Abstract
Background
Omega-3 polyunsaturated fatty acids [ω-3 PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] from fish oil ameliorate cardiovascular diseases. However, little is known about the effects of ω-3 PUFAs on cardiac fibrosis, a major cause of diastolic dysfunction and heart failure. The current study assessed the effects of ω-3 PUFAs on cardiac fibrosis.
Methods and Results
We assessed left ventricular (LV) fibrosis and pathology in mice subjected to transverse aortic constriction (TAC) subsequent to the consumption of a fish oil or a control diet. In control mice, four weeks of TAC induced significant cardiac dysfunction, cardiac fibrosis and cardiac fibroblast activation (proliferation and transformation into myofibroblasts). Dietary supplementation with fish oil prevented TAC-induced cardiac dysfunction and cardiac fibrosis, and blocked cardiac fibroblast activation. In heart tissue, TAC increased active TGF-β1 levels and phosphorylation of Smad2. In isolated adult mouse cardiac fibroblasts, TGF-β1 induced cardiac fibroblast transformation, proliferation, and collagen synthesis. EPA and DHA increased cGMP levels and blocked cardiac fibroblast transformation, proliferation, and collagen synthesis. EPA and DHA blocked phospho-Smad2/3 nuclear translocation. DT3, a PKG inhibitor, blocked the anti-fibrotic effects of EPA and DHA. EPA and DHA increased phospho-eNOS and eNOS protein levels and nitric oxide production.
Conclusions
ω-3 fatty acids prevent cardiac fibrosis and cardiac dysfunction by blocking TGF-β1-induced phospho-Smad2/3 nuclear translocation through activation of the cGMP/PKG pathway in cardiac fibroblasts.
Keywords: transverse aortic constriction, ω-3 fatty acids, cardiac fibrosis, cGMP/PKG
Introduction
Heart failure is the leading cause of cardiovascular morbidity and mortality worldwide.1 Up to 50% of heart failure patients have diastolic dysfunction.2 One of the main causes of diastolic dysfunction is cardiac fibrosis characterized by a pathological accumulation of fibrillar collagen throughout the myocardium, which results in reduced cardiac muscle compliance, impaired filling, and ultimately leads to heart failure.3–4
In the heart, collagen is primarily produced by cardiac fibroblasts. In response to stress, profibrotic cytokines are released leading to cardiac fibroblast proliferation and transformation into myofibroblasts. Myofibroblasts have contractile fibers containing alpha-smooth muscle actin (α-SMA) and are responsible for the excessive accumulation of extracellular matrix under pathological conditions.3, 5 Therefore, abrogation of cardiac fibroblast transformation into myofibroblasts is one strategy for suppressing cardiac fibrotic remodeling which can lead to heart failure.
Cardiac fibroblast transformation is primarily induced by transforming growth factor (TGF)-β1,6–7 which, under pathological conditions, can result in excessive collagen production.8 TGF-β1 binds to type II (TGFβRII) and type I (TGFβRI) receptors which phosphorylate TGFβRI-associated Smad2 and Smad3. Phosphorylated Smad2/3 binds Smad4, and translocates into the nucleus where it promotes gene transcription.8 Therefore, inhibition of phosphorylation and/or nuclear translocation of Smad2/3 is a potential target for suppressing the fibrotic effects of TGF-β1.9 The cGMP/protein kinase G (PKG) signaling pathway inhibits TGF-β1-induced cardiac fibrosis by blocking TGF-β1-induced nuclear translocation of phospho-Smad3 through PKG-induced phosphorylation of Ser309 and Thr388 sites in the MH2 domain of the Smad3 protein.9–10
Omega-3 polyunsaturated fatty acids [ω-3 PUFAs, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] protect against coronary artery disease and heart failure.11–13 The GISSI heart failure trial indicated that ω-3 PUFAs (EPA+DHA, 0.85 g/day) significantly reduced mortality in patients with chronic heart 3 failure.14 However, there is little information regarding the impact of ω-3 PUFAs on the progression of cardiac fibrosis and diastolic heart failure. Interestingly, EPA and DHA increase cGMP levels in neonatal rat cardiac myocytes.15 This suggests that ω-3 PUFAs may have an anti-fibrotic effect in the heart through the cGMP/PKG pathway.
The present study demonstrates that ω-3 PUFAs inhibit the cardiac fibrotic response and prevent progression of cardiac dysfunction by suppression of cardiac fibroblast proliferation, transformation and collagen production. These anti-fibrotic effects of ω-3 PUFAs are exerted through the activation of the cGMP/PKG pathway which blocks the TGF-β1-stimulated nuclear translocation of phospho-Smads.
Methods
Detailed methods are available in the online-only Data Supplement.
Animals
Male C57BL/6 mice (5 weeks, Jackson Laboratory) were fed a control or a fish oil supplemented diet for eight weeks (Dyets; Bethlehem, PA). Both diets contained 4% by weight test oil (control diet: 40 g corn oil per kg; fish oil supplemented diet: 12 g menhaden oil plus 28 g corn oil per kg), but were otherwise identical. The fish oil diet provided 1% energy as EPA+DHA. After 8 weeks on the assigned diet, mice were randomly allocated into 4 groups using a 2×2 factorial design and subjected to Transverse aortic constriction (TAC) surgery (groups: control/sham, control/TAC, fish oil/sham, fish oil/TAC; n=22–25/group). Following surgery, mice were continually fed the assigned diets and sacrificed after 3, 7, or 28 days.
Transverse Aortic Constriction
Transverse aortic constriction surgery was performed without intubation under anesthesia with isoflurane, as previously described.16–17
Measurement of Cardiac Function
Echocardiography and Hemodynamics were performed as described previously.18
Measurement of the Omega-3 Content
Omega-3 content in red blood cells and cardiac tissues was measured as previously described.19 A similar analysis was performed on cultured cardiac fibroblasts.
Isolation and Culture of Adult Mouse Cardiac Fibroblasts
Adult mouse cardiac fibroblasts were isolated using a previously described procedure17 with modifications.
Cell Proliferation and Collagen Synthesis in Isolated Adult Cardiac Fibroblasts
Cell proliferation was determined by counting of living cells. Collagen synthesis was measured by incorporation of 3H-proline.
Real-Time Polymerase Chain Reaction (PCR)
Gene expressions were determined by quantitative real-time PCR performed with TaqMan Gene Expression Assay kit.
Western blot, Immunohistochemistry and Immunocytochemistry
Western blot, Immunohistochemistry and Immunocytochemistry were performed as standard procedures detailed in the Methods section of online-only Data Supplement.
