Abstract
Caulobacter crescentus divides asymmetrically and creates distinct polar membrane surfaces that partition during the cell cycle to distinct cell progeny. Blocking membrane synthesis prevented transcription from selective promoters involved in asymmetric cell division. Transcription from sigma-54-dependent flagellar promoters was blocked completely; however, transcription from the CtrA response regulator-dependent flagellar promoters was activated but reduced. Transcription from the ccrM (DNA methylation) promoter and the che (chemosensory) promoter was also blocked completely. Transcription from a strong promoter at the chromosome replication origin was first stopped then induced by blocked membrane synthesis. We propose a feedback control coupling membrane synthesis to transcription that selectively supports membrane-associated processes such as flagellar assembly, chemosensory biogenesis and chromosome replication.
Keywords: cell cycle/feedback/membranes/polarity/transcription
Introduction
The complexities of biological membranes are only partially explained by their established properties (Dowhan, 1997). In bacteria, the lack of conspicuous microfilament and motor proteins implies that membranes assume additional, but poorly understood roles in cellular organization. Bacteria that divide asymmetrically offer an especially attractive system for studying the relationships between membrane metabolism and cellular organization, because these bacteria must have precise mechanisms that distinguish opposite polar membrane surfaces (Shapiro and Losick, 1997; Wu and Newton, 1997).
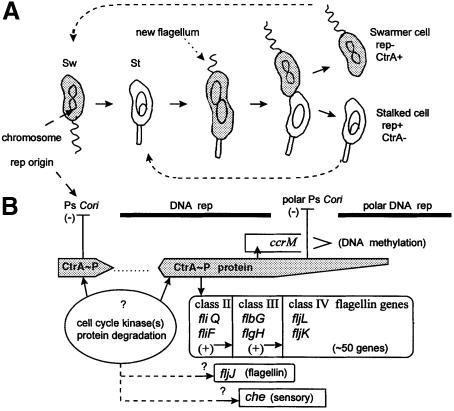
Caulobacter crescentus divides asymmetrically and creates unique polar surfaces during its dimorphic cell cycle (Figure 1). The ‘swarmer cell pole’ has a unique flagellum and chemosensory proteins (Brun et al., 1994). The opposite ‘stalked cell pole’ lacks these components, but maintains a tubular extension of the cell wall (the stalk). C.crescentus'distinct cell poles are formed prior to cell division that yields distinct swarmer cell and stalked cell progeny (Figure 1). These cells have different behaviors and different genetic programs (Brun et al., 1994). Stalked cells are non-motile. Swarmer cells swim and chemotax. Stalked cells immediately initiate chromosome replication and they divide asymmetrically. Swarmer cells delay chromosome replication until they differentiate into stalked cells (Figure 1).
Fig. 1. Caulobacter crescentus dimorphic cell cycle and regulatory systems. (A) The motile swarmer (Sw) loses its flagellum as it differentiates into a sessile stalked cell (St) where chromosome replication and asymmetric cell division take place. A new flagellum is synthesized and assembled at the new swarmer cell pole. The shaded cytoplasm indicates both the presence and the phosphorylation of the CtrA–P protein. (B) Interrelationships between CtrA protein and major cell cycle events. The arrows indicate the interactions described in the text.
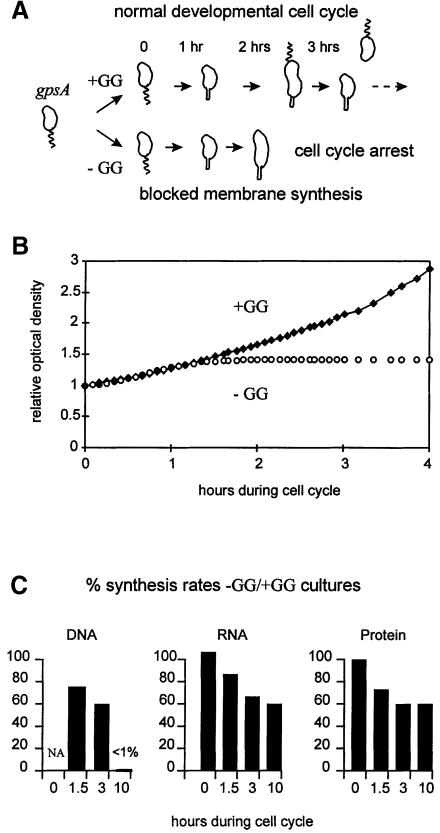
Blocking phospholipid synthesis permits the study of physiological processes that require membrane biogenesis (Mindich, 1975; Dowhan, 1997). The gpsA gene product provides biosynthetic sn-glycerol-3-phosphate whose subsequent acylation is the committed step for phospholipid biosynthesis (Ray and Cronan, 1987). gpsA mutations were isolated in a number of bacteria (Mindich, 1975), including C.crescentus (Contreras et al., 1980). In each case, null gpsA alleles confer glycerol and/or glycerol-3-phosphate auxotrophy, and membrane phospholipid synthesis stops abruptly upon withdrawal of the glycerol supplement. This treatment has significant consequences for the C.crescentus cell cycle (Figure 2A). For example, cell division and the initiation of a second round of chromosome replication both require phospholipid synthesis (O'Neill and Bender, 1989; Loewy et al., 1990).
Fig. 2. Blocked membrane synthesis through glycerol and glycerol-3-phosphate (GG) withdrawal from the synchronized gpsA swarmer cells (strain GM1461), and the consequences on (A) cell cycle progression, (B) cell growth at relative optical density of 660 nm and (C) macromolecule synthesis. This basic protocol was repeated for each of the subsequent experiments in Figures 3–7.
