Abstract
Background
Estimates of the decline in CD4 cell counts in untreated HIV-infected patients are important for patient care and public health. We analyzed declines in the Cape Town AIDS Cohort and the Swiss HIV Cohort Study.
Methods
We used mixed-effects models and joint models that allow for the correlation between CD4 declines and survival, stratifying analyses by the initial count (50-199, 200-349, 350-499, 500-750 cells/μL). Results are presented as the average decline in CD4 count with 95% confidence intervals (CIs) during the first year after the initial CD4 count.
Results
784 South African (629 Non-white) and 2030 Swiss (218 Non-white) HIV-1 infected patients contributed 13,388 CD4 cell counts. Declines in CD4 cell counts were steeper in white patients, patients with higher initial CD4 counts, and older patients. Declines ranged from 38 (95% CI 24 to 54) cells/μL in non-white SHCS patients aged 15-39 years with an initial CD4 count of 200-349 cells/μL to 210 (95% CI 143 to 268) cells/μL in white CTAC patients aged 40 years or over with an initial CD4 count of 500-750 cells/μL.
Conclusions
Both in Switzerland and South Africa CD4 declines of white HIV-1 infected patients were steeper than in non-white patients.
Keywords: CD4 cell count, HIV-1, ethnicity, South Africa, Switzerland
Introduction
An understanding of the factors that influence CD4 T-cell counts (CD4 counts) and their decline in untreated HIV-infected persons is of importance for clinical management of HIV disease, for example to inform guidelines on when to initiate antiretroviral therapy (ART). Such information is also important in the context of public health: the distribution of CD4 cell count declines is required to model time to AIDS and ART eligibility and to project the course of the epidemic and the need for treatment at the population level.
Whilst the decline in CD4 counts has been extensively studied in cohorts from North America [1] and Europe [2;3] there is little data from patients with estimated dates of seroconversion in sub-Saharan Africa [4], where ART has become more widely available in recent years [5]. Data from the Cape Town AIDS Cohort, a cohort of seroprevalent patients from Cape Town, South Africa, suggested that mean CD4 declines ranged from 21 to 47 cells/mL per year depending on initial CD4 strata [6]. Reported declines from industrialized countries are steeper (1-3) but results may not be directly comparable since the statistical methodology used to model the trajectories varied. Also, declines may have been underestimated in the Cape Town cohort because patients with steeper trajectories are more likely to die and have fewer CD4 counts than those with less steep declines [7;8].
We analyzed CD4 cell count declines in the Cape Town AIDS cohort and the Swiss HIV Cohort Study and assessed the effects of ethnicity, sex, age and cohort on the rate of CD4 cell count decline, taking into account the correlation between survival and CD4 decline.
Methods
Cohorts and eligibility criteria
The Cape Town AIDS Cohort
The Cape Town AIDS Cohort (CTAC) is an observational cohort of patients who received care from public sector clinics affiliated with the University of Cape Town, South Africa. The cohort has been described in detail elsewhere [9;10]. The clinics mainly served indigent communities, with a predominance of heterosexually transmitted infections[10].
A total of 2,086 HIV-infected patients were enrolled in the cohort between 1984 and 2000. Demographic information collected on the first visit included date of birth, sex, marital status, ethnicity/ethnic background, sexual preference and HIV risk factors. HIV diagnosis was confirmed on two separate blood specimens by enzyme-linked immuno-sorbent assay and/or Western blot. Laboratory data were collected approximately every 6 months and included CD4 cell count. Some patients with a diagnosis of AIDS or with CD4 count less than 200 cells/μL received cotrimoxazole prophylaxis after 1993 [11]. In addition, zidovudine monotherapy was used, although infrequently.
The Swiss HIV Cohort Study
Set up in 1988, the Swiss HIV Cohort Study (SHCS) is a national prospective cohort study of HIV-infected patients followed up at outpatient departments of seven University and Cantonal outpatient clinics in Basel, Bern, Geneva, Lausanne, Zurich, Lugano and St. Gallen, Switzerland. The study is described in detail elsewhere [12;13]. Information on demographics, mode of HIV acquisition, risk behaviors, clinical events, laboratory results, and treatments is collected at registration and then at intervals of six months. CD4 counts and other laboratory parameters are measured at least every three months. Non-white patients participating in the SHCS are predominantly migrants from sub-Saharan Africa, who are an increasingly important patient group in Switzerland [14].
