Abstract
BACKGROUND
Primary care providers (PCPs) must balance treatment of chronic non-cancer pain with opioid analgesics with concerns about opioid misuse.
OBJECTIVE
We co-enrolled community-based indigent adults and their PCPs to determine PCPs’ accuracy of estimating opioid analgesic misuse and illicit substance use.
DESIGN
Patient-provider dyad study.
PARTICIPANTS
HIV-infected, community-based indigent adults (‘patients’) and their PCPs.
MAIN MEASURES
Using structured interviews, we queried patients on use and misuse of opioid analgesics and illicit substances. PCPs completed patient- and provider-specific questionnaires. We calculated the sensitivity, specificity, and measures of agreement between PCPs’ judgments and patients’ reports of opioid misuse and illicit substance use. We examined factors associated with PCPs’ thinking that their patients had misused opioid analgesics and determined factors associated with patients’ misuse.
KEY RESULTS
We had 105 patient-provider dyads. Of the patients, 21 had misused opioids and 45 had used illicit substances in the past year. The sensitivity of PCPs’ judgments of opioid analgesic misuse was 61.9% and specificity, 53.6% (Kappa score 0.09, p = 0.10). The sensitivity of PCPs’ judgments of illicit substance use was 71.1% and specificity, 66.7% (Kappa score 0.37, p <0.001). PCPs were more likely to think that younger patients (Adjusted odds ratio (AOR) 0.89, 95% CI 0.84-0.97), African American patients (AOR 2.53, 95% CI 1.05-6.07) and those who had used illicit substances in the past year (AOR 3.33, 95% CI 1.35-8.20) had misused opioids. Younger (AOR 0.94, 95% CI 0.86-1.02) and African American (AOR 0.71, 95% CI 0.25-1.97) patients were not more likely to report misuse, whereas persons who had used illicit substances were (AOR 3.01, 95% CI 1.04-8.76).
CONCLUSION
PCPs’ impressions of misuse were discordant with patients’ self-reports of opioid analgesic misuse. PCPs incorrectly used age and race as predictors of misuse in this high-risk cohort.
KEY WORDS: opioid misuse, substance use, PCP judgments, chronic pain
INTRODUCTION
Chronic non-cancer pain (CNCP) is common among primary care patients. There has been a rise in prescription opioid analgesic use for the treatment of CNCP1,2, and a rise in opioid analgesic misuse, with a prevalence of 9% to 41% in patients treated with opioid analgesics for CNCP3–6. Patients with HIV/AIDS have a high prevalence of chronic pain (prevalence 40%-60%), mental health, and substance use disorders11–14. On the one hand, mental health and substance use disorders are associated with opioid analgesic misuse7–9, and on the other hand, they are also risk factors for under treatment of pain in HIV-infected patients10,11. Clinicians must rely on existing tools and their judgment of patients’ likelihood of misuse before continuing to prescribe opioid analgesics. Studies have shown that clinicians judge non-white patients to have a higher risk for prescription opioid misuse when compared to white patients15,16. However, existing data do not support these beliefs7,8.
There are several risk stratification tools that rely on patients’ self-reports of misuse17–22; none have adequate sensitivity and specificity to guide treatment decisions23. Clinicians can use urine toxicology screens, pill and patch counts to measure adherence and monitor for use of non-prescribed opioid analgesics or illicit substances24. A recent systematic review found insufficient evidence for routine urine toxicology testing because of the difficulty interpreting negative tests, which could indicate diversion, inadequate treatment of pain, or inadequate test sensitivity24–26. Another element of risk assessment is clinicians’ impressions. Few studies have examined clinicians’ impressions of misuse27, and none have directly compared clinicians’ opinions of misuse to patients’ self-reports.
We conducted a patient–provider dyad study, co-enrolling members of a representative community-based cohort of indigent adults with HIV-infection and their primary care providers (PCPs) to determine the concordance and discordance of PCPs’ judgments about prescription opioid misuse and illicit substance use compared to patients’ self-reports. We hypothesized that PCPs’ estimates of misuse would have a low concordance with patients’ self-reports. We hypothesized that PCPs would overestimate risk in African American patients, while underestimating risk in white patients.
METHODS
Study Patients and Sampling
We enrolled patient participants (“patients”) into the dyad study from a two-year longitudinal study of pain, use and misuse of opioid analgesics in a community-based sample of indigent adults (Pain Study). We recruited members of the Pain Study from the pre-existing Research on Access to Care in the Homeless (REACH) study, a longitudinal study of HIV-infected adults in San Francisco who were systematically sampled from homeless shelters, free meal programs, and low-rent single-room occupancy hotels28. We offered participation, regardless of pain status, to all active REACH members between September 2007 and June 2008 (n = 337); 296 (87.8%) completed enrollment and baseline activities.