In C.crescentus, only selective cell cycle protein synthesis is blocked when the gpsA mutants block membrane biogenesis. While bulk protein synthesis continues, the synthesis of the 70 kDa hook and the 25 and 27 kDa flagellin proteins is selectively blocked (Shapiro et al., 1982). These flagellar components are only synthesized during discrete periods of the cell cycle (Brun et al., 1994; Wu and Newton, 1997) as part of the cell division program that creates a new polar flagellum (Figure 1). These three proteins are synthesized from the flbG, fljL and fljK transcription operons, respectively, shown in Figure 1B as representatives of class III and class IV genes within the flagellar genetic hierarchy (Brun et al., 1994; Wu and Newton, 1997).
The CtrA protein is a major cell cycle transcription regulator on top of this genetic hierarchy (Quon et al., 1996). CtrA is regulated through phosphorylation by cell cycle histidine kinases and stalked cell-specific protein degradation (Domian et al., 1997). Therefore, CtrA is absent from the new stalked cell (St) and from the stalked cell compartment of the dividing cell (Figure 1). CtrA was originally identified as the activator of early transcribed (class II) flagellar genes, and ctrA is regarded as a class I flagellar gene (Quon et al., 1996). The class II, class III and class IV genes form a hierarchy where the expression of genes in one class depends on the functional expression of all the genes in the preceding class (Shapiro and Losick, 1997; Wu and Newton, 1997). The fljJ (29 kDa flagellin) and che (chemosensory operon) genes are also transcribed selectively in stalked cells, and their proteins are also targeted specifically to the swarmer cell pole (Loewy et al., 1987; Alley et al., 1992). However, regulators for the fljJ and che promoters have not been identified, and these promoters have not been placed within the genetic hierarchy (Figure 1B).
CtrA also regulates DNA metabolism. It activates the transcription of the ccrM DNA methyltransferase gene, but this occurs later during the cell cycle (Figure 1) than for the class II flagellar genes (Reisenauer et al., 1999). The chromosome replication origin (Cori) contains five CtrA-binding sites (Quon et al., 1998), including two binding sites overlapping the strong (Ps) promoter (Marczynski et al., 1995). CtrA represses Ps Cori transcription in the swarmer cell and at the swarmer cell pole and, accordingly, punctuates the chromosome replication periods (Figure 1).
This study demonstrates that selective cell cycle transcription requires membrane synthesis. Transcription is blocked only from certain promoters. This implies selective requirements for membrane synthesis by transcription regulators. We discuss feedback control mechanisms involving the genetic pathways illustrated in Figure 1.
Results
Blocked membrane synthesis, cell cycle growth and macromolecule synthesis
To analyze how cell cycle events depend on membrane synthesis, synchronous gpsA swarmer cells were isolated, washed free of the glycerol and glycerol-3-phoshate (GG) supplement and released into duplicate cultures either with (+GG) or without (–GG) supplement (Figure 2A). The +GG cells behave indistinguishably from the wild-type cells, but membrane synthesis stops abruptly in the –GG cells (Loewy et al., 1990). Although they differentiate into stalked cells (Contreras et al., 1980; O'Neill and Bender, 1989) and initiate DNA synthesis, these –GG cells fail to complete DNA replication and they arrest uniformly as non-motile but elongated stalked cells (Loewy et al., 1990).
Figure 2C demonstrates that appreciable RNA and protein synthesis continue during the cell cycle block imposed by removing the GG supplement. DNA, RNA and protein synthesis rates were measured at 0, 1.5, 3 and 10 h into the cell cycle and normalized per cell optical density. To facilitate comparison, the synthetic rates were presented as percentage ratios between the –GG and +GG cultures. Although cell growth stopped in the –GG culture (Figure 2B), the RNA and protein synthesis continued in both –GG and +GG cultures. Even after 10 h (∼3 cell division cycles in +GG), RNA and protein synthesis rates remained high in the –GG culture, at least at 50% levels, relative to the growing +GG culture. Relative DNA synthesis, comparable after 1.5 and 3 h into the cell cycle, was not detectable (<1%) in the –GG culture after 10 h. This is consistent with a uniform cell cycle arrest (Figure 2A).
Blocked membrane synthesis does not prevent the transcription of most genes
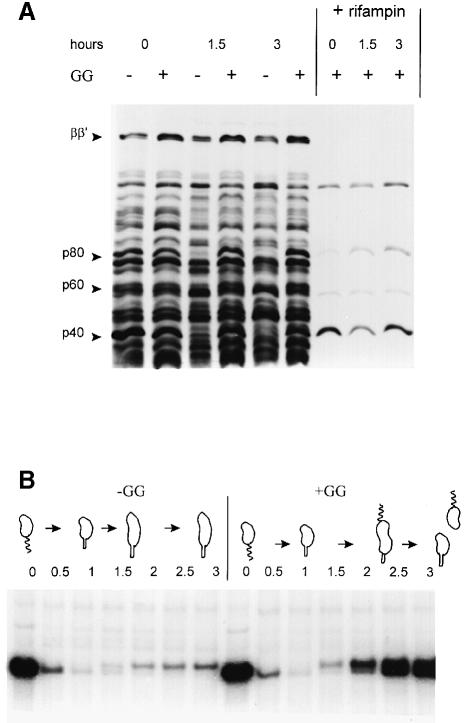
Figure 3A demonstrates that after 0, 1.5 and 3 h into the cell cycle, side by side –GG and +GG cultures synthesized most of the same proteins. Three exceptions are p80, p60 and p40 (80, 60 and 40 kDa unidentified proteins) absent only in the –GG cells at 1.5 and 3 h. In contrast, RNA polymerase subunits (ββ′), as well as most of the unidentified proteins, were synthesized in both –GG and +GG cultures. Newly synthesized and unstable mRNA programs most of this protein synthesis. A 10 min pre-incubation with rifampin abolished the synthesis of all but four proteins (Figure 3A). Therefore, although blocking membrane synthesis arrests cell cycle progression, only the transcription of selective genes is blocked under these conditions.
