Abstract
Background
A subset of atopic dermatitis (AD) subjectsare susceptible to serious infections with herpes simplex virus, called eczema herpeticum or vaccina virus, called eczema vaccinatum.
Objective
This National Institute of Allergy and Infectious Disease-funded, multicenter study was performed to establish a database of clinical information and biological samples on subjects with AD with and without a history of eczema herpeticum (ADEH+ and ADEH-, respectively) and healthy controls (CTL). Carefully phenotyping of AD subsets may suggest mechanisms responsible for disseminated viral infections and help identify at-risk individuals.
Methods
We analyzed the data from 901 subjects (ADEH+ n=134, ADEH- n=419, CTL n=348) enrolled between 5.11.2006 and 9.16.2008 at seven US medical centers.
Results
ADEH+ subjects had more severe disease based on scoring systems (Eczema Area and Severity Index and Rajka-Langeland), body surface area affected and biomarkers (circulating eosinophil counts, serum IgE, TARC and CTACK) than ADEH- subjects (p<0.001). ADEH+ subjects were also more likely to have a history of food allergy (69 vs 40%; p<0.001) or asthma (64 vs 44%; p<0.001) and were more commonly sensitized to many common allergens (p<0.001). Cutaneous infections with S. aureus or molluscum contagiosum virus were more common in ADEH+ (78% and 8%, respectively) than in ADEH-subjects (29% and 2%; p<0.001).
Conclusion
AD subjects who develop ADEH have more severe, Th2-polarized disease with greater allergen sensitization and more commonly have food allergy and/or asthma. They are also much more likely to experience cutaneous infections with S. aureus or molluscum contagiosum.
Keywords: Atopic dermatitis, herpes simplex virus, eczema herpeticum, eczema vaccinatum, biomarkers, staphylococcus aureus
Introduction
The overall objective of the National Institute of Allergy and Infectious Diseases (NIAID)-funded Atopic Dermatitis and Vaccinia Immunization Network (ADVN) is to investigate the mechanism(s) responsible for the susceptibility of atopic dermatitis (AD) subjects to viral infections. The most severe example is eczema vaccinatum (EV), which occurs following exposure to the smallpox vaccine.2 Fortunately, cases of EV have occurred only rarely since the risk of vaccinating high-risk subjects was appreciated. However, 7 to 10% of AD subjects have difficulty containing other cutaneous viral infections caused by herpes simplex virus (HSV) and molluscum contagiosum virus (MCV).1 The most commonly recognized viral complication in AD subjects is eczema herpeticum (EH) caused by an extensive cutaneous infection with HSV. EH can be complicated by keratoconjunctivitis and viremia, and sometimes leads to multiple organ involvement with meningitis and encephalitis.3 The central hypothesis of the ADVN Registry study is that AD subjects who have had EH (ADEH+) have a unique phenotype that can be recognized by a careful history and physical exam and/or by serum biomarkers. This information may also be useful to identify AD subjects who are at risk for EV, the more life-threatening viral complication that would be highly relevant if variola was weaponized and obligatory smallpox vaccination strategies had to be employed.2 This is the first study to characterize the phenotype and biomarkers of two ethnically diverse American ADEH+ populations and is the most comprehensive study performed to date based on both the number of subjects recruited, serum/plasma collected and detailed disease characterization (including the completion of a 29 page case report form).
Most cases of EH are caused by HSV-1. Because HSV seropositivity is high in the general population (∼20% of children and ∼60% of the adult population) it is unlikely that EH episodes are simply a function of viral exposure.4 An analysis of AD cases with a history of eczema herpeticum (ADEH+) examined at a single German University between 1959 to 1986 demonstrates a significant increase in the incidence of this complication, from a rate of 0.6 cases/year to greater than 15 cases/year.5 This increase is not likely explained by an increased prevalence of AD, since this would predict a mere doubling or tripling of the cases. Rather, it suggests that AD has evolved into a disease with greater susceptibility to infection than was observed previously, which may be the result of changes in the environment, susceptibility genes or treatment approaches. Consequently, there is growing concern that smallpox vaccination would pose a greater problem than would be explained by the increasing AD prevalence data alone.
The increased susceptibility of AD subjects to EH may be determined by multiple factors including a Th2 predominance and relative Th1 deficiency. Collectively this leads to the diminished production of antimicrobial peptides (AMP) and reduced skin barrier proteins, which is more pronounced in AD subjects with severe, allergen-driven (or extrinsic) disease. To more definitively characterize the epidemiological, clinical and laboratory characteristics of African American (AA) and European American (EA) AD subjects with a history of EH, we established a Registry of ADEH+, ADEH- and healthy controls (CTL) and are reporting our findings from 901 subjects that have been recruited at seven academic centers within the United States.
Methods
The study was approved by the institutional review boards at seven US Academic centers. Information about the ADVN structure, statistical and data coordinating center (SDCC), and a more detailed study outline can be found in the Online Repository.
Standard Diagnostic Criteria and Study Procedures
Standard diagnostic criteria were developed for this Registry study where all subjects were between 1 to 80 years of age (see Table E1 in the Online Repository). AD was diagnosed by standard criteria with the additional requirement for subjects less than 4 years of age that the disease needed to be present for at least six months prior to study enrollment to minimize the likelihood of recruiting children with other eczematous disorders that commonly mimic AD.7 ADEH+ was defined as AD subjects with at least one EH episode that had a diameter ≥ 5 cm documented either 1) by a physician at an ADVN study site or 2) by an outside provider and HSV infection was confirmed by either polymerase chain reaction (PCR), Tzanck smear, immunofluorescence and/or culture. ADEH- was defined as AD subjects with no history of EH as obtained from patient and/or caregiver. AD subjects whose EH history was equivocal were not enrolled. Healthy CTL subjects were defined as having no personal or family history of atopic diseases and no personal history of chronic skin or systemic diseases.
All study participants underwent a detailed history, physical examination, disease severity assessments and blood draw. Disease severity was assessed by the Rajka-Langeland and the Eczema Area and Severity Index (EASI) scoring systems. EASI is a standardized grading system (range of score, 0-72) that assesses erythema, excoriation, lichenification, infiltration and/or papulation.8 The Rajka-Langeland score (RLS) rates extent, course, and itch intensity separately and yields a score from 0-9).9 The RLS system provides a broad and somewhat historical view of a subject's AD severity, whereas the EASI provides a more sensitive measure of disease severity at the time of enrollment. Blood samples were sent to Quest Diagnostics Laboratory for a complete blood count (CBC) with differential and to the Dermatology, Allergy and Clinical Immunology Laboratory (DACI) at JHAAC for a serum total IgE, multiallergen and individual using the UniCap 250 system (Pharmacia and Upjohn). All remaining serum and plasma samples were catologued and stored at URMC at -80°C.
Biomarker Analysis
The DACI laboratory performed the following tests on serum samples from all ADEH+ and ADEH- subjects: total IgE (kU/L) and allergen-specific Phadia ImmunoCAP® including food (FX5E), mite-roach (HX2), animal dander (EX2), weed (WX1), grass (GX2), tree (TX3), tree (RTX10), mold (MX2), and specific Phadia ImmunoCAP® including staph enterotoxin A (SEA; AM80), staph enterotoxin B (SEB; BM81) and staph toxic shock syndrome toxin-1 (TSST-1; RM226). CTL subjects had total IgE levels and a multiallergen RAST called a Phadiatop™ performed. Total and allergen-specific IgE levels were determined from serum samples using the UniCap 250 system (Pharmacia and Upjohn, Kalamazoo, MI); samples were measured in duplicate. The total eosinophil count (cells/mm3) was calculated from the CBC with differential.