One year into the Pain Study, we began enrolling primary care providers (PCPs) identified by Pain Study patients. We recruited the PCP that each study patient identified, if the patient gave informed consent and the identified PCP met our definition (physician, Nurse Practitioner (NP), or Physician Assistant (PA) providing longitudinal, comprehensive care).
All study protocols were reviewed and approved by the University of California, San Francisco (UCSF), Institutional Review Board; we obtained a Certificate of Confidentiality from the National Institute on Drug Abuse. We told both patients and PCPs that the information they provided would be kept anonymous and not shared with the other party.
Study Procedures
We conducted data collection from the patients at the UCSF Clinical and Translational Research Institute’s Tenderloin Clinical Research Center (TCRC), a community-based university-research site. Trained research assistants administered the Pain Study and REACH study questionnaires every three months. At the baseline Pain Study interview, a trained interviewer administered the Diagnostic Interview Schedule—IV (DIS-IV) substance modules (cocaine, methamphetamine, and heroin)29. At each REACH study quarterly visit, trained interviewers obtained information on current illicit substance use. At each Pain Study quarterly visit, patients answered questions about prescription opioid analgesic misuse using Audio Computer Assisted Self-Interview (ACASI) technology30.
We recruited and collected data from PCPs by mail, using a modified version of Dillman’s Tailored Design Method31. PCPs completed a short, provider-specific questionnaire and a separate patient-specific questionnaire for each of their dyad-study patients. PCPs were encouraged, but not required, to access their patients’ medical records while completing the patient-specific questionnaires.
We reimbursed patients $20.00 for the baseline Pain Study interview, $5.00 for each Pain Study quarterly interview, and $20.00 for each REACH study quarterly interview. PCPs received a $10.00 gift certificate for each completed questionnaire.
Variable Definitions
Patients
At the baseline Pain Study and REACH study visits, patients self-reported information on demographics (age and sex), race/ethnicity (white, African American, Hispanic, or mixed/other), residential stability, jail and prison stays, mean monthly income, insurance status (Medicaid/ Medicare, AIDS Drug Assistance Program (ADAP), or uninsured), and lifetime tobacco history (smoked at least 100 cigarettes in lifetime). We administered the Beck Depression Inventory – II (BDI) and classified depression as moderate to severe (BDI score >19) or none or mild (BDI score ≤19)32,33. We administered the Brief Pain Inventory (BPI)34, and using patients’ self-reports of average intensity of pain in the past 7 days and cut-point analysis, we classified pain as mild (0-3), moderate (4-5), or severe (6-10)35.
We created an inventory of opioid analgesic misuse behaviors that have been previously described in the literature14,36–38. We defined opioid analgesic misuse as getting high, altering the route, selling, stealing, forging prescriptions, trading street drugs for opioids, and exchanging opioids for sex in the past 90 days. We used patients’ ACASI self-reports of misuse from four quarterly interviews to create a dichotomous variable that measured past-year misuse. We created a variable that combined responses for getting high, altering the route or selling prescription opioids (“one or more misuse behaviors”) to mirror what we had asked PCPs.
We obtained self-reports of illicit substance use (cocaine, methamphetamine, and heroin) in the past 90 days from the REACH quarterly interviews, and created a dichotomous variable of any illicit substance use in the past year based on four quarterly interviews. We diagnosed lifetime substance abuse or dependence for cocaine, methamphetamine, and heroin using the DIS-IV instrument29. We used the DIS-IV question, “Which one of these have you used more than five times when they were not prescribed for you, or for longer than prescribed, to feel more active or alert, or to feel good or high?” to define lifetime substance use of cocaine, methamphetamine, and heroin.
Primary Care Providers
In the provider-specific questionnaires, PCPs answered questions on demographics (age and sex), race/ethnicity (white, African American, Hispanic, or Asian/Pacific Islander), clinical training (physician, NP or PA), specialty for physicians (internists, family practice or other), subspecialty for internists (Infectious disease (ID) or HIV), and years in clinical training (4 to 9 years, 10 to 19 years, ≥20 years).
In the patient-specific questionnaire, PCPs reported whether they had prescribed that patient an opioid analgesic, and whether they had ordered urine or blood toxicology screens. Using a 5-point Likert scale, PCPs reported whether their study patient had misused opioid analgesics by getting high, altering the route, or selling these medications. We classified responses as affirmative (maybe, probably or definitely) versus negative (probably not or definitely not). We asked PCPs’ to estimate (yes versus no or don’t know) whether the dyad-study patients they were responding for had used individual illicit substances (cocaine, methamphetamine, and heroin). We created a variable that measured PCPs’ composite affirmative responses of any illicit substance use in the past year for each patient under their care.