Fig. 3. Protein synthesis during the cell cycle with and without (–GG and +GG) blocked membrane synthesis. (A) Fluorogram of total 35S-labeled proteins, extracted by boiling cells in protein loading buffer and resolved by 6% SDS–PAGE. The synchronized cultures (Figure 2A) were sampled and labeled for 5 min with [35S]methionine, or sampled and incubated with 10 μg/ml rifampin prior to the 35S pulse label, at the times indicated. (B) Fluorogram of immuno-precipitated 35S-labeled flagellin proteins. Soluble proteins were extracted, pre-cleared with Staphylococcus cells, immunoprecipitated with rabbit antiserum and resolved by 10% SDS–PAGE. These synchronized cultures (Figure 2A) likewise were sampled and pulse-labeled with [35S]methionine at the cell cycle times indicated.
Selective flagellin protein synthesis requires membrane synthesis
The –GG gpsA cells become non-motile, and blocked 25 and 27.5 kDa flagellin protein synthesis was reported in unsynchronized cultures (Shapiro et al., 1982). The immune precipitates in Figure 3B demonstrate that the –GG cell cycle arrest selectively blocks protein synthesis of the 25 and 27.5 kDa flagellins, but not the synthesis of the 29 kDa flagellin. The filament (propeller) is composed of 29, 27.5 and 25 kDa flagellin proteins (Brun et al., 1994). As shown in Figure 3B, swarmer cells at 0 h synthesized large amounts of flagellins, but stalked cells at 1.0 h synthesized no flagellin proteins. In the +GG culture, flagellin synthesis resumed as the stalked cells proceeded to divide asymmetrically. However, in the –GG culture, only the synthesis of the 29 kDa flagellin protein was resumed. The transcription promoter for the 29 kDa flagellin gene is distinct from the sigma-54 promoters for the 27.5 and 25 kDa flagellin genes (Brun et al., 1994). Therefore, selective protein synthesis in Figure 3 implies that selective promoters require membrane synthesis.
Selective flagellin gene transcription requires membrane synthesis
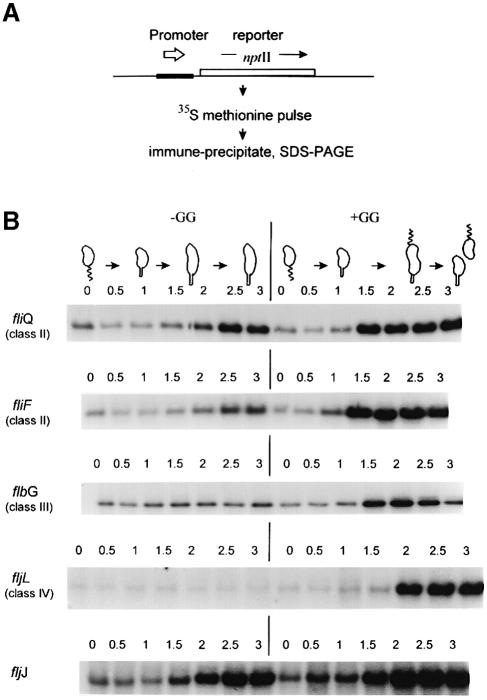
Figure 4 clearly demonstrates that the fljJ (29 kDa flagellin) promoter is activated, but that the fljL (27.5 kDa flagellin) promoter remains inactive in the –GG culture. To exclude post-transcription mechanisms, we assayed transcription by the protocol in Figure 4A. Test promoters were fused to the nptII reporter gene with its own Shine and Dalgarno sequence. Therefore, reporter protein synthesis was only directed by transcription from the test promoter. The NptII protein is very stable in C.crescentus (Champer et al., 1987), and this transcription reporter method avoids RNA degradation artifacts and inherent mRNA instability (Figure 3A). Test promoter constructs, on stable broad host-range plasmids (Table I), were introduced into the C.crescentus gpsA strain GM1461 for the following experiments.
Fig. 4. Transcription from flagellar promoters during the cell cycle with and without (–GG and +GG) blocked membrane synthesis. (A) Protocol for measuring transcription from promoters fused to the nptII reporter gene, which lacks its own promoter but contains an efficient ribosome-binding site. (B) Five separate cell cycle transcription experiments based on the blocked membrane synthesis protocol (Figure 2A). Each panel is an SDS–PAGE fluorogram revealing NptII reporter protein synthesis sampled at the times (h) indicated above each lane. GM1461 contained one of the following transcription reporter plasmids: pSN1 (fliQ), pHX280 (fliF), pHX120 (flgH), pGM210 (fljL) or pZL1451 (fljJ); see Table I.