Serum concentrations of cutaneous T cell-attracting chemokine (CTACK/CCL27), thymus and activation-regulated chemokine (TARC/CCL17), IP-10 (CXCL10) and IFNβ were measured in a subset of ADEH+ and ADEH-subjects who were age- and gender-matched (see below). Each sample was run by enzyme-linked immunosorbant assay (ELISA) (R & D Systems) in duplicate and the minimum detectable concentration for these cytokines was 1.6, 7.0, 1.7 and 12.5 pg/ml, respectively.
HSV-1 and -2 Serology
HSV-1 IgG and HSV-2 IgG antibody testing was performed on serum samples (Quest Diagnostics Laboratory). The reference ranges for the tests are as follows: <0.90 = Negative, 0.90 – 1.10 = Equivocal and >1.10 = Positive.
Statistical Analysis
All analyses utilized the full sample of ADVN Registry subjects, for the indicated diagnostic groups, who completed the ADVN Registry protocol by 9.16.08, unless otherwise specified. Comparisons between ADEH+ and ADEH-groups for categorical endpoints were assessed using Fisher's Exact Test. These included categorical demographic variables (e.g., gender), categorical IgE antibody results (classified based on values above or below 0.35 kUA/L, the lower limit of detection), self-reported history of asthma or food allergy, and categorical body surface area affected by eczema (more or less than 35%). History of S. aureus infection was collected as “Any previous infection (Y/N)?” combined with the text entered into the follow up question indicating specific infections. Similarly, comparisons of categorical endpoints across ADEH+, ADEH- and CTL groups were made using pairwise Fisher's Exact Tests, including self-reported history of human papilloma virus (HPV), molluscum contagiosum skin infections, HSV eye and skin infections, and history of S. aureus infection. Comparisons across the ADEH+, ADEH- and CTL groups for continuous endpoints were made with the full sample using two-sample t-tests. These endpoints included allergen-specific IgE values > 0.35 kUA/L, total IgE and eosinophil count and disease severity measures. Additionally, correlations of the EASI score with total IgE and eosinophil count were calculated via Pearson's correlation coefficients and presented in scatterplots. Log10 transformations of continuous endpoints were applied when necessary.
To adjust for the effects of age and gender on comparisons between ADEH+ and ADEH- subjects a matched sample was generated by selecting ADEH- subjects to gender- and age (within 5 years) match a subset of ADEH+ subjects. Relationships between ADEH+ and ADEH- for continuous endpoints were then assessed using paired t-tests, and binary endpoints were tested using McNemar's tests. The correlations between EASI score and CTACK, TARC and IP-10 were calculated using Pearson's correlation coefficients and presented in scatterplots. Correlations between Rajka-Langeland scores and the biomarkers listed above were also computed.
All p-values reported were considered descriptive. No adjustments for multiple comparisons were made. SAS® version 9.1 was used for all analyses.
Results
Demographics
A total of 901 subjects were enrolled in the three diagnostic groups, ADEH+, ADEH- and CTL (See Table E2 in the Online Repository). Both AD subgroups (ADEH+ and ADEH-) were younger than the CTL group (p<0.001) and the ADEH+ group was younger than the ADEH- group (p<0.001). There was a greater percentage of females in the ADEH- (68%; p<0.001) compared to ADEH+ (50%) and CTL (54%) groups.
Nearly 50% of ADEH+ subjects had more than one episode of EH and 4.5% reported greater than five episodes. Ten percent of ADEH+ subjects reported that a first-degree family member also had EH, compared to 1% of ADEH- and 0% of LCTL. The vast majority (94%) of ADEH+ subjects developed AD before five years of age in contrast to only 59% of ADEH- subjects (p<0.001). More ADEH+ subjects (58%) said “Yes” in response to the question, “Do you have keratosis pilaris, hyperlinear palms or ichthyosis?” compared to the ADEH-group (42%, p=0.005). Both groups (ADEH+ and ADEH-) reported a similar frequency (4 to 5%) of alopecia areata.
EH and Disease Severity
Disease severity was significantly greater in ADEH+ compared to ADEH-subjects using several objective measures of AD severity. Both the EASI and Rajka-Langeland scores were higher in ADEH+ subjects, even after adjusting for age (p<0.001; Figure 1A,B). Greater severity among the ADEH+ group was also reflected in serum IgE and circulating eosinophil counts (cells/mm3) compared to both ADEH- and CTL and this difference was also unaffected by age adjustment (p<0.001; Figure 1C,D). ADEH+ had greater surface area of involvement with 32% having ≥ 35% BSA compared to only 9% of ADEH-subjects (p<0.001). Not surprisingly, serum IgE and eosinophil counts from both ADEH+ and ADEH- subjects correlated with EASI scores (r=0.54 and r=0.48 respectively, p<0.001; Figure 2A,B) and Rajka-Langeland score (r=0.49 and r=0.41 respectively, p<0.001; data not shown).
Figure 1.
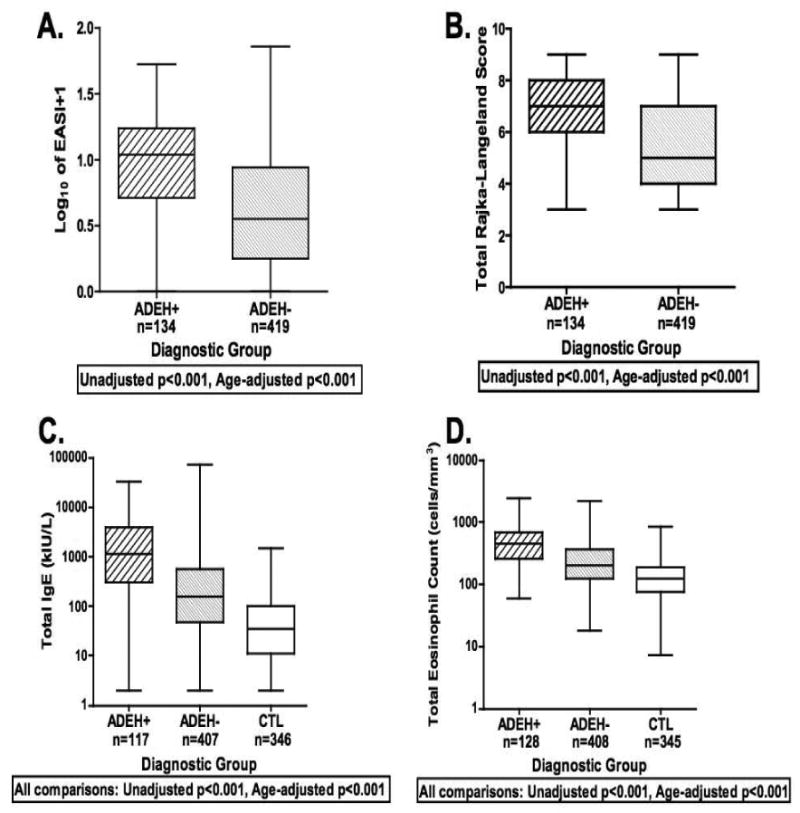
Boxplots of EASI (A) and Rajka-Langeland (B) severity scores and serum total IgE (C) and total eosinophil counts (D). The statistics are reported for all datapoints (as shown in these graphs) as well as age-adjusted cohorts.
Figure 2.
