Abstract
Objective
We have previously shown that surrounding fat causes an increase of up to 21% in bone mineral density (BMD) measured by Lunar ‘Intelligent DXA’ (iDXA), one of the latest generation dual energy X-ray absorptiometry (DXA) scanners [1]. The purpose of our study was to see if it was possible to avoid this artifact when measuring the BMD of metacarpals II, III, and IV by digital X-ray radiogrammetry (DXR).
Methods
We took X-rays of the bones of a cadaveric left hand which were immobilized in a wooden cradle to preserve an approximate in vivo configuration. The X-rays were digitized into Digital Imaging and Communications in Medicine (DICOM) files which were analyzed using dxr-online (dxr-online, Sectra, Sweden) which uses the same DXR-BMD algorithm previously used by Pronosco X-posure v2 and Sectra Osteoporosis package. The X-rays were repeated four times. We then surrounded the bones with a layer of lard, and again X-rayed four times. This process was repeated with the bones were covered by two layers, and then three layers of lard.
Results
The measured DXR-BMD increased by a maximum of 0.44% when the metacarpals were covered by either two or three layers of lard compared with when the metacarpals were not covered by lard.
Conclusion
The measurement of metacarpal BMD measured by DXR is minimally affected by surrounding lard. The measurement of metacarpal BMD by DXR seems to be a way of avoiding the artifactual change in BMD caused by fat, when it is measured by DXA.
Keywords: Radiogrammetry, BMD, Fat, DXR
Introduction
Today, the most widely used technique for measuring BMD is DXA. It has proved useful for following the BMD of individuals longitudinally, to see how osteoporosis is evolving, and how it is responding to treatment.
However we have previously shown that surrounding lard causes an increase of up to 21% in BMD measured by dual energy X-ray absorptiometry (DXA) [1]. Bolotin et al [2] showed that only relatively small extraosseous soft tissue inhomogeneities within the region of interest (ROI) of DXA scans causes substantial inaccuracies in addition to those resulting from uniformly distributed soft tissues around the bone. The extent of these in vivo inaccuracies depended on the mean extraossous fat to lean areal density ratio and its degree of nonuniformity within the ROI. They found that in many cases, inaccuracies can be as large as 20–50%, particularly in osteopenic, osteoporotic, and elderly patients. In another in vitro study using a different phantom configuration, we have found that iDXA overestimated fat by 2 - 29%, and underestimated BMD by 7.2% with the thickest layer of lard [3]. Thus the measurements of BMD, bone mineral content (BMC), and fat by iDXA are changed by the configuration of the phantom in relation to fat, or bone in relation to fat, emphasizing that the configuration of fat and bone have a marked influence on the results of fat and bone reported by iDXA. This accords with the findings of Bolotin et al when they used the older DXA machine to measure phantoms of fat, bone, and lean arranged in different configurations [2]. Weigart and Cann compared spinal T scores determined by DXA with spinal T scores determined by quantitative CT (QCT) in control and obese groups. Their results suggested that DXA scanning in obese patients can have substantial errors, overestimating BMD by 1 or 2 T score units and causing misdiagnosis of the patient if DXA is used as the primary diagnostic methods [4]. Others have reported similar findings [5 - 6].
We were interested in finding a technique for measuring BMD which is not altered by surrounding fat. DXR-BMD is based on the measurement of cortical thickness which is a geometric measurement that does not depend on the absorption of X-rays. Thus it seemed possible that there might not be measurement artifact when using DXR-BMD.
Materials and Methods
We used hand bones from a cadaveric skeleton. They were put in a usual anatomic configuration and placed in a cradle carved out of balsa wood, and stabilized by a molded plastic cover. The underside of the cradle was flat, enabling it to be placed firmly on an X-ray plate from which it was separated by about 0.5 – 1.0 cm, approximately the same distance that would separate hand bones from an X-ray plate in vivo.