Measurement of TGF-β1, Cyclic GMP and Nitrite/Nitrate Concentrations
Active form of TGF-β1 levels were measured by ELISA kit (R&D Systems, Minneapolis, MN). Cyclic GMP levels were quantified using the acetylation protocol for a competitive Cyclic GMP EIA kit (Cayman Chemical, Ann Arbor, MI). Nitrite/Nitrate concentrations were determined using a Total Nitrate/Nitrite Fluorometric Assay kit (Cayman Chemical).
Statistics
Results are reported as means ± SEM. Mean values were compared by one-way or two-way ANOVA followed by Tukey’s post-hoc test or Dunnett’s post-hoc test as appropriate. P<0.05 was considered significant. For analysis of fractional shortening (Figure 1B), longitudinal analysis with restricted maximum likelihood estimation method and an unstructured correlation matrix for the repeated measurements was used (SAS version 9.2; Cary, NC).
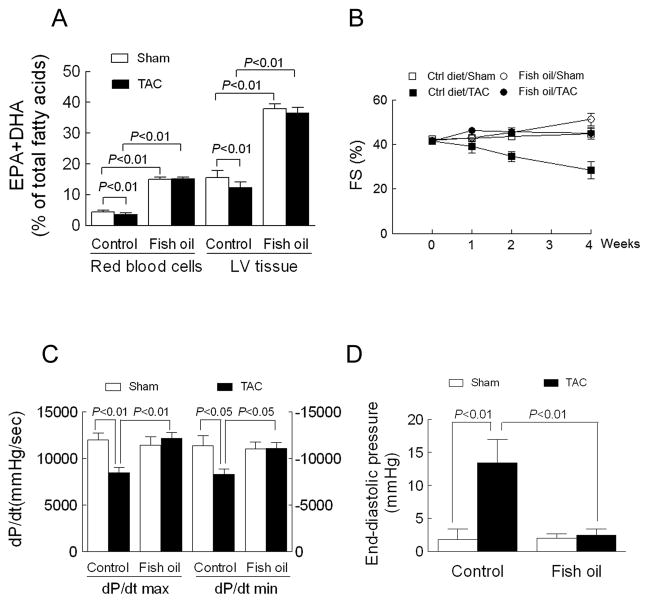
Figure 1. Fish oil prevents pressure overload-induced cardiac dysfunction.
(A) The omega-3 content (EPA+DHA % of total fatty acids) of red blood cells or left ventricle (LV) determined by gas chromatography 4 wk following aortic constriction surgery (TAC). (B) Fractional shortening (FS) determined by echocardiography before and 1-, 2-and 4 wk after TAC. For analysis of FS, a repeated measures model was used. FS in Ctrl diet/Sham animals was not changed (0.7 ± 0.8%, P>0.2) compared to baseline, nor was FS in Fish oil/Sham animals (1.5 ± 1.1%, P=0.2). TAC had the effect of decreasing FS by −4.4 ± 1.0% per week (P=0.0003), and Fish oil reversed the TAC effect by 3.1 ± 1.5% per week (p=0.043), almost three-quarters of the effect of TAC surgery. (C and D) Contractility (± dP/dt) and end-diastolic pressure determined from hemodynamic measurements 4 wk after TAC. Data are presented as mean ± SEM with n=6 in control/sham and fish oil/sham groups and n=8–9 in control/TAC and fish oil/TAC groups. In A, C and D, data were analyzed by two-way ANOVA with Tukey’s post-hoc test.
Results
Fish Oil Prevents Pressure Overload-Induced Cardiac Dysfunction
Before inducing pressure overload, mice were fed a diet supplemented with ω-3 PUFAs for 8 weeks. This increased the ω-3 content (EPA+DHA % of total fatty acids) in both red blood cells (3.4 fold) and left ventricle (2.4 fold) relative to mice on the control diet (Figure 1A and Supplementary Table 1–2). Aortic constriction decreased the ω-3 content in red blood cells and heart in mice fed the control diet (p<0.01), whereas it had no effect on the fish oil groups (Figure 1A and Supplementary Table 1–2). As expected, in mice fed the control diet, aortic constriction induced contractile dysfunction by four weeks following surgery as evidenced by a 38% decrease in fractional shortening, decreases of 29% in dP/dt max and 27% in dP/dt min, and a 6.5-fold increase in end-diastolic pressure (all at least p<0.05 relative to sham). Conversely, contractile dysfunction was corrected following aortic constriction in mice fed the fish oil diet (Figures 1B–D). There was no significant difference in pressure gradients induced by aortic constriction between the control diet and fish oil diet groups (Supplementary Table 3). In short, dietary supplementation with fish oil protects against pressure overload-induced contractile dysfunction.
Fish Oil Prevents Pressure Overload-Induced Cardiac Fibrosis in Left Ventricle
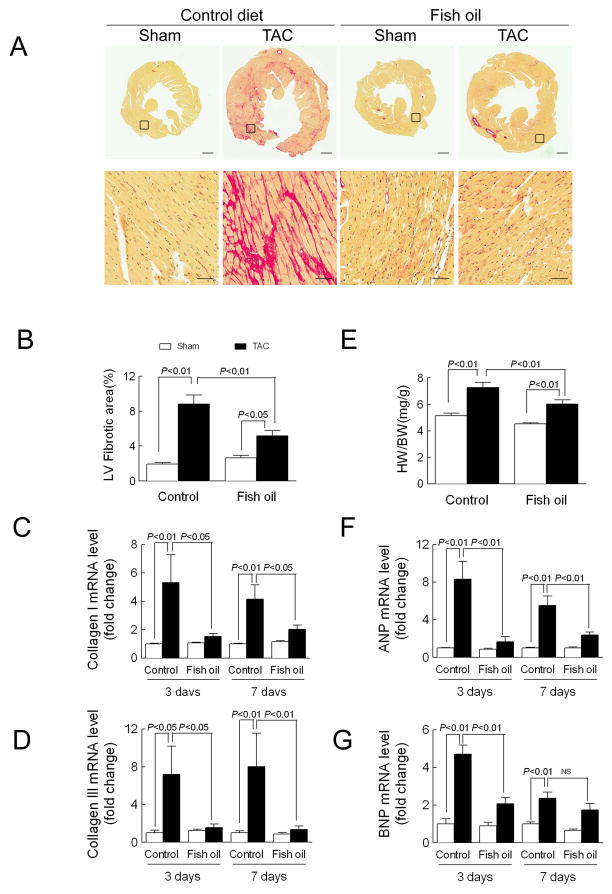
To determine if dietary supplementation with fish oil prevents pressure overload-induced cardiac fibrosis, tissues were stained with picro-sirius red to detect collagen distribution in LV cross sections four weeks after surgery. In mice fed the control diet, aortic constriction induced significant fibrosis. The collagen content was increased 4.6-fold over sham (Figures 2A–B). This was accompanied by a significant increase in collagen I and III expression at both three and seven days following surgery (collagen I: 5.3-fold at 3 days, 4.2-fold at 7 days; collagen III: 7.2-fold at 3 days, 8.0-fold at 7 days) (Figures 2C–D). However, in mice fed the fish oil diet, fibrosis was increased only 2.0-fold over sham and there was no induction of collagen expression, resulting in 63% less fibrosis compared to the mice fed the control diet. In summary, fish oil feeding prevents fibrosis induced by pressure overload.