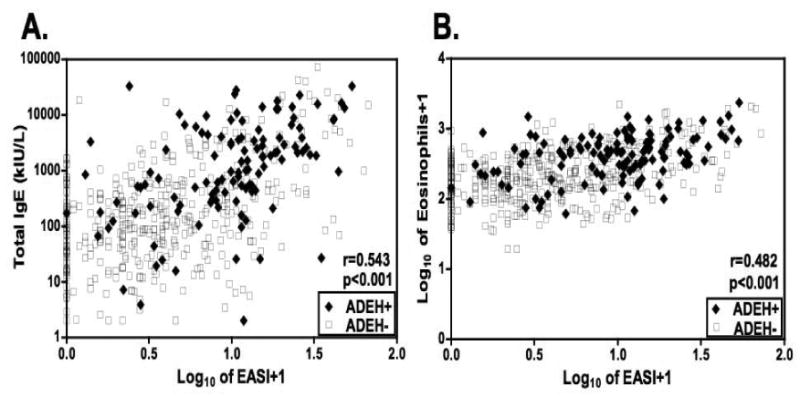
Correlations between the log10-transformed EASI scores and (A) serum IgE and (B) log10-transformed total eosinophil counts in AD subjects. [(A) n=117 for ADEH+ (filled diamond) and n=407 for ADEH- (open square); (B) n=128 for ADEH+ (filled diamond) and n=408 for ADEH- (open square)].
EH and History of Atopic Disorders
Significantly more ADEH+ subjects (69%) reported a history of food allergy than ADEH- subjects (40%, p<0.001; Figure 3A). Remarkably similar findings were observed for asthma with 64% of ADEH+ subjects reporting a positive history compared to 44% of ADEH- subjects (p<0.001; Figure 3B).
Figure 3.
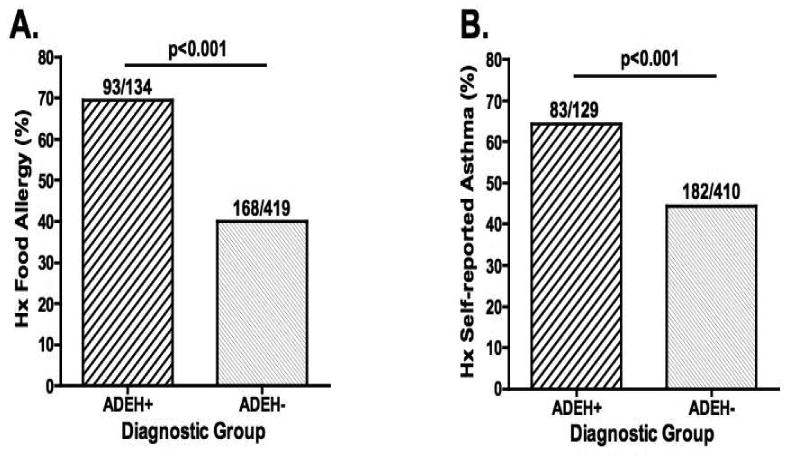
Percentage of AD subjects or caregivers who self-report a history of or current food allergy or asthma.
EH and Allergen Sensitization
The fact that total serum IgE values were significantly higher in the ADEH+ compared to ADEH- group (Figure 1C) suggested that there might be differences in allergen-specific sensitization. To address this we measured the following on all AD subjects: multiallergen ImmunoCAP® (Food/FX5E, mite/roach mix/HX2, animal dander/EX2, weed/WX1, grass/GX), tree/TX3, tree/RTX10, mold/MX2), and specific ImmunoCAP® for staph enterotoxin A (SEA; AM80), staph enterotoxin B (SEB; BM81) and staph toxic shock syndrome toxin-1 (TSST-1; RM226). The log10-transformed ImmunoCAP® values that were greater than -0.4559 (log10 of 0.35 kUA/L) are shown as a Gaussian distribution for all of the ImmunoCAP® results that were significantly different between AD subgroups (Figure 4). The animal dander mix ImmunoCAP®, which measures reactivity to cat dander and epithelium, dog dander, guinea pig, rat and mouse epithelium, was significantly greater in ADEH+ (Log mean ± SD; 1.58 ± 0.88 kUA/L) than ADEH- subjects (0.96 ± 0.91 kUA/L; p<0.001) (Figure 4A). The food ImmunoCAP® measures the reactivity to six food allergens including egg-white, milk, fish, wheat, peanut and soybean and was significantly greater in ADEH+ (1.13 ± 1.04 kUA/L) than ADEH- subjects (0.68 ± 0.98 kUA/L; p<0.001) (Figure 4B). The mite-cockroach ImmunoCAP®, which measures reactivity to house dust [Hollister Stier], Dermatophagoides pteronyssinus, Dermatophagoides farinae and Blatella germanica was significantly greater in ADEH+ (1.33 ± 0.90 kUA/L) than ADEH- subjects (1.02 ± 0.92 kUA/L; p=0.006) (Figure 4C). The grass ImmunoCAP® measures reactivity to Bermuda, rye, timothy, Kentucky blue, Johnson grass and Bahia and was significantly greater in ADEH+ (1.13 ± 0.80 kUA/L) than ADEH- subjects (0.91 ± 0.79 kUA/L; p=0.021) (Figure 4D). The weed mix ImmunoCAP® measures reactivity to common ragweed, mugwort, English plantain, lamb's quarters and Russian thistle and was significantly greater in ADEH+ (0.71 ± 0.70 kUA/L) than ADEH- subjects (0.52 ± 0.64 kUA/Lp=0.029) (Figure 4E). The mold mix ImmunoCAP® measures reactivity to Penicillium notatum, Cladosporium herbarum (Hormodendrum), Aspergillus fumigatus, Candida albicans, Alternaria alternata/tenuis, and Helminthosporium halodes and was significantly greater in ADEH+ (0.68 ± 0.60 kUA/L) than ADEH- subjects (0.51 ± 0.56 kUA/L; p=0.047) (Figure 4F). Using this analytical approach there were no differences between ADEH+ and ADEH- subjects for the two tree ImmunoCAPs® (TX3 and RTX10) or the S. aureus toxin (SEA, SEB and TSST-1)-specific ImmunoCAPs® (data not shown).
Figure 4.
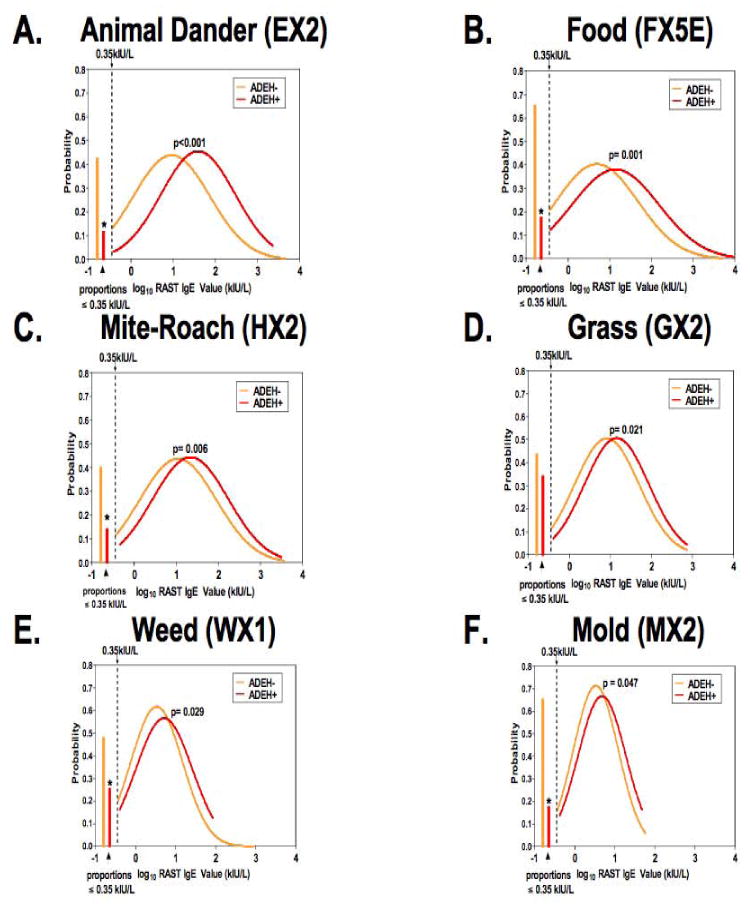
Six allergen-specific ImmunoCAPs® performed on AD subjects were depicted as a Gaussian distribution with ADEH+ (red) curves shifted to the right compared to ADEH- (orange) subjects are shown in A-E. On the far left of each graph is the proportion of ADEH- (orange) and ADEH+ (red) with values ≤ 0.35 kUA/L. When ImmunoCAP® results [(including SEA, SEB and TSST-1 (not shown)] were treated as a binary trait, a greater proportion of ADEH+ had positive results than ADEH- subjects with the exception of Grass (D). *p-value<0.001 for ADEH+ vs ADEH- on binary outcome (≤0.35, >0.35 kUA/L).
