association; the assays themselves measure close localization, but not necessarily direct binding. In contrast, a second set of studies utilizing a bioluminescence resonance energy transfer (BRET) approach to measure association of GFP-β-arr2 and CB1-Rluc, found little agonist-induced BRET signals, indicating these proteins interact with low affinity and/or do not associate under physiological conditions (Vrecl et al. 2009). In order for the authors to obtain signals high enough to analyze CB1 agonist-dependent β-arrestin 2 translocation, chimeric structures were required, where the CB1 receptor carboxyl-terminal tail after the H8 region (i.e. after residue G417) was replaced with the carboxyl-terminus of the vasopressin V2 receptor (Vrecl et al. 2009). This chimera bound β-arrestin 2 much more efficiently (~8-fold) as compared to wild-type CB1-GFP receptor. The authors proposed that the reduced activity of the full-length CB1 receptor for β-arrestin 2 associations as compared to other class A GPCRs may be indicative of class B-like arrestin interactions (i.e. the CB1 receptor may prefer β-arrestin 1; Vrecl et al. 2009). Another interpretation is that the GFP tag placed on the CB1 receptor interfered with β-arrestin binding. More detailed study of the β-arrestin binding site is required for full interpretation.
The only study to date that reports direct association of β-arrestin binding to the CB1 receptor utilized an NMR approach, studying the association of purified human β-arrestin 1 with a diphosphorylated peptide (phosphorylated at S425 and S429) corresponding to CB1 residues T418-N437 (Bakshi et al. 2007). Broadening of the NMR signal, as well as small changes in chemical shifts occurred following the addition of β-arrestin 1 to the CB1 peptide, indicative of an exchange between free and bound peptide. Furthermore, an increase in the number and intensity of NOE peaks was indicative of complex formation. The CB1 peptide was found to undergo a conformational change following interaction with β-arrestin 1, forming two helical segments (L423-G428 and D429-L433), with residues amino terminal and carboxyl-terminal to these residues exhibiting random coil. Glycine, an amino acid known to introduce flexibility in α-helices, acts as a hinge at position 428, providing flexibility to the relative orientation of the helical regions. Similar structural changes were observed for a peptide of rhodopsin corresponding to the region carboxyl-terminal to H8 when bound to arrestin 1 (Kisselev et al. 2004a; Kisselev et al. 2004b). These data indicate that a direct binding event occurs between β-arrestin 1 and a portion of the phosphorylated carboxyl-terminus of CB1 (Fig. 4). Further study is required to determine if 1) β-arrestin 2 also binds to the CB1 receptor, and if so, where and how, 2) if additional β-arrestin binding sites exist on the CB1 receptor, and 3) the role of putative regulatory regions in β-arrestin 1,2 binding and affinity.
Cannabinoid Receptor Interacting Protein (CRIP1a/b)
CRIP1a and CRIP1b are newly identified proteins that are alternatively-spliced variants of the same gene, generating two mRNAs that encode for proteins 164 (CRIP1a) and 128 (CRIP1b) amino acids in length (Niehaus et al. 2007). These CB1-interacting proteins were discovered utilizing a yeast two-hybrid assay of a human brain cDNA library, with a peptide encompassing the last 55 residues of the CB1 carboxyl-terminal tail as bait. Both CRIP1a and b co-immunoprecipate with CB1 from CHAPS solubilized rat brain membrane homogenates and can be isolated in a pull-down assay using a GST-CB1 carboxyl-terminal tail construct, further supporting a direct binding event between these proteins. Furthermore, both CRIP1a and CRIP1b are found to co-localize at the plasma membrane with, and trafficked to, the same subcellular compartment as the CB1 receptor, suggesting that spatially these proteins can interact in vivo (Niehaus et al. 2007).
Currently the functional relevance of the CRIP1b protein is unknown. In contrast, in SCG neurons coexpressing both CRIP1a and the CB1 receptor, the effects of SR141716A-induced increases in Ca2+ current are attenuated relative to expression of CB1 alone, suggesting that CRIP1a plays a role in regulating CB1-mediated tonic/constitutive inhibition of voltage-gated Ca2+ channels (Niehaus et al. 2007) and thus is involved in the downregulation of CB1 receptor function. Although the exact binding site for CRIP1a has not been determined, when coexpressed in SCG neurons with a CB1 receptor mutant containing a deletion of the last 9 residues of the CB1 carboxyl-terminus, SR141716A effects on Ca2+ currents are restored (Niehaus et al. 2007). With the finding that the last 9 amino acids of CB1 are necessary for CRIP1b interaction in yeast two-hybrid assays, these data suggest that CRIP1a binds to a motif within residues 464–472 of the CB1 carboxyl-terminal tail (Fig. 4).
G protein-coupled receptor-associated sorting protein (GASP1)
GASP1 binds to the carboxyl-terminus of various GPCRs, modulating their post-endocytic sorting (Abu-Helo and Simonin 2010; Heydorn et al. 2004; Moser et al. 2010). Initial studies suggest GASP1 also regulates CB1 degradation, and in animal models is necessary for the development of tolerance (both behaviorally and in the population of surface receptors; Martini et al. 2007; Martini et al. 2010; Tappe-Theodor et al. 2007). However, the CB1-GASP1 binding site remains elusive. A direct interaction was proposed following the successful co-immunoprecipitation of GASP1 with the full-length CB1 receptor from HEK293 membrane homogenates (Martini et al. 2007; Tappe-Theodor et al. 2007). Attempts at isolating the residues involved in binding, however, have yielded seemingly conflicting results. Studies utilizing a construct of GST fused to the last 14 amino acids of the human CB1 receptor and in vitro translated GASP1, found that GASP1 and the GST-CB1 construct could be co-isolated in GST pull-down assays (Martini et al. 2007), implicating the last 14 residues in GASP1 binding. In contrast, a CB1 receptor mutant lacking the last 13 residues was found to co-immunoprecipitate with cGASP1 (a dominant negative mutant containing the last 459 residues of GASP1; Tappe-Theodor et al. 2007) suggesting additional binding domains on the CB1 receptor. These studies indicate that although the last 14 residues may be sufficient for GASP1 binding, these residues may contain a component of a single GASP1 binding site that is comprised of additional CB1 residues or is one of multiple individual GASP1 binding sites.