Figure 2. Fish oil prevents pressure overload-induced cardiac fibrosis in mice.
(A) Fibrosis measured in ventricular cross sections stained with picro-sirius red 4 wk following aortic constriction surgery (TAC). Upper: Entire LV section. Scale bar=500 μm. Lower: High magnification of indicated collagen area. Scale bar=100 μm. (B) Left ventricular fibrosis quantified using Image J and expressed as the fibrosis area/total area. (E) Heart weight to body weight ratio (HW/BW) calculated 4 wk after TAC. In B and E, n=6 in Ctrl diet/Sham and Fish oil/Sham groups; n=9 in Ctrl diet/TAC and Fish oil/TAC groups. Left ventricular expression of (C) collagen I, (D) collagen III, (F) atrial natriuretic peptide (ANP), and (G) brain natriuretic peptide (BNP) analyzed by real-time PCR 3-and 7-d after TAC (n=5 each group). Data are presented as mean ± SEM. Data were analyzed by two-way ANOVA with Tukey’s post-hoc test. 23
A previous report indicated that dietary supplementation with fish oil reduced hypertrophy following aortic constriction in rats.20 Here, aortic constriction induced a hypertrophic response as evidenced by a 42% increase in heart weight-to-body weight (HW/BW) ratio in mice fed the control diet and a 31% increase in HW/BW ratio in mice fed the fish oil diet. However, after adjusting for the effect of fish oil, aortic constriction increased the HW/BW ratio by 1.9±0.3 in both control and fish oil groups (Figure 2E). Conversely, in mice fed the control diet, aortic constriction significantly induced expression of the hypertrophic marker genes atrial and brain natriuretic peptide, which was not observed in mice fed the fish oil diet (Figures 2F–G).
Fish Oil Blocks Pressure Overload-Induced Non-Myocyte Proliferation and Myofibroblast Transformation
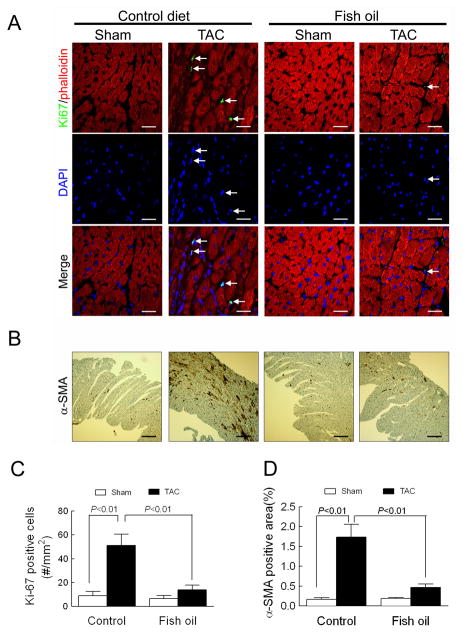
To determine how fish oil feeding affected cardiac fibroblast proliferation and transformation after aortic constriction, we stained ventricular sections for interstitial expression of Ki67 (excluding myocytes, which were counter-stained with phalloidin) as a marker of non-myocyte proliferation and for expression of α-SMA as a marker of fibroblast transformation (excluding the vascular staining) (Figures 3A–B). In mice fed the control diet, aortic constriction induced a significant increase in the number of interstitial Ki67 positive cells and α-SMA positive area, whereas dietary supplementation with fish oil prevented non-myocyte proliferation and fibroblast transformation (Figures 3C–D). These findings indicate that fish oil prevents fibroblast proliferation and transformation, which can explain why fish oil prevented fibrosis and contractile dysfunction in this model.
Figure 3. Fish oil blocks pressure overload-induced non-myocyte proliferation and myofibroblast transformation.
(A) Fibroblast proliferation measured in ventricular sections stained with an antibody to Ki67 (green), phalloidin (red, labels cardiac myocytes), and DAPI (blue, label nuclei) 4 wk following TAC. Arrows show interstitial co-labeling of Ki67 and DAPI. Scale bar=20 μm. (B) Myofibroblast transformation measured in ventricular sections stained with an antibody to α-SMA 4 wk after TAC. Scale bar=200 μm. Quantification of (C) the number of Ki67 positive cells from 15–20 fields per heart and (D) the percent area of α-SMA positive staining in whole sections. Data are presented as mean ± SEM with n=4 per group. Data were analyzed by two-way ANOVA with Tukey’s post-hoc test.
Fish Oil Does Not Block Pressure Overload-Induced TGF-β1 Production or Phosphorylation of Smad2
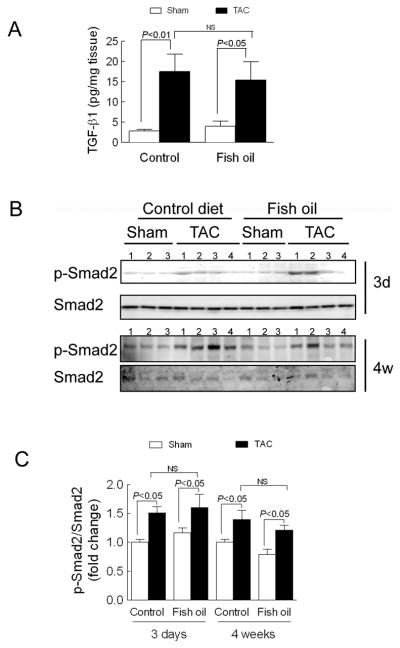
To determine if dietary supplementation with fish oil interfered with TGF-β1 signaling following aortic constriction, we measured active form of TGF-β1 levels three days after surgery and Smad2 phosphorylation levels three days and four weeks after surgery. Interestingly, aortic constriction increased TGF-β1 levels and Smad2 phosphorylation to a similar degree in both groups (Figures 4A–C). Therefore, dietary supplementation with fish oil does not interrupt TGF-β1 production and Smad2 phosphorylation induced by aortic constriction. We also tested Smad3 phosphorylation levels at three days and four weeks post-TAC, but Smad3 phosphorylation was not detected at either time point in the heart tissues (data not shown).
Figure 4. Fish oil does not block pressure overload-induced TGF-β1 production or phosphorylation of Smad2.
