SYNOPSIS
Objective
This study examined prostate-specific antigen (PSA) test use among men and identified sociodemographic and health-related characteristics associated with its use over time.
Methods
The National Health Interview Survey collected information on PSA test use among 16,058 men ≥40 years of age in 2000 and 2005. We examined two outcomes: (1) having had a recent (within two years) screening PSA test and (2) having had three or more PSA tests in the past five years (to indicate frequent use).
Results
Marital status, family history of prostate cancer, and having seen a doctor in the past year differed over time in the unadjusted model. In the adjusted model, recent PSA screening decreased from 2000 to 2005 for single, never-married men, but increased for men with chronic diseases. Frequent PSA test use increased for men with a family history of prostate cancer, men with chronic diseases, and men who had seen a physician in the past year. Significant correlates of higher recent PSA test use included being older, married, and of black race/ethnicity; having higher levels of education and income, health-care coverage, and a usual place of health care; and increased comorbidity.
Conclusion
Major organizations are not in agreement about the efficacy of prostate cancer screening; however, men ≥40 years of age continue to use the PSA test. Both recent screening and frequent testing showed variability during the study period and may have implications for the ongoing randomized clinical trials that are expected to clarify whether early detection of prostate cancer with PSA testing increases survival.
Prostate cancer is the most common cancer among men in the United States and the second-leading cause of cancer deaths in this population after lung cancer.1,2 The disease poses a burden for older men in general and black men in particular. Additionally, there are often morbidity-related issues from this disease and its treatment that affect men's quality of life.3,4
The prostate-specific antigen (PSA) test is routinely used as a screening tool to assist in the diagnosis of prostate cancer, and routine PSA-based screening has led to a dramatic increase in prostate cancer detection.5 There is, however, some disagreement about the efficacy of such screening, as it has not been demonstrated in randomized clinical trials to improve survival.6 In spite of the disagreement, PSA remains an important prognostic marker among men diagnosed with prostate cancer. While its sensitivity and specificity in detecting prostate cancer may not be optimal, it has reported clinical validity and is an important predictor of outcome.7,8 Testing for PSA has profoundly affected the diagnosis, treatment, and monitoring of prostate cancer and has allowed physicians to detect prostate tumors while they are small, typically low-grade, and treatable.9,10 There is, however, some debate that screening for these types of prostate cancer contributes to overdiagnosis or finding and treating cancers that may be insignificant compared with the risk of possible side effects from the treatment.9,11,12
Prostate cancer screening using the PSA test has varied by race/ethnicity, and some studies have suggested that black men have lower screening rates for prostate cancer than white men.13,14 However, recent studies have found that PSA test use for screening has increased, especially among younger black men.15,16 In addition to race/ethnicity, correlates of PSA test use include older age, higher socioeconomic status, being married, having a family history of prostate cancer, having health insurance coverage, and having a usual source of health care.17,18
The purpose of this study was to examine the use of the PSA test among men from a national survey conducted in 2000 and 2005 by sociodemographic and health characteristics. This analysis explored use of the PSA test for screening purposes within the past two years and the number of PSA tests during the past five years to determine correlates of both recency and frequency of PSA testing among men in the U.S.
METHODS
We examined data from the National Health Interview Survey (NHIS), an annual health survey conducted by the National Center for Health Statistics. The survey includes core questions about the respondents' health, access to and use of health services, as well as demographic and socioeconomic characteristics. It also contains one or more annual supplements addressing particular health issues. In 2000 and 2005, the survey collected information related to cancer prevention and control.19,20 Trained U.S. Census Bureau interviewers conducted in-person interviews. Black and Hispanic households were oversampled to obtain more precise estimates.19,20 Response rates for the core survey and the cancer control supplement were 88.9% and 72.1%, respectively, in 2000, and 86.5% and 69.0%, respectively, in 2005.
Study population
Only NHIS respondents who were male and ≥40 years of age were asked about their PSA test use. We focused on these men for our analysis. Many organizations that support prostate cancer screening recommend that annual testing begin at 50 years of age.21 However, because black men and men with a family history of prostate cancer are at higher risk at younger ages, some organizations recommend offering PSA testing at an earlier age for these men.2 Prior studies related to physician practices also indicate that many physicians begin prostate cancer screening at earlier ages,16,22 and this sample allowed for examination of this pattern. We excluded men who self-reported a previous diagnosis of prostate cancer, as they were likely to have had PSA testing as part of their routine care.