Statistical Analysis
We compared dyad-study patients’ self-reports of opioid analgesic misuse or illicit substance use with their corresponding PCPs’ assessments. We matched the date that the PCP completed the patient-specific questionnaire to the closest-occurring quarterly interview completed by the patient, and included three prior quarterly interviews to create a one-year assessment time period.
We restricted our analysis to patients who were prescribed an opioid analgesic in the past year based on PCPs’ reports. We calculated the sensitivity and specificity of PCPs’ opinions of opioid analgesic misuse and illicit substance use compared to patients’ self-reports, and used the Kappa statistic to measure concordance between PCPs’ opinions and patients’ self-reports.
We used logistic regression to examine patient factors that were associated with PCPs’ thinking that their patients had misused prescription opioid analgesics in the past year. We used patients’ age, sex, race (African American versus non-African American) and history of illicit substance use in the past year as independent variables as these have been shown to predict opioid misuse or to be associated with PCPs’ perception of misuse in prior studies. We then determined if any of these factors were independent predictors of patients’ self-reports of past-year misuse. All analyses were conducted using Stata software, version 11.
RESULTS
Sample Characteristics
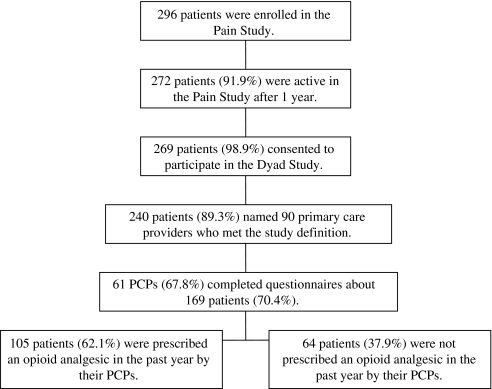
Of the 296 patients initially enrolled in the parent Pain Study, 272 (91.9%) were active in follow-up during data collection for the dyad study, of whom 269 (98.9%) provided written consent allowing us to contact their PCP (Fig. 1). Of these, 240 (89.3%) named a provider who fit the definition of PCP and had confirmable contact information. The 240 patients identified 90 unique PCPs from 23 clinical settings. We obtained responses from 61 PCPs (67.8%) who responded on 169 patients (70.4%). Of these 169 patients, 105 (62.1%) were prescribed an opioid analgesic by PCPs in the past year. Of the 105 patients, 80.8% (n = 84) were prescribed an opioid analgesic for treatment of chronic pain with the intention of taking the medication regularly for more than one month. PCPs reported ordering urine or blood toxicology screens on 27.6% of patients in the past year. We reported results on 105 patient-provider dyads.
Figure 1.
Patient flow.
The mean age of the patients who were prescribed an opioid analgesic in the past year was 50 (Table 1). The majority of patients were male (61.9%), and 48.6% were African American. Almost half (44.2%) had completed high school. Patients who were prescribed an opioid analgesic in the past year did not differ significantly from those who were not, except for reports of severe pain in the past week (58.1% vs. 32.8%; p < 0.004).
Table 1.
Patient Characteristics by Prescription Opioid Analgesic Use in the Past Year
| Characteristic | Total | Prescribed Opioid | Not Prescribed Opioid | P-Value |
|---|---|---|---|---|
| N = 169 | N = 105 | N = 64 | ||
| N (%) | N (%) | N (%) | ||
| Age, Mean (SD) | 50.4 (7.26) | 50.5 (6.95) | 50.2 (7.80) | 0.79 |
| Female | 59 (34.9) | 40 (38.1) | 19 (29.7) | 0.27 |
| Race/Ethnicity | ||||
| White | 60 (35.5) | 39 (37.1) | 21 (32.8) | 0.49 |
| African American | 79 (46.8) | 51 (48.6) | 28 (43.8) | |
| Hispanic | 17 (10.1) | 8 (7.6) | 9 (14.1) | |
| Other/Mixed | 13 (7.7) | 7 (6.7) | 6 (9.4) | |
| Education | ||||
| Less Than High School | 49 (29.2) | 32 (30.8) | 17 (26.7) | 0.82 |
| High School | 75 (44.6) | 46 (44.2) | 29 (45.3) | |
| More Than High School | 44 (26.2) | 26 (25.0) | 18 (28.1) | |
| Residential History | ||||
| Lifetime History of Homelessness | 136 (81.9) | 87 (84.5) | 49 (77.8) | 0.28 |
| Lifetime History of Being Jailed | 149 (88.2) | 96 (91.4) | 53 (82.8) | 0.09 |
| Lifetime History of Being in Prison | 25 (15.7) | 17 (17.4) | 8 (13.1) | 0.48 |
| Mean Monthly Income | $919 | $930 | $900 | 0.59 |
| Insurance | ||||