(A) Ventricular levels of active form of TGF-β1 measured by ELISA 3 d following aortic constriction surgery (TAC) (n=5–6 mice per group). (B) Levels of phospho-and total-Smad-2 measured by Western blot 3 d and 4 wk after TAC. (C) Densitometric quantification of Smad-2 phosphorylation 3 d and 4 wk after TAC (n=6–8 mice per group). Data are presented as mean ± SEM. Data were analyzed by two-way ANOVA with Tukey’s post-hoc test.
EPA and DHA Inhibit the TGF-β1-Stimulated Fibrotic Response in Isolated Adult Mouse Cardiac Fibroblasts
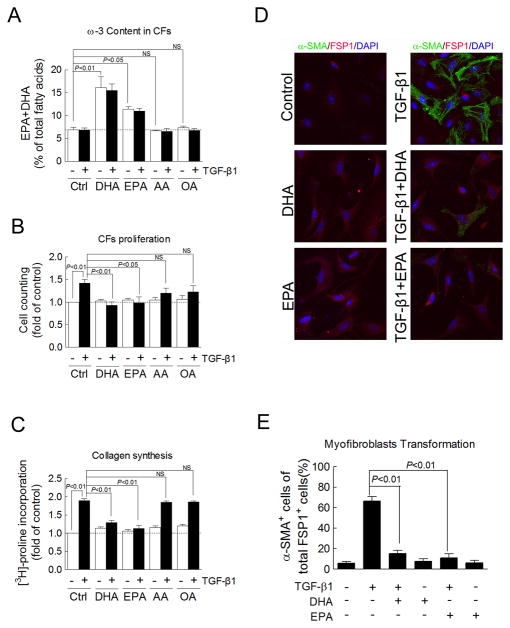
To explain the anti-fibrotic effects of dietary fish oil we observed in vivo, we investigated EPA-and DHA-(the two primary ω-3 PUFAs in fish oil) mediated inhibition of pro-fibrotic TGF-β1 signaling in isolated adult mouse cardiac fibroblasts. EPA (10 μM) and DHA (10 μM) significantly increased the ω-3 content by 2.3-fold and 1.65-fold, respectively. Neither arachidonic acid (ω-6 PUFA) nor oleic acid (ω-9 PUFA) altered the ω-3 content (Figure 5A). TGF-β1 (1 ng/ml) significantly increased proliferation by 142% (P<0.01, Figure 5B), collagen synthesis by 190% (P<0.01, Figure 5C), and myofibroblast transformation by 66% (P<0.01, Figure 5D–E), whereas, EPA and DHA at 10 μM had no effect on these parameters. However, both EPA and DHA (10 μM) completely blocked TGF-β1-induced cardiac fibroblast proliferation, prevented collagen synthesis, and significantly decreased myofibroblast transformation (all P<0.01 vs. TGF-β1-treated cardiac fibroblasts, Figures 5B–E). Other fatty acids, arachidonic acid (10 μM) and oleic acid (10 μM), did not affect TGF-β1-induced collagen synthesis in cardiac fibroblasts (Figures 5B–C). These results demonstrate that the anti-fibrotic effects of ω-3 PUFAs are mediated by suppression of TGF-β1-induced fibrosis in cardiac fibroblasts.
Figure 5. EPA and DHA inhibit the TGF-β1-stimulated fibrotic response in isolated adult mouse cardiac fibroblasts.
(A) The omega-3 content measured in cultured cardiac fibroblasts treated for 48 hr with omega-3 fatty acids EPA and DHA (10 μM), arachidonic acid (AA, 10 μM), and the oleic acid (OA, 10 μM), and treated for the final 24 hr with TGF-β1 (1 ng/ml) (n=3). (B) Cardiac fibroblast proliferation measured by counting live cells using trypan blue exclusion in cardiac fibroblasts treated as in A (n=3–4). (C) Collagen synthesis determined by [3H]-proline incorporation in cardiac fibroblasts treated as in A (n=9–42). 24 (D) Myofibroblast transformation measured by α-SMA staining with counterstains for fibroblast specific protein 1 (FSP1, red) and nuclei (DAPI, blue) in cardiac fibroblasts treated as in A. (E) Quantification of α-SMA positive cells as a percentage of total FSP1 positive cells (4 slides for each group). Data are presented as mean ± SEM. Data were analyzed by one-way ANOVA with Dunnett’s post-hoc test in A-C and Tukey’s post-hoc test in E.
EPA and DHA Inhibit the TGF-β1-Stimulated Fibrotic Response Through the cGMP/PKG Pathway
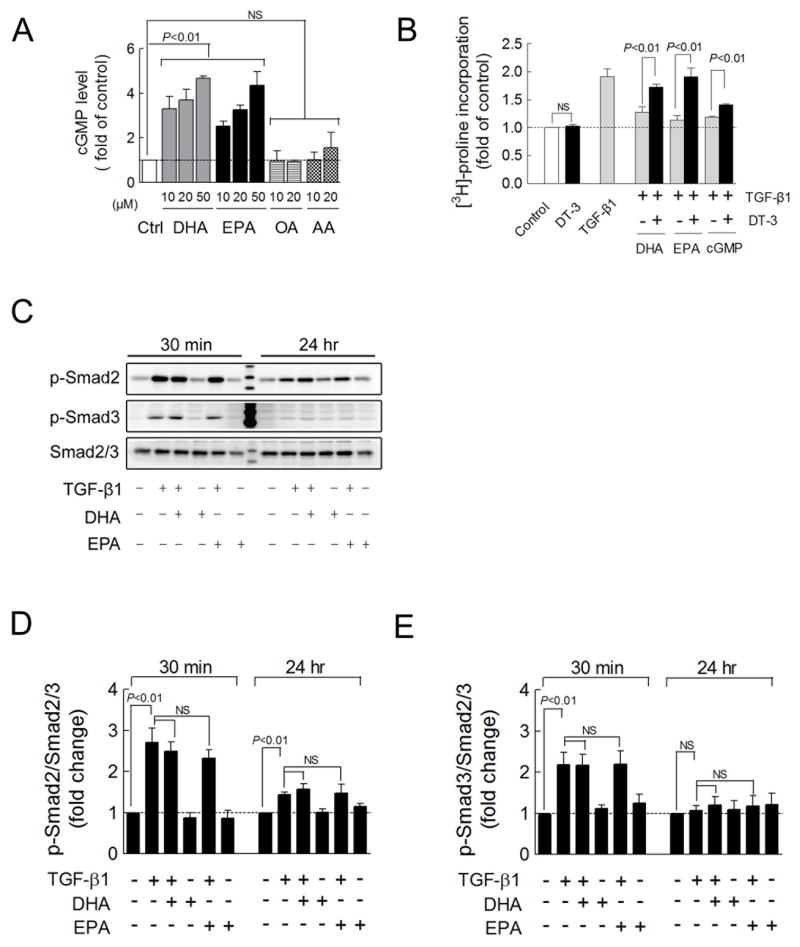
The cGMP/PKG signaling pathway plays a counter-regulatory role against TGF-β1-induced cardiac fibrosis.9–10 We therefore tested the hypothesis that ω-3 PUFAs activate cGMP/PKG signaling and block pro-fibrotic TGF-β1 signaling. In cultured cardiac fibroblasts, both EPA and DHA (10–50 μM) induced dose-dependent increases in cGMP production (all P<0.01), but neither of the control fatty acids, arachidonic acid nor oleic acid (10 or 20 μM), were able to induce cGMP production (Figure 6A). Furthermore, EPA (10 μM) and DHA (10 μM) as well as 8-bromo-cGMP (1 mM), which activates PKG, completely blocked the TGF-β1-induced collagen synthesis (Figure 6B). Conversely, a peptide-based inhibitor of PKG, DT-3 (1 μM) significantly diminished the anti-fibrotic effect of EPA, DHA and cGMP (Figure 6B). In summary, these results indicate that ω-3 PUFAs inhibition of pro-fibrotic TGF-β1 signaling is mediated by ω-3 PUFA-induced cGMP/PKG signaling.