The NHIS 2000 sample included 32,374 respondents, and the NHIS 2005 sample included 31,428 respondents. Participants were excluded if they were not male (2000: n=18,388; 2005: n=17,666), if they were <40 years of age (2000: n=5,889; 2005: n=5,272), or if they reported a diagnosis of prostate cancer (2000: n=225; 2005: n=304). Therefore, the analysis sample included a total of 16,058 respondents—7,872 men in 2000 and 8,186 men in 2005—weighted to represent more than 55 million men.
Data collection
During both survey years, respondents were asked if they had ever undergone a PSA test and, if they had, the length of time since their most recent test. They were also asked how many PSA tests they had received in the past five years. From these questions, we created two PSA test use measures indicating (1) recent PSA test for screening purposes (as a routine test or screening test, or because of family history of prostate cancer) and (2) frequent use of the PSA test, defined as having had three or more tests in the previous five years. Recent PSA tests included tests within two years prior to each survey. PSA tests for any purpose were included in the frequent-use measure, as respondents were not asked the reasons for having a PSA test other than for the most recent one.
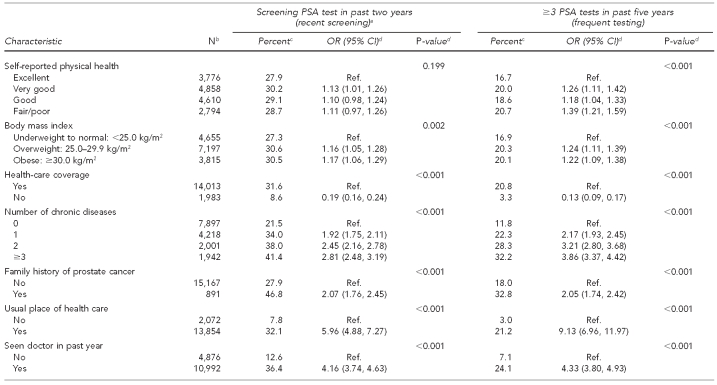
We also used several variables in our analyses that were identified in the literature as possibly being related to PSA test use. They included age of respondent, race/ethnicity, marital status, education and income levels, region of the U.S., self-reported physical health, body mass index (BMI), health-care coverage, number of chronic diseases, family history of prostate cancer, usual place of health care, and having seen a doctor in the past year (Table 1).
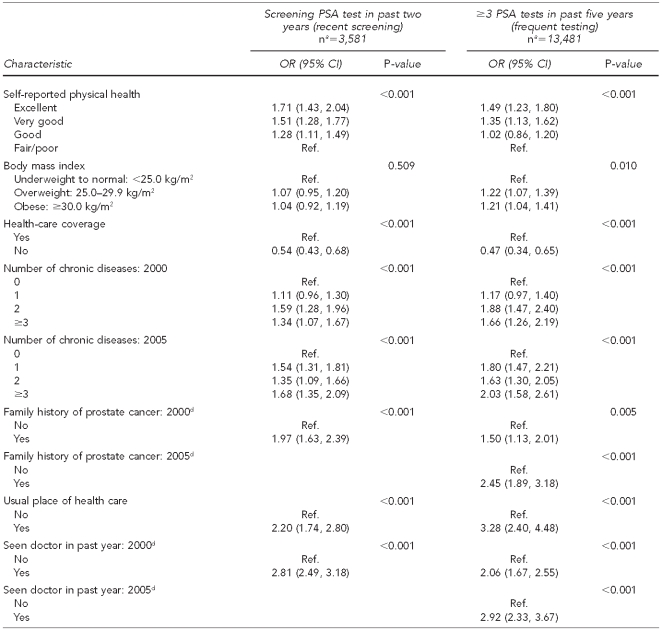
Table 1.
PSA test use among men ≥40 years of age with no prostate cancer (n=16,058), by demographic and health characteristics: NHIS 2000 and 2005
aScreening PSA test indicates that the reason for most recent PSA test within two years was routine screening.
bNumbers that do not add up to total indicate missing values.
cPercentages are weighted to reflect the entire population.
dUnadjusted ORs and p-values obtained from logistic regression using non-missing values. P-values compare percentages within levels of each category.
eIncome level of <$35,000 includes those who reported income as ≥$20,000.