Patients
We included all patients aged 15 years (CTAC) or 16 years (SHCS) and over with at least two CD4 counts while not on ART and ART-naive. Patients whose initial CD4 count was greater than 750 cells/μL were left censored up to the first count below 750 because their raised CD4 count might have been due to recent seroconversion, and CD4 declines unrepresentative of the chronic phase of the infection. Patients with initial CD4 count less than 50 cells/μL were also excluded because the assumed linearity of decline of CD4 on the log scale may not hold below this low level. Finally, we excluded patients from the SHCS whose transmission risk group was injection drug use, since CTAC patients were infected through sexual transmission.
Statistical Methods
We used log transformed CD4 in order to linearize the relationship with time and make the distribution more symmetric. We considered using square root transformed CD4, which is commonly used to model trajectories [15], however models using the log transformed data were a better fit to the data than those using the square root transformation and effect estimates may be more easily back transformed to the original scale. We measured time from the first available CD4 count below 750 and stratified analyses by the initial count (50-199, 200-349, 350-499, 500-750 cells/μL) to allow for different slopes in CD4 declines across CD4 strata. We excluded CD4 counts measured greater than four years after the first measurement because models give more weight to patients with many measurements, which might introduce bias since patients surviving longer than four years since the first measurement are more likely to be slow progressors. We also excluded CD4 counts after ART initiation and deaths more than 4 years after the initial CD4 measurement.
We used mixed effects models with random effects for intercept and slope for CD4 measurements to allow for the fitted curve to vary between individuals, and fixed effects for intercept and gradient terms for sex, age (<40, ≥40), ethnicity (White, Non-white) and initial CD4 strata. Univariable models were fitted for each potential predictor of CD4 decline. We then fitted a multivariable model that mutually adjusted for all other variables separately for each cohort. We used the multivariable model to estimate the average decline in CD4 count for different groups of patients for each cohort. The distribution of CD4 declines was then estimated using the best linear unbiased predictions (BLUP) of the random effects. The final model was a joint model for CD4 trajectory and survival time [8] that allowed for the correlation between the CD4 intercept and slope parameters and the log survival time. The CD4 model was the model described above and the survival model assumed a lognormal distribution of survival times with frailty. Results are presented as average declines in CD4 counts during the first year after the initial CD4 measurement with 95% confidence intervals (CIs). These were calculated as the difference between the midpoint of the initial CD4 count strata and the estimated value one year later, which is calculated as the product of the exponentiated coefficients from the model and the midpoint value (i.e. the calculation uses the ratio of geometric means on the back-transformed log CD4). The estimates of CD4 decline are corrected for the bias introduced by informative censoring due to death and hence are the estimates that hypothetically the whole cohort would have in the absence of mortality. The comparisons of the CD4 declines between the two cohorts are therefore not affected by differences in mortality.
Results
Of the 2086 patients enrolled in CTAC, 1766 had two or more visits, and 784 of these (37.6%) had two or more eligible CD4 cell count measurements and were included in the study. CTAC patients who were excluded from the study were similar with respect to age, sex, ethnicity, sexual preference, or mean initial CD4 cell count, but had shorter mean follow-up time than those included in the study (18.7 months versus 28.5 months). In the SHCS, there were 7153 patients enrolled before 2000 who were not infected by intravenous drug use; 2030 patients (28.4%) had two or more eligible CD4 cell counts and were included in the study. SHCS patients who were excluded from the study had lower initial median CD4 counts (220 versus 450 cell/μl), were more likely to be heterosexual (45% versus 42%) and had shorter mean follow-up time than those included in the study (4.2 years versus 8.5 years), but were similar with respect to age and sex.
A total of 2814 patients and 13,388 CD4 cell counts were included in analyses. Table 1 shows demographic and clinical characteristics of CTAC and SHCS patients by ethnicity. CTAC had a high proportion of non-white patients (81.2%) whereas in SHCS the majority of patients were white (89.3%). In both cohorts the proportion of patients who were female was much higher for Non-whites compared with Whites. The median CD4 count at enrolment was lower for Non-whites compared with Whites in both cohorts, and was lower in CTAC compared with SHCS. Patients in CTAC were more likely to have enrolled with a CD4 count below 200 cells/μl than above 500 cells/μl, whereas the opposite was true for the SHCS.