| Medicaid/Medicare | 89 (52.7) | 57 (54.3) | 32 (50.0) | 0.58 |
| AIDS Drug Assistance Program | 69 (42.1) | 40 (39.6) | 29 (46.0) | 0.42 |
| Uninsured | 10 (5.9) | 5 (4.8) | 5 (7.8) | 0.42 |
| Depression | ||||
| Moderate to Severe (BDI Score >19) | 47 (26.6) | 33 (31.4) | 12 (18.8) | 0.07 |
| Average Intensity of Chronic Pain, Past 7 Days | ||||
| Mild | 44 (26.0) | 20 (19.1) | 24 (37.5) | 0.004 |
| Moderate | 43 (25.4) | 24 (22.9) | 19 (29.7) | |
| Severe | 82 (48.5) | 61 (58.1) | 21 (32.8) | |
| Smoking | ||||
| Lifetime Smoking History | 142 (84.0) | 89 (84.8) | 53 (82.8) | 0.74 |
| Lifetime History of Substance Abuse or Dependence* | ||||
| Cocaine | 97 (59.2) | 59 (58.4) | 38 (60.3) | 0.81 |
| Methamphetamine | 67 (40.6) | 38 (37.3) | 29 (46.0) | 0.27 |
| Heroin | 49 (29.9) | 29 (28.7) | 20 (31.8) | 0.68 |
| Lifetime History of Substance Use † | ||||
| Cocaine | 128 (77.1) | 81 (78.6) | 47 (74.6) | 0.55 |
| Methamphetamine | 95 (57.2) | 56 (54.4) | 39 (61.9) | 0.34 |
| Heroin | 75 (45.2) | 49 (47.6) | 26 (41.3) | 0.43 |
* DIS-IV diagnosis of lifetime cocaine, methamphetamine and heroin abuse or dependence
† DIS-IV diagnosis of lifetime cocaine, methamphetamine and heroin use
The mean age of PCPs was 47 (Table 2). Among the PCPs, 45.9% were female, and 80.3% were white. The majority (81.9%) of PCPs were physicians, 16.4% were NPs, and 1.6% were PAs. Among physicians, 80.0% were internists. Of the internists, 37.5% (n = 15) were ID subspecialists and 17.5% (n = 7) were HIV specialists.
Table 2.
Characteristics of Primary Care Providers (N = 61)
| Characteristic | N (%) |
|---|---|
| Age, Mean (SD) | 46.7 (8.3) |
| Female | 28 (45.9) |
| Race/Ethnicity | |
| White | 49 (80.3) |
| African American | 1 (1.6) |
| Hispanic | 5 (8.2) |
| Asian or Pacific Islander | 6 (9.8) |
| Clinical Training | |
| Physician | 50 (81.9) |
| Nurse Practitioner | 10 (16.3) |
| Physician Assistant | 1 (1.6) |
| Specialty for Physicians | |
| Internal Medicine | 40 (80.0) |
| Family Practice | 7 (14.0) |
| Other | 3 (5.0) |
| Subspecialty for Physicians | |
| Infectious Disease | 15 (37.5) |
| HIV | 7 (17.5) |
| Other | 2 (5.0) |
| Years in Clinical Practice | |
| 4-9 years | 11 (18.0) |
| 10-19 years | 31 (50.8) |
| ≥20 years | 19 (31.2) |
Prevalence of Opioid Analgesic Misuse and PCPs’ Judgments of Misuse
Among the 105 patients who were prescribed an opioid analgesic, 21 (20.0%) self-reported getting high, altering the route or selling prescription opioids in the past year (Table 3). Of these 21 patients, 8 were African Americans (38.1%), 11 were whites (52.4%), and 2 were Hispanic/other race (9.5%) (p = 0.45).
Table 3.
Self-Reported Prevalence of Prescription Opioid Analgesic Misuse in the Past Year (N = 105)
| N (%) | |
|---|---|
| Used opioid analgesic to get high | 13 (12.4) |
| Sold opioid analgesic | 10 (9.5) |
| Snorted, crushed, injected or smoked opioid analgesic | 5 (4.8) |
| Licked or dissolved opioid analgesic | 4 (3.8) |
| Altered the route of opioid analgesic | 8 (7.6) |
| Exchanged opioid analgesic for sex or drugs | 5 (4.8) |
| Stole opioid analgesic from an individual | 5 (4.8) |
| Stole opioid analgesic from a pharmacy, hospital or clinic | 2 (1.9) |
| Forged a prescription for opioid analgesic | 4 (3.8) |
| Traded street drugs to get opioid analgesic | 5 (4.8) |
| Getting high or altering the route or selling opioid analgesic | 21 (20.0) |
PCPs estimated that half (49.5%) of the patients got high, altered the route, or sold opioid analgesics in the past year. The sensitivity of PCPs’ opinions of their patients misusing opioid analgesics in the past year was 61.9% (95% CI 38.4% - 81.9%) and the specificity was 53.6% (95% CI 42.4% - 64.5%) (Table 4). PCPs did not identify 38.1% (8/21) of patients who reported misusing opioid analgesics, and mis-identified 46.4% (39/84) who did not report doing so. There was no concordance between PCPs’ opinions and patients’ self-reports of misuse behaviors in the past year (Kappa score 0.09, p = 0.10).