Factor associated with neutral sphingomyelinase activation (FAN)
Cannabinoids have recently been found to initiate growth arrest and apoptosis in transformed neuronal and nonneuronal cells as well as serve a protective role in healthy neurons exposed to toxic insults. These effects of cannabinoids are thought to be mediated, at least in part, through activation of a non-G protein-mediated signal transduction pathway, the less-explored sphingomyelin metabolic pathway (reviewed in Guzman et al. 2002; Velasco et al. 2005). Activation of the sphingomyelin metabolic pathway leads to ceramide generation, either through sphingomyelin hydrolysis or through ceramide synthesis de novo (Guzman et al. 2002; Velasco et al. 2005). In primary astrocytes and C6 glioma cells, THC has been found to induce the breakdown of sphingomyelin and intracellular ceramide accumulation in both a time and dose-dependent manner (Sanchez et al. 1998a; Sanchez et al. 1998b; Blazquez et al. 1999; Galve-Roperh et al. 2000). These effects of CB1 agonist are blocked by SR141617A, but not the CB2 antagonist SR144528 or PTX (Sanchez et al. 2001), indicating CB1-specificity and confirming that ceramide accumulation is not Gαi/o-mediated. Although little is known about the mechanism by which CB1 receptor activation leads to ceramide accumulation, successful co-immunoprecipitation studies with the CB1 receptor have implicated the adaptor protein factor associated with neutral sphingomyelinase activation (FAN) as a key mediator of this signaling cascade (Sanchez et al. 2001), and thus association with FAN allows the CB1 receptor to function through a non-G protein-mediated signalling pathway. Transfection of dominant-negative FAN into the CB1 endogenously-expressing cell line ECV304 results in a reduced level of CB1 agonist-induced sphinogomyelin hydrolysis (Sanchez et al. 2001), providing further evidence that FAN acts as an adaptor protein in CB1-mediated ceramide accumulation.
To date, a direct interaction between FAN and CB1 has not been reported, nor has FAN activity been identified as being mediated specifically by the CB1 carboxyl-terminus. However, examination of FAN interactions with other receptors suggests a putative CB1 binding site. For example, the domains of the tumor necrosis factor (TNF) receptor that couple to sphingomyelinase activation have been identified (Kolesnick and Kronke, 1998; Adam-Klages et al. 1998), and are composed of a stretch of nine amino acids including residues DSAHK (Adam-Klages et al. 1998). The CB1 carboxyl-terminal tail contains a highly homologous region comprised of a DCLHK sequence from residues 431–435 (Figs. 3, 4). This sequence is highly conserved across rat, human, mouse, and cat CB1 receptors. Further study of this motif remains to be performed to assess its potential as a FAN-binding site.
Concluding remarks
Recent advances have found significant structure within the CB1 carboxyl terminus and interactions of accessory proteins with this region have been found to be critical for mediating key points of the receptor life cycle. Nonetheless, an understanding of the extent to which the CB1 cellular fate is governed by the carboxyl-terminus is still in its infancy. In the future it will be important to elucidate additional protein binding partners and their involvement in CB1 receptor function. For instance, a plethora of additional proteins in CB1 receptor trafficking, desensitization, and recycling have been implicated including clathrin, dynamin, rab 4, rab 5, esp15, caveolin-1, and AP-3 (Hsieh et al. 1999; Daigle et al. 2008b; Leterrier et al. 2004; Leterrier et al. 2006; Bari et al. 2008; Rozenfeld and Devi 2008), yet the specific CB1-binding domains or motifs to which they interact have not been identified. Other future studies include delineating the pharmacological and physiological relevance of the H8 and H9 regions, as well as to identify interactions of these domains with potential binding partners and the remainder of the receptor. It will also be interesting to see if, like β-arrestin 1 (Bakshi et al. 2007), other accessory proteins induce or alter carboxyl-terminal structure upon binding, and how the binding of one protein affects the interactions with others. Further insight into this critical region and its accessory proteins will advance our understanding of CB1 receptor function and potentially identify novel drug targets for CB1-mediated diseases.
Acknowledgements
This work was supported in part by the National Institute on Drug Abuse (F32DA028080 to RS and DA020763 to DAK). The content is the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of abbreviations
- BRET
Bioluminescence resonance energy transfer
- CB1
cannabinoid type-1 receptor
- CB2
cannabinoid type-2 receptor
- CD
circular dichroism
- FAN
factor associated with neutral sphingomyelinase activation
- GPCR
G protein-coupled receptor
- GIRK
G protein-gated inward-rectifying K+ channel
- GRK
G-protein receptor kinase
- H8
helix 8
- H9
helix 9
- MAPK
mitogen-activated protein kinase
- MD
molecular dynamics
- SR141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- NMR
nuclear magnetic resonance
- SCG
superior cervical ganglion
- THC
Δ9-tetrahydrocannabinol
- TM
transmembrane domain
Footnotes
The authors declare no conflict of interest.