We alsoperformed descriptive analyses of each of the ImmunoCAP® measurements as a binary trait with values reported as the proportion ≤ 0.35 kUa/L (or negative) shown in the left aspect of each graph (Figure 4). The percentage of ADEH+ subjects with a negative ImmunoCAP® was significantly less than ADEH- subjects for all ImmunoCAP® tests performed except Grass (GX2; Figure 4D). Although not shown, when using this statistical approach the S. aureus-specific ImmunoCAPs® [SEA (AM80), SEB (BM81) and TSST-1 (RM226)] were positive in a greater proportion of ADEH+ compared to ADEH-subjects (p<0.001).
Serum IgE and Phadiatop™ results on CTL population
As shown in Figure 1C, CTL had a mean total IgE of 36.4 ± 1.2 kU/Lwhich was significantly (p<0.001) lower than both ADEH+ (1041.5 ± 83.6 kU/L) and ADEH- (175.3 ± 7.6 kU/L) populations with and without age-adjustment. The Phadiatop™ was the only RAST assay performed on the CTL group and measures 15 common allergens covering weeds, grasses, trees, epidermals, mites and molds with results reported in kUA/L. The Phadiatop™ was positive (>0.35 kUA/L) in 165/346 (48%) of CTL subjects with a mean (± SD) value in those with positive results of 10.6 ± 17.6 kUA/L.
EH and History of Cutaneous Infections
ADEH+ subjects more frequently reported a history of cutaneous infections with S. aureus (78%) and molluscum contagiosum (8%) than either ADEH- (29% and 2%, respectively) or CTL (1% and 0%, respectively) populations (Figure 5B,D). Human papilloma virus (HPV) infections were more frequent in both AD subgroups compared to CTL but there was no difference between ADEH+ and ADEH- subjects (Figure 5C). Approximately one year after initiating the Registry study we added a question to the CRF to evaluate subjects history of HSV ocular infections. We found that significantly more ADEH+ subjects (16%; p<0.001) reported a history of ocular infection(s) compared to ADEH- (1%) and CTL (0%; Figure 5A). We reviewed subjects' dental histories focusing on gingivitis, periodontal disease, extractions, root canals and number of cavities and found no significant difference among our three groups (ADEH+, ADEH- and CTL) based on any of these parameters of oral health and hygiene.
Figure 5.
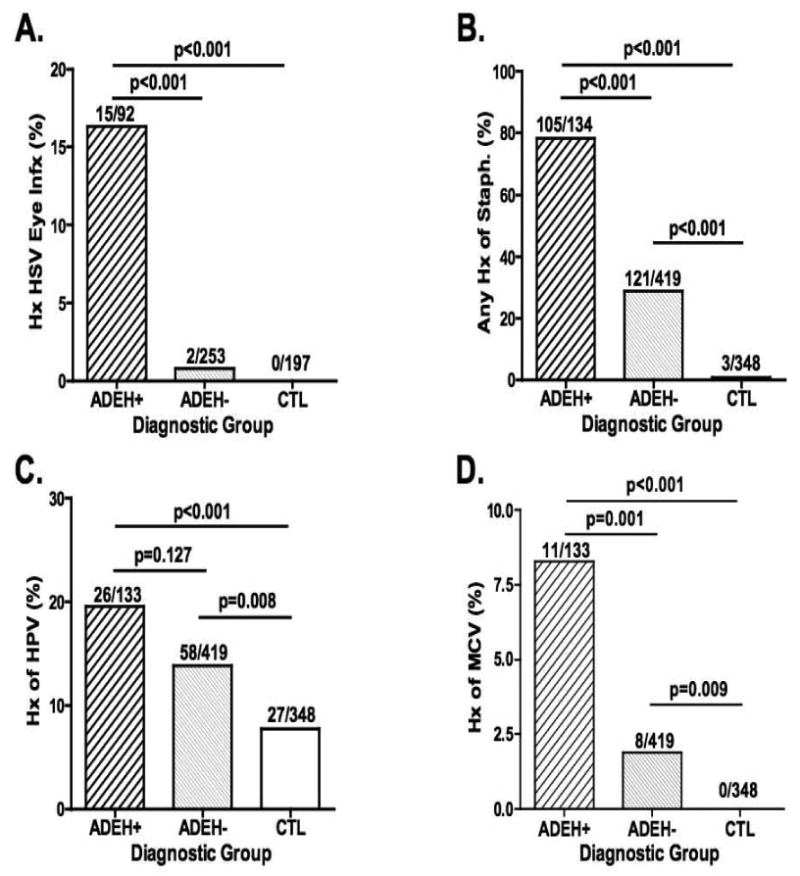
Percentage of subjects (ADEH+, ADEH- and CTL) who self-report a history of ocular infections with herpes simplex virus (A), S. aureus skin infections (B), Human papilloma virus skin infections (C) and molluscum contagiosum virus infections (D).
EH and HSV serology
A higher proportion of the ADEH+ group had seropositive results for HSV-1 (92.9%) than either ADEH- (52.1%, p < 0.001) or CTL (54.2%, p < 0.001). HSV-1 positivity was slightly higher for ADEH+ subjects with > 1 episode (95.5%) when compared to subjects with 1 episode (81.8%) but this did not reach statistical significance (p= 0.178). The ADEH+ group had lower proportion of HSV-2 seropositive subjects (8.8%) than either ADEH- (36.3%, p < 0.001) or CTL (31.3%, p < 0.001), which likely reflects the differences in mean age of these groups. For overall HSV status, the ADEH+ group had higher proportion of seropositive results (94.7%) than either ADEH- (65.9%, p < 0.001) or CTL (66.4%, p < 0.001)(See Table E3 in the Online Repository). The ADEH- and CTL groups were not statistically different from each other. Six ADEH+ subjects were not seropositive for either HSV-1 or -2.
HSV-1 and -2 status was also treated as a binary trait and compared in ADEH+ and ADEH- groups using 51 age- and gender-matched pairs and McNemar's test (see Table E4 in the Online Repository). There was significant discordance (p<0.0001) between ADEH+ and ADEH- members of the pairs with respect to HSV status (including HSV-1, HSV-2 and both HSV-1 and -2).