Four sets of X-rays were taken of the hand bones when uncovered, and then covered by one, two, and finally three layers of lard. A Fuji FCR5000 processing machine was used at 50 kV. 2.5 mAs without a grid, 100 cm source image distance, fine focus 0.6 mm. Each set of X-rays was repeated four times. The X-rays were digitized into DICOM files which were sent to dxr-online, Sectra; the DXR computer algorithm was used to calculate the mean cortical thickness and DXR-BMD of metacarpals II, III, and IV.
Statistical Analysis
Mean and standard deviation were calculated for each DXR-BMD result with no lard and with one, two, and three layers of lard covering the metacarpals. The t-test was used to test the null hypothesis that the mean values were equal to each other. Separate analyses were performed for each measured value.
Results
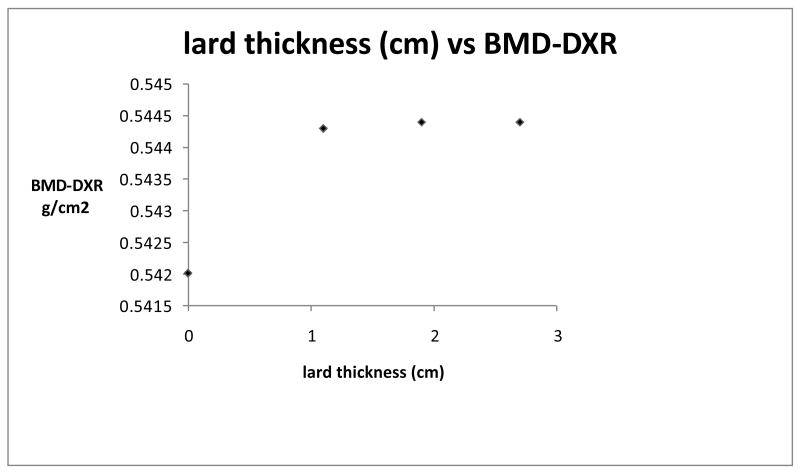
Figure 1 shows a plot of mean DXR-BMD of the metacarpals vs thickness of lard covering the hand bones. There was a statistical difference (p = 0.03) between DXR-BMD with 1.9 cm of lard vs no lard, although the difference was only 0.44%. The standard deviations of these two measurements were very small (0.0014 and 0.0015 respectively) and this would account for the low p value.
Figure 1.
mean DXR-BMD of the metacarpals vs thickness of lard covering the hand bones.
BMD 0.544 (1.9 or 2.7 cm lard thickness) is 0.4% > BMD 0.542 (no lard)
Table 1 gives the details of the lard thicknesses and the measured BMD.
Table 1. Relationship of lard thickness with measured BMD.
| Thickness of individual Lard strips (cm) | Measured BMD-DXR (g/cm2) | SD |
|---|---|---|
| 0 | 0.5420 | 0.0014 |
| 1.1 | 0.5443 | 0.0031 |
| 1.9 | 0.5444 | 0.0015 |
| 2.7 | 0.5444 | 0.0030 |
Thickness of each lard strip was measured 12 times
Strip 1 average: 0.7 cm SD 0.35
Strip 2 average: 0.7 cm SD 0.08
Strip 3 average: 0.8 cm SD 0.33
The large SDs reflect the relative unevenness of the lard strips (which were prepared by a butcher).