References
- Abadji V, Lucas-Lenard JM, Chin C, Kendall DA. Involvement of the carboxyl terminus of the third intracellular loop of the cannabinoid CB1 receptor in constitutive activation of Gs. J. Neurochem. 1999;72:2032–2038. doi: 10.1046/j.1471-4159.1999.0722032.x. [DOI] [PubMed] [Google Scholar]
- Abdulaev NG, Ridge KD. Light-induced exposure of the cytoplasmic end of transmembrane helix seven in rhodopsin. Proc. Natl Acad. Sci. USA. 1998;95:12854–12859. doi: 10.1073/pnas.95.22.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abood ME, Martin BR. Neurobiology of marijuana abuse. Trends Pharmacol. Sci. 1992;13:201–206. doi: 10.1016/0165-6147(92)90064-d. [DOI] [PubMed] [Google Scholar]
- Abu-Helo A, Simonin F. Identification and biological significance of G protein-coupled receptor associated sorting proteins (GASPs) Pharmacol. Ther. 2010;126:244–250. doi: 10.1016/j.pharmthera.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Adam-Klages S, Schwandner R, Adam D, Kreder D, Bernardo K, Kronke M. Distinct adapter proteins mediate acid versus neutral sphingomyelinase activation through the P55 receptor for tumor necrosis factor. J. Leukoc. Biol. 1998;63:678–682. doi: 10.1002/jlb.63.6.678. [DOI] [PubMed] [Google Scholar]
- Ahn KH, Nishiyama A, Mierke DF, Kendall DA. Hydrophobic residues in helix 8 of cannabinoid receptor 1 are critical for structural and functional properties. Biochemistry. 2010;49:502–511. doi: 10.1021/bi901619r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn KH, Pellegrini M, Tsomaia N, Yatawara AK, Kendall DA, Mierke DF. Structural analysis of the human cannabinoid receptor one carboxyl-terminus identifies two amphipathic helices. Biopolymers. 2009;91:565–573. doi: 10.1002/bip.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anavi-Goffer S, Fleischer D, Hurst DP, et al. Helix 8 Leu in the CB1 cannabinoid receptor contributes to selective signal transduction mechanisms. J. Biol. Chem. 2007;282:25100–25113. doi: 10.1074/jbc.M703388200. [DOI] [PubMed] [Google Scholar]
- Bakshi K, Mercier RW, Pavlopoulos S. Interaction of a fragment of the cannabinoid CB1 receptor C-terminus with arrestin-2. FEBS Lett. 2007;581:5009–5016. doi: 10.1016/j.febslet.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari M, Oddi S, De SC, Spagnolo P, Gasperi V, Battista N, Centonze D, Maccarrone M. Type-1 cannabinoid receptors colocalize with caveolin-1 in neuronal cells. Neuropharmacology. 2008;54:45–50. doi: 10.1016/j.neuropharm.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Thomas BF, McMahon LR. Cannabinoid CB1 receptor antagonists as potential pharmacotherapies for drug abuse disorders. Int. Rev. Psychiatry. 2009;21:134–142. doi: 10.1080/09540260902782786. [DOI] [PubMed] [Google Scholar]
- Bechinger B, Kim Y, Chirlian LE, Gesell J, Neumann JM, Montal M, Tomich J, Zasloff M, Opella SJ. Orientations of amphipathic helical peptides in membrane bilayers determined by solid-state NMR spectroscopy. J. Biomol. NMR. 1991;1:167–173. doi: 10.1007/BF01877228. [DOI] [PubMed] [Google Scholar]
- Bermak JC, Li M, Bullock C, Zhou QY. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat. Cell Biol. 2001;3:492–498. doi: 10.1038/35074561. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Sanchez C, Daza A, Galve-Roperh I, Guzman M. The stimulation of ketogenesis by cannabinoids in cultured astrocytes defines carnitine palmitoyltransferase I as a new ceramide-activated enzyme. J. Neurochem. 1999;72:1759–1768. doi: 10.1046/j.1471-4159.1999.721759.x. [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Chang LK, Kwan J, Martin GR. Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J. Pharmacol. Exp. Ther. 1998;287:884–888. [PubMed] [Google Scholar]
- Bouaboula M, Perrachon S, Milligan L, et al. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- Bramblett RD, Panu AM, Ballesteros JA, Reggio PH. Construction of a 3D model of the cannabinoid CB1 receptor: determination of helix ends and helix orientation. Life Sci. 1995;56:1971–1982. doi: 10.1016/0024-3205(95)00178-9. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Walker JM, Huang SM, Roy MB, Childers SR. Cannabinoid signaling in rat cerebellar granule cells: G-protein activation, inhibition of glutamate release and endogenous cannabinoids. Neuropharm. 2004;47:81–91. doi: 10.1016/j.neuropharm.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Robin HC. Is GPR55 an anandamide receptor? Vitam. Horm. 2009;81:111–137. doi: 10.1016/S0083-6729(09)81005-4. [DOI] [PubMed] [Google Scholar]
- Butler H, Korbonits M. Cannabinoids for clinicians: the rise and fall of the cannabinoid antagonists. Eur. J. Endocrinol. 2009;161:655–662. doi: 10.1530/EJE-09-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai K, Klein-Seetharaman J, Farrens D, Zhang C, Altenbach C, Hubbell WL, Khorana HG. Single-cysteine substitution mutants at amino acid positions 306–321 in rhodopsin, the sequence between the cytoplasmic end of helix VII and the palmitoylation sites: sulfhydryl reactivity and transducin activation reveal a tertiary structure. Biochem. 1999;38:7925–7930. doi: 10.1021/bi9900119. [DOI] [PubMed] [Google Scholar]