PSA = prostate-specific antigen
NHIS = National Health Interview Survey
OR = odds ratio
CI = confidence interval
Ref. = referent group
kg/m2 = kilograms/meter squared
Data analysis
The NHIS used a stratified, multistage cluster sample.19,20 We analyzed data using the SURVEYFREQ and SURVEYLOGISTIC procedures in SAS® version 9.223 to account for the stratified sampling design. We obtained sample weights from the NHIS public-use data file and divided by two for the combined years of data.19,20 We examined the weighted percentages of men who reported a screening PSA test within the past two years (recent screening) and men who had undergone three or more PSA tests within the past five years (frequent testing) by sociodemographic, health, and health-care characteristics. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess variability in the estimates of the percentages and to make general comparisons within and across groups.
We also examined interactions between each factor and time to determine whether PSA test use varied over time by specific characteristics. Overall statistical significance of the factors in predicting PSA test use was determined using the Wald Chi-square test. We conducted multivariate logistic regression analysis of the combined 2000 and 2005 data to obtain adjusted estimates of odds of each PSA test use measure by year and by sociodemographic and health-related factors. All statistical tests were two-sided with a significance level of α=0.05.
RESULTS
The combined number of men in the sample was 16,058. The large majority of these men were non-Hispanic white, married, had health-care coverage and a usual source of health care, and had seen a doctor within the past year (Table 1). For the recent-screening outcome (having had a screening PSA test in the past two years), we noted statistically significant differences across categories within year, age, race/ethnicity, marital status, education and income levels, geographic region, BMI, number of chronic diseases, family history of cancer, having health-care coverage, having a usual place of health care, and having seen a doctor in the past year.
For the frequent-testing outcome (having had three or more PSA tests in the past five years), differences across categories within variables were similar to the recent-screening outcome, except for self-reported physical health, which was found to be significant for this variable, and income, which was not significant (Table 1).
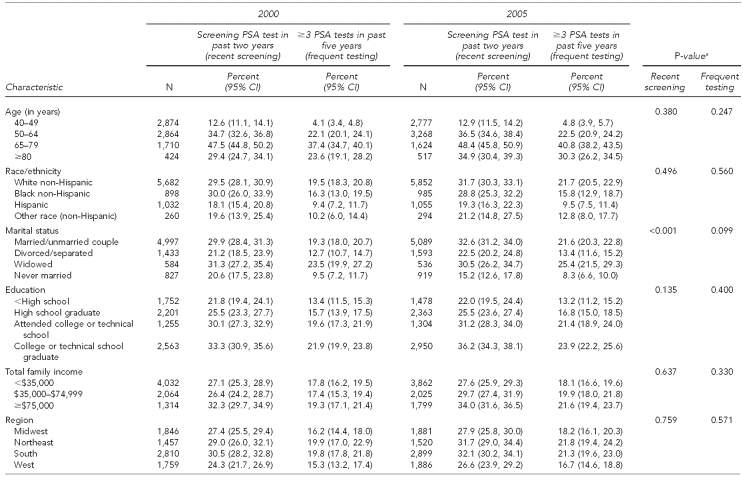
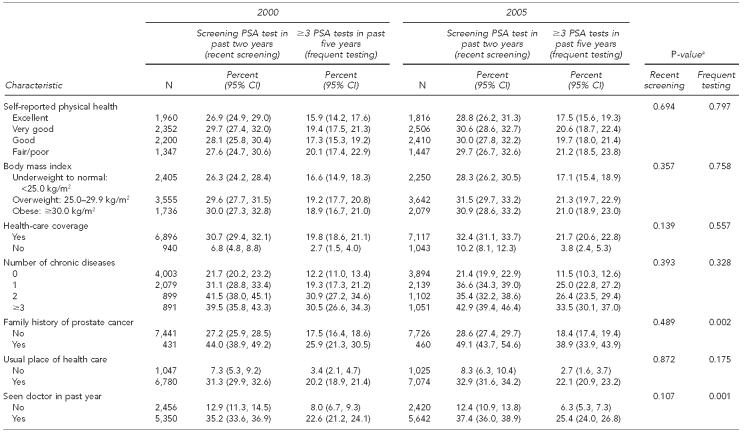
Table 2 examines both outcomes by year to determine trends by specific characteristics. From 2000 to 2005, we noted variations in receipt of a recent screening PSA test for the variable marital status. Recent screening PSA tests declined in the single, never-married category from 20.6% in 2000 to 15.2% in 2005, while use among those married increased from 29.9% to 32.6% in the same periods.