Table 1. Demographic and clinical characteristics of study patients from the Cape Town AIDS Cohort and the Swiss HIV Cohort Study by ethnicity.
| Cape Town AIDS Cohort N = 784 |
Swiss HIV Cohort Study N = 2030 |
|||
|---|---|---|---|---|
| White | Non-white | White | Non-white | |
| N = 155 (19.8%) | N = 629 (81.2%) | N = 1812 (89.3%) | N = 218 (10.7%) | |
| No. of CD4 counts (%) | 606 (23) | 1988 (77) | 9624 (89) | 1171 (11) |
| No. of deaths during follow up (%) | 37 (24) | 120 (19) | 183 (10) | 6 (3) |
| Median No. of CD4 counts per person (IQR) | 5 (3-8) | 3 (2-5) | 8 (5-13) | 9 (4-16) |
| Years of follow up (IQR) | 1.16 (0.50-2.09) | 1.03 (0.57-1.95) | 1.33 (0.53-2.45) | 0.65 (0.27-1.73) |
| Median age (IQR) | 33 (28-42) | 31 (25-37) | 33 (28-41) | 29 (25-33) |
| Age ≥40 years n (%) | 43 (28) | 119 (19) | 511 (28) | 22 (10) |
| Female n (%) | 14 (9) | 364 (58) | 379 (21) | 139 (64) |
| CD4 count at enrolment (cells/μl) | ||||
| Median (IQR) | 321 (188-495) | 279 (174-423) | 460 (310-620) | 415 (279-580) |
| 50-199 | 45 (29) | 193 (31) | 184 (10) | 31 (14) |
| 200-349 | 43 (28) | 201 (32) | 391 (22) | 49 (22) |
| 350-499 | 34 (22) | 133 (21) | 506 (28) | 60 (28) |
| 500-750 | 33 (21) | 102 (16) | 731 (40) | 78 (36) |
determined by first recorded CD4 below 750
IQR; interquartile range
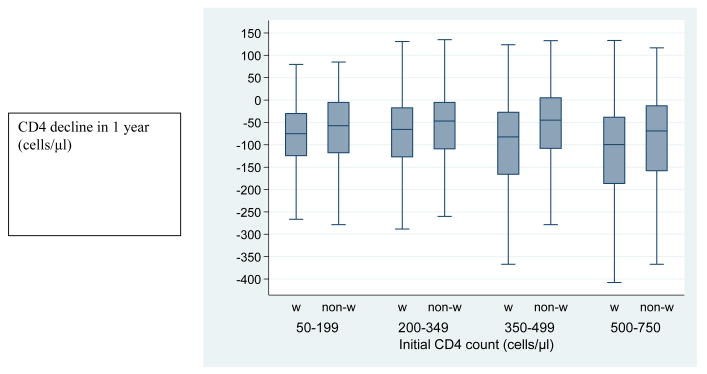
Declines in CD4 cell counts in the first year after the initial CD4 measurement were similar in men and women, but steeper in patients with higher initial CD4 counts, in white patients and in older patients. Therefore we did not include a term for sex in the final model. Table 2 shows declines estimated from joint models converted to the original CD4 scale for each cohort by ethnicity and age groups. Declines ranged from 38 (95% CI 24 to 54) cells/μL in non-white SHCS patients aged 15-39 years with an initial CD4 count of 200-349 cells/μL to 210 (95% CI 143 to 268) cells/μL in white CTAC patients aged 40 years or over with an initial CD4 count of 500-750 cells/μL. Figure 1 illustrates the distribution of the decline in CD4 count in 1 year by initial CD4 strata and ethnicity for both cohorts combined.