Figure 6. EPA and DHA inhibit the TGF-β1-stimulated fibrotic response through the cGMP/PKG pathway.
(A) cGMP levels measured by ELISA in cultured cardiac fibroblasts treated for 48 hr with EPA and DHA (10–50 μM), using AA (10–20 μM), and OA (10–20 μM) (n=3–7) as control. (B) Collagen synthesis determined by [3H]-proline incorporation in cardiac fibroblasts treated for 48 hr with EPA (10 μM), DHA (10 μM), 8-bromo-cGMP (1 mM), and/or the guanylyl cyclase inhibitor DT-3 (1 μM) and for the final 24 hr with TGF-β1 (1 ng/ml) (n=4–20). (C) Smad2 and Smad3 phosphorylation (Smad2 Ser465/467; Smad3 Ser423/425) detected by Western blot in cultured cardiac fibroblasts treated for 24 hr with EPA or DHA (10 μM) and for an additional 30 min or 24 hr with fatty acids and TGF-β1 (1 ng/ml). (D, E) Quantification of Smad2 or Smad3 phosphorylation relative to total Smad2/3 (n=3). Data are presented as mean ± SEM. Means were compared by each pair Student t test in B, one-way ANOVA with Dunnet’s post-hoc test in A and Tukey’s post-hoc test in D, E.
Smad2 and Smad3 phosphorylation and subsequent translocation to the nucleus are required for TGF-β1 signaling. Since EPA and DHA demonstrated an inhibitory effect on TGF-β1-induced fibrosis (Figure 5B–E), we tested the effects of EPA and DHA on the TGF-β1-induced phosphorylation of Smad2 and Smad3. In cultured cardiac fibroblasts, TGF-β1 treatment for 30 minutes induced robust phosphorylation of Smad2 (2.7 fold, P<0.01) and Smad3 (2.2 fold, P<0.01). Longer exposure (24 hours) to TGF-β1 induced phosphorylation of Smad2 but not Smad3 (Figure 6C–E). However, EPA and DHA (10 μM) failed to block TGF-β1-induced phosphorylation of Smad2 and Smad3 (Figure 6C–E) at either 30 minutes or 24 hours, which was consistent with our finding that fish oil did not inhibit TAC-induced phosphorylation of Smad2 in vivo (Figure 4B–C). Thus, the inhibitory effect of EPA and DHA on TGF-β1-induced cardiac fibrosis is not due to inhibition of Smad phosphorylation.
EPA and DHA Block Nuclear Translocation of Phosphorylated Smad2 and Smad3 Through the cGMP/PKG Pathway
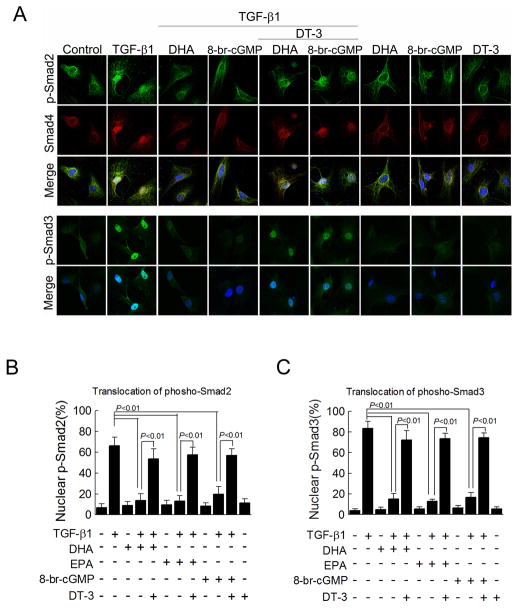
Since TGF-β1-induced phosphorylation of Smads was not blocked by DHA and EPA, we analyzed the ability of EPA and DHA to block TGF-β1-induced nuclear translocation of phospho-Smad2 and-Smad3. In cultured cardiac fibroblasts, TGF-β1 increased the nuclear localization of phospho-Smad2 (66%, P<0.01) and phospho-Smad3 (83%, P<0.01) (Figure 7). TGF-β1-induced nuclear translocation of both Smads was significantly blocked by EPA and DHA (phospho-Smad2: reduced to 15% with DHA; to 13% with EPA and phospho-Smad3: reduced to 14% with DHA; to 13% with EPA) (Figure 7). Furthermore, 8-bromo-cGMP (1 mM) blocked TGF-β1-induced translocation of both phospho-Smad2 and-Smad3, whereas DT-3 reversed the effects of EPA and DHA on nuclear translocation of Smads in cardiac fibroblasts treated with TGF-β1 (Figure 7). Therefore, EPA and DHA inhibition of TGF-β1-induced fibrosis is mediated by blockage of phospho-Smad nuclear translocation.
Figure 7. EPA and DHA block nuclear translocation of phosphorylated Smad2 and Smad3 through the cGMP/PKG pathway.