Table I. Bacterial strains and plasmids.
| Genotype or description | Reference or source | |
|---|---|---|
| E.coli | ||
| S17-1 | E.coli 294::RP4-2 (Tc::Mu) (Km::Tn7) | Simon et al. (1983) |
| C.crescentus | ||
| NA1000 | CB15N synchronizable wild type | Evinger and Agabian (1977) |
| AE5168 | CB15 gpsA505 | Contreras et al. (1980) |
| SC1090 | CB15 purA::Tn5 | B.Ely |
| GM1461 | CB15N gpsA505 | this work |
| Plasmids | ||
| pRK290/20R | pRK290 with polylinker | J.W.Gober |
| pKIC7 | promoterless nptII cloning vector, Ap | M.R.K.Alley |
| pSN1 | P fliQ::nptII in pRK290a | A.Dingwall |
| pHX280 | P fliF::nptII in pRK290a | H.Xu |
| pHX120 | P flbG::nptII in pRK290 | H.Xu |
| pADN-1 | P flgH::nptII in pRK290a | A.Dingwall |
| pZL1451 | P fljJ::nptII in pRK290a | Loewy et al. (1987) |
| pGM210b | P fljL::nptII in pRK290a | J.W.Gober |
| pRKlac290 | RK2-based lacZ transcription reporter | J.W.Gober |
| pRCZ1 | P che::lacZ, RK2-based, Km, Tc | Alley et al. (1991) |
| pGZ22 | P ccrM::lacZ in pRK290 | C.Stephens |
| pGM986c | P tac::lacZ in pRK290, lacIQ | this work |
| ppGM976d | pRKlac290 Cori BamHI to Ps::lacZ | Marczynski et al. (1995) |
| pGM1028e | pRKlac290 Cori BamHI to PsΔ::lacZ | Marczynski et al. (1995) |
| pRK290-Tn5ΩMP | CcrM methylation-sensitive XhoI, ClaI sites | Zweiger et al. (1994) |
aNote new nomenclature: fliF, flgH changes from flaO, flbN, and fliQ, fljJ, fljL changes from flaS, flgJ, flgL now match the E.coli nomenclature (http://www.cosm.sc.edu/caulobacter/map.html).
bAn 830 bp HindIII–SalI fragment upstream of fljL cloned into pKIC7, then transferred as a HindIII fragment into pRK290/20R.
cThe regulated P tac promoter and lacIQ gene derive from pBRG1410 on an EcoRI fragment inserted into pRKlac290.
dPs, strong promoter overlapping CtrA-binding sites (a) and (b), Cori DNA end point positions 243–998 in the HindIII and BamHI sites of pRKlac290.
ePsΔ, strong promoter downstream deletion of CtrA-binding site (a) and 1 bp of site (b), Cori DNA end point positions 273–998 in the BamHI site of pRKlac290.
Selective blockage of the flagellar transcription hierarchy
We applied the transcription reporter assay (Figure 4A) to promoters for genes at all levels of the C.crescentus flagellar hierarchy (Figure 1B). When C.crescentus commits to remaking a new polar flagellum (Figure 1), the earliest transcribed genes, dedicated to flagellar biogenesis, belong to class II, represented by fliQ and fliF. Both class II promoters were activated in the –GG synchronized cultures (Figure 4B). However, class III and class IV promoters, represented by flbG and fljL in Figure 4B, were not activated. Although class III and IV promoters induce at 1.5 and 2 h in the +GG cultures, there was no change from baseline transcription in the –GG cultures. Note that these transcription reporters have varying degrees of background transcription (seen in all lanes) from vector DNA sequences. Since background transcription remains constant in the –GG cultures (flbG and fljL in Figure 4B), this also argues that these C.crescentus cell cycle promoters have a special dependence on membrane synthesis.
Chemosensory operon transcription requires membrane synthesis
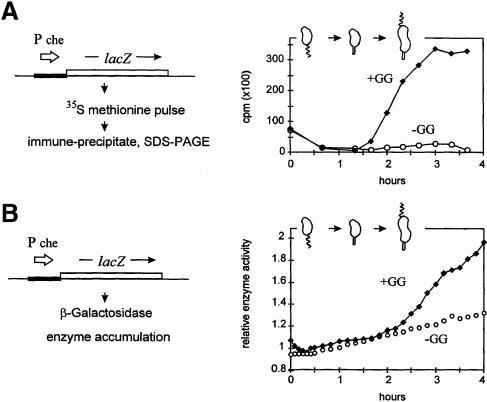
The assay protocol in Figure 5A is essentially the same as that used above in Figure 4A, except that the lacZ gene replaced the nptII reporter. This also allowed us to assay transcription by the accumulation of β–galactosidase activity (Figure 5B). Figure 5A demonstrates that significant LacZ synthesis rates are only induced in the +GG culture. Figure 5B confirms that significant β–galactosidase accumulation likewise only occurred in the +GG culture. Enzyme accumulation followed the increased synthetic rates at 2 h. Note that this lacZ transcription reporter, like the nptII reporters (Figure 4), also has background transcription from vector DNA sequences. In the –GG cultures, background transcription produced a steady linear accumulation of enzyme activity (Figure 5B). This also argues that the C.crescentus che promoter has a special dependence on membrane synthesis, not shared by the constitutive vector promoter(s), and also not shared by the cell cycle class II and fljJ promoters (Figure 4).