Table 2.
Comparison of 2000 and 2005 trends in PSA test use among men ≥40 years of age: NHIS 2000 and 2005
aP-value compares receipt of PSA test within subgroups in 2000 and 2005 from weighted logistic regression; test of interaction between characteristic and time.
PSA = prostate-specific antigen
NHIS = National Health Interview Survey
CI = confidence interval
kg/m2 = kilograms/meter squared
Similarly, the receipt of three or more PSA tests in the past five years increased among those with a known family history of prostate cancer and those who had seen a doctor in the past year, while remaining relatively stable among those without these characteristics. For this outcome, no other variables were found to differ significantly from 2000 to 2005.
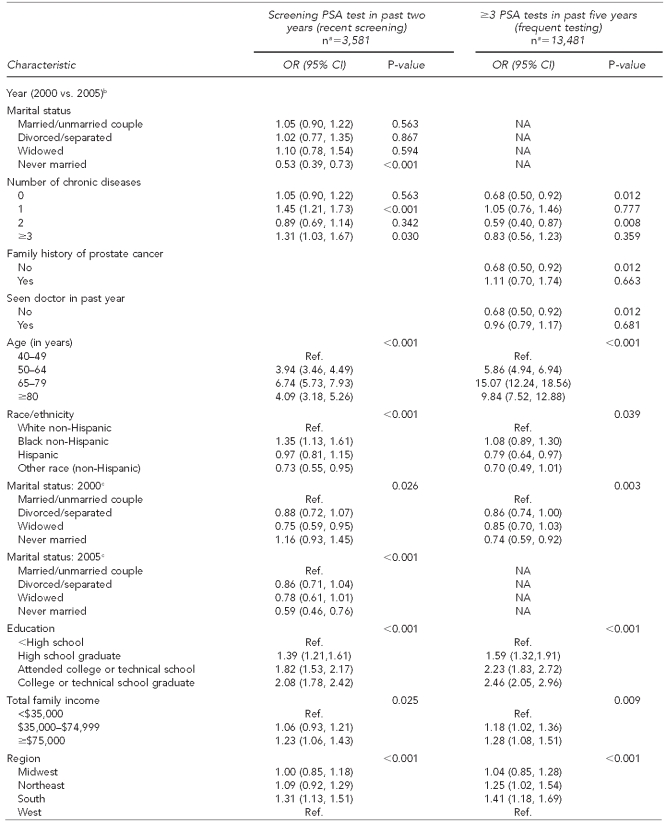
After adjusting for all characteristics in the multivariate model (Table 3), higher odds of having had a recent PSA screening were associated with being 50–64 years of age (OR=3.94; 95% CI 3.46, 4.49), 65–79 years of age (OR=6.74; 95% CI 5.73, 7.93), and ≥80 years of age (OR=4.09; 95% CI 3.18, 5.26); black race/ethnicity (OR=1.35; 95% CI 1.13, 1.61); higher levels of education and income; living in the southern region of the U.S. (OR=1.31; 95% CI 1.13, 1.51); good and better self-reported health status; two or more chronic diseases (2000) and one or more chronic diseases (2005); having a family history of prostate cancer (2000) (OR=1.97; 95% CI 1.63, 2.39); having a usual place of health care (OR=2.20; 95% CI 1.74, 2.80); and having seen a doctor in the past year (OR=2.81; 95% CI 2.48, 3.18). Non-Hispanic men of other race/ethnicity (OR=0.73; 95% CI 0.55, 0.95), widowed men (OR=0.75; 95% CI 0.59, 0.95), never-married men (2005) (OR=0.59; 95% CI 0.46, 0.76), and men without health-care coverage (OR=0.54; 95% CI 0.43, 0.68) had lower odds of having had a recent screening compared with their referents.
Table 3.
Multivariate logistic regression model of PSA test use among men ≥40 years of age with no prostate cancer, NHIS 2000 and 2005
aUnweighted
bNo significant interactions with year for family history of prostate cancer and having seen a doctor in the past year for recent-screening outcome; no significant interactions with year for marital status for frequent-testing outcome
cORs for marital status for receipt of ≥3 PSA tests in the past five years were the same for 2000 and 2005; no significant interaction.
dORs for family history of prostate cancer and seen doctor in the past year for receipt of a screening PSA test in the past two years were the same for 2000 and 2005; no significant interaction.