Table 4.
Primary Care Providers’ Judgment of Opioid Analgesic Misuse and Illicit Substance Use in the Past Year Compared to Patients’ Self-Reports (N = 105)
| Sensitivity %(TP/TP+FN) * | Specificity %(TN/TN+FP) * | Agreement % | Kappa Coefficient | |
|---|---|---|---|---|
| Opioid Analgesic Misuse | ||||
| Getting high on opioids | 38.5 (5/13) | 67.4 (62/92) | 63.8 | 0.03 |
| Altering the route of opioids | 37.5 (3/8) | 92.8 (90/97) | 88.6 | 0.27 † |
| Selling opioids | 50.0 (5/10) | 56.8 (54/95) | 56.2 | 0.03 |
| One or More Misuse Behaviors | 61.9 (13/21) | 53.6 (45/84) | 55.2 | 0.09 |
| Illicit Substance Use | ||||
| Cocaine | 74.3 (26/35) | 71.2 (47/66) | 72.3 | 0.43‡ |
| Methamphetamine | 56.5 (13/23) | 87.3 (69/79) | 80.4 | 0.44‡ |
| Heroin | 27.3 (3/11) | 93.6 (87/93) | 86.5 | 0.23 † |
| Used One or More Illicit Substances | 71.1 (32/45) | 66.7 (40/60) | 68.6 | 0.37‡ |
* Abbreviations: TP, True Positive; TN, True Negative; FP, False Positive; FN, False Negative.
† p < 0.01
‡ p < 0.001
Prevalence of Illicit Substance Use and PCPs’ Judgments of Substance Use
More than a third of the patients (n = 45, 42.9%) self-reported using one or more illicit substances in the past year: 34.3% (n = 36) self-reported using cocaine, 24.8% (n = 26) methamphetamine, and 10.5% (n = 11) heroin (Table 4). African Americans (37.8%) were as likely as whites (44.4%) to self-report using one or more illicit substances in the past year (p = 0.14).
PCPs estimated that 49.5% had used one or more illicit substances in the past year. The sensitivity of PCPs’ opinions of patients using one or more substances in the past year was 71.1% (95% CI 55.7% - 83.6%) and the specificity was 66.7% (95% CI 53.3% - 78.3%) (Table 4). PCPs overestimated illicit substance use in 33.3% (20/60) and underestimated in 28.9% (13/45) of patients. PCPs’ impressions were concordant with patients’ self-reports (Kappa score 0.37, p < 0.001).
Factors Associated with PCPs’ Thinking that Their Patients had Misused Opioids
In a multivariable regression model after adjusting for patients’ age, sex, race, and history of illicit substance use in the past year, PCPs were more likely to think that younger patients (Adjusted Odds Ratio (AOR) 0.89, 95% CI 0.84-0.97), African American patients (AOR 2.53, 95% CI 1.05-6.07), and those who had used illicit substances in the past year (AOR 3.33, 95% CI 1.35-8.20) had misused opioids (Table 5). Younger (AOR 0.94, 95% CI 0.86-1.02) and African American (AOR 0.71, 95% CI 0.25-1.97) patients were not more likely to report misusing opioids; but those who had used illicit substances in the past year had three-fold higher odds of misuse than those who had not (AOR 3.01, 95% CI 1.04-8.76).
Table 5.
Factors Associated with Primary Care Providers’ Thinking that Their Patients had Misused Opioid Analgesics in the Past Year (N = 105)
| Factors | Unadjusted Odds Ratio 95% CI | Adjusted Odds Ratio 95% CI |
|---|---|---|
| Age | 0.92 (0.86-0.98) * | 0.89 (0.84-0.97) † |
| Sex (ref. Women) | 1.34 (0.61-2.96) | 1.02 (0.42-2.43) |
| African American (ref. non-African American) | 1.78 (0.82-3.85) | 2.53 (1.05-6.07) ‡ |
| History of substance use in the past year | 2.10 (0.96-4.61) | 3.33 (1.35-8.20) § |
* p < 0.01
† p < 0.004
‡ p < 0.04
§ p < 0.009
DISCUSSION
We found that PCPs judgments of opioid analgesic misuse were discordant with patients’ ACASI self-reports. PCPs were more likely to overestimate misuse in younger and African American patients. PCPs determinations of misuse were more accurate among patients with histories of illicit substance use. To our knowledge, this is the first study that compares PCPs’ estimates of prescription opioid misuse with patients’ ACASI self-reports. Our study highlights the challenges of determining and monitoring opioid misuse.