Fig. 5. Transcription from the che (chemosensory operon) promoter during the cell cycle with and without (–GG and +GG) blocked membrane synthesis. Comparison of two protocols for measuring transcription from a promoter fused to the lacZ reporter and an efficient ribosome-binding site. In both experiments, C.crescentus strain GM1461 contained plasmid pRCZ1; see Table I. (A) Transcription assayed by β–galactosidase protein synthesis. Scintillation counting measured radioactivity from cut 35S-labeled bands. (B) Transcription assayed by β–galactosidase accumulation. The cartoon indicates the cell types present in the +GG culture based on the protocol in Figure 2A.
The P tac promoter induces during the gpsA cell cycle arrest
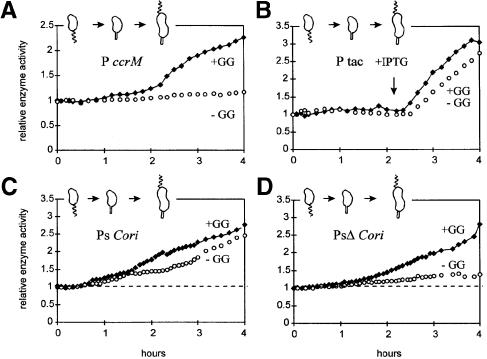
A general inability to induce transcription, irrespective of promoter structure, is excluded by the experiments in Figure 6B. The Escherichia coli P tac promoter is well regulated by the E.coli lacI repressor in C.crescentus. The gratuitous inducer (isopropyl-β-d-thiogalactopyranoside; IPTG) was added at 2.2 h, and this produced comparably vigorous β–galactosidase synthesis in both +GG and –GG cultures (Figure 6B).
Fig. 6. Transcription during the cell cycle with and without (–GG and +GG) blocked membrane synthesis. C.crescentus strain GM1461 contained one of the following lacZ transcription reporter plasmids: (A) pGZ22 (ccrM), (B) pGM986 (P tac), (C) pGM976 (Ps Cori) or (D) pGM1028 (PsΔ Cori); see Table I. IPTG (1 mM) was added at 2.2 h to the cultures in (B). Transcription from these lacZ reporters was assayed, as in Figure 5B, by β–galactosidase accumulation.
Chromosome origin (Cori) promoter transcription during the gpsA cell cycle arrest
The Cori strong (Ps) promoter is repressed and stimulated sequentially during the gpsA cell cycle arrest. The +GG synchronized culture presents Ps expression patterns consistent with published results (Marczynski et al., 1995). β–galactosidase expression from Ps was off in swarmer cells, on in stalked cells and further increased at 1.5 h in the pre-divisional cells (Figure 6C). The parallel –GG culture presents an interesting variation of this pattern. Normal β–galactosidase expression from Ps stopped at 1.5 h, then Ps induced at 2.5 h and, despite the –GG arrest, Ps continued vigorous transcription (Figure 6C).
Induction during the –GG cell cycle arrest requires the wild-type Ps promoter structure. PsΔ removes one CtrA-binding site and the –10 element (Table I). The PsΔ promoter is transcribed preferentially towards the end of the cell cycle (Marczynski et al., 1995). Accordingly, β–galactosidase rises at ∼1.5 h, but only in the +GG culture (Figure 6D). The parallel –GG culture showed only a gradual linear accumulation of β–galactosidase activity, consistent with background transcription. Therefore, during the gpsA cell cycle arrest, the wild-type Ps promoter is repressed at 1.5 h but stimulated at 2.5 h, and this stimulation requires promoter sequences deleted in the PsΔ promoter (Figure 6C and D).
Blocked membrane synthesis prevents ccrM transcription and DNA methylation
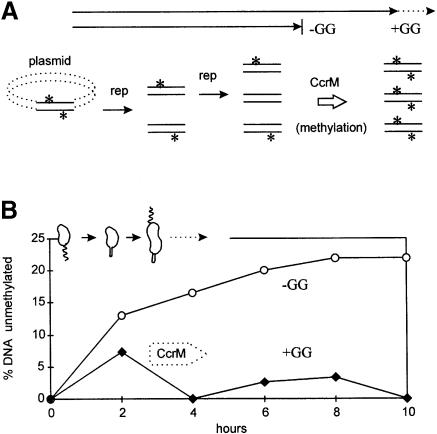
The P ccrM transcription reporter in Figure 6A induced β–galactosidase in the +GG culture, but only produced background enzyme activity in the parallel –GG culture. We confirmed the absence of CcrM by the absence of plasmid DNA methylation. In swarmer cells, both plasmid and chromosome DNA are methylated on both DNA strands. Following replication, the DNA is methylated on only one strand (Zweiger et al., 1994). Plasmids can replicate a second time during the cell cycle and produce unmethylated DNA on both strands (Figure 7A). When CcrM is produced near the end of the cell cycle, all DNA is again methylated on both strands. However, without CcrM, unmethylated DNA accumulates due to promiscuous plasmid replication.
Fig. 7. Plasmid DNA methylation by CcrM during the cell cycle with and without (–GG and +GG) blocked membrane synthesis. (A) Unmethylated plasmid DNA accumulates by plasmid replication in the absence of DNA methylation (asterisk) by the CcrM methyltransferase, normally present after 2 h in dividing cells. (B) Accumulation of unmethylated plasmid DNA on both strands. GM1461 contained pRK290-Tn5ΩMP. This plasmid has CcrM methylation sequences (GANTC) that overlap ClaI restriction sites. DNA methylation was assayed by acquired sensitivity to ClaI digestion. The cartoon shows cell types present in the +GG culture based on the protocol in Figure 2A.