PSA = prostate-specific antigen
NHIS = National Health Interview Survey
OR = odds ratio
CI = confidence interval
NA = not applicable
kg/m2 = kilograms/meter squared
Ref. = reference group
Results for the frequent-testing outcome (having had three or more PSA tests in the past five years) mirrored those for the recent-screening outcome (having had a screening PSA test in the past two years) with a few exceptions. Frequent PSA test use was lower for Hispanic men (OR=0.79; 95% CI 0.64, 0.97) compared with non-Hispanic white men, and for never-married men (OR=0.74; 95% CI 0.59, 0.92) compared with married men. Frequent PSA test use was higher for both overweight (OR=1.22; 95% CI 1.07, 1.39) and obese (OR=1.21; 95% CI 1.04, 1.41) men compared with underweight men and men with a normal BMI. Men with a family history of prostate cancer had higher odds of frequent PSA testing than men without a family history of prostate cancer in both years; however, the magnitude was greater in 2005 (OR=2.45; 95% CI 1.89, 3.18) (Table 3).
Multivariate logistic regression revealed significant interactions with year (2000 vs. 2005, Table 3) for marital status and for number of chronic diseases when we examined the recent-screening outcome. Similarly, we found significant interactions with year for the frequent-testing outcome for number of chronic diseases, family history, and having seen a doctor in the past year. Therefore, all results for year trends are reported based on these categories.
Single, never-married men had a 47% decrease in odds of a recent screening PSA test from 2000 to 2005, while individuals with one chronic disease had a 45% increase in odds of a recent screening PSA test during the same period. Additionally, men with three or more chronic diseases had a 31% increase in odds of recent screening from 2000 to 2005. Trends were different for frequent PSA testing, which declined among individuals with no chronic disease (32% lower odds) and among individuals with two chronic diseases (41% lower odds) from 2000 to 2005. We also saw declines in frequent PSA testing for individuals with no family history of prostate cancer (32% lower odds) and those who had not seen a doctor in the past year (32% lower odds), while individuals with a family history of prostate cancer and those who had seen a doctor in the past year showed no change during the study period.
DISCUSSION
Many studies have examined PSA test use patterns, but few have examined PSA test use over time.17,24,25 The PSA test (along with the digital rectal examination) is associated with early detection of prostate cancer.2 Some organizations, such as the American Cancer Society, recommend that prostate cancer screening tests—including the PSA test—be offered to men ≥50 years of age, and earlier for men at higher risk, such as black men and men with a positive family history of prostate cancer.2 The U.S. Preventive Services Task Force (USPSTF) concluded that the evidence was insufficient to advocate for or against routine prostate cancer screening using the PSA test and digital rectal examination.26 Most organizations recommend some form of shared decision-making between physician and patient.21
Actual test use may not conform to the USPSTF's recommendation against screening in men ≥75 years of age.26 In the adjusted model, both outcomes (recent screening and frequent PSA test use) increased for all age categories except ≥80 years of age. This pattern is expected, as more opportunities to discuss and undergo a PSA test present themselves with advanced age. For men ≥80 years of age, this pattern also shows lower screening and frequent PSA test use compared with men 65–79 years of age, but higher use than the referent group (i.e., men 40–49 years of age).
Black men were more likely to have had a recent screening PSA test compared with white men, but there was no difference between these racial/ethnic groups for the frequent-testing outcome. In a trend study that examined the period 1995–2004, primary care providers ordered PSA tests for age-appropriate men at an increase of 8% or more per year, with more dramatic increases seen in black men and men with health-care insurance.6 This finding may mean, in part, that physicians are becoming aware of the higher incidence and mortality of prostate cancer among black men compared with white men.
Although no difference was found between white and Hispanic men for the recent-screening outcome, Hispanic men were less likely to have had frequent PSA test use compared with white men. Presently, Hispanic men are at lower risk of both prostate cancer incidence and mortality compared with white men.2
Findings from this study also offered information about marital status and PSA test use. Recent PSA test use declined from 2000 to 2005 for men who were single and never married. This pattern of decline in recent PSA screening among single, never-married men suggests that men in this group should discuss PSA testing with their physicians. The pattern of unmarried men having low PSA test use has also been found in other studies.27,28 It appears that marriage may be associated with higher use of preventive health-care services.29
In our study, both the recent-screening and frequent-testing outcomes were associated with higher levels of education and income. Also, the variables residing in the southern region and having health-care coverage, one or more chronic diseases, good or better health status, a usual place of health care, a known family history of prostate cancer, and seen a doctor in the past year were associated with both higher recent PSA screenings and more frequent test use compared with their referents. In a different national study, older age, higher levels of education, and having health insurance and a usual source of care were positively associated with having a PSA test.17
Overall, the number of chronic diseases played an important role for both recent PSA screening and frequent PSA testing during 2000 and 2005. During the five-year period, the number of chronic diseases was associated with an increase in recent PSA screening; however, it was associated with a decrease in the frequent-testing outcome. For the recent-screening outcome, having one or more chronic diseases may have provided greater opportunity for more interaction with the health-care system and, thus, more opportunities for screening.