The difference between DXR-BMD with 1.1 cm of lard vs no lard was 0.42% p = 0.13
The difference between DXR-BMD with 1.9 cm of lard vs no lard was 0.44% p = 0.03
The difference between DXR-BMD with 2.7 cm of lard vs no lard was 0.44% p = 0.118
Discussion
The measuring of cortical thickness as a surrogate for BMD was introduced in 1960 by Barnett and Nordin [7]; it was supplanted by DXA in the 1970s; this was quicker, simpler, and able to measure BMD at 3 different sites – lumbar spine, hip, and forearm. DXA was a more convenient technique than measuring cortical thickness which required a radiologist to measure the thickness of the bone cortex seen on an X-ray. Around 2000, digital X-ray radiogrammetry (DXR-BMD) was developed. This technique was based on measuring the cortical thickness of various bones including the middle three metacarpals - using a computer algorithm to avoid observer error. Jorgensen et al found that the coefficient of variation of DXR-BMD in a group of pre-menopausal women was 0.68%, and the CV in a group of post-menopausal women was 0.61% [8]. DXR-BMD is not subject to observer error (as were the original cortical thickness measurements of Barnett and Nordin); there are approximately 180 measurements per cm in the ROI of each metacarpal. Around 1000 measurements contribute to the total measurement of the three metacarpals.
The reason that fat causes artifactual alterations in measured BMD is that it absorbs X-rays albeit to a lesser extent than bone. There is no way for the amount of fat in the path of the X-rays measuring the bone density to be accurately known, and as the reported BMD is a function of the amount of absorption of X-rays, the reported result will be affected according to the amount of fat overlying the region of interest (ROI).
As DXA is inaccurate for measuring BMD in obese subjects, we were looking for an alternative method for measuring BMD. We believe that this is the first report to demonstrate that the measurement of BMD by DXR is minimally affected by fat.
Overall, DXR-BMD is as predictive of future fracture as is BMD measured by DXA. In a prospective study of 9704 elderly women, Bouxsein et al found that DXR-BMD of metacarpals II – IV obtained from hand radiographs was as predictive of subsequent fracture of vertebrae and wrist as BMD of these sites - measured by DXA. [9]. Bach-Mortensen et al in a study of 1,370 post menopausal women reported similar findings [10]. Ravna et al reported an association between body size and bone mass [11]. Others have reported an association between body size, body weight, BMD [12 - 13].
In contrast to these studies showing a positive association between obesity and BMD, Hsu et al showed that increased fatness is associated with reduced bone density in a cross-sectional study of 7137 men, 4585 premenopausal women, and 2248 post menopausal women aged 25-64 yrs [14]. Total-body and hip bone mineral content (BMC), bone mineral density (BMD) and body composition were measured by DXA. They found that BMD was associated with both body weight and lean body mass. They concluded that the risks of osteoporosis, osteopenia, (and non-spine fractures) were significantly higher for subjects with higher percentage body fat mass (FM), independent of body weight, physical activity and age. Thus in this study, FM had a negative effect on bone mass in contrast with the positive effect of weight itself. Wang et al also found that lean body mass has a greater effect than fat mass on bone mass [15].
In view of the inaccuracy of BMD measured by DXA in obese subjects, it is not clear to what extent these associations represent a real effect of obesity on BMD rather than being the result of an artifact in the measurement of BMD.
It would seem that the relationship of BMD to obesity needs to be revisited by using a technique for measuring BMD which is not artifactually altered by fat. There seems to be a place for the use of DXR-BMD in measuring BMD in subjects and patients of differing degrees of fatness. In the present epidemic of obesity, DXR-BMD may prove to be an important contribution to the measurement of BMD.
Contributor Information
Edward Colt, Department of Endocrinology and Obesity Research Center, St Luke's Roosevelt Hospital and Columbia University, New York, New York.
Johan Kälvesten, Sectra, Imtec AB, Sweden.
Kenneth Cook, Department of Radiology, St Luke's Roosevelt Hospital and Columbia University, New York, New York.
Nata Khramov, Department of Radiology, St Luke's Roosevelt Hospital and Columbia University, New York, New York.
Fahad Javed, Department of Medicine, St Luke's Roosevelt Hospital and Columbia University, New York, New York.