- Chen CY, Dion SB, Kim CM, Benovic JL. Beta-adrenergic receptor kinase. agonist-dependent receptor binding promotes kinase activation. J. Biol. Chem. 1993;268:7825–7831. [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G, Guo J, Makriyannis A. The conformation of the cytoplasmic Helix 8 of the CB1 cannabinoid receptor using NMR and circular dichroism. Biochim. Biophys. Acta. 2005;1668:1–9. doi: 10.1016/j.bbamem.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Choi G, Landin J, Xie XQ. The cytoplasmic helix of cannabinoid receptor CB2, a conformational study by circular dichroism and (1)H NMR spectroscopy in aqueous and membrane-like environments. J. Pept. Res. 2002;60:169–177. doi: 10.1034/j.1399-3011.2002.21012.x. [DOI] [PubMed] [Google Scholar]
- Claeysen S, Sebben M, Becamel C, Bockaert J, Dumuis A. Novel brain-specific 5-HT4 receptor splice variants show marked constitutive activity: role of the C-terminal intracellular domain. Mol. Pharmacol. 1999;55:910–920. [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog. Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Coutts A, Anavi-Goffer S, Ross RA, MacEwan DJ, Mackie K, Pertwee RG, Irving AJ. Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J. Neurosci. 2001;21:2425–2433. doi: 10.1523/JNEUROSCI.21-07-02425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowsik SM, Lucke C, Ruterjans H. Lipid-induced conformation of substance P. J. Biomol. Struct. Dyn. 1997;15:27–36. doi: 10.1080/07391102.1997.10508942. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL, Robinson JK, Felder CC, Devane WA, Axelrod J. Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol. Biochem. Behav. 1993;46:967–972. doi: 10.1016/0091-3057(93)90230-q. [DOI] [PubMed] [Google Scholar]
- D'Antona AM, Ahn KH, Kendall DA. Mutations of CB1 T210 produce active and inactive receptor forms: correlations with ligand affinity, receptor stability, and cellular localization. Biochemistry. 2006;45:5606–5617. doi: 10.1021/bi060067k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle TL, Kearn CS, Mackie K. Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology. 2008a;54:36–44. doi: 10.1016/j.neuropharm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle TL, Kwok ML, Mackie K. Regulation of CB1 Cannabinoid receptor internalization by a promiscuous phosphorylation-dependent mechanism. J. Neurochem. 2008b;106:70–82. doi: 10.1111/j.1471-4159.2008.05336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet AD, Woods SC. Minireview: endocannabinoids and their receptors as targets for obesity therapy. Endocrinology. 2009;150:2531–2536. doi: 10.1210/en.2009-0046. [DOI] [PubMed] [Google Scholar]
- Demchyshyn LL, McConkey F, Niznik HB. Dopamine D5 receptor agonist high affinity and constitutive activity profile conferred by carboxyl-terminal tail sequence. J. Biol. Chem. 2000;275:23446–23455. doi: 10.1074/jbc.M000157200. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Marini P, Matias I, Moriello AS, Starowicz K, Cristino L, Nigam S, Di MV. Mechanisms for the coupling of cannabinoid receptors to intracellular calcium mobilization in rat insulinoma beta-cells. Exp. Cell Res. 2007;313:2993–3004. doi: 10.1016/j.yexcr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dong C, Zhou F, Fugetta EK, Filipeanu CM, Wu G. Endoplasmic reticulum export of adrenergic and angiotensin II receptors is differentially regulated by Sar1 GTPase. Cell Signal. 2008;20:1035–1043. doi: 10.1016/j.cellsig.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernay MT, Zhou F, Wu G. A conserved motif for the transport of G protein-coupled receptors from the endoplasmic reticulum to the cell surface. J. Biol. Chem. 2004;279:30741–30750. doi: 10.1074/jbc.M313881200. [DOI] [PubMed] [Google Scholar]
- Ernst OP, Meyer CK, Marin EP, Henklein P, Fu WY, Sakmar TP, Hofmann KP. Mutation of the fourth cytoplasmic loop of rhodopsin affects binding of transducin and peptides derived from the carboxyl-terminal sequences of transducin alpha and gamma subunits. J. Biol. Chem. 2000;275:1937–1943. doi: 10.1074/jbc.275.3.1937. [DOI] [PubMed] [Google Scholar]
- Felder CC, Nielsen A, Briley EM, et al. Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS Lett. 1996;393:231–235. doi: 10.1016/0014-5793(96)00891-5. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Zhang J, Barak LS, Caron MG. Molecular mechanisms of G protein-coupled receptor desensitization and resensitization. Life Sci. 1998;62:1561–1565. doi: 10.1016/s0024-3205(98)00107-6. [DOI] [PubMed] [Google Scholar]
- Free RB, Hazelwood LA, Cabrera DM, Spalding HN, Namkung Y, Rankin ML, Sibley DR. D1 and D2 dopamine receptor expression is regulated by direct interaction with the chaperone protein calnexin. J. Biol. Chem. 2007;282:21285–21300. doi: 10.1074/jbc.M701555200. [DOI] [PubMed] [Google Scholar]
- Freedman NJ, Lefkowitz RJ. Desensitization of G protein-coupled receptors. Recent Prog. Horm. Res. 1996;51:319–351. [PubMed] [Google Scholar]