(A) Phospho-Smad localization detected by staining for phospho-Smad2 (green, first row), Smad4 (red, second row) or phospho-Smad3 (green, fourth row) with a nuclear counter stain (DAPI, merged images third and fifth rows) in cultured cardiac fibroblasts treated for 24 hr EPA (10 μM) (images not shown), DHA (10 μM), 8-bromo-cGMP (1 mM), and/or the guanylyl cyclase inhibitor DT-3 (1 μM) and for an 25 additional 30 min with fatty acids and TGF-β1 (1 ng/ml). (B, C) Quantification of the percent of nuclei positive for phosphorylated Smad2 and Smad3 (counting 50–200 cells per field, n=5–33 in B and n=4–31 in C). Data are presented as mean ± SEM. Data were analyzed by one-way ANOVA with Tukey’s post-hoc test.
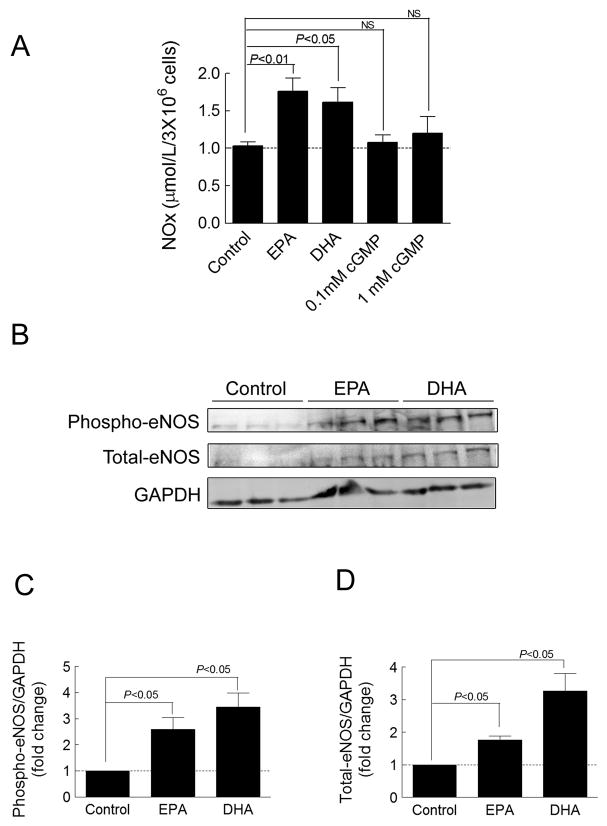
EPA and DHA Increased NOx Production, phospho-eNOS and eNOS Expression in Cardiac Fibroblasts
Nitric oxide (NO) increases cGMP production through activation of soluble guanylyl cyclase (sGC)21–22 and ω-3 PUFAs increase NO synthesis in humans.23 Therefore, we tested the hypothesis that EPA and DHA increase NO production in cardiac fibroblasts. Because NO is rapidly metabolized, we measured the total intracellular level of nitrite (NO2 −) and nitrate (NO3−), stable NO metabolites, as an index of total NO production. Twenty-four hours of treatment with EPA and DHA (10 μM) significantly increased nitrite and nitrate levels (NOx) in cardiac fibroblasts (70% in EPA and 65% in DHA). We also examined whether NO production was induced with an increase in intracellular levels of cGMP. As expected, the cGMP analog, 8-br-cGMP, (0.1 and 1 mM) did not induce NO production in cardiac fibroblasts (Figure 8A). This demonstrates that the increase of NO is not a result of increased intracellular cGMP.
Figure 8. EPA and DHA increase NOx production, phospho-eNOS and eNOS expression in cardiac fibroblasts.
(A) The levels of nitrite/nitrate (NOx) in the cell were determined using a Total Nitrate/Nitrite Fluorometric method in cultured cardiac fibroblasts treated for 24 hr with EPA (10 μM), DHA (10 μM), 8-bromo-cGMP (0.1 mM), or 8-bromo-cGMP (1 mM) (n=4–14). (B) Phospho-eNOS and eNOS detected by western blot in cultured cardiac fibroblasts treated for 24 hr with EPA or DHA (10 μM). (C, D) Quantification of phospho-eNOS or eNOS relative to GAPDH (n=7–9). Data are presented as mean ± SEM. Data were analyzed by one-way ANOVA with Tukey’s post-hoc test.
To determine if EPA and DHA enhance expression of phospho-eNOS and eNOS, we measured phospho-eNOS and eNOS protein levels in cardiac fibroblasts. 11 Treatment with 10 μM DHA or EPA significantly increased phospho-eNOS and eNOS protein levels in cardiac fibroblasts (Figure 8B–D). These results demonstrate that EPA and DHA increase phospho-eNOS and eNOS expression, thereby promoting NO production in cardiac fibroblasts.
To examine other possible mechanisms involved in cGMP production and degradation,21–22 we tested the effects of EPA and DHA on particulate guanylyl cyclase activity, cGMP-specific phosphodiesterases activity, and mRNA levels of ANP and BNP in cardiac fibroblasts. We found that EPA (10 μM) and DHA (10 μM) did not increase particulate guanylyl cyclase activity and did not inhibit cGMP-specific phosphodiesterases activity (data not shown). We also found that EPA (10 μM) and DHA (10 μM) significantly decreased ANP mRNA and did not change the BNP mRNA in cardiac fibroblasts (data not shown).
Discussion
In the present study, we investigated the effects of ω-3 PUFAs on the cardiac fibrotic response. Fischer 24 reported that a diet supplemented with ω-3 PUFAs inhibits left ventricle perivascular fibrosis in rats carrying human rennin and angiotensinogen transgenes, and Medeiros 25 reported that ω-3 PUFAs and palm oil (which is devoid of ω-3 PUFAs) reduce cardiac fibrosis in diabetic SHR rats. However, in the above hypertension models, ω-3 PUFAs significantly decreased the systemic arterial blood pressure. Therefore, from the above studies, we cannot conclude that the anti-fibrotic effect of ω-3 PUFAs is blood pressure independent. At the same time, both studies were comparing the group fed an ω-3 PUFA-supplemented diet to a control group fed a diet containing less total fatty acids, which makes the results more difficult to interpret. In the present study, which used an isocaloric control diet containing equal levels of total fatty acids, ω-3 PUFAs decreased pressure overload-induced cardiac fibrosis by 63% and consequently preserved cardiac function without changing the blood pressure or pressure gradients (Supplementary Table 3). Therefore, our results demonstrate an anti-fibrotic effect of ω-3 PUFAs independent of afterload.
We also identified the in vivo mechanism for this anti-fibrotic effect. Here, we found that in the heart, aortic constriction significantly increased the levels of active TGF-β1 protein, phosphorylation of Smad2, transformation of cardiac fibroblasts, non-myocyte proliferation, and the levels of collagen I and III mRNA. We also found that ω-3 PUFAs prevented the increase of transformation of cardiac fibroblasts, non-myocyte proliferation, and collagen I and III mRNA without altering the levels of active TGF-β1 protein and phosphorylation of Smad2. Therefore, our findings indicate that ω-3 PUFAs inhibit pressure overload-induced cardiac fibrosis by preventing cardiac fibroblast transformation, proliferation, and consequently collagen I and III gene expression. Our findings also indicate that ω-3 PUFAs do not inhibit TGF-β1 fibrotic signaling at the level of the TGF-β1 ligand, TGF-β1 receptors or phosphorylation of Smad2.