References
- 1.Javed F, Yu W, Thornton J, Colt E. Effect of fat on measurement of bone mineral density. Int J Body Comp Res. 2009;7(1):37–40. [PMC free article] [PubMed] [Google Scholar]
- 2.Bolotin HH, Sievanen H, Grashuis JL. Patient specific DXA bone mineral density inaccuracies: Quantitative effects of nonuniform extraosseous fat distributions. J Bone Miner Res. 2003;18:1020–7. doi: 10.1359/jbmr.2003.18.6.1020. [DOI] [PubMed] [Google Scholar]
- 3.Javed F, Wang J, Colt E, et al. The validity of iDXA in determining bone mineral content (BMC), bone mineral density (BMD) and body fat (BF) with changes in subcutaneous tissue thickness (STT) Int J Body Comp Res. 2008;6(74) 15-P. [Google Scholar]
- 4.Weigert JM, Cann CE. Dual energy X-ray absorptiometry (DXA) in obese patient: Are normal values really normal? J Women's Imaging. 1999;1:11–7. [Google Scholar]
- 5.Guiglielmi G, Grimston SK, Fischer KC, et al. Osteoporosis. Diagnosis with lateral and posteroanterior dual X-ray absorptiometry compared with quantitative CT. Radiology. 1994;192:845–50. doi: 10.1148/radiology.192.3.8058958. [DOI] [PubMed] [Google Scholar]
- 6.Grampp S, Genant HK, Mathur A, et al. Comparison of noninvasive bone mineral measurement in assessing age-related loss, fracture discrimination, and diagnostic classification. J Bone Miner Res. 1997;12:697–711. doi: 10.1359/jbmr.1997.12.5.697. [DOI] [PubMed] [Google Scholar]
- 7.Barnett E, Nordin B. The radiological diagnosis of osteoporosis: a new approach. Clin Radiol. 1960;1:166–174. doi: 10.1016/s0009-9260(60)80012-8. [DOI] [PubMed] [Google Scholar]
- 8.Jorgensen JT, Andersen PB, Rosholm A, et al. Digital X-ray radiogrammetry:a new appendicular bone densitometric method with high precision. Clin Physiol. 2000;20:330–5. doi: 10.1046/j.1365-2281.2000.00268.x. [DOI] [PubMed] [Google Scholar]
- 9.Bouxsein ML, Palermo L, Yeung C, et al. Digital X-ray Radiogrammetry Predicts Hip, Wrist and Vertebral Fracture Risk in Elderly Women: A Prospective Analysis from the Study of Osteoporositc Fractures. Osteoporosis Int. 2002;13:358–365. doi: 10.1007/s001980200040. [DOI] [PubMed] [Google Scholar]
- 10.Bach-Mortensen P, Hyldstrup L, Appleyard M, et al. Digital X-Ray Radiogrammetry Identifies Women at Risk of Osteoporotic Fracture: Results from a Prospective study. Calcif Tissue Int. 2006;79:1–6. doi: 10.1007/s00223-005-0260-z. [DOI] [PubMed] [Google Scholar]
- 11.Ravn P, Cizza G, Bjarnason NH, et al. Low body mass index is an important risk factor for low bone mass and and increased bone loss in early post menopausal women. J Bone Miner Res. 1999;14:1622–1627. doi: 10.1359/jbmr.1999.14.9.1622. [DOI] [PubMed] [Google Scholar]
- 12.Edelstein SL, Barrett-Connor E. Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol. 1993;138:160–9. doi: 10.1093/oxfordjournals.aje.a116842. [DOI] [PubMed] [Google Scholar]
- 13.Felson DT, Zhang Y, Hannan MT, et al. Effects of weight and body mass index on bone mineral density in men and women:the Framingham study. J Bone Miner Res. 1993;8:567–73. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- 14.Hsu YH, Venners SA, Terwedow HA, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–54. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 15.Wang MC, Bachrach LK, Van Loan M, et al. The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone (NY) 2005;37:474–81. doi: 10.1016/j.bone.2005.04.038. [DOI] [PubMed] [Google Scholar]