Table 2. Estimated 1-year CD4 decline according to baseline CD4 stratum, ethnicity and age in the Cape Town AIDS Cohort and the Swiss HIV Cohort Study.
| Initial CD4 count (cells/μl) | Cape Town AIDS Cohort | Swiss HIV Cohort Study | ||||||
|---|---|---|---|---|---|---|---|---|
| White | Non-white | White | Non-white | |||||
| Age group | N | CD4 decline (cells/μl) |
N | CD4 decline (cells/μl) |
N | CD4 decline (cells/μl) |
N | CD4 decline (cells/μl) |
| 15-39 yrs | ||||||||
| 50-199 | 26 | 52 (42 - 60) | 154 | 47 (40 – 54) | 120 | 46 (40 - 51) | 25 | 39 (31 - 46) |
| 200-349 | 31 | 65 (38 - 89) | 157 | 50 (31 - 69) | 276 | 59 (50 - 67) | 43 | 38 (24 - 54) |
| 350-499 | 22 | 70 (21 - 110) | 109 | 44 (8 - 81) | 353 | 84 (70 - 94) | 55 | 52 (29 - 74) |
| 500-750 | 33 | 123 (59 - 180) | 90 | 92 (36 - 143) | 552 | 98 (87 - 113) | 73 | 54 (18 - 87) |
| ≥40 years | ||||||||
| 50-199 | 19 | 65 (55 - 73) | 39 | 61 (52 - 68) | 83 | 48 (42 - 54) | 7 | 41 (33 - 49) |
| 200-349 | 11 | 101 (75 - 124) | 40 | 91 (67 - 112) | 119 | 65 (54 - 75) | 6 | 48 (29 - 63) |
| 350-499 | 8 | 131 (84 - 172) | 20 | 113 (70 - 151) | 130 | 94 (81 - 107) | 4 | 63 (37 - 91) |
| 500-750 | 5 | 210 (143 - 268) | 20 | 185 (118 - 242) | 179 | 118 (98 - 138) | 5 | 71 (30 - 108) |
Declines from the within group median in the first year after the initial CD4 count measurement with 95% confidence intervals are shown. Results are from adjusted joint model taking survival time into account.
Figure 1. Distribution of CD4 declines by initial CD4 count and ethnicity for the combined CTAC and SHCS population.
Box-and-whisker diagrams showing the median and quartiles (box with horizontal line) and the smallest and largest decline that are not outliers (upper and lower whiskers). w = White, non-w = Non-white.
Outliers are defined as points more than 1.5 times the interquartile range above the 75th percentile or below the 25th percentile
The model coefficients with 95% CIs on the log transformed CD4 scale for gradient and intercept terms from the unadjusted univariable models, the mutually adjusted full multivariable models and the joint models are available on the web (www…). In the joint model, adjusted estimate of survival was longer in SHCS than in CTAC. In both cohorts, older patients had shorter survival than younger patients and there was a strong association of survival with initial CD4 strata, the lower the CD4 strata the shorter the survival time. In the SHCS, Non-whites and females survived longer than Whites and males, but these associations were not seen in CTAC. Compared with the standard mixed-effects multivariable model, the joint model estimated less steep CD4 declines for the highest CD4 strata in which there is the best survival in the CTAC cohort. In the SHCS, the estimates from both models were similar, probably due to fewer deaths being recorded.
Discussion
We compared the CD4 cell count declines in untreated patients in a European and African setting, analyzing two cohorts from Cape Town, South Africa, and Switzerland. Both in South Africa and Switzerland Non-whites had slower CD4 declines than Whites, and older patients had faster declines. Furthermore, the CD4 decline was more rapid in patients with higher initial CD4 counts than in patients with lower counts.
We applied the same analytical approach to the data from the CTAC and SHCS cohorts and results are therefore directly comparable. We have taken account of the demographic characteristics in the cohorts by excluding injection drug users, adjusting models for age and sex. We focused on estimating short-term CD4 trajectories within four years of the first measurement to reduce bias due to slow progressors having more CD4 counts than fast progressors. Finally, we examined the effect of deaths on estimated CD4 decline and found that CD4 declines in the higher CD4 strata are over-estimated by the standard mixed-effects model. The joint model takes into account survival time and deaths and adjusts for the steeper decline of CD4 counts in very ill patients. A previous analysis of the CTAC data by Holmes and colleagues [6] estimated the CD4 decline to be 47.1 cells/μL per annum for patients with initial CD4 counts above 500 cells/μL, 30.6 for those with CD4 counts between 351 and 500 cells/μL and 20.5 cells/μL in patients with counts between 201 and 350 cells/μL [6]. Our estimates are somewhat higher. The different methodological approach may at least partially account for the difference between our estimates and those of Holmes and colleagues [6].