Using cultured adult mouse cardiac fibroblasts, we further identified the anti-fibrotic signaling of ω-3 PUFAs and its interaction with TGF-β1’s fibrotic signaling. In our in vitro study, we saw increased ω-3 content and cGMP levels in DHA-and EPA-treated cardiac fibroblasts. These findings indicate that EPA and DHA can be incorporated into cardiac fibroblasts, and activate cGMP signaling in cardiac fibroblasts. We also observed that EPA and DHA prevented TGF-β1-induced proliferation, transformation and collagen synthesis in cardiac fibroblasts and TGF-β1-induced Smad-responsive promoter activity (Supplementary Figure 1). We also found that DT3, a highly selective PKG inhibitor, blocked the inhibitory effects of EPA and DHA on TGF-β1-induced cardiac fibroblast proliferation, transformation and collagen synthesis in cardiac fibroblasts. These findings suggest that EPA and DHA act through the cGMP/PKG pathway to achieve their anti-fibrotic effect in cardiac fibroblasts.
Cyclic GMP has an inhibitory effect on the TGF-β1-induced fibrotic response.9–10, 26 Li 10 reported that disruption of nuclear translocation of Smad3 accounts for the anti-fibrotic effect of cGMP/PKG signaling against the TGF-β1 signaling pathway. In our observations, EPA and DHA increased cGMP levels in cardiac fibroblasts in a dose-dependent manner. EPA and DHA as well as 8-br-cGMP prevented TGF-β1-induced nuclear translocation of phospho-Smad2 and phospho-Smad3 without affecting Smad2 and Smad3 phosphorylation in cardiac fibroblasts. In addition, DT3 reversed the inhibitory effects of 8-Br-cGMP, EPA and DHA on the TGF-β1-induced nuclear translocation of phospho-Smad2 and phospho-Smad3. These findings indicate that in cardiac fibroblasts, EPA and DHA block TGF-β1-induced nuclear translocation of phospho-Smad2 and phospho-Smad3 through activation of the cGMP/PKG pathway (Supplementary Figure 2).
In the cell, NO-sensitive soluble guanylyl cyclase and natriuretic peptide-sensitive particulate guanylyl cyclase produce cGMP, whereas cGMP-specific phosphodiesterases hydrolyze cGMP.21–22 DHA increases NO synthesis and phospho-eNOS and eNOS expression levels.27 NO promotes cGMP production through activation of nitric oxide-sensitive soluble guanylyl cyclase.21–22 In this study, we found that EPA and DHA significantly increased expression levels of phospho-eNOS and eNOS and increased nitrite/nitrate levels in cardiac fibroblasts. We also found that EPA and DHA did not increase particulate guanylyl cyclase activity or ANP/BNP production. Additionally, EPA and DHA did not inhibit cGMP-specific phosphodiesterase activity. Therefore, we postulate that EPA and DHA increase intracellular levels of cGMP by increasing phospho-eNOS and eNOS protein levels and NO production in cardiac fibroblasts.
TGF-β1-induced phosphorylation of ERK1/2 is also involved in the TGF-β1-induced fibrotic response in fibroblasts.28 To determine whether EPA and DHA affect TGF-β1-induced phosphorylation of ERK1/2, we tested phospho-ERK levels in cardiac fibroblasts after one hour treatment of TGF-β1 with or without pretreatment of EPA and DHA. TGF-β1 (1 ng/ml) induced significant phosphorylation of ERK1/2 in cardiac fibroblasts whereas EPA and DHA did not block this effect (Supplementary Figure 3).
In summary, we discovered an anti-fibrotic effect of EPA and DHA and established that EPA and DHA exert their anti-fibrotic effect through activation of the cGMP/PKG pathway. We extended the current understanding of the mechanism of cGMP’s anti-fibrotic effects by demonstrating that activation of PKG blocks TGF-β1-induced nuclear translocation of phospho-Smad2 in cardiac fibroblasts. Our results also suggest that ω-3 PUFAs increase cGMP levels in cardiac fibroblasts by increasing phospho-eNOS and eNOS protein levels and NO production.
About 50% of heart failure cases are due to diastolic dysfunction.2 One of the main causes of diastolic heart failure is cardiac fibrosis, and at present, there are no therapies available to prevent or treat cardiac fibrosis.4, 8, 29 Given the clinically proven safety and tolerance of oily fish, fish oil and the DHA+EPA preparations, a new clinical study of the therapeutic effect of ω-3 PUFAs on cardiac fibrosis and diastolic heart failure is warranted.
Study Limitations
This study examined the preventive effects of ω-3 PUFAs on a pressure overloaded animal model which only mimics some aspects of the disease as it occurs in human. In this animal model, pressure overload induced an acute cardiac injury with a rapid progression which is more severe compared to human hypertension conditions. Another limitation of this study is that even though we fed ω-3 PUFAs at a reasonable level (1% energy), the RBC levels achieved were much higher compared to humans taking two grams of EPA and DHA daily.30 Therefore, our findings should be explored with lower ω-3 PUFA doses.
CLINICAL PERSPECTIVE.
Heart failure is the leading reason for hospital admissions and is the most expensive Medicare expenditure. About half of heart failure cases are due to diastolic dysfunction. One of the main causes of diastolic dysfunction is cardiac fibrosis, and there are no therapies available to prevent or treat cardiac fibrosis. Transforming growth factor (TGF)-β1-induced cardiac fibroblast transformation and proliferation are the key events lead to cardiac fibrosis. This study shows that ω-3 PUFAs prevent pressure overload-induced cardiac fibrosis and subsequent cardiac dysfunction. This study also demonstrates that in cardiac fibroblast, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) increase cGMP levels by increasing phospho-eNOS and eNOS protein levels and nitric oxide production, and exerts their anti-fibrotic effect through activation of the cGMP/PKG pathway and subsequent blocking of TGF-β1-induced nuclear translocation of phospho-Smad2 and phospho-Smad3. This study defines the beneficial effects of ω-3 PUFAs on cardiac fibrosis and cardiac dysfunction, and clarifies the underlying mechanisms. In addition, this study provides the basis for extending the application of ω-3 PUFAs, which appear to be exceptionally safe and well tolerated, to the prevention of cardiac fibrosis.
