Abstract
Typically, burn wound infections are classified by the organisms present in the wound within the first several days following injury or later, by routine surveillance cultures. With universal acceptance of early excision and grafting, classification of burn wound colonization in unexcised burn wounds is less relevant shifting clinical significance to open burn-related surgical wound infections (SWI). To better characterize SWIs and their clinical relevance, we identified the pathogens responsible for SWIs, their impact on rates of regrafting, and the relationship between SWI and nosocomial infection (NI) pathogens. Epidemiologic and clinical data for 71 adult patients with ≥20% TBSA burn were collected. Following excision and grafting, if a grafted site had clinical characteristics of infection, a wound culture swab was obtained and organism identified. Surveillance cultures were not obtained. SWI pathogen, anatomic location, post-burn day of occurrence and need for regrafting were compiled. A positive culture obtained from an isolated anatomic location at any time point after excision and grafting of that location was considered a distinct infection. Pathogens responsible for NIs (urinary tract infections, pneumonia, bloodstream and catheter-related bloodstream infections, pseudomembranous colitis and donor site infections) and their post-burn day were identified. The profiles of SWI pathogens and NI pathogens were then compared. Of the 71 patients included, 2 withdrew, 6 had no excision or grafting performed and 1 had incomplete data. Of the 62 remaining, 24 (39%) developed a SWI. In these 24 patients, 70 distinct infections were identified of which 46% required regrafting. Candida species (24%), Pseudomonas aeruginosa (22%), Serratia marcescens (11%) and Staphylococcus aureus (11%) comprised the majority of pathogens. The development of a SWI with the need for regrafting increased overall length of stay, area of autograft, number of operative events and was closely associated with the number of NIs. The % TBSA burn and depth of the burn were the main risk factors for SWI with need for regrafting. The SWI pathogen was identified as a NI pathogen 56% of the time with no temporal correlation between shared SWI and NI pathogens. SWIs are commonly found in severely burned patients and are associated with regrafting. As a result, patients with SWIs are subjected to increased operative events, autograft placement, and increased length of hospitalization. Additionally, the presence of a SWI may be a risk factor for development of NIs.
Keywords: burn, wound infection, excision and grafting, nosocomial infection
INTRODUCTION
Early excision and grafting of the burn wound has become standard of care for the management of a burn injury (1, 2). Early excision and grafting reduces surgical blood loss (3, 4), improves mortality (2, 5) and reduces length of hospitalization (2, 6). Since the surgically untreated burn wound is present for a shorter period of time following the burn injury, studies describing the clinical characteristics of burn wounds should differentiate between the surgically treated and untreated burn wound (7).
For the most part, studies on burn wound infections have focused on the colonization of the burn wound prior to excision and grafting (7, 8) or have not distinguished between colonization of burn eschar and surgically repaired burn wounds (9–11). These studies have confirmed the well-established trend that Gram positive organisms first colonize the wounds and are later replaced with Gram negative organisms (12–14). While this work provides a well-established portrait of burn wound colonization, it does not provide any information about the pathogen profile unique to surgically treated wound infections (SWI). Specifically, these studies have not incorporated the use of more defined terminology (invasive infection in unexcised burn wounds v. open burn-related surgical wound infections) to distinguish infection in unexcised burn wounds and SWIs (15, 16).
With the acceptance of early excision and grafting, the focus on overall burn wound infections should shift to the surgical wound. However, no attempt at describing or characterizing surgical burn wound infections or their clinical characteristics have been made. Therefore, our primary objective was to classify SWIs in severely burned patients based on organism and anatomic location. Our secondary objective was to determine the clinical characteristics associated with these infections and their clinical implications including association with nosocomial infections and need for regrafting.
METHODS
Patient Selection
This study is a retrospective review of a prospectively collected database. Secondary use of the Inflammation and Host Response to Injury database, a multi-center study which includes epidemiologic and clinical data from several burn centers, was performed (17). Burn patients from our institution were selected from the larger database. Consent for epidemiologic data retrieval was obtained upon patient arrival to the burn unit. Patients were enrolled from March 2003 until March 2008. Adult (>18 years old) patients having ≥20% TBSA burn were included. This study was approved by our hospital’s Institutional Review Board.
Clinical Characteristics of a Surgical Wound Infection
SWIs were first identified clinically, and the pathogen confirmed with culture swab growth of moderate or many colonies. Clinically suspicious graft sites were those with purulent discharge, poor graft take and/or discoloration of the graft and increased surrounding erythema (15, 16). Swab cultures were obtained from the area of greatest discharge or discoloration at the site. Cultures were then plated in our microbiology lab, growth assessed by an independent laboratory technician and reported as none, few, moderate or many colonies. An SWI was confirmed only after a wound had autograft coverage. Routine, surveillance biopsies or culture swabs of grafts were not obtained. Antibiograms of isolates were not collected as part of this database. Wound cultures which grew coagulase-negative Staphylococcus were not considered pathogens in a SWI. All Candida and Aspergillus species were grouped as Candida species or Aspergillus species, respectively.