Our study also found that men who were overweight (BMI 25.0–29.9 kilograms/meters squared [kg/m2]) or obese (BMI ≥30.0 kg/m2) reported more frequent PSA tests than men with normal BMI (<25.0 kg/m2). Similar positive associations between PSA testing and BMI have been shown in prior studies of black and white men.30,31 High BMI has been associated with higher-grade tumors, poorer outcomes, and higher mortality due to prostate cancer;32 yet, the reason for higher testing or screening use among overweight and obese men has not been clearly established.33 It may be that more frequent testing in these men may be driven by more visits to health-care providers, due to other existing comorbid conditions.
Our finding of a positive association between family history of prostate cancer and PSA testing has also been confirmed in other studies.17,18 However, one recent study examining the probability of ever having a PSA test found an association for white men but not black men; that is, black men with a family history of prostate cancer were not more likely to have ever had a PSA test than black men with no family history of prostate cancer.34 Also, another study found that there was a significant association between both age and family history and recent PSA testing; that is, the association between family history of prostate cancer and recent PSA testing was significant for men ≥50 years of age but not for younger men.35 In our study, more frequent PSA test use decreased during the study period among men with no family history of prostate cancer.
Strengths and limitations
This study had several strengths. Data were from large, nationally representative surveys. These cross-sectional surveys, conducted five years apart, collected information on several aspects of PSA test use, including recent (within the past two years) PSA test use for screening purposes, as well as the number of PSA tests taken in the past five years. These categories allowed examination of more than one form of utilization. The NHIS 2000 and 2005 data collections oversampled both black and Hispanic populations to produce more precise estimates of use among these understudied populations. We were also able to examine interactions between year and factors that may help to clarify the use of PSA tests.
The study also had several limitations. The main limitation was the reliance on self-report of PSA test use. Studies have shown less reliability of self-report when compared with medical records.36,37 Despite the lower reliability, self-reports are useful and show great potential in conducting health research.38 There also may have been an underestimation of how much time had passed since a respondent's last PSA test.39 Also, the NHIS questions asked the reason for the most recent PSA test only, so we do not know for sure if the previous tests were used for PSA screening or for some other purpose.
Additionally, we were able to offer little information on the other race (non-Hispanic) group, which included Asian Americans, American Indians/Alaska Natives, Hawaiian/Pacific Islanders, and other groups, due to small sample sizes. Findings reflected a five-year pattern only, and these results may be subject to change when additional years of data are examined. Finally, the survey response rates declined slightly from 2000 (72.1%) to 2005 (69.0%). Sampling weights reflective of the national population were used during both years of data collection; therefore, this slight decline in response rates should have had no effect on our results.
CONCLUSIONS
Findings from this study could have important implications for medical and public health professionals. Using nationally representative data, the study adds some clarity to PSA test use among men of black, white, and Hispanic race/ethnicity (the latter of which has been understudied in the prostate cancer literature) and other factors, including having seen a doctor in the past year and having a family history of prostate cancer. The fact that there has been variability in factors (increases in some and decreases in others) associated with recent screening PSA test use and frequent PSA test use alerts us that PSA testing is highly complex and invokes us to examine this variation in different ways for better understanding. Results from ongoing randomized, controlled trials may help to clarify the importance of the increase or decrease in PSA test use.
Despite inconclusive evidence that PSA test use is beneficial, physicians and patients may be recognizing that (1) frequent visits, especially preventive health visits, are important, and (2) men with a known family history of prostate cancer are at increased risk for the disease. Both factors provide opportunity for discussions about prostate cancer and decisions about screening.
Footnotes
This research was supported by the Department of Defense, U.S. Army Medical Research and Materiel Command, contract #W81XWH-07-1-0350 and grant #PC060224.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Department of Defense, U.S. Army Medical Research and Materiel Command.