We selected patients who were indigent and living in an urban poor neighborhood to reflect a group of patients thought to be at increased risk of misuse. Despite this, PCPs were more likely to think that African American patients had misused opioid analgesics, even though they were no more likely to report misuse. Patients’ race was shown to be associated with clinicians’ perceptions of patients’ likelihood of risk behaviors16. White patients are more likely to be prescribed opioid analgesics for the treatment of chronic pain than non-whites;15 though, no evidence points to a higher risk of misuse by non-whites7,8. Studies suggest that providers invoke racially based stereotypes when faced with clinical ambiguity or complex medical decisions39. In the absence of adequate training or objective measures to determine misuse, PCPs may have unknowingly relied on racially based clinical stereotypes when making these assessments16. PCPs’ lack of knowledge and training on determining opioid misuse may increase the probability of use of these stereotypes to determine patient behaviors.
PCPs were more likely to think that younger patients had a higher risk of misuse. Younger age was found to be a consistent predictor of opioid misuse among the general patient population7,8,37. However, younger age was not associated with misuse in our study. Uniformly applying established predictors without contextualizing to the target patient population might result in erroneous conclusions.
PCPs’ identification of illicit substance use was better than that of prescription opioid analgesic misuse. Tools such as urine toxicology screens are easier to interpret for presence of illicit substances than for assessing prescription opioid misuse. Illicit substance use in the past year was associated with patients’ self-reports of opioid misuse. These findings are supported by prior studies that showed that illicit substance use was one of the most reliable predictors of future prescription opioid misuse in clinic-based samples7,9.
Given the difficulty in making these assessments, several risk stratification tools have been developed to help providers identify patients at risk for misuse, though none of these tools have been validated in high-risk patients seen in primary care clinics17,18,21–23. In the absence of a single, gold-standard test for determining opioid analgesic misuse, PCPs need to be conscious of their preconceptions and substantiate their clinical judgments of misuse with all available tools. Studies that examine the predictive validity as well as applicability of these tools to primary care providers caring for high-risk patients are needed.
The high-risk patients in our study were representative of those who are disproportionately affected by chronic pain and co-occurring substance use disorders 4,40 and are routinely treated in primary care practices that lack the resources of specialty pain clinics. Therefore, identifying better strategies to monitor opioid misuse and providing additional resources for patients with co-occurring substance use disorders is important given rising rates of opioid misuse and the serious consequences of overdose41,42.
We note several limitations. We relied on patients’ self report as the ‘gold standard’, which may have underestimated the prevalence of opioid analgesic misuse and illicit substance use. This may have lead to misclassification bias. We tried to minimize underreporting by using ACASI technology, which reduces barriers in reporting sensitive information30,43. While self-reports may have lead to underreporting, other methods such as urine toxicology screens are similarly imperfect. A recent systematic review concluded that there was inadequate evidence to support using urine toxicology tests to determine opioid misuse, and highlighted the difficulty of using ‘objective’ data for measuring misuse24,25. Despite our overall good response rate for PCPs, we did not have information on non-responders, thus introducing a potential for selection bias. Our patients were poor, HIV-infected, from one urban area, and had high rates of chronic pain and illicit substance use, limiting the generalizability of our findings. However, these findings may be applicable to other urban, poor, non-HIV infected patient populations with a high burden of chronic pain, depression, and substance use disorders44–46.
Our study had several strengths. Patients were recruited systematically from high-risk communities, and from these, we selected those who were prescribed opioid analgesics. We minimized recall bias by obtaining self-reports in the past 90 days instead of the past year. Patients reported misuse using ACASI at a community-based research site separate from clinical settings. We recruited PCPs from diverse clinical settings including general and HIV specific community health clinics, private practices and university-affiliated medical practices.
We found that PCPs’ assessments of opioid misuse were discordant with patients’ self-reports and appeared to factor patients’ age and race incorrectly. As use and misuse of these medications continue to rise, PCPs will need more reliable means of determining misuse. Increasing awareness among PCPs about the harmful effects of racially based heuristic shortcuts on clinical assessments of misuse and providing education on contextualizing risk and incorporating individual patient experiences may improve the accuracy of estimates of misuse.
Acknowledgments
Contributors
The authors would like to thank Jennifer Mattson, BA for her invaluable assistance in preparing this manuscript and Jane Liebschutz, MD for her thoughtful comments on the manuscript. We would like to thank the research assistants for conducting the patient interviews. We would also like to thank all the patients and their PCPs for their contribution to this study.