- Fride E, Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur. J. Pharmacol. 1993;231:313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- Fritze O, Filipek S, Kuksa V, Palczewski K, Hofmann KP, Ernst OP. Role of the conserved NPxxY(x)5,6F motif in the rhodopsin ground state and during activation. Proc. Natl. Acad. Sci. USA. 2003;100:2290–2295. doi: 10.1073/pnas.0435715100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Sanchez C, Cortes ML, Gomez d P, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000;6:313–319. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski G, Offertaler L, Wagner JA, Kunos G. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 2009;89:105–111. doi: 10.1016/j.prostaglandins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace CR, Cowsik SM, Shim JY, Welsh WJ, Howlett AC. Unique helical conformation of the fourth cytoplasmic loop of the CB1 cannabinoid receptor in a negatively charged environment. J. Struct. Biol. 2007;159:359–368. doi: 10.1016/j.jsb.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol. Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M, Sanchez C, Galve-Roperh I. Cannabinoids and cell fate. Pharmacol. Ther. 2002;95:175–184. doi: 10.1016/s0163-7258(02)00256-5. [DOI] [PubMed] [Google Scholar]
- Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of beta-arrestin at 1.9 A: Possible mechanism of receptor binding and membrane translocation. Structure. 2001;9:869–880. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- Heydorn A, Sondergaard BP, Ersboll B, Holst B, Nielsen FC, Haft CR, Whistler J, Schwartz TW. A Library of 7TM Receptor C-Terminal Tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP) J. Biol. Chem. 2004;279:54291–54303. doi: 10.1074/jbc.M406169200. [DOI] [PubMed] [Google Scholar]
- Higashijima T, Burnier J, Ross EM. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. Mechanism and structural determinants of activity. J. Biol. Chem. 1990;265:14176–14186. [PubMed] [Google Scholar]
- Hillard CJ, Muthian S, Kearn CS. Effects of CB(1) Cannabinoid receptor activation on cerebellar granule cell nitric oxide synthase activity. FEBS Lett. 1999;459:277–281. doi: 10.1016/s0014-5793(99)01253-3. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb. Exp. Pharmacol. 2005;168:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol. Pharmacol. 1986;29:307–313. [PubMed] [Google Scholar]
- Howlett AC, Song C, Berglund BA, Wilken GH, Pigg JJ. Characterization of CB1 cannabinoid receptors using receptor peptide fragments and site-directed antibodies. Mol. Pharmacol. 1998;53:504–510. doi: 10.1124/mol.53.3.504. [DOI] [PubMed] [Google Scholar]
- Hsieh C, Brown S, Derleth C, Mackie K. Internalization and recycling of the CB1 cannabinoid receptor. J. Neurochem. 1999;73:493–501. doi: 10.1046/j.1471-4159.1999.0730493.x. [DOI] [PubMed] [Google Scholar]
- Inglese J, Freedman NJ, Koch WJ, Lefkowitz RJ. Structure and mechanism of the G protein-coupled receptor kinases. J. Biol. Chem. 1993;268:23735–23738. [PubMed] [Google Scholar]
- Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, Ijzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jala VR, Shao WH, Haribabu B. Phosphorylation-independent beta-arrestin translocation and internalization of leukotriene B4 receptors. J. Biol. Chem. 2005;280:4880–4887. doi: 10.1074/jbc.M409821200. [DOI] [PubMed] [Google Scholar]
- Jin W, Brown S, Roche JP, Hsieh C, Celver JP, Kovoor A, Chavkin C, Mackie K. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J. Neurosci. 1999;19:3773–3780. doi: 10.1523/JNEUROSCI.19-10-03773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Cornell RB. Amphitropic proteins: regulation by reversible membrane interactions. Mol. Membr. Biol. 1999;16:217–235. doi: 10.1080/096876899294544. [DOI] [PubMed] [Google Scholar]
- Jung H, Windhaber R, Palm D, Schnackerz KD. Conformation of a beta-adrenoceptor-derived signal transducing peptide as inferred by circular dichroism and 1H NMR spectroscopy. Biochemistry. 1996;35:6399–6405. doi: 10.1021/bi952575s. [DOI] [PubMed] [Google Scholar]
- Kisselev OG, Downs MA, McDowell JH, Hargrave PA. Conformational changes in the phosphorylated C-terminal domain of rhodopsin during rhodopsin arrestin interactions. J. Biol. Chem. 2004a;279:51203–51207. doi: 10.1074/jbc.M407341200. [DOI] [PubMed] [Google Scholar]
- Kisselev OG, McDowell JH, Hargrave PA. The arrestin-bound conformation and dynamics of the phosphorylated carboxy-terminal region of rhodopsin. FEBS Lett. 2004b;564:307–311. doi: 10.1016/S0014-5793(04)00226-1. [DOI] [PubMed] [Google Scholar]
- Kobilka BK, Dixon RA, Frielle T, Dohlman HG, Bolanowski MA, Sigal IS, Yang-Feng TL, Francke U, Caron MG, Lefkowitz RJ. CDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc. Natl. Acad. Sci. U S A. 1987;84:46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu. Rev. Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- König B, Arendt A, McDowell JH, Kahlert M, Hargrave PA, Hofmann KP. Three cytoplasmic loops of rhodopsin interact with transducin. Proc. Natl. Acad. Sci. U S A. 1989;86:6878–6882. doi: 10.1073/pnas.86.18.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Nappey V, Kieffer BL, Chavkin C. Mu and delta opioid receptors are differentially desensitized by the coexpression of beta-adrenergic receptor kinase 2 and beta-arrestin 2 in Xenopus Oocytes. J. Biol. Chem. 1997;272:27605–27611. doi: 10.1074/jbc.272.44.27605. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu. Rev. Pharmacol. Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Kumar RN, Chambers WA, Pertwee RG. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anaesthesia. 2001;56:1059–1068. doi: 10.1046/j.1365-2044.2001.02269.x. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Labasque M, Reiter E, Becamel C, Bockaert J, Marin P. Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol. Biol. Cell. 2008;19:4640–4650. doi: 10.1091/mbc.E08-04-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner JE, Hille B, Mackie K. The Cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc. Natl. Acad. Sci. U S A. 2005;102:19144–19149. doi: 10.1073/pnas.0509588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le FB, Forget B, Aubin HJ, Goldberg SR. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict. Biol. 2008;13:239–252. doi: 10.1111/j.1369-1600.2008.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, Bonnard D, Carrel D, Rossier J, Lenkei Z. Constitutive endocytic cycle of the CB1 cannabinoid receptor. J. Biol. Chem. 2004;279:36013–36021. doi: 10.1074/jbc.M403990200. [DOI] [PubMed] [Google Scholar]
- Leterrier C, Laine J, Darmon M, Boudin H, Rossier J, Lenkei Z. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J. Neurosci. 2006;26:3141–3153. doi: 10.1523/JNEUROSCI.5437-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Edwards PC, Burghammer M, Villa C, Schertler GF. Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol. 2004;343:1409–1438. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- Liggett SB, Ostrowski J, Chesnut LC, Kurose H, Raymond JR, Caron MG, Lefkowitz RJ. Sites in the third intracellular loop of the alpha 2A-adrenergic receptor confer short term agonist-promoted desensitization. Evidence for a receptor kinase-mediated mechanism. J. Biol. Chem. 1992;267:4740–4746. [PubMed] [Google Scholar]
- Maneuf YP, Brotchie JM. Paradoxical action of the cannabinoid WIN 55,212-2 in stimulated and basal cyclic AMP accumulation in rat globus pallidus slices. Br. J. Pharmacol. 1997;120:1397–1398. doi: 10.1038/sj.bjp.0701101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Sim-Selley LJ, Selley DE. Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol. Sci. 2004;25:325–330. doi: 10.1016/j.tips.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Martin BR, Welch SP, Abood M. Progress toward understanding the cannabinoid receptor and its second messenger systems. Adv. Pharmacol. 1994;25:341–397. doi: 10.1016/s1054-3589(08)60437-8. [DOI] [PubMed] [Google Scholar]
- Martini L, Thompson D, Kharazia V, Whistler JL. Differential regulation of behavioral tolerance to WIN55,212-2 by GASP1. Neuropsychopharmacology. 2010;35:1363–1373. doi: 10.1038/npp.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini L, Waldhoer M, Pusch M, Kharazia V, Fong J, Lee JH, Freissmuth C, Whistler JL. Ligand-induced down-regulation of the cannabinoid 1 receptor Is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. 2007;21:802–811. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McDonald NA, Henstridge CM, Connolly CN, Irving AJ. An essential role for constitutive endocytosis, but not activity, in the axonal targeting of the CB1 cannabinoid receptor. Mol. Pharmacol. 2007;71:976–984. doi: 10.1124/mol.106.029348. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Mikasova L, Groc L, Choquet D, Manzoni OJ. Altered surface trafficking of presynaptic cannabinoid type 1 receptor in and out synaptic terminals parallels receptor desensitization. Proc. Natl. Acad. Sci. USA. 2008;105:18596–18601. doi: 10.1073/pnas.0805959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Kargl J, Whistler JL, Waldhoer M, Tschische P. G protein-coupled receptor-associated sorting protein 1 regulates the postendocytic sorting of seven-transmembrane-spanning G protein-coupled receptors. Pharmacology. 2010;86:22–29. doi: 10.1159/000314161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Cowsik SM, Lynn AM, Welsh WJ, Howlett AC. Regulation of Gi by the CB1 cannabinoid receptor C-terminal juxtamembrane region: structural requirements determined by peptide analysis. Biochemistry. 1999;38:3447–3455. doi: 10.1021/bi981767v. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Howlett AC. CB1 Receptor-G protein association. Subtype selectivity is determined by distinct intracellular domains. Eur. J. Biochem. 2001;268:499–505. doi: 10.1046/j.1432-1327.2001.01810.x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, McIntosh HH, Houston DB, Howlett AC. The CB(1) cannabinoid receptor juxtamembrane C-terminal peptide confers activation to specific G proteins in brain. Mol. Pharmacol. 2000;57:162–170. [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kouyama T. Crystal structure of squid rhodopsin. Nature. 2008;453:363–367. doi: 10.1038/nature06925. [DOI] [PubMed] [Google Scholar]
- Navarro G, Aymerich MS, Marcellino D, et al. Interactions between calmodulin, adenosine A2A, and dopamine D2 receptors. J. Biol. Chem. 2009;284:28058–28068. doi: 10.1074/jbc.M109.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J, Lewis DL. Structural domains of the CB1 cannabinoid receptor that contribute to constitutive activity and G-protein sequestration. J. Neurosci. 2001a;21:8758–8764. doi: 10.1523/JNEUROSCI.21-22-08758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J, Lewis DL. The proximal and distal C-terminal tail domains of the CB1 cannabinoid receptor mediate G protein coupling. Neuroscience. 2001b;107:161–167. doi: 10.1016/s0306-4522(01)00335-9. [DOI] [PubMed] [Google Scholar]