EH and Biomarkers
Little is known about the effect of age and gender on serum levels of CTACK (CCL27) and TARC (CCL17). Therefore we evaluated only age-matched (within 5 years) and gender-matched samples from the AD subgroups. We found that serum levels of CTACK (CCL27) were significantly increased in the ADEH+ compared to ADEH- subjects (mean ± SD; 1233.0 ± 2298.9 vs 595.2 ± 310.5 pg/ml, respectively; p=0.019) (Figure 6A). Similarly, serum TARC (CCL17) levels were elevated in ADEH+ subjects (3211.5 ± 5741.2 vs 805.5 ± 806.4 pg/ml, respectively; p=0.019) (Figure 6B). CTACK and TARC values correlated with measures of AD severity including EASI (p<0.001; Figure 6C,D) and Rajka-Langeland scores (data not shown). We noted no differences in serum levels of IP-10 (CXCL10; n=13 per group) and IFNβ (n=46 per group; see Figure E1 in the Online Repository). Only serum IP-10 levels weakly correlated with AD severity as assessed by either EASI (r=0.22, p=0.04) or Rajka-Langeland (r=0.24, p=0.02) (see Figure E1 in the Online Repository).
Figure 6.
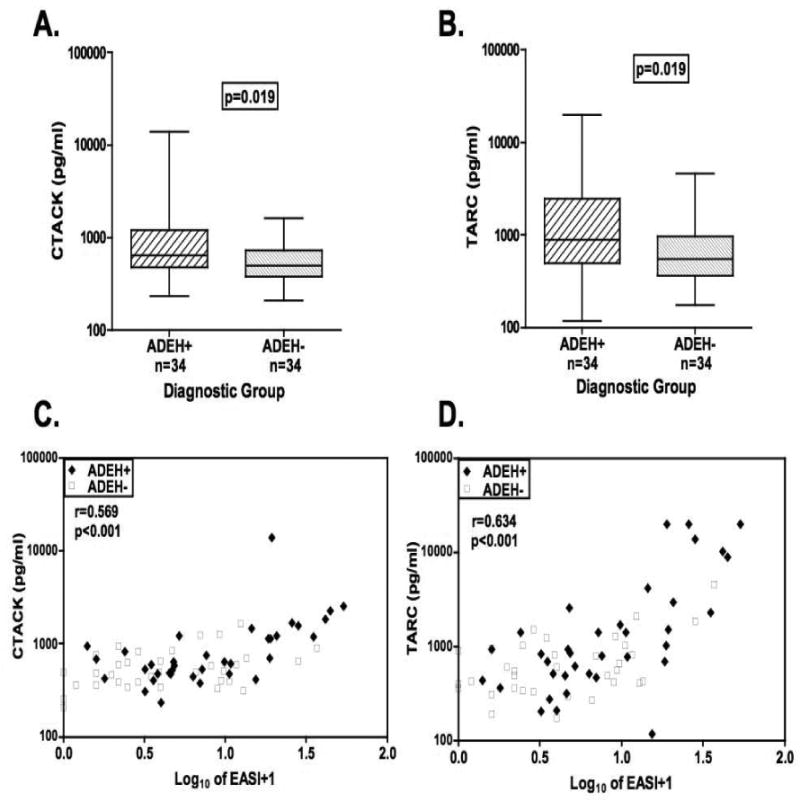
Boxplots of serum CTACK (CCL27; A) and TARC (CCL17; B) levels in age- and gender-matched ADEH+ and ADEH- cohorts. Correlations between the log10-transformed EASI scores and the (C) serum levels of CTACK and (D) TARC in AD subjects. [n=34 for ADEH+ (filled diamond) and n=34 for ADEH-(open square)].
Discussion
This is the largest study to date and the only study conducted in the US to comprehensively characterize AD subjects who develop EH (ADEH+). Ours is the first to report that ADEH+ subjects have an enhanced susceptiblity for developing infections with microbes that commonly affect the skin and eye. Not surprisingly, almost half of the ADEH+ subjects had a specific IgE to one or more of the S. aureus toxins (SEA, SEB or TSST-1) compared to one-fifth of the ADEH- group. This was consistent with our observation that ADEH+ patients had a higher prevalence of Staphylococcus aureus skin infections than the ADEH- subjects. In general, ADEH+ subjects were poly-sensitized and mounted greater IgE responses per allergen than ADEH- subjects, which were also reflected in their total IgE levels and the fact that they commonly suffered from other atopic diseases. Based on a current hypothesis that argues that allergen sensitization in AD subjects occurs primarily through the skin and is enhanced by epidermal barrier defects our findings strongly implicate epidermal barrier and innate immune defects as risk factors for EH.10,11 Our study also found that ADEH+ subjects have more severe disease, characterized by earlier age of onset. We have strengthened the evidence that EH subjects have more Th2-polarized disease (or less Th1 cytokines) by demonstrating their serum levels of the Th2 chemokine, TARC/CCL17 are higher and their peripheral eosinophilia is greater. The greater Th2 polarity noted in ADEH+ subjects was also reflected in their greater allergen sensitization.
The demographics of the subgroups (ADEH+, ADEH- and CTL) revealed significant differences in age and gender (see Table E2 in the Online Repository). Therefore, where appropriate, we adjusted for age and gender in our analysis (e.g. EASI, RL, total IgE, total eosinophil count, serum biomarkers). All diagnostic groups had the same age restrictions (1 to 80 yrs), although the ADEH+ subjects were significantly younger than the ADEH- and CTL (p<0.001; see Table E2 in the Online Repository), is likely the consequence of two factors. The first being that EH episodes typically occur early in life and therefore it was easier to find the necessary documentation of EH if the subject had experienced this complication more recently (see Table E1 in the Online Repository). The second factor was that the ADVN Genetics study that followed the Registy study restricted the age of ADEH- and CTL groups to 18 to 80 yrs to provide greater assurance that these populations had been exposed to HSV and minimizing the possibility that the difference between ADEH+ and ADEH-subjects simply reflected viral exposure. Most ADVN sites tried to enroll subjects in both Registry and Genetic studies and that would result in older subjects in ADEH- and CTL groups (see Table E2 in the Online Repository). We believe that having recruited older ADEH- subjects was a strength in that their likelihood of being misclassified would be diminished because most episodes of EH occur within the first three decades of life.5 There were no restrictions on gender and the ADEH+ group was equally represented by males and females. This result is consistent with previous reports showing no gender bias in EH.3 It is unclear why ADEH- subjects were more commonly female (p<0.001; see Table E2 in the Online Repository). The differences in ethnicity and race observed are likely due to the restrictions placed on the ADEH- and CTL groups that were enacted approximately one year after commencement of Registry recruitment to ensure that these two groups would also qualify for the ADVN Genetics study. For both studies, the ADEH- and CTL subjects had to self-report as non-Hispanic and either AA or EA to meet enrollment criteria. These restrictions were put in place because the ADVN Genetics study focused its initial analysis on these ethnic and racial groups to allow for smaller sample sizes while maintaining power to detect differences between ADEH+ and ADEH- populations (manuscript submitted).