Supplementary Material
Acknowledgments
We thank the Physiology and Cell Culture Cores at Sanford Research/USD for technical support and James V. Pottala for his assistance with the longitudinal analysis.
Source of Funding
This study was supported by NIH National Center for Research Resources Grant 2P20RR017662-06A1 and by the South Dakota 2010 Initiative Research Centers Program.
Footnotes
Disclosures: WSH and GCS have relations with companies that sell omega-3 products. WSH and GCS are on the speaker’s bureau for GlaxoSmithKline (GSK) and have received grants from GSK. WSH is a scientific advisor to GSK. WSH is also a consultant for and has received research grants from Monsanto. In addition, WSH is the owner of a company that offers blood omega-3 tests (OmegaQuant Analytics).
References
- 1.Braunwald E, Bristow MR. Congestive heart failure: Fifty years of progress. Circulation. 2000;102:IV14–23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 2.Gary R, Davis L. Diastolic heart failure. Heart Lung. 2008;37:405–416. doi: 10.1016/j.hrtlng.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Berk BC, Fujiwara K, Lehoux S. Ecm remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998;16:1031–1041. doi: 10.1097/00004872-199816070-00018. [DOI] [PubMed] [Google Scholar]
- 5.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: Therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 6.Kai H, Kuwahara F, Tokuda K, Imaizumi T. Diastolic dysfunction in hypertensive hearts: Roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens Res. 2005;28:483–490. doi: 10.1291/hypres.28.483. [DOI] [PubMed] [Google Scholar]
- 7.Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–135. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- 8.Leask A. Tgfbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res. 2007;74:207–212. doi: 10.1016/j.cardiores.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Buxton IL, Duan D. Cyclic gmp/protein kinase g phosphorylation of smad3 blocks transforming growth factor-beta-induced nuclear smad translocation: A key antifibrogenic mechanism of atrial natriuretic peptide. Circ Res. 2008;102:151–153. doi: 10.1161/CIRCRESAHA.107.170217. [DOI] [PubMed] [Google Scholar]
- 10.Li P, Wang D, Lucas J, Oparil S, Xing D, Cao X, Novak L, Renfrow MB, Chen YF. Atrial natriuretic peptide inhibits transforming growth factor beta-induced smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circ Res. 2008;102:185–192. doi: 10.1161/CIRCRESAHA.107.157677. [DOI] [PubMed] [Google Scholar]
- 11.Levitan EB, Wolk A, Mittleman MA. Fish consumption, marine omega-3 fatty acids, and incidence of heart failure: A population-based prospective study of middle-aged and elderly men. Eur Heart J. 2009;30:1495–1500. doi: 10.1093/eurheartj/ehp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–2021. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, Inaba Y, Tanabe N, Tamakoshi A. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of japanese men and women the jacc (japan collaborative cohort study for evaluation of cancer risk) study. J Am Coll Cardiol. 2008;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the gissi-hf trial): A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 15.Picq M, Dubois M, Grynberg A, Lagarde M, Prigent AF. Specific effects of n-3 fatty acids and 8-bromo-cgmp on the cyclic nucleotide phosphodiesterase activity in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 1996;28:2151–2161. doi: 10.1006/jmcc.1996.0207. [DOI] [PubMed] [Google Scholar]
- 16.O’Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, Cotecchia S, Rokosh DG, Grossman W, Foster E, Simpson PC. The alpha(1a/c)-and alpha(1b)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J Clin Invest. 2003;111:1783–1791. doi: 10.1172/JCI16100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, Tecott LH, Baker AJ, Foster E, Grossman W, Simpson PC. Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest. 2006;116:1005–1015. doi: 10.1172/JCI22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang YD, Kuzman JA, Said S, Anderson BE, Wang X, Gerdes AM. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation. 2005;112:3122–3130. doi: 10.1161/CIRCULATIONAHA.105.572883. [DOI] [PubMed] [Google Scholar]
- 19.Duda MK, O’Shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, Chess DJ, Azimzadeh AM, Harris WS, Sharov VG, Sabbah HN, Stanley WC. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009;81:319–327. doi: 10.1093/cvr/cvn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duda MK, O’Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Dietary supplementation with omega-3 pufa increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res. 2007;76:303–310. doi: 10.1016/j.cardiores.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritchie RH, Irvine JC, Rosenkranz AC, Patel R, Wendt IR, Horowitz JD, Kemp-Harper BK. Exploiting cgmp-based therapies for the prevention of left ventricular hypertrophy: No* and beyond. Pharmacol Ther. 2009;124:279–300. doi: 10.1016/j.pharmthera.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Tsai EJ, Kass DA. Cyclic gmp signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther. 2009;122:216–238. doi: 10.1016/j.pharmthera.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris WS, Rambjor GS, Windsor SL, Diederich D. N-3 fatty acids and urinary excretion of nitric oxide metabolites in humans. Am J Clin Nutr. 1997;65:459–464. doi: 10.1093/ajcn/65.2.459. [DOI] [PubMed] [Google Scholar]
- 24.Fischer R, Dechend R, Qadri F, Markovic M, Feldt S, Herse F, Park JK, Gapelyuk A, Schwarz I, Zacharzowsky UB, Plehm R, Safak E, Heuser A, Schirdewan A, Luft FC, Schunck WH, Muller DN. Dietary n-3 polyunsaturated fatty acids and direct renin inhibition improve electrical remodeling in a model of high human renin hypertension. Hypertension. 2008;51:540–546. doi: 10.1161/HYPERTENSIONAHA.107.103143. [DOI] [PubMed] [Google Scholar]
- 25.Medeiros FJ, Mothe CG, Aguila MB, Mandarim-de-Lacerda CA. Long-term intake of edible oils benefits blood pressure and myocardial structure in spontaneously hypertensive rat (shr) and streptozotocin diabetic shr. Prostaglandins Other Lipid Mediat. 2005;78:231–248. doi: 10.1016/j.prostaglandins.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic gmp phosphodiesterase 5a prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 27.Stebbins CL, Stice JP, Hart CM, Mbai FN, Knowlton AA. Effects of dietary decosahexaenoic acid (dha) on enos in human coronary artery endothelial cells. J Cardiovasc Pharmacol Ther. 2008;13:261–268. doi: 10.1177/1074248408322470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Sun SQ, Hassid A, Ostrom RS. Camp inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and smad signaling in cardiac fibroblasts. Mol Pharmacol. 2006;70:1992–2003. doi: 10.1124/mol.106.028951. [DOI] [PubMed] [Google Scholar]
- 29.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–163. doi: 10.1161/01.cir.89.1.151. [DOI] [PubMed] [Google Scholar]
- 30.Harris WS, Von Schacky C. The omega-3 index: A new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.