Unmethylated plasmid DNA accumulated in the –GG but not in the +GG synchronized cultures (Figure 7B). Unmethylated plasmid DNA was produced at 2 h in both cultures. CcrM production completely methylated this DNA at 4 h in the +GG culture. However, more unmethylated plasmid DNA accumulated in the –GG culture, and continued to accumulate for 10 h. Considering the sensitivity of this methylation assay (Zweiger et al., 1994), it is unlikely that CcrM methyltransferase is produced during the gpsA cell cycle arrest.
Discussion
We demonstrated that selective genetic transcription requires membrane synthesis. Table II summarizes our results. Transcription reporters that produce stable cytoplasmic proteins, NptII protein or β–galactosidase (Figures 4–6) unambiguously separated regulation by transcription initiation from post-transcription mechanisms (Gober et al., 1991). Most importantly, Table II derives from experiments with parallel –GG and +GG cultures where the supplement for membrane synthesis was the only variable.
Table II. Summary of genetic transcriptiona during the gpsA cell cycle block.
| |
|
Inductiona |
Regulator(s) |
| Flagellar hierarchy | |||
| class I | ctrA | +b | CtrA |
| class II | fliQ | + | CtrA |
| fliF | + | CtrA | |
| class III | flbG | – | sigma-54 |
| flgH | – | sigma-54 | |
| class IV | fljL | – | sigma-54 |
| Independent/cell cycle | |||
| flagellin | fljJ | + | ? |
| chemotaxis | che | – | ? |
| P tac | + | LacI | |
| DNA metabolism | |||
| methylation | ccrM | – | CtrA |
| Cori RNA | Ps | +c | CtrA |
| PsΔ | – | CtrA | |
How might cells coordinate genetic transcription with membrane metabolism?
Part of our data can be rationalized by incomplete CtrA phosphorylation. During the gpsA (–GG) cell cycle block, class II fliQ and fliF promoter transcription is activated but clearly reduced relative to the +GG control (Figure 4B). Class II flagellar promoters require CtrA for transcription both in vivo (Quon et al., 1996) and in vitro (Wu et al., 1998). CtrA protein is replenished and phosphorylated in stalked cells (Figure 1), coincident with class II promoter transcription (Reisenauer et al., 1999). Immune blot experiments indicate that CtrA protein is replenished during the gpsA (–GG) cell cycle block (results not shown), suggesting that reduced fliQ and fliF transcription is due primarily to reduced phosphorylation.
The inactivity of the ccrM promoter might also result from lowered CtrA phosphorylation during the gpsA cell cycle block. This result seemed paradoxical, since the ccrM promoter resembles the class II flagellar promoters. However, the ccrM promoter binds phosphorylated CtrA with at least a 10–fold lower affinity than the fliQ class II promoter, and this may account for the delayed activation of the ccrM promoter relative to the fliQ promoter (Reisenauer et al., 1999). According to our hypothesis, CtrA is present at 2 h during the gpsA cell cycle block (Figure 6A), but it is not phosphorylated sufficiently to activate the ccrM promoter. Accordingly, after 2 h during the gpsA cell cycle block, there is no inherent barrier to activating the P tac promoter (Figure 6B), because it utilizes a completely different mechanism.
We are testing our hypothesis with the CtrAD51E pseudo-phosphorylated allele (Domian et al., 1997) in an attempt to bypass the gpsA transcription block. Preliminary results indicate that artificial expression of the CtrAD51E protein advances the normal induction of P ccrM transcription, but fails to induce P ccrM during the gpsA (–GG) cell cycle block (results not shown). CtrA may not be the only transcription factor dependent on membrane synthesis.
How might the flagellar hierarchy be coordinated with membrane metabolism?
Promoters in the flagellar hierarchy can be grouped into CtrA-dependent promoters that activate transcription during the gpsA cell cycle block, and sigma-54-dependent promoters that do not (Table II). While CtrA-dependent promoters are small (<45 bp plus the CtrA-binding site), the sigma-54-dependent promoters are large and require distant enhancers (Brun et al., 1994). It is not clear why class III and class IV genes fail to be transcribed during the gpsA cell cycle block. The flagellar hierarchy is governed by at least four, as yet poorly understood checkpoint control systems (Wu and Newton, 1997). The functional expression of all genes in one class is required for the expression of genes in the subsequent class. Therefore, the class III and class IV genes might fail to be transcribed during the gpsA cell cycle block because of insufficient expression of one, or insufficient cumulative expression of all the class II gene products. In the class II fliF operon, for example, these gene products include FlbD and its cognate kinase (Wingrove and Gober, 1996). The gpsA cell cycle block may prevent or reduce the phosphorylation of FlbD, as well as of CtrA and similar response regulator proteins.
Transcription from the chromosome replication origin and membrane metabolism
Ps repression in swarmer cells (Marczynski et al., 1995) by CtrA protein binding (Quon et al., 1998) may determine the active (rep+) versus inactive (rep–) chromosome states (Figure 1). Ps revealed an unexpected, but a CtrA-binding site-dependent response to the gpsA cell cycle block. Ps failed to induce transcription at 1.5 h in the gpsA cell cycle block (Figure 6C); however, it clearly induced transcription at 2.5 h and, by 4 h, vigorous Ps transcription nearly compensated for the delay. The PsΔ also failed to induce transcription at 1.5 h (Figure 6D) but, unlike Ps, PsΔ remained inactive. PsΔ completely removes CtrA-binding site (a) over the Ps –10 element. Phosphorylated CtrA protein binds very poorly to the sequences retained in PsΔ (R.Siam and G.T.Marczynski, submitted). These observations imply that CtrA fails to activate PsΔ transcription after 2.5 h, while CtrA activates Ps transcription at 2.5 h in the gpsA cell cycle block.