Our results show that EH recurs in about half of subjects, which is more than was noted in previous publications which reported recurrence in 13 to 16% of cases.3,5 The mean age of our ADEH+ subjects is comparable to previous publications and therefore this is unlikely to explain this difference. About 95% of ADEH+ subjects had a positive serology for HSV-1 or -2 or both (see Table E3 in the Online Repository). The majority (91%) of ADEH+ subjects were positive for HSV-1 with only 9% positive for HSV-2, confirming that most EH episodes are caused by HSV-1. This degree of HSV-1 seroprevalence is markedly higher than the most recent US National Health and Nutrition Examination Surveys (NHANES) data where the seroprevalence in the 20 to 30 years age group is only 52%.4 This also suggests that EH is not likely due to a diminished immunoglobulin response to HSV and is consistent with a prospective study demonstrating similar T cell and immunoglobulin responses to a diptheria-tetanus-toxoid immunization in atopic compared to noatopic subjects,=6 In contrast, only about half of ADEH- and CTL subjects were HSV-1 positive, although 31 to 36% were HSV-2 positive, which likely reflects their older age (36.0 and 38.4 yrs, respectively). Only six ADEH+ subjects had a negative serology to both HSV-1 and -2. Whether this indicates that these subjects have been missclassified as ADEH+ or the fact that some subjects were enrolled during their first EH episode and therefore their IgG response had not yet developed is not known.
One of the more remarkable findings was that ADEH+ subjects also suffered from other cutaneous infections, such as those caused by S. aureus (78% vs. 29%, ADEH+ vs. ADEH-; p<0.001), molluscum contagiosum (8% vs. 2%, p=0.001), and history of HSV infection of the eye (16% vs. 0.8%, p<0.001)(Figure 5). However, they did not have a greater incidence of dental infections (gingivitis or periodontitis) or skin infections with human papilloma virus. The frequency of S. aureus infections in ADEH+ subjects is much higher than the 30% reported in AD and observed in our ADEH- subgroup.12 This work suggests that some global defect in cutaneous immune responses to microbes may be present in subjects with a history of EH that is relevant for both viral and bacterial infections of the skin and possibly the eyes. Interestingly, this susceptibility to S. aureus infections was also reflected in the frequency of positive Immunocap® tests to specific staphylococcal toxins ([SEA: 43% vs. 19%, ADEH+ vs. ADEH-; p=0.001], [SEB: 43% vs. 20%, -; p=0.001], [TSST-1: 44% vs. 21%, p=0.001]).
Another signature of the ADEH+ subgroup was the breadth and magnitude of their allergen responsiveness. AD subjects underwent eight Immunocap® teststo animal dander, food, mite-cockroach, grass, weed, mold, tree in addition to the S. aureus toxins. When Immunocap® results were evaluated as Gaussian curves, ADEH+ had greater reactivity to six out of the eight allergen-specific Immunocaps® compared to ADEH- subjects (Figure 4). When Immunocap® results were analyzed as binary traits (positive vs negative), all allergens were more frequently positive in ADEH+ subjects except grass (Figure 4). The most significant differences were observed for food and perennial allergens (animal dander and mite-cockroach) suggesting that these allergens would be most predictive of ADEH+ subjects. Importantly, the greater reactivity to food allergens observed in ADEH+ subjects corroborates self-reported histories of food allergy, which were higher in this group (Figure 3). Although this is the most extensive assessment of allergen sensitization in ADEH+ subjects, a smaller study by Peng et al., showed similar findings for five allergen-specific RASTs.13
The greater allergen sensitization observed in ADEH+ subjects likely reflects greater Th2 polarity. To address this we evaluated several Th2 biomarkers including total IgE, eosinophil count and TARC/CCL17 (Figure 1 and 6).14, 15 TARC is a Th2-chemokine which binds to CCR4 which is highly expressed on skin-homing lymphocytes. AD subjects express high levels of TARC in lesional skin and serum levels may reach the ng/ml range as was the case in our subjects.16, 17 CTACK/CCL27 plays a role in the homeostatic migration of memory T cells to the skin. But CTACK is not selective for a T cell subset as serum levels are elevated in both AD and psoriasis, although CTACK levels have only been shown to correlate with disease severity in AD as was the case in our subjects (Figure 6).18, 19 All three Th2 biomarkers were elevated in ADEH+ compared to ADEH- subjects (p≤0.02), firmly establishing the importance of Th2 cytokines as a risk factor for widespread HSV infections in AD subjects. Wollenberg et al, demonstrated that high IgE levels were a risk factor for EH among 45 ADEH+ cases.3 Furthermore, the strong correlation between total IgE, eosinophilia and TARC with disease severity suggests that the degree of Th2 polarization is an important predictor of AD disease activity.
We found a history of food allergy and asthma was more frequently elicited from ADEH+ (69.4% and 64.3%, respectively) than ADEH- subjects (40.1% and 44.4%, respectively; p<0.001)(Figure 3). Wollenberg et al., noted a similar trend with greater reports of asthma and hay fever in EH subjects, but these were not statistically different from their control AD population.3 The food allergy prevalence of our AD subjects (40 to 69%) was higher than previous reports that estimate IgE-mediated food allergy prevalence in children with moderate-to-severe AD to be about 30%.20 It is important to note that historical accounts of food allergy significantly overestimate the true prevalence sometimes by as much as 2 to 3-fold.22 Nevertheless, we had about 75% concordance with history of food allergy and food FX5E Immunocap® result (as a binary trait). Asthma prevalence in this ADVN group are also higher than the general U.S. population, where US prevalence estimates from 1995 were 5.7% with slightly greater values for children than adults, and higher among African Americans compared to European Americans. Asthma prevalence in children with AD is estimated to be about 25 to 30% which is less than what we observed in the ADVN AD subgroups.21 This difference may reflect the inaccuracies of self-reporting or may suggest that the AD subjects recruited out of tertiary referral centers may in fact have more severe disease that is more frequently complicated by reactive airways. In summary, ADEH+ are more likely to have other atopic diseases than ADEH- subjects. Additionally, both AD groups report rates of food allergy and asthma that were greater than would have been predicted from published studies. This latter point may reflect a recall bias by subjects and their caregivers. We speculate that this may reflect greater disease severity of subjects recruited from tertiary referral centers for AD. Future studies proposed as part of ADVN will need to validate these historical findings.
To evaluate whether EH susceptibility could be related to a relative reduction in Th1-associated cytokines we measured IFNβ and the interferon-induced chemokine, IP-10 in age and gender-matched serum samples (see Figure E1 in the Online Repository). IFNβ values were highly variable but no difference was observed between the AD subgroups. Similarly, there were no differences in IP-10 between the AD subgroups. Peng et al. found that IFNβ was reduced in ADEH+ compared to ADEH- subjects using a similar sample size.13 They did not find any differences in serum IFNα or IFNγ between AD subgroups. These findings would suggest that the T cell defect in ADEH+ subjects is primarily the enhanced expression of Th2 cytokines and not diminished Th1 cytokines. Th2 cytokines are thought to be permissive to microbial invasion on the basis of their inhibitory actions on antimicrobial proteins, epidermal barrier proteins and cell-mediated immunity.23-31
Our study confirmed and extended the finding that EH develops in AD subjects with greater disease severity. We found that the vast majority (94%) of ADEH+ subjects developed AD before 5 yrs of age. Multiple markers of AD severity including biomarkers (total IgE, peripheral eosinophil counts, TARC and CTACK), two well-accepted clinical scoring systems (EASI and RL) and BSA affected were all significantly greater in ADEH+ subjects. Most of the biomarkers are thought to dynamically reflect disease severity with the exception of total IgE which because of its long T1/2 is more reflective of chronic changes in disease severity. Importantly, these observations were evident even after controlling for age and gender (total IgE, eosinophil counts, TARC and CTACK). Two previous publications have noted the association with early age of onset and IgE levels.3,13 Peng et al. demonstrated that AD subjects with a history of EH had a slightly increased severity score using the SCORAD assessment (p<0.05).13 In our study we found that a number of the biomarkers such as TARC, CTACK, total IgE, eosinophil count and IP-10 correlated significantly with EASI and are listed in order of the strength of this correlation (Figure 2, 6 and Supplemental Figure 1).