Burn wounds are excised and grafted with autograft as soon as safely possible following the burn. Wounds are treated with a topical antimicrobial solution (bacitracin/polymixin or mafenide acetate) as clinically indicated based on the depth of the wound or additional character changes to the wound. Preferrably, wounds are autografted initially, but when donor site is limited, temporary allograft coverage may be used. SWIs are treated either with a change in topical antimicrobial or removal of the graft, preparation of the wound bed and regrafting if a significant area of the graft is lost. Systemic antibiotics are administered when systemic signs of infection are present.
The body was divided into five areas in order to determine anatomic location of these infections (head and neck, anterior torso, posterior torso, upper extremities and lower extremities). Distinct infections were defined as a positive culture obtained from a clinically suspicious wound and from an isolated anatomic location at any time point after excision and grafting of that site. The SWIs requiring regrafting were also identified and their organisms and anatomic locations determined. The post burn days (PBD) of all SWIs were recorded. To follow trends in organism class, organisms were also classified based on Gram staining and whether a fungi/yeast (Candida, Aspergillus and Calcofluor).
Clinical characteristics collected for all patients include demographics (age, Baux score, sex,), size of burn and time to operating room (OR) (% total body surface area burn (TBSA) including % 2nd and 3rd degree and PBD of first OR event), co-morbidities (diabetes, smoking history, BMI) and outcome (survival, length of hospitalization, cm2 autograft, number of OR events, presence of a nosocomial infection and number of nosocomial infections). The trend of clinical characteristics for patients with and without a SWI was compared to determine both risk factors for the development of a SWI and consequences of SWIs. Also, those who developed a SWI were further segregated based on whether or not a regrafting procedure was needed, and the trend of clinical characteristics was determined to identify risk factors for regrafting.
Nosocomial infection data included pneumonia, urinary tract infections, bloodstream and catheter-related bloodstream infections, pseudomembranous colitis and donor site infections. Donor site infections were considered nosocomial infections as they are the result of the manipulation of virgin tissue. Diagnostic criteria for each nosocomial infection followed the American Burn Association consensus guidelines (18) and Inflammation and Host Response to Injury criteria (19). For patients with a SWI, the onset of the SWI was correlated with the onset of a nosocomial infection with the same organism to determine the temporal relationship between the two infections.
Statistics
Data are reported as mean ± SE. For the comparison of clinical characteristics, differences between continuous groups were measured using a Mann-Whitney U test and data are reported as p-value with significance attributed to p<0.05. To measure differences between dependent groups, the Fischer exact test was used and data are reported as p-value and odds ratio (OR). Statistics were calculated using InStat3 (GraphPad Software, Inc, LaJolla, CA).
RESULTS
Patient Profile
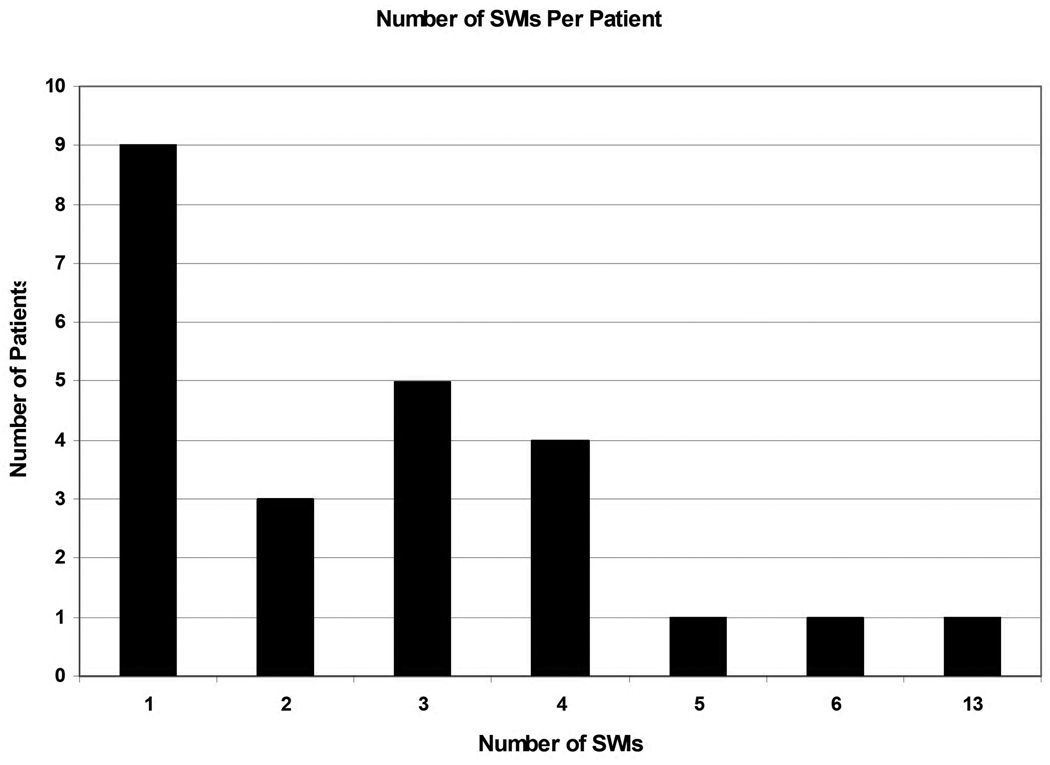
There were 71 total patients in the database. Two patients withdrew from the study. One patient was excluded for incomplete data. Six additional patients did not require excision and grafting either due to early death or withdrawal of care. Of the remaining 62 patients who did have at least one excision and grafting procedure performed, 24 (39%) developed a SWI. The clinical characteristics for these 62 patients included are supplied in Table 1. The majority of patients had 4 or less SWIs with most (38%;9/24) patients having only 1 SWI (Figure 1)
Table 1. Clinical Characteristics of All Patients.