REFERENCES
- 1.U.S. Cancer Statistics Working Group. United States cancer statistics: 2002 incidence and mortality. Atlanta: Department of Health and Human Services (US), Centers for Disease Control and Prevention, and National Cancer Institute; 2005. [Google Scholar]
- 2.American Cancer Society. Cancer facts and figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 3.Bellizzi KM, Latini DM, Cowan JE, Duchane J, Carroll PR. Fear of recurrence, symptom burden, and health-related quality of life in men with prostate cancer. Urology. 2008;72:1269–73. doi: 10.1016/j.urology.2007.12.084. [DOI] [PubMed] [Google Scholar]
- 4.Dandapani SV, Sanda MG. Measuring health-related quality of life consequences from primary treatment for early-stage prostate cancer. Semin Radiat Oncol. 2008;18:67–72. doi: 10.1016/j.semradonc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amling CL. Prostate-specific antigen and detection of prostate cancer: what have we learned and what should we recommend for screening? Curr Treat Options Oncol. 2006;7:337–45. doi: 10.1007/s11864-006-0001-1. [DOI] [PubMed] [Google Scholar]
- 6.Farwell WR, Linder JA, Jha AK. Trends in prostate-specific antigen testing from 1995 through 2004. Arch Intern Med. 2007;167:2497–502. doi: 10.1001/archinte.167.22.2497. [DOI] [PubMed] [Google Scholar]
- 7.Freedland SJ, Terris MK, Presti JC, Jr, Amling CL, Kane CJ, Trock B, et al. Obesity and biochemical outcome following radical prostatectomy for organ confined disease with negative surgical margins. J Urol. 2004;172:520–4. doi: 10.1097/01.ju.0000135302.58378.ae. [DOI] [PubMed] [Google Scholar]
- 8.Crawford ED, Abrahamsson PA. PSA-based screening for prostate cancer: how does it compare with other cancer screening tests? Eur Urol. 2008;54:262–73. doi: 10.1016/j.eururo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8:268–78. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 10.Aus G, Bergdahl S, Lodding P, Lilja H, Hugosson J. Prostate cancer screening decreases the absolute risk of being diagnosed with advanced prostate cancer—results from a prospective, population-based randomized controlled trial. Eur Urol. 2007;51:659–64. doi: 10.1016/j.eururo.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Etzioni R, Penson DF, Legler JM, Tommaso DD, Boer R, Gann PH, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–90. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 12.Pelzer AE, Horninger W. How should PSA screening efforts be focused to prevent underdiagnosis and overdiagnosis of prostate cancer? Nat Clin Pract Urol. 2008;5:172–3. doi: 10.1038/ncpuro1051. [DOI] [PubMed] [Google Scholar]
- 13.Gwede CK, McDermott RJ. Prostate cancer screening decision making under controversy: implications for health promotion practice. Health Promot Pract. 2006;7:134–46. doi: 10.1177/1524839904263682. [DOI] [PubMed] [Google Scholar]
- 14.Gilligan T, Wang PS, Levin R, Kantoff PW, Avorn J. Racial differences in screening for prostate cancer in the elderly. Arch Intern Med. 2004;164:1858–64. doi: 10.1001/archinte.164.17.1858. [DOI] [PubMed] [Google Scholar]
- 15.Ross LE, Berkowitz Z, Ekwueme DU. Use of the prostate-specific antigen test among U.S. men: findings from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:636–44. doi: 10.1158/1055-9965.EPI-07-2709. [DOI] [PubMed] [Google Scholar]
- 16.Purvis Cooper C, Merritt TL, Ross LE, John LV, Jorgensen CM. To screen or not to screen, when clinical guidelines disagree: primary care physicians' use of the PSA test. Prev Med. 2004;38:182–91. doi: 10.1016/j.ypmed.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Finney Rutten LJ, Meissner HI, Breen N, Vernon SW, Rimer BK. Factors associated with men's use of prostate-specific antigen screening: evidence from Health Information National Trends Survey. Prev Med. 2005;40:461–8. doi: 10.1016/j.ypmed.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Ross LE, Coates RJ, Breen N, Uhler RJ, Potosky AL, Blackman D. Prostate-specific antigen test use reported in the 2000 National Health Interview Survey. Prev Med. 2004;38:732–44. doi: 10.1016/j.ypmed.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, National Center for Health Statistics (US) 2000 National Health Interview Survey (NHIS) public-use data release: NHIS survey description. Hyattsville (MD): NCHS; 2002. [cited 2009 Sep 23]. Also available from: URL: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2000/srvydesc.pdf. [Google Scholar]
- 20.Centers for Disease Control and Prevention, National Center for Health Statistics (US) 2005 National Health Interview Survey (NHIS) public-use data release: NHIS survey description. Hyattsville (MD): NCHS; 2006. [cited 2009 Sep 23]. Also available from: URL: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2005/srvydesc.pdf. [Google Scholar]
- 21.National Guideline Clearinghouse. Guideline synthesis: screening for prostate cancer. [cited 2010 Jun 3]. Available from: URL: http://www.guideline.gov/syntheses/synthesis.aspx?id=16398.