Landmark studies have demonstrated that AD subjects, particularly those with more severe disease, may have a loss of function mutations in the filaggrin gene (FLG) as has been observed in ichthyosis vulgaris (IV).32 For this reason we asked subjects or their caregivers if they had a history of any of the features found in subjects with both IV and AD (e.g. keratosis pilaris, hyperlinear palms or ichthyosis). More ADEH+ (58%) reported having one or more of these features than ADEH- subjects (42%; p<0.005). Recent studies suggest that IV, diagnosed by ichthyotic changes on the anterior tibial region, can be observed in up to 32% of AD subjects.33 Although keratosis pilaris and hyperlinear palms are less specific for IV, they are more commonly observed in AD/IV subjects (53 and 81%, respectively) than AD subjects without IV (28 and 43%; p<0.001).33
Finally, we measured serum total IgE and a multi-allergen ImmunoCAP® assay called Phadiatop™ on our CTL group, to provide some measure of the allergen sensitization and Th2 polarity of this group that had no personal or family history of atopic disorders (see Table E1 in the Online Repository). Although total IgE levels were within age-specific normal values and substantially lower than the values seen in both AD subgroups (p<0.001), 48% of CTL subjects had a positive Phadiatop™. We did not perform a Phadiatop™ on AD subjects so we cannot make direct comparisons with other Registry groups. This percentage was higher than that reported in a large Italian and Swiss population where the prevalence of positive Phadiatop™ ranged from 24 to 29%, respectively.34,35 Nevertheless, NHANES III demonstrated that more than 50% of the population has a positive skin test response to at least one allergen.36 Our findings agree with previous literature suggesting that total serum IgE values are a more sensitive screening assay for atopic diseases in adults than Phadiatop™.37
In conclusion, we have found that AD subjects who are susceptible to EH are characterized by more severe disease, early age of onset, more frequent history of other atopic disorders, greater Th2 polarity, allergen sensitization to many common allergens and more frequent skin infections with other microbes. Collectively, this provides a reasonable snapshot of the at-risk AD subject and may help identify individuals who are at greatest risk for more life-threatening infections with vaccinia (EV) or variola (smallpox). One of the most profound findings is the remarkably high rate of skin infections with S. aureus reported by ADEH+ subjects. Further work is warranted to identify additional biomarkers that can be assessed rapidly and will be both sensitive and specific for ADEH+ subjects.
Supplementary Material
Table 1. ADVN Registry Study Demographics.
| Endpoint | Stat | ADEH+ | ADEH- | CTL | Comparison | P-value |
|---|---|---|---|---|---|---|
| Age (yrs) | N | 134 | 419 | 348 | Overall Test | <0.001 |
| Mean (SD) | 21.54 (20.4) | 32.84 (15.3) | 38.34 (12.4) | ADEH+ vs. ADEH- | <0.001 | |
| Median | 11.9 | 31.1 | 37 | ADEH+ vs. CTL | <0.001 | |
| Min, Max | (1, 80.7) | (1.2, 73.6) | (12.4, 78.1) | ADEH- vs. CTL | <0.001 | |
| Female | N (%) | 67 (50%) | 286 (68.3%) | 189 (54.3%) | Overall Test | <0.001 |
| Male | N (%) | 67 (50%) | 133 (31.7%) | 159 (45.7%) | ADEH+ vs.ADEH- | <0.001 |
| ADEH+ vs. CTL | 0.416 | |||||
| ADEH- vs. CTL | <0.001 | |||||
| Hispanic or Latino | N (%) | 7 (5.2%) | 4 (1.0%) | 4 (1.2%) | Overall Test | 0.002 |
| Not Hispanic or Latino | N (%) | 127 (94.8%) | 415 (99.0%) | 344 (98.8%) | ADEH+ vs. ADEH- | 0.006 |
| ADEH+ vs. CTL | 0.013 | |||||
| ADEH- vs. CTL | 0.999 | |||||
| African American | N (%) | 21 (15.7%) | 192 (45.8%) | 161 (46.3%) | Overall Test | <0.001 |
| European American | N (%) | 99 (73.9%) | 217 (51.8%) | 186 (53.4%) | ADEH+ vs. ADEH- | <0.001 |
| Other | N (%) | 14 (10.4%) | 10 (2.4%) | 1 (0.3%) | ADEH+ vs. CTL | <0.001 |
| ADEH- vs. CTL | 0.051 | |||||
Acknowledgments
We would like to acknowledge several groups whose steadfast efforts made this study possible:
ADVN Coordinators (Patricia Taylor NP, Trista Berry BS, Susan Tofte FNP, Shahana Baig-Lewis MPH, Peter Brown BS, Lisa Heughan BA, CCRC, Meggie Nguyen BS, Doru Alexandrescu MD, Lorianne Stubbs RC, Deborra James RN, CCRC, Reena Vaid MD, Diana Lee MD), ADVN regulatory advisors (Judy Lairsmith RN and Lisa Leventhal, MSS, CIM, CIP), biological sample tracking (JHU - Tracey Hand MSc, Jessica Scarpola and Muralidhar Bopparaju MSc, and URMC - Mary Bolognino MS, Paul Spear BS and Lisa Latchney MS), NIAID-DAIT support (Marshall Plaut MD and Joy Laurienzo Panza RN, BSN), DACI Laboratory (Robert Hamilton, PhD), Rho,⎝ Inc. (Brian Armstrong MPH) and last but by no means least all the patients who participated in this study.
Funding: The Atopic Dermatitis and Vaccinia Network NIH/NIAID contract N01 AI40029 and NO1 AI40033.