- Niehaus JL, Liu Y, Wallis KT, et al. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol. Pharmacol. 2007;72:1557–1566. doi: 10.1124/mol.107.039263. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Okuno T, Yokomizo T, Hori T, Miyano M, Shimizu T. Leukotriene B4 receptor and the function of its helix 8. J. Biol. Chem. 2005;280:32049–32052. doi: 10.1074/jbc.R500007200. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Pals-Rylaarsdam R, Hosey MM. Two homologous phosphorylation domains differentially contribute to desensitization and internalization of the M2 muscarinic acetylcholine receptor. J. Biol. Chem. 1997;272:14152–14158. doi: 10.1074/jbc.272.22.14152. [DOI] [PubMed] [Google Scholar]
- Pan X, Ikeda SR, Lewis DL. SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoid receptor activity. Mol. Pharmacol. 1998;54:1064–1072. doi: 10.1124/mol.54.6.1064. [DOI] [PubMed] [Google Scholar]
- Parker EM, Ross EM. Truncation of the extended carboxyl-terminal domain increases the expression and regulatory activity of the avian beta-adrenergic receptor. J. Biol. Chem. 1991;266:9987–9996. [PubMed] [Google Scholar]
- Perry SJ, Lefkowitz RJ. Arresting developments in heptahelical receptor signaling and regulation. Trends Cell Biol. 2002;12:130–138. doi: 10.1016/s0962-8924(01)02239-5. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Stevenson LA, Griffin G. Cross-tolerance between delta-9-tetrahydrocannabinol and the cannabimimetic agents, CP 55,940, WIN 55,212-2 and anandamide. Br. J. Pharmacol. 1993;110:1483–1490. doi: 10.1111/j.1476-5381.1993.tb13989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piserchio A, Zelesky V, Yu J, Taylor L, Polgar P, Mierke DF. Bradykinin B2 receptor signaling: structural and functional characterization of the C-terminus. Biopolymers. 2005;80:367–373. doi: 10.1002/bip.20220. [DOI] [PubMed] [Google Scholar]
- Porter AC, Felder CC. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol. Ther. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Prioleau C, Visiers I, Ebersole BJ, Weinstein H, Sealfon SC. Conserved helix 7 tyrosine acts as a multistate conformational switch in the 5HT2C receptor. Identification of a novel "locked-on" phenotype and double revertant mutations. J. Biol. Chem. 2002;277:36577–36584. doi: 10.1074/jbc.M206223200. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;4:713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Calandra B, Shire D, Bouaboula M, Oustric D, Barth F, Casellas P, Ferrara P, Le FG. Characterization of two cloned human CB1 cannabinoid receptor Isoforms. J. Pharmacol. Exp. Ther. 1996;278:871–878. [PubMed] [Google Scholar]
- Robert J, Clauser E, Petit PX, Ventura MA. A novel C-terminal motif is necessary for the export of the vasopressin V1b/V3 receptor to the plasma membrane. J. Biol. Chem. 2005;280:2300–2308. doi: 10.1074/jbc.M410655200. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J. 2008;22:2311–2322. doi: 10.1096/fj.07-102731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E, Vu HK, Larsson N, Groblewski T, Hjorth S, Elebring T, Sjogren S, Greasley PJ. Identification and characterisation of a novel splice variant of the human CB1 receptor. FEBS Lett. 2005;579:259–264. doi: 10.1016/j.febslet.2004.11.085. [DOI] [PubMed] [Google Scholar]
- Salom D, Lodowski DT, Stenkamp RE, Le Trong I, Golczak M, Jastrzebska B, Harris T, Ballesteros JA, Palczewski K. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc. Nat. Acad. Sci. USA. 2008;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Galve-Roperh I, Canova C, Brachet P, Guzman M. Delta9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 1998a;436:6–10. doi: 10.1016/s0014-5793(98)01085-0. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Galve-Roperh I, Rueda D, Guzman M. Involvement of sphingomyelin hydrolysis and the mitogen-activated protein kinase cascade in the delta9-tetrahydrocannabinol-induced stimulation of glucose metabolism in primary astrocytes. Mol. Pharmacol. 1998b;54:834–843. doi: 10.1124/mol.54.5.834. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Rueda D, Segui B, Galve-Roperh I, Levade T, Guzman M. The CB(1) cannabinoid receptor of astrocytes is coupled to sphingomyelin hydrolysis through the adaptor protein fan. Mol. Pharmacol. 2001;59:955–959. doi: 10.1124/mol.59.5.955. [DOI] [PubMed] [Google Scholar]
- Savinainen JR, Saario SM, Niemi R, Jarvinen T, Laitinen JT. An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors. Br. J. Pharm. 2003;140:1451–1459. doi: 10.1038/sj.bjp.0705577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- Schertler GF. Signal Transduction: the rhodopsin story continued. Nature. 2008;453:292–293. doi: 10.1038/453292a. [DOI] [PubMed] [Google Scholar]
- Shi C, Szczesniak A, Mao L, Jollimore C, Coca-Prados M, Hung O, Kelly MEM. A3 adenosine and CB1 receptors activate a PKC-sensitive Cl-current in human nonpigmented ciliary epithelial cells via a Gβγ-coupled MAPK signaling pathway. Br. J. Pharm. 2003;139:475–486. doi: 10.1038/sj.bjp.0705266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JY. Transmembrane helical domain of the cannabinoid CB1 receptor. Biophys. J. 2009;96:3251–3262. doi: 10.1016/j.bpj.2008.12.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura T, Hiraki K, Takahashi N, Hori T, Ago H, Masuda K, Takio K, Ishiguro M, Miyano M. Crystal structure of squid rhodopsin with intracellularly extended cytoplasmic region. J. Biol. Chem. 2008;283:17753–17756. doi: 10.1074/jbc.C800040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shire D, Carillon C, Kaghad M, Calandra B, Rinaldi-Carmona M, Le FG, Caput D, Ferrara P. An amino-terminal variant of the central cannabinoid receptor resulting from alternative splicing. J. Biol. Chem. 1995;270:3726–3731. doi: 10.1074/jbc.270.8.3726. [DOI] [PubMed] [Google Scholar]