Our study has a number of limitations. There were few white patients in CTAC and few non-white patients in SHCS. The study thus had limited power to examine whether the effect of ethnicity differed between the two settings. Also, follow-up was short for many patients in CTAC and therefore there are few CD4 measurements for most patients. A further problem with analyzing CD4 trajectories in seroprevalent cohorts is the lack of the time of infection, which would be the natural point in time to line up the trajectories. This is to some extent overcome by stratifying on initial CD4 count and reflects what the treating physicians see in practice and are interested in, namely estimates of short-term CD4 decline from the current value. The validity of using first CD4 count measurement as a surrogate for time from infection has been questioned in survival analyses from the Concerted Action on Seroconversion to AIDS and Death in Europe (CASCADE) study, with time to death as the outcome [16], which have shown variation in CD4 set point associated with rate of subsequent decline in CD4. Our analyses, which examined CD4 decline according to initial CD4 strata, may thus be grouping together patients who have different lengths of time since infection. Furthermore, the mean time since infection for each initial CD4 strata could vary by cohort or ethnicity.
The data that are available from patients with well-documented seroconversion are limited, and particularly limited in resource-limited settings: a recent collaborative analysis of time to ART treatment eligibility was based on just over 2000 seroconverters from five cohorts from sub-Saharan Africa and Thailand [4]. In contrast, the CASCADE collaboration of cohorts in Europe, Canada and Australia is based on over 17000 patients with documented seroconversion [16]. There are also disadvantages to seroconverter cohorts, which are generally not representative of the HIV-infected population but include many patients infected through intravenous drug use or the transfusion of blood products. Also, independently of the route of transmission, patients whose seroconversion was documented because they experienced symptomatic illness are likely to have steeper CD4 declines and more rapid clinical progression compared with those who were asymptomatic [17;18].
Other seroprevalent cohorts comparing white and black patients also found slower declines in the black group [19-21]. A recent analysis of the SHCS showed similar differences in CD4 cell count declines between patients of African and European descent, but declines in general were less pronounced, probably because the analysis was restricted to patients in CDC clinical stage A with at least five CD4 cell counts and did not take into account informative censoring due to death [21]. Interestingly, in CASCADE, non-white, compared with white ethnicity, was associated with higher odds of spontaneously achieving undetectable viremia and, in those who did control viral load, with a longer period of undetectable viremia [22]. Analyses of seroprevalent cohorts such as the CTAC and SHCS are helpful to complement and extend the evidence that is available from seroconverter cohorts. In particular, our results are relevant when modeling time to ART eligibility and the need for ART at the population level, and estimating and projecting the future course of the AIDS epidemic [23].
Whilst the decline in CD4 cell count is a determinant of the time from HIV-1 infection to AIDS or death, other factors include the level of CD4 before infection in the population and the rapid drop in CD4 in the weeks immediately after seroconversion. A study of CD4 count declines in seroincident and seroprevalent individuals in Tanzania showed that the median initial CD4 count of the seroincident individuals who seroconverted within 1 year of the first CD4 measurement was around 500 cells/μL whereas in non-infected individuals it was around 800 cells/μL, indicating that about 300 cells/μL were lost soon after seroconversion [24]. Reference ranges for CD4 counts in people not infected by HIV vary across geographical regions, gender and age and racial groups [25;26], as well as by lifestyle and biological factors such as smoking or contraceptive use [27]. A study of Swiss blood donors found that the reference range was higher in women than men, and lower at older ages [28]. African studies showed heterogeneity across populations, with, for example, markedly lower CD4 counts in Ethiopians [29], and also found higher counts in women than men [25;30].
The association between ethnicity and CD4 count declines may have several explanations. Ethnicity reflects social and economic position of participants within their respective cohorts. In CTAC, non-white participants tended to be of lower socioeconomic position than white participants due to historical limitations on socioeconomic opportunity. In the SHCS, the non-white participants are migrants from sub-Saharan Africa who are also generally of lower socioeconomic position than white participants. In untreated patients socioeconomic position may affect CD4 declines through increased exposure to opportunistic infections and resultant immune activation and by influencing access and adherence to prophylactic therapies and other relevant health behaviors. In the Swiss cohort, but not in South Africa, the slower decline of CD4 counts appears to have translated into better survival in Non-whites compared to Whites. Of note, once enrolled in the SHCS access to ART and prognosis of non-white participants is equivalent to white participants [14]. Socioeconomic conditions, health-seeking behaviors, access to health care, and exposure to pathogens are more important determinants of mortality in South Africa [31].