Funders
This study was funded by the Pain Study grant from the National Institute on Drug Abuse R01DA022550, and the REACH study grant from the National Institute of Mental Health R01MH54907. Additional funding includes the Department of Health and Human Services – Health Resources and Services Administration, Primary Care Research Fellowship grant D55HP05165 (Dr. Vijayaraghavan). The Tenderloin Center for Clinical Research was supported by the University of California, San Francisco (UCSF), Clinical and Translational Institute grant, NIH/NCRR UCSF-CTSI UL1 RR024131. The funders had no role in the design or conduct of the study or the preparation of the manuscript.
Previous Presentation
Results from this study were presented at the Annual Meeting of the Society of General Internal Medicine Conference; April 29, 2010; Minneapolis, Minnesota.
Conflict of Interest
None disclosed.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Gilson AM, Ryan KM, Joranson DE, Dahl JL. A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997-2002. J Pain Symptom Manage. 2004;28(2):176–88. doi: 10.1016/j.jpainsymman.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1–8. doi: 10.1097/AJP.0b013e3181b99f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manchikanti L, Pampati V, Damron KS, Fellows B, Barnhill RC, Beyer CD. Prevalence of opioid abuse in interventional pain medicine practice settings: a randomized clinical evaluation. Pain Physician. 2001;4(4):358–65. [PubMed] [Google Scholar]
- 4.Manchikanti L, Cash KA, Damron KS, Manchukonda R, Pampati V, McManus CD. Controlled substance abuse and illicit drug use in chronic pain patients: an evaluation of multiple variables. Pain Physician. 2006;9(3):215–25. [PubMed] [Google Scholar]
- 5.Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17(3):173–9. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chelminski PR, Ives TJ, Felix KM, et al. A primary care, multi-disciplinary disease management program for opioid-treated patients with chronic non-cancer pain and a high burden of psychiatric comorbidity. BMC Health Serv Res. 2005;5(1):3. doi: 10.1186/1472-6963-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ives TJ, Chelminski PR, Hammett-Stabler CA, et al. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355–62. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Michna E, Ross EL, Hynes WL, et al. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J Pain Symptom Manage. 2004;28(3):250–8. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Breitbart W, Rosenfeld BD, Passik SD, McDonald MV, Thaler H, Portenoy RK. The undertreatment of pain in ambulatory AIDS patients. Pain. 1996;65(2–3):243–9. doi: 10.1016/0304-3959(95)00217-0. [DOI] [PubMed] [Google Scholar]
- 11.Breitbart W, Passik S, McDonald MV, et al. Patient-related barriers to pain management in ambulatory AIDS patients. Pain. 1998;76(1–2):9–16. doi: 10.1016/S0304-3959(98)00018-9. [DOI] [PubMed] [Google Scholar]
- 12.Hewitt DJ, McDonald M, Portenoy RK, Rosenfeld B, Passik S, Breitbart W. Pain syndromes and etiologies in ambulatory AIDS patients. Pain. 1997;70(2–3):117–23. doi: 10.1016/S0304-3959(96)03281-2. [DOI] [PubMed] [Google Scholar]
- 13.Lebovits AH, Smith G, Maignan M, Lefkowitz M. Pain in hospitalized patients with AIDS: analgesic and psychotropic medications. Clin J Pain. 1994;10(2):156–61. doi: 10.1097/00002508-199406000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Passik SD, Kirsh KL, Donaghy KB, Portenoy RK. Pain and aberrant drug-related behaviors in medically ill patients with and without histories of substance abuse. Clin J Pain. 2006;22(2):173–81. doi: 10.1097/01.ajp.0000161525.48245.aa. [DOI] [PubMed] [Google Scholar]
- 15.Burgess DJ, Crowley-Matoka M, Phelan S, et al. Patient race and physicians’ decisions to prescribe opioids for chronic low back pain. Soc Sci Med. 2008;67(11):1852–60. doi: 10.1016/j.socscimed.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 16.van Ryn M, Burke J. The effect of patient race and socio-economic status on physicians’ perceptions of patients. Soc Sci Med. 2000;50(6):813–28. doi: 10.1016/S0277-9536(99)00338-X. [DOI] [PubMed] [Google Scholar]