- 22.Stroud L, Ross LE, Rose SW. Formative evaluation of the prostate cancer screening practices of African-American physicians. J Natl Med Assoc. 2006;98:1637–43. [PMC free article] [PubMed] [Google Scholar]
- 23.SAS Institute, Inc. SAS®: Version 9.2. Cary (NC): SAS Institute, Inc.; 2008. [Google Scholar]
- 24.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–40. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 25.Nelson DE, Kreps GL, Hesse BW, Croyle RT, Willis G, Arora NK, et al. The Health Information National Trends Survey (HINTS): development, design, and dissemination. J Health Commun. 2004;9:443–60. doi: 10.1080/10810730490504233. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Preventive Services Task Force. Screening for prostate cancer: U.S Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185–91. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 27.Ross LE, Uhler RJ, Williams KN. Awareness and use of the prostate-specific antigen test among African-American men. J Natl Med Assoc. 2005;97:963–71. [PMC free article] [PubMed] [Google Scholar]
- 28.Haque R, Van Den Eeden SK, Jacobsen SJ, Caan B, Avila CC, Slezak J, et al. Correlates of prostate-specific antigen testing in a large multiethnic cohort. Am J Manag Care. 2009;15:793–9. [PubMed] [Google Scholar]
- 29.Walter FM, Emery J. Perceptions of family history across common diseases: a qualitative study in primary care. Fam Pract. 2006;23:472–80. doi: 10.1093/fampra/cml006. [DOI] [PubMed] [Google Scholar]
- 30.Fowke JH, Signorello LB, Chang SS, Matthews CE, Buchowski MS, Cookson MS, et al. Effects of obesity and height on prostate-specific antigen (PSA) and percentage of free PSA levels among African-American and Caucasian men. Cancer. 2006;107:2361–7. doi: 10.1002/cncr.22249. [DOI] [PubMed] [Google Scholar]
- 31.Fontaine KR, Heo M, Allison DB. Obesity and prostate cancer screening in the USA. Public Health. 2005;119:694–8. doi: 10.1016/j.puhe.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Fesinmeyer MD, Gulati R, Zeliadt S, Weiss N, Kristal AR, Etzioni R. Effect of population trends in body mass index on prostate cancer incidence and mortality in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:808–15. doi: 10.1158/1055-9965.EPI-08-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9:1039–47. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drake BF, Lathan CS, Okechukwu CA, Bennett GG. Racial differences in prostate cancer screening by family history. Ann Epidemiol. 2008;18:579–83. doi: 10.1016/j.annepidem.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross LE, Berkowitz Z, Ekwueme DU. Use of the prostate-specific antigen test among U.S. men: findings from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:636–44. doi: 10.1158/1055-9965.EPI-07-2709. [DOI] [PubMed] [Google Scholar]
- 36.Jordan TR, Price JH, King KA, Masyk T, Bedell AW. The validity of male patients' self-reports regarding prostate cancer screening. Prev Med. 1999;28:297–303. doi: 10.1006/pmed.1998.0430. [DOI] [PubMed] [Google Scholar]
- 37.Volk RJ, Cass AR. The accuracy of primary care patients' self-reports of prostate-specific antigen testing. Am J Prev Med. 2002;22:56–8. doi: 10.1016/s0749-3797(01)00397-x. [DOI] [PubMed] [Google Scholar]
- 38.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4:2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 39.Gordon NP, Hiatt RA, Lampert DI. Concordance of self-reported data and medical record audit for six cancer screening procedures. J Natl Cancer Inst. 1993;85:566–70. doi: 10.1093/jnci/85.7.566. [DOI] [PubMed] [Google Scholar]