It is also possible that our results relate to host genetic differences: Non-whites may have adapted to frequent infectious diseases by selection over many generations for the ability to survive despite chronic immune activation [20]. Of note, the low immune activation phenotype is also found in HIV-1 infected patients with slow disease progression in the Western world [32], and in asymptomatic nonhuman African primates infected with Simian immunodeficiency viruses (SIVs) [33]. A recent study suggested that genetically determined divergent Toll-like receptor signaling and interferon production distinguishes pathogenic (“immune activated”) from non-pathogenic infection in the animal model [34]. In Caucasians genetic polymorphisms explained about 15% of the variation in viral load set points during the asymptomatic period of infection [35]. Of note, a recent analysis of the SHCS found that the CD4 cell count decline was less steep in patients of African descent compared to patients of European descent independently of whether patients were infected with HIV-1 subtype B or subtype C [21]. The slower CD4 cell count decline in patients of African descent is therefore unlikely to be due to infection with less virulent subtypes.
In conclusion, further studies on reference ranges of CD4 counts and on rates of declines in CD4 counts, in different countries and populations are needed to inform the development of guidelines for when to start ART, and to improve projections of the epidemic, particularly in resource-limited settings. The methodology used in the present study, which addressed a number of issues not generally considered in previous studies, might serve as a model for future studies.
Supplementary Material
Additional web table: Model coefficients with 95% confidence intervals on log transformed CD4 scale for gradient and intercept terms and survival time (years) for mortality model from a) unadjusted univariable random effets (RE) models b) mutually adjusted multivariable RE model and c) joint marker and mortality RE model (JMRE).
Acknowledgments
We are grateful to Jonathan Sterne for helpful comments on an earlier draft of this manuscript. This study was funded within the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation. The members of the Swiss HIV Cohort Study are M. Battegay, E. Bernasconi, J. Böni, HC Bucher, Ph. Bürgisser, A. Calmy, S. Cattacin, M. Cavassini, R. Dubs, M. Egger, L. Elzi, M. Fischer, M. Flepp, A. Fontana, P. Francioli (President of the SHCS), H. Furrer (Chairman of the Clinical and Laboratory Committee), C. Fux, M. Gorgievski, H. Günthard (Chairman of the Scientific Board), H. Hirsch, B. Hirschel, I. Hösli, Ch. Kahlert, L. Kaiser, U. Karrer, C. Kind, Th. Klimkait, B. Ledergerber, G. Martinetti, B. Martinez, N. Müller, D. Nadal, F. Paccaud, G. Pantaleo, A. Rauch, S. Regenass, M. Rickenbach (Head of Data Center), C. Rudin (Chairman of the Mother & Child Substudy), P. Schmid, D. Schultze, J. Schüpbach, R. Speck, P. Taffé, A. Telenti, A. Trkola, P. Vernazza, R. Weber, S. Yerly.