- 17.Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004;112(1–2):65–75. doi: 10.1016/j.pain.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432–42. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 19.Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):131–46. doi: 10.1016/j.jpain.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Compton PA, Wu SM, Schieffer B, Pham Q, Naliboff BD. Introduction of a self-report version of the prescription drug use questionnaire and relationship to medication agreement noncompliance. J Pain Symptom Manage. 2008;36(4):383–95. doi: 10.1016/j.jpainsymman.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16(6):355–63. doi: 10.1016/S0885-3924(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 22.Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the current opioid misuse measure. Pain. 2007;130(1–2):144–56. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008;24(6):497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- 24.Pergolizzi J, Pappagallo M, Stauffer J, et al. The role of urine drug testing for patients on opioid therapy. Pain Pract. 2010. [Epub ahead of print] [DOI] [PubMed]
- 25.Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712–20. doi: 10.7326/0003-4819-152-11-201006010-00004. [DOI] [PubMed] [Google Scholar]
- 26.Reisfield GM, Bertholf R, Barkin RL, Webb F, Wilson G. Urine drug test interpretation: what do physicians know? J Opioid Manag. 2007;3(2):80–6. doi: 10.5055/jom.2007.0044. [DOI] [PubMed] [Google Scholar]
- 27.Wasan AD, Butler SF, Budman SH, Benoit C, Fernandez K, Jamison RN. Psychiatric history and psychologic adjustment as risk factors for aberrant drug-related behavior among patients with chronic pain. Clin J Pain. 2007;23(4):307–15. doi: 10.1097/AJP.0b013e3180330dc5. [DOI] [PubMed] [Google Scholar]
- 28.Robertson MJ, Clark RA, Charlebois ED, et al. HIV seroprevalence among homeless and marginally housed adults in San Francisco. Am J Public Health. 2004;94(7):1207–17. doi: 10.2105/AJPH.94.7.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38(4):381–9. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 30.Riley ED, Chaisson RE, Robnett TJ, Vertefeuille J, Strathdee SA, Vlahov D. Use of audio computer-assisted self-interviews to assess tuberculosis-related risk behaviors. Am J Respir Crit Care Med. 2001;164(1):82–5. doi: 10.1164/ajrccm.164.1.2101091. [DOI] [PubMed] [Google Scholar]
- 31.Dillman DA. Mail and Telephone Surveys: The Total Design Method. New York: Wiley; 1978. [Google Scholar]
- 32.Beck A, Steer R, Brown G. Manual for Beck Depression Inventory-II. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- 33.Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory-Second Edition in a sample of college students. Depress Anxiety. 2004;19(3):187–9. doi: 10.1002/da.20002. [DOI] [PubMed] [Google Scholar]
- 34.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–18. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–84. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 36.Arvanitis ML, Satonik RC. Transdermal fentanyl abuse and misuse. Am J Emerg Med. 2002;20(1):58–9. doi: 10.1053/ajem.2002.29562. [DOI] [PubMed] [Google Scholar]
- 37.Chabal C, Erjavec MK, Jacobson L, Mariano A, Chaney E. Prescription opiate abuse in chronic pain patients: clinical criteria, incidence, and predictors. Clin J Pain. 1997;13(2):150–5. doi: 10.1097/00002508-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Dunbar SA, Katz NP. Chronic opioid therapy for nonmalignant pain in patients with a history of substance abuse: report of 20 cases. J Pain Symptom Manage. 1996;11(3):163–71. doi: 10.1016/0885-3924(95)00165-4. [DOI] [PubMed] [Google Scholar]
- 39.Van Ryn M. Research on the provider contribution to race/ethnicity disparities in medical care. Med Care. 2002;40(1 Suppl):I140–51. doi: 10.1097/00005650-200201001-00015. [DOI] [PubMed] [Google Scholar]
- 40.Potter JS, Prather K, Weiss RD. Physical pain and associated clinical characteristics in treatment-seeking patients in four substance use disorder treatment modalities. Am J Addict. 2008;17(2):121–5. doi: 10.1080/10550490701862902. [DOI] [PubMed] [Google Scholar]
- 41.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613–20. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 43.Metzger DS, Koblin B, Turner C, et al. Randomized controlled trial of audio computer-assisted self-interviewing: utility and acceptability in longitudinal studies. HIVNET Vaccine Preparedness Study Protocol Team. Am J Epidemiol. 2000;152(2):99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- 44.Yancy WS, Jr, Macpherson DS, Hanusa BH, et al. Patient satisfaction in resident and attending ambulatory care clinics. J Gen Intern Med. 2001;16(11):755–62. doi: 10.1111/j.1525-1497.2001.91005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiebach NH, Wong JG. Taking care of patients in resident clinics: where do we stand? J Gen Intern Med. 2001;16(11):787–9. doi: 10.1111/j.1525-1497.2001.10917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brook RH, Fink A, Kosecoff J, et al. Educating physicians and treating patients in the ambulatory setting. Where are we going and how will we know when we arrive? Ann Intern Med. 1987;107(3):392–8. doi: 10.7326/0003-4819-107-2-392. [DOI] [PubMed] [Google Scholar]