- Siffroi-Fernandez S, Giraud A, Lanet J, Franc JL. Association of the thyrotropin receptor with calnexin, calreticulin and BiP. Efects on the maturation of the receptor. Eur. J. Biochem. 2002;269:4930–4937. doi: 10.1046/j.1432-1033.2002.03192.x. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J. Neurosci. 1996;16:8057–8066. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. The pharmacological activity of anandamide, a putative endogenous cannabinoid in mice. J. Pharmacol. Exp. Ther. 1994;270:219–227. [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Suvorova ES, Gripentrog JM, Jesaitis AJ, Miettinen HM. Agonist-dependent phosphorylation of the formyl peptide receptor is regulated by the membrane proximal region of the cytoplasmic tail. Biochim. Biophys. Acta. 2009;1793:406–417. doi: 10.1016/j.bbamcr.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift S, Leger AJ, Talavera J, Zhang L, Bohm A, Kuliopulos A. Role of the PAR1 receptor 8th helix in signaling: the 7-8-1 receptor activation mechanism. J. Biol. Chem. 2006;281:4109–4116. doi: 10.1074/jbc.M509525200. [DOI] [PubMed] [Google Scholar]
- Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin’s carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein ligh chain Tctex-1. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, Swiercz J, Mackie K, Monyer H, Parolaro D, Whistler J, Kuner T, Kuner R. A molecular basis of analgesic tolerance to cannabinoids. J. Neurosci. 2007;27:4165–4177. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapaidze N, Keith DE, Cvejic S, Evans CJ, Devi LA. Sequestration of the delta opioid receptor. Role of the C terminus in agonist-mediated internalization. J. Biol. Chem. 1996;271:29279–29285. doi: 10.1074/jbc.271.46.29279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tsuga H, Kameyama K, Haga T, Honma T, Lameh J, Sadee W. Internalization and down-regulation of human muscarinic acetylcholine receptor M2 subtypes. Role of third intracellular M2 loop and G protein-coupled receptor kinase 2. J. Biol. Chem. 1998;273:5323–5330. doi: 10.1074/jbc.273.9.5323. [DOI] [PubMed] [Google Scholar]
- Tyukhtenko S, Tiburu EK, Deshmukh L, Vinogradova O, Janero DR, Makriyannis A. NMR solution structure of human cannabinoid receptor-1 helix 7/8 peptide: candidate electrostatic interactions and microdomain formation. Biochem. Biophys. Res. Commun. 2009;390:441–446. doi: 10.1016/j.bbrc.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfers AL, McMurry JL, Miller A, Wang L, Kendall DA, Mierke DF. Cannabinoid receptor-G protein interactions: G(Alphai1)-bound structures of IC3 and a mutant with altered G protein specificity. Protein Sci. 2002;11:2526–2531. doi: 10.1110/ps.0218402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lee MM, Blomenrohr M, van der Doelen AA, Wat JW, Smits N, Hanson BJ, van Koppen CJ, Zaman GJ. Pharmacological characterization of receptor redistribution and beta-arrestin recruitment assays for the cannabinoid receptor 1. J. Biomol. Screen. 2009;14:811–823. doi: 10.1177/1087057109337937. [DOI] [PubMed] [Google Scholar]
- Vasquez C, Lewis DL. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J. Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco G, Galve-Roperh I, Sanchez C, Blazquez C, Haro A, Guzman M. Cannabinoids and ceramide: two lipids acting hand-by-hand. Life Sci. 2005;77:1723–1731. doi: 10.1016/j.lfs.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Vrecl M, Norregaard PK, Almholt DL, Elster L, Pogacnik A, Heding A. Beta-arrestin-based bret2 screening assay for the •non•-beta-arrestin binding CB1 receptor. J. Biomol. Screen. 2009;14:371–380. doi: 10.1177/1087057109333101. [DOI] [PubMed] [Google Scholar]
- Walsh D, Nelson KA, Mahmoud FA. Established and potential therapeutic applications of cannabinoids in oncology. Support Care Cancer. 2003;11:137–143. doi: 10.1007/s00520-002-0387-7. [DOI] [PubMed] [Google Scholar]
- Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a beta1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J, Han S, Kim S, Jacobson KA, Li JH. Conformational changes involved in G-protein-coupled-receptor activation. Trends Pharmacol. Sci. 2008;29:616–625. doi: 10.1016/j.tips.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XQ, Chen JZ. NMR Structural comparison of the cytoplasmic juxtamembrane domains of G-protein-coupled CB1 and CB2 receptors in membrane mimetic dodecylphosphocholine micelles. J. Biol. Chem. 2005;280:3605–3612. doi: 10.1074/jbc.M410294200. [DOI] [PubMed] [Google Scholar]
- Yasuda D, Okuno T, Yokomizo T, Hori T, Hirota N, Hashidate T, Miyano M, Shimizu T, Nakamura M. Helix 8 of leukotriene B4 type-2 receptor is required for the folding to pass the quality control in the endoplasmic reticulum. FASEB J. 2009;23:1470–1481. doi: 10.1096/fj.08-125385. [DOI] [PubMed] [Google Scholar]