What is the regulatory significance of altered PsΔ transcription (Figure 6C and D) during the gpsA cell cycle block? If CtrA only served to repress the Ps and PsΔ Cori promoters, the loss of CtrA binding would allow transcription from both promoters after the gpsA cell cycle block. However, CtrA may also be an activator of transcription from Ps, especially considering that CtrA binding at Ps is highly cooperative and phosphorylation dependent (R.Siam and G.T.Marczynski, submitted). The PsΔ mutation also delays Cori plasmid replication during the cell cycle (Marczynski et al., 1995). Therefore, although the details are not yet understood, we believe that the gpsA block reflects the complex regulation of the Ps Cori promoter and its dual response to CtrA-P protein.
Why should cells coordinate transcription with membrane metabolism?
The bacterial membrane may participate actively in organizing the bacterial cell. Our studies have perhaps revealed a feedback control whereby continued membrane synthesis is required to sustain RNA synthesis for processes, especially flagellar assembly and chromosome replication, which are associated intimately with the membrane. The logic of the feedback control dictates that, for example, membrane proteins are only made when a membrane surface is prepared to receive them. Both C.crescentus and E.coli place flagellar and chemosensory proteins into special polar membrane domains (Alley et al., 1992; Maddock and Shapiro, 1993). Therefore, our studies demonstrate a new level of regulation that overlaps established regulatory systems (Figure 1), currently under active investigation, which may reveal how bacteria develop and organize their cellular structures.
Materials and methods
Bacterial strains, plasmids and growth conditions
Table I lists our strains and plasmids. Escherichia coli S17–1 (Simon et al., 1983) was used to mobilize pRK290-based plasmids into C.crescentus strain GM1461 by conjugation (Ely, 1991). The GM1461 gpsA synchronizable strain was constructed by phage Cr30 transduction of the gpsA505 allele into NA1000 via linkage to purA::Tn5 (SC1090) and prototrophic selection (Ely, 1991). Caulobacter crescentus cells were grown in PYE medium or in M2G (synthetic 0.2% glucose) medium (Ely, 1991) supplemented with antibiotics: 20 μg/ml nalidixic acid (post-conjugation) and 1.5 μg/ml tetracycline (plasmid selection) when appropriate. All plates and liquid cultures contained 1 mM glycerol and 1 mM sn-glycerol-3-phosphate (+GG) except where indicated (–GG).
Synchronized C.crescentus growth and analysis
Synchronized swarmer cells (GM1461) were prepared from cultures grown to OD660 = 0.5–1.0 in M2G +GG media. Swarmer cells (judged >95% pure by light microscopy) were isolated by the Ludox (DuPont) gradient method (Evinger and Agabian, 1977), and released in fresh M2G media (+GG or –GG) at 30°C, OD660 = 0.05–0.10 (Figure 2). DNA and RNA synthesis rates (Figure 2C) were measured by pulse labeling with [α–32P]dGTP or [α–32P]rGTP (3000 Ci/mmol; Amersham), respectively, as described (Marczynski et al., 1995). Protein synthesis rates (Figure 2C) and total protein synthesis (Figure 3A) were measured by 5 min pulse labeling of 2 ml culture samples with 10 μCi of [35S]methionine (TranS-Label; Amersham), as described (Champer et al., 1987). 35S-labeled soluble protein extracts (0.5 μCi) were prepared and immune precipitated with rabbit anti-flagellin serum (Figure 3B) or with rabbit anti-NptII (neomycin phosphotransferase) serum (Figure 4), as described (Champer et al., 1987). For Figure 5A, β–galactosidase protein was labeled similarly and immune precipitated with anti-LacZ antibodies (5′ to 3′; West Chester, PA), as described (Alley et al., 1991). SDS–PAGE fluorography was performed by a 30 min fixation with Amplify (Amersham) prior to gel drying and exposure to X-ray film. β–galactosidase assays (Figures 5 and 6) were performed as described (Miller, 1972). Immune blotting was performed from 1.0 ml cell samples with the Immun-Lite Chemiluminescent assay kit (Bio-Rad) employing rabbit anti-CtrA, as described (Domian et al., 1997). For Figure 7, C.crescentus plasmid DNA was prepared, digested with ClaI and analyzed by Southern blotting, as described (Zweiger et al., 1994).
Acknowledgments
Acknowledgements
We thank Dr Brenda Loewy for generating our initial interest in this work, which was started in Dr Lucy Shapiro's laboratory under an American Cancer Society Fellowship to G.T.M. We also thank M.R.K.Alley, Andrew Dingwall, Bert Ely, James Gober, Ann Reisenauer, Lucy Shapiro, Craig Stephens, Hong Xu and Gary Zweiger for providing antibodies, strains and plasmids. This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Grant OGPO-184894-96 and an MRC Scholarship Award 273-46 to G.T.M., and a Fonds pour la Formation de Chercheurs et l'Aide a la Recherche (FCAR) PhD Fellowship (990885) to A.K.C.B.