Footnotes
Conflict of interest statement: None of the authors have any conflict of interest
Reference List
- 1.Gottlieb GS, Sow PS, Hawes SE, et al. Equal plasma viral loads predict a similar rate of CD4+ T cell decline in human immunodeficiency virus (HIV) type 1- and HIV-2-infected individuals from Senegal, West Africa. J Infect Dis. 2002 Apr 1;185(7):905–14. doi: 10.1086/339295. [DOI] [PubMed] [Google Scholar]
- 2.Phillips AN, Pezzotti P, Lepri AC, et al. Cd4 Lymphocyte Count As A Determinant of the Time from Hiv Seroconversion to Aids and Death from Aids - Evidence from the Italian Seroconversion Study. AIDS. 1994 Sep;8(9):1299–305. doi: 10.1097/00002030-199409000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Lepri AC, Sabin CA, Phillips AN, Lee CA, Pezzotti P, Rezza G. The rate of CD4 decline as a determinant of progression to AIDS independent of the most recent CD4 count. Epidemiology and Infection. 1998 Oct;121(2):369–76. doi: 10.1017/s095026889800140x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wandel S, Egger M, Rangsin R, et al. Duration from seroconversion to eligibility for antiretroviral therapy and from ART eligibility to death in adult HIV-infected patients from low and middle-income countries: collaborative analysis of prospective studies. Sex Transm Infect. 2008 Aug;84(Suppl 1):i31–i36. doi: 10.1136/sti.2008.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Progress report 2008. Geneva: World Health Organization, UNAIDS, Unicef; 2008. Towards universal access. Scaling up priority HIV/AIDS interventions in the health sector. [Google Scholar]
- 6.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: Implications for prophylaxis and treatment. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2006 Aug 1;42(4):464–9. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 7.Duvignac J, Thiebaut R. CD4 natural history and informative censoring in sub-Saharan Africa. J Acquir Immune Defic Syndr. 2006;43(3):380–1. doi: 10.1097/01.qai.0000242452.12898.ba. [DOI] [PubMed] [Google Scholar]
- 8.Touloumi G, Pocock SJ, Babiker AG, Darbyshire JH. Estimation and comparison of rates of change in longitudinal studies with informative dropouts. Statistics in Medicine. 1999 May 30;18(10):1215–33. doi: 10.1002/(sici)1097-0258(19990530)18:10<1215::aid-sim118>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Badri M, Wood R. Usefulness of total lymphocyte count in monitoring highly active antiretroviral therapy in resource-limited settings. AIDS. 2003 Mar 7;17(4):541–5. doi: 10.1097/00002030-200303070-00009. [DOI] [PubMed] [Google Scholar]
- 10.Badri M, Ehrlich R, Pulerwitz T, Wood R, Maartens G. Tuberculosis should not be considered an AIDS-defining illness in areas with a high tuberculosis prevalence. International Journal of Tuberculosis and Lung Disease. 2002 Mar;6(3):231–7. [PubMed] [Google Scholar]
- 11.Badri M, Ehrlich R, Wood R, Maartens G. Initiating co-trimoxazole prophylaxis in HIV-infected patients in Africa: an evaluation of the provisional WHO/UNAIDS recommendations. AIDS. 2001 Jun 15;15(9):1143–8. doi: 10.1097/00002030-200106150-00009. [DOI] [PubMed] [Google Scholar]
- 12.Egger M, Hirschel B, Francioli P, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. Swiss HIV Cohort Study. BMJ. 1997 Nov 8;315(7117):1194–9. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudre P, Rickenbach M, Taffe P, Janin P, Volkart AC, Francioli P. Clinical epidemiology and research on HIV infection in Switzerland: the Swiss HIV Cohort Study 1988-2000. Schweiz Med Wochenschr. 2000 Oct 14;130(41):1493–500. [PubMed] [Google Scholar]
- 14.Staehelin C, Rickenbach M, Low N, et al. Migrants from Sub-Saharan Africa in the Swiss HIV Cohort Study: access to antiretroviral therapy, disease progression and survival. AIDS. 2003 Oct 17;17(15):2237–44. doi: 10.1097/00002030-200310170-00012. [DOI] [PubMed] [Google Scholar]
- 15.Taffe P, May M. A joint back calculation model for the imputation of the date of HIV infection in a prevalent cohort. Stat Med. 2008 Oct 15;27(23):4835–53. doi: 10.1002/sim.3294. [DOI] [PubMed] [Google Scholar]
- 16.Porter K, Babiker A, Walker S, et al. Effect of ignoring the time of HIV seroconversion in estimating changes in survival over calendar time in observational studies: results from CASCADE. AIDS. 2000 Sep 8;14(13):1899–906. doi: 10.1097/00002030-200009080-00003. [DOI] [PubMed] [Google Scholar]