Abbreviations
- AD
atopic dermatitis
- ADVN
Atopic Dermatitis Vaccinia Network
- ADEH+
atopic dermatitis with a history of eczema herpeticum
- ADEH-
atopic dermatitis without a history of eczema herpeticum
- AMP
antimicrobial peptide
- ASC
animal study consortium
- BSA
body surface area
- CBC
complete blood count
- CRF
case report form
- CSC
clinical study consortium
- CTACK (CCL27)
cutaneous T-cell attracting chemokine
- CTL
healthy controls
- DACI
Dermatology, Allergy and Clinical Immunology Laboratory
- DAIT
Division of Allergy, Immunology and Transplantation at NIAID branch
- EASI
Eczema Area and Severity Index
- EDC
electronic data capture
- EH
eczema herpeticum
- ELISA
enzyme-linked immunosorbant assay
- EV
eczema vaccinatum
- FLG
filaggrin gene
- HBD
human β-defensin
- HPV
human papilloma virus
- HSV
herpes simplex virus
- IFN
interferon
- IgE
immunoglobulin E
- IL
interleukin
- IRB
institutional review board
- I-TAC (CXCL11)
interferon-inducible T cell alpha chemoattractant
- IP-10 (CXCL11)
interferon-inducible protein
- JHAAC
Johns Hopkins Asthma and Allergy Center
- JHU
Johns Hopkins University
- LF
lactoferrin
- MCV
molluscum contagiosum virus
- MIG (CXCL9)
Monokine induced by IFN-gamma
- MIP-3α (CCL20)
macrophage inflammatory protein-3alpha
- MRSA
methicillin-resistant Staphylococcus aureus
- MTA
Material Transfer Agreement
- NA
nonatopic
- NIAID
National Institute of Allergy and Infectious Diseases
- NIH
National Institutes of Health
- PBMC
peripheral blood mononuclear cell
- PCR
polymerase chain reaction
- PI
principal investigator
- RAST
radioallergosorbent test
- RLS
Rajka-Langeland Score
- SDCC
statistical and data coordinating center
- SEA
Staphylococcus aureus enterotoxin A
- SEB
Staphylococcus aureus enterotoxin B
- SCORAD
severity scoring of atopic dermatitis
- SSL
secure sockets link
- TARC (CCL17)
thymus- and activation-regulated chemokine
- Th
T helper cell
- TSST-1
toxic shock syndrome toxin-1
Footnotes
Conflicts of Interest: None Declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wetzel S, Wollenberg A. Eczema herpeticatum. Hautarzt. 2004;55:646–52. doi: 10.1007/s00105-004-0744-1. [DOI] [PubMed] [Google Scholar]
- 2.Vora S, Damon I, Fulginiti V, Weber SG, Kahana M, Stein SL, Gerber SI, Garcia-Houchins S, Lederman E, Hruby D, Collins L, Scott D, Thompson K, Barson JM, Regnery R, Hughes C, Daum RS, Li Y, Zhao H, Smith S, Braden Z, Karem K, Olson V, Davidson W, Trinidade G, Bolken T, Jordan R, Tien D, Marcinak J. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555–61. doi: 10.1086/587668. [DOI] [PubMed] [Google Scholar]
- 3.Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49:198–205. doi: 10.1067/s0190-9622(03)00896-x. [DOI] [PubMed] [Google Scholar]
- 4.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 5.Bork K, Brauninger W. Increasing incidence of eczema herpeticum: analysis of seventy-five cases. J Am Acad Dermatol. 1988;19:1024–9. doi: 10.1016/s0190-9622(88)70267-4. [DOI] [PubMed] [Google Scholar]
- 6.Holt PG, Rudin A, Macaubas C, Holt BJ, Rowe J, Loh R, Sly PD. Development of immunological memory against tetanus toxoid and pertactin antigens from the diptheria-tetanus-pertussis vaccine in atopic versus nonatopic children. J Allergy Clin Immunol. 2000;105:1117–22. doi: 10.1067/mai.2000.105804. [DOI] [PubMed] [Google Scholar]
- 7.Eichenfield LF, Hanifin JM, Luger TA, Stevens SR, Pride HB. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003;49:1088–95. doi: 10.1016/s0190-9622(03)02539-8. [DOI] [PubMed] [Google Scholar]
- 8.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11–8. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 9.Rajka G, Langeland T. Grading of the severity of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1989;144:13–4. doi: 10.2340/000155551441314. [DOI] [PubMed] [Google Scholar]
- 10.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122:261–6. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122:689–93. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Christophers E, Henseler T. Contrasting disease patterns in psoriasis and atopic dermatitis. Arch Dermatol Res. 1987;279(Suppl):S48–51. doi: 10.1007/BF00585919. [DOI] [PubMed] [Google Scholar]
- 13.Peng WM, Jenneck C, Bussmann C, Bogdanow M, Hart J, Leung DY, et al. Risk factors of atopic dermatitis patients for eczema herpeticum. J Invest Dermatol. 2007;127:1261–3. doi: 10.1038/sj.jid.5700657. [DOI] [PubMed] [Google Scholar]
- 14.Vestergaard C, Bang K, Gesser B, Yoneyama H, Matsushima K, Larsen CG. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J Invest Dermatol. 2000;115:640–6. doi: 10.1046/j.1523-1747.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- 15.Kimata H. Selective enhancement of production of IgE, IgG4, and Th2-cell cytokine during the rebound phenomenon in atopic dermatitis and prevention by suplatast tosilate. Ann Allergy Asthma Immunol. 1999;82:293–5. doi: 10.1016/S1081-1206(10)62611-7. [DOI] [PubMed] [Google Scholar]
- 16.Uchida T, Suto H, Ra C, Ogawa H, Kobata T, Okumura K. Preferential expression of T(h)2-type chemokine and its receptor in atopic dermatitis. Int Immunol. 2002;14:1431–8. doi: 10.1093/intimm/dxf109. [DOI] [PubMed] [Google Scholar]
- 17.Kakinuma T, Nakamura K, Wakugawa M, Mitsui H, Tada Y, Saeki H, et al. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001;107:535–41. doi: 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- 18.Kakinuma T, Saeki H, Tsunemi Y, Fujita H, Asano N, Mitsui H, et al. Increased serum cutaneous T cell-attracting chemokine (CCL27) levels in patients with atopic dermatitis and psoriasis vulgaris. J Allergy Clin Immunol. 2003;111:592–7. doi: 10.1067/mai.2003.114. [DOI] [PubMed] [Google Scholar]
- 19.Hijnen D, De Bruin-Weller M, Oosting B, Lebre C, De Jong E, Bruijnzeel-Koomen C, et al. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell- attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J Allergy Clin Immunol. 2004;113:334–40. doi: 10.1016/j.jaci.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8. doi: 10.1542/peds.101.3.e8. [DOI] [PubMed] [Google Scholar]
- 21.Kapoor R, Menon C, Hoffstad O, Bilker W, Leclerc P, Margolis DJ. The Prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68–73. doi: 10.1016/j.jaad.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 22.Sampson HA. The evaluation and management of food allergy in atopic dermatitis. Clin Dermatol. 2003;21(3):183–92. doi: 10.1016/s0738-081x(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 23.Gordon YJ, Huang LC, Romanowski EG, Yates KA, Proske RJ, McDermott AM. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res. 2005;30:385–94. doi: 10.1080/02713680590934111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006;117:836–41. doi: 10.1016/j.jaci.2005.12.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–8. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 27.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–5. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim BE, Leung DY, Streib JE, Boguniewicz M, Hamid QA, Howell MD. Macrophage inflammatory protein 3alpha deficiency in atopic dermatitis skin and role in innate immune response to vaccinia virus. J Allergy Clin Immunol. 2007;119:457–63. doi: 10.1016/j.jaci.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, et al. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008;128:2248–58. doi: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- 30.Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332–7. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–6. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 33.Bremmer SF, Hanifin JM, Simpson EL. Clinical detection of ichthyosis vulgaris in an atopic dermatitis clinic: implications for allergic respiratory disease and prognosis. J Am Acad Dermatol. 2008;59:72–8. doi: 10.1016/j.jaad.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Tschopp JM, Sistek D, Schindler C, Leuenberger P, Perruchoud AP, Wuthrich B, et al. Current allergic asthma and rhinitis: diagnostic efficiency of three commonly used atopic markers (IgE, skin prick tests, and Phadiatop). Results from 8329 randomized adults from the SAPALDIA Study. Swiss Study on Air Pollution and Lung Diseases in Adults. Allergy. 1998;53:608–13. doi: 10.1111/j.1398-9995.1998.tb03937.x. [DOI] [PubMed] [Google Scholar]
- 35.Matricardi PM, Nisini R, Pizzolo JG, D'Amelio R. The use of Phadiatop in mass-screening programmes of inhalant allergies: advantages and limitations. Clin Exp Allergy. 1990;20:151–5. doi: 10.1111/j.1365-2222.1990.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 36.Arbes Samuel J, Jr, Gergen Peter J, Elliott Leslie, Zeldin Darryl C. Prevalences of positive skin test responses to 10 common allergens in the US population: Results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–83. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Wuthrich B, Schindler C, Medici TC, Zellweger JP, Leuenberger P. IgE levels, atopy markers and hay fever in relation to age, gender and smoking status in a normal adult Swiss population. SAPALDIA (Swiss Study on Air Pollution and Lung Diseases in Adults) Team. Int Arch Allergy Immunol. 1996;111:396–402. doi: 10.1159/000237398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