References
- Alley M.R.K., Gomes, S.L., Alexander, W. and Shapiro, L. (1991) Genetic analysis of a temporally transcribed chemotaxis gene cluster in Caulobacter crescentus. Genetics, 129, 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley M.R.K., Maddock, J.R. and Shapiro, L. (1992) Polar localization of a bacterial chemoreceptor. Genes Dev., 6, 825–836. [DOI] [PubMed] [Google Scholar]
- Brun Y.V., Marczynski, G.T. and Shapiro, L. (1994) The expression of asymmetry during Caulobacter cell differentiation. Annu. Rev. Biochem., 63, 419–450. [DOI] [PubMed] [Google Scholar]
- Champer R., Dingwall, A. and Shapiro, L. (1987) Cascade regulation of Caulobacter flagellar and chemotaxis genes. J. Mol. Biol., 194, 71–80. [DOI] [PubMed] [Google Scholar]
- Contreras I., Weissborn, A., Amemiya, K., Mansour, J., Henry, S., Shapiro, L. and Bender, R. (1980) The effect of termination of membrane phospholipid synthesis on cell cycle-dependent events in Caulobacter. J. Mol. Biol., 138, 401–409. [DOI] [PubMed] [Google Scholar]
- Domian I.J., Quon, K.C. and Shapiro, L. (1997) Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1 to S transition in a bacterial cell cycle. Cell, 90, 415–424. [DOI] [PubMed] [Google Scholar]
- Dowhan W. (1997) Molecular basis for membrane phospholipid diversity: why are there so many lipids?Annu. Rev. Biochem., 66, 199–232. [DOI] [PubMed] [Google Scholar]
- Ely B. (1991) Genetics of Caulobacter crescentus. Methods Enzymol., 204, 372–384. [DOI] [PubMed] [Google Scholar]
- Evinger M. and Agabian, N. (1977) Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J. Bacteriol., 132, 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober J.W., Champer, R., Reuter, S. and Shapiro, L. (1991) Expression of positional information during cell differentiation in Caulobacter. Cell, 64, 381–391. [DOI] [PubMed] [Google Scholar]
- Loewy B., Marczynski, G.T., Dingwall, A. and Shapiro, L. (1990) Regulatory interactions between phospholipid synthesis and DNA replication in Caulobacter crescentus. J. Bacteriol., 172, 5523–5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy Z.G., Bryan, R.A., Reutter, S.H. and Shapiro, L. (1987) Control of synthesis and positioning of a Caulobacter crescentus flagellar protein. Genes Dev., 1, 626–635. [DOI] [PubMed] [Google Scholar]
- Maddock J.R. and Shapiro, L. (1993) Polar localization of the chemoreceptor complex in the Escherichia coli cell. Science, 259, 1717–1723. [DOI] [PubMed] [Google Scholar]
- Marczynski G.T., Lentine, K. and Shapiro, L. (1995) A developmentally regulated chromosomal origin of replication uses essential transcription elements. Genes Dev., 9, 1543–1557. [DOI] [PubMed] [Google Scholar]
- Miller J.H. (1972) Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Mindich L. (1975) Studies on bacterial membrane biogenesis using glycerol auxotrophs. In Tzagoloff,A. (ed.), Membrane Biogenesis: Mitochondria, Chloroplasts and Bacteria. Plenum Publishing, New York, NY. [Google Scholar]
- O'Neill E.A. and Bender, R.A. (1989) Cell-cycle-dependent polar morphogenesis in Caulobacter crescentus: roles of phospholipid, DNA, and protein syntheses. J. Bacteriol., 171, 4814–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quon K.C., Marczynski, G.T. and Shapiro, L. (1996) Cell cycle control by an essential bacterial two-component signal transduction protein. Cell, 84, 83–93. [DOI] [PubMed] [Google Scholar]
- Quon K.C., Yang, B., Domian, I.J., Shapiro, L. and Marczynski, G.T. (1998) Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc. Natl Acad. Sci. USA, 95, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T.K. and Cronan, J.E. (1987) Acylation of glycerol 3-phosphate is the sole pathway of de novo phospholipid synthesis in Escherichia coli. J. Bacteriol., 169, 2896–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisenauer A., Quon, K. and Shapiro, L. (1999) The CtrA response regulator mediates temporal control of gene expression during the Caulobacter cell cycle. J. Bacteriol., 181, 2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. and Losick, R. (1997) Protein localization and cell fate in bacteria. Science, 276, 712–718. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Mansour, J., Shaw, P. and Henry, S. (1982) Synthesis of specific membrane proteins is a function of DNA replication and phospholipid synthesis in Caulobacter crescentus. J. Mol. Biol., 159, 303–322. [DOI] [PubMed] [Google Scholar]
- Simon R., Priefer, U. and Puler, A. (1983) A broad host range mobilization system for in vivo genetic engineering. Biotechnology, 1, 784–791. [Google Scholar]
- Wingrove J.A. and Gober, J.W. (1996) Identification of an asymmetrically localized sensor histidine kinase responsible for temporally and spatially regulated transcription. Science, 274, 597–601. [DOI] [PubMed] [Google Scholar]
- Wu J. and Newton, A. (1997) Regulation of Caulobacter flagellar gene hierarchy; not just for motility. Mol. Microbiol., 24, 233–239. [DOI] [PubMed] [Google Scholar]
- Wu J., Ohta, N. and Newton, A. (1998) An essential, multicomponent signal transduction pathway required for cell cycle regulation in Caulobacter. Proc. Natl Acad. Sci. USA, 95, 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweiger G., Marczynski, G.T. and Shapiro, L. (1994) A Caulobacter DNA methyltransferase that functions only in the predivisional cell. J. Mol. Biol., 235, 472–485. [DOI] [PubMed] [Google Scholar]