- 17.Lindback S, Brostrom C, Karlsson A, Gaines H. Does symptomatic primary HIV-1 infection accelerate progression to CDC stage IV disease, CD4 count below 200 × 10(6)/l, AIDS, and death from AIDS? BMJ. 1994 Dec 10;309(6968):1535–7. doi: 10.1136/bmj.309.6968.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanhems P, Lambert J, Cooper DA, et al. Severity and prognosis of acute human immunodeficiency virus type 1 illness: a dose-response relationship. Clin Infect Dis. 1998 Feb;26(2):323–9. doi: 10.1086/516289. [DOI] [PubMed] [Google Scholar]
- 19.Easterbrook PJ, Farzadegan H, Hoover DR, et al. Racial differences in rate of CD4 decline in HIV-1-infected homosexual men. AIDS. 1996 Sep;10(10):1147–55. [PubMed] [Google Scholar]
- 20.Mekonnen Y, Geskus RB, Hendriks JC, et al. Low CD4 T cell counts before HIV-1 seroconversion do not affect disease progression in Ethiopian factory workers. J Infect Dis. 2005 Sep 1;192(5):739–48. doi: 10.1086/432545. [DOI] [PubMed] [Google Scholar]
- 21.Muller V, von Wyl V, Yerly S, et al. African descent is associated with slower CD4 cell count decline in treatment-naive patients of the Swiss HIV Cohort Study. AIDS. 2009 Jun 19;23(10):1269–76. doi: 10.1097/QAD.0b013e32832d4096. [DOI] [PubMed] [Google Scholar]
- 22.Madec Y, Boufassa F, Porter K, Meyer L. Spontaneous control of viral load and CD4 cell count progression among HIV-1 seroconverters. AIDS. 2005 Nov 18;19(17):2001–7. doi: 10.1097/01.aids.0000194134.28135.cd. [DOI] [PubMed] [Google Scholar]
- 23.Ghys PD, Walker N, McFarland W, Miller R, Garnett GP. Improved data, methods and tools for the 2007 HIV and AIDS estimates and projections. Sex Transm Infect. 2008 Aug;84 1:i1–i4. doi: 10.1136/sti.2008.032573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urassa W, Bakari M, Sandstrom E, et al. Rate of decline of absolute number and percentage of CD4 T lymphocytes among HIV-1-infected adults in Dar es Salaam, Tanzania. AIDS. 2004 Feb 20;18(3):433–8. doi: 10.1097/00002030-200402200-00009. [DOI] [PubMed] [Google Scholar]
- 25.Aina O, Dadik J, Charurat M, et al. Reference values of CD4 T lymphocytes in human immunodeficiency virus-negative adult Nigerians. Clin Diagn Lab Immunol. 2005 Apr;12(4):525–30. doi: 10.1128/CDLI.12.4.525-530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee BW, Yap HK, Chew FT, et al. Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: From birth to adulthood. Cytometry. 1996 Mar 15;26(1):8–15. doi: 10.1002/(SICI)1097-0320(19960315)26:1<8::AID-CYTO2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 27.Maini MK, Gilson RJC, Chavda N, et al. Reference ranges and sources of variability of CD4 counts in HIV-seronegative women and men. Genitourinary Medicine. 1996 Feb;72(1):27–31. doi: 10.1136/sti.72.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. European Journal of Haematology. 2004 Mar;72(3):203–12. doi: 10.1046/j.0902-4441.2003.00199.x. [DOI] [PubMed] [Google Scholar]
- 29.Wolday D, Tsegaye A, Messele T. Low absolute CD4 counts in Ethiopians. Ethiop Med J. 2002 Apr;40 1:11–6. [PubMed] [Google Scholar]
- 30.Ampofo W, Torpey K, Mukadi YD, et al. Normal CD4+ T lymphocyte levels in HIV seronegative individuals in the Manya/Yilo Krobo communities in the Eastern region of Ghana. Viral Immunol. 2006;19(2):260–6. doi: 10.1089/vim.2006.19.260. [DOI] [PubMed] [Google Scholar]
- 31.Del Amo J, Petruckevitch A, Phillips A, et al. Disease progression and survival in HIV-1-infected Africans in London. AIDS. 1998 Jul 9;12(10):1203–9. doi: 10.1097/00002030-199810000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Choudhary SK, Vrisekoop N, Jansen CA, et al. Low immune activation despite high levels of pathogenic human immunodeficiency virus type 1 results in long-term asymptomatic disease. J Virol. 2007 Aug;81(16):8838–42. doi: 10.1128/JVI.02663-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007 Nov;117(11):3148–54. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandl JN, Barry AP, Vanderford TH, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008 Oct;14(10):1077–87. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 35.Fellay J, Shianna KV, Ge D, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007 Aug 17;317(5840):944–7. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional web table: Model coefficients with 95% confidence intervals on log transformed CD4 scale for gradient and intercept terms and survival time (years) for mortality model from a) unadjusted univariable random effets (RE) models b) mutually adjusted multivariable RE model and c) joint marker and mortality RE model (JMRE).