Clinical Characteristics of All Patients. Data are listed as mean ± standard error if applicable and then either the absolute number of patients or range of values in parenthesis.
| Parameter (n=62 patients) | Mean ± SE (Range) |
|---|---|
| Demographics | |
| Age | 44.82 ± 2.1 (18–76) |
| Baux Score | 83.07 ± 2.6 (38–132) |
| Sex (male) | 65% (40) |
| Burn Size and Time to 1st OR | |
| % TBSA Burn | 38.24 ± 2.0 (20–87) |
| % 2nd Degree | 6.70 ± 1.0 (0–33) |
| % 3rd Degree | 31.61 ± 2.0 (6–75) |
| Days to 1st OR | 6.87 ± 0.6 (2–28) |
| Comorbidities | |
| Diabetes (yes) | 11% (7) |
| Smoker (yes) | 42% (26) |
| BMI | 26.00 ± 0.7 (16–47) |
| Outcome | |
| Survival (yes) | 84% (52) |
| Length of Stay (days) | 54.79 ± 6.2 (9–230) |
| Area of autograft (cm2) | 5184 ± 366 (600–15000) |
| Number of OR Events | 7.1 ± 0.8 (1–30) |
| Development of a Nosocomial Infection (yes) |
74% (45) |
| Number of Nosocomial Infections |
2.7 ± 0.4 (0–18) |
Figure 1.
Number of SWIs for patients who developed a SWI.
Surgical Wound Infection Characteristics
For the 24 patients who developed SWIs, there were 70 distinct infections. The organisms responsible for these infections and their frequency are listed in Table 2. Candida species was the most common (24%) organism found in these distinct infections. Pseudomonas aeruginosa (23%), Serratia marcescens (11%) and Staphylococcus aureus (11%) were the most common bacteria. Gram negative organisms were the most common class of pathogen accounting for 49% (34/70) of all infections. Fungi accounted for 36% (25/70) while Gram positive organisms only accounted for 15% (11/70).
Table 2. % of each organism found in a distinct surgical wound infection.
% of each organism found in a distinct surgical wound infection. 70 total SWIs were identified from suspicious wounds and growing >105 organisms.
| Organism | % of all distinct infections (n=70 distinct infections) |
|---|---|
| Candida species | 24% (17) |
| Pseudomonas aeruginosa | 23% (16) |
| Serratia marcescens | 11% (8) |
| Staphylococcus aureus | 11% (8) |
| Aspergillus species | 10% (7) |
| Acinetobacter baumannii | 7% (5) |
| Proteus mirabilis | 4% (3) |
| Enterococcus faecalis | 3% (2) |
| Escherichia coli | 1% (1) |
| Calcofluor | 1% (1) |
| Enterobacter species | 1% (1) |
| Stenotrophomonas maltophilia | 1% (1) |
The anatomic location of all SWIs and the organisms present in these infections are provided in Table 3. The majority of SWIs were found on the lower extremities (46%). The remainder of SWIs were evenly distributed along the head and neck, anterior torso, posterior torso and upper extremities. There does not appear to be any obvious trend in the location of these SWI pathogens although more Gram positive organisms were found on the head and neck and upper extremities while more Gram negatives were found on the lower extremities. There was no association between fungal/yeast infections and anatomic site.
Table 3. Specific Organisms and Class of Organisms Associated with Anatomic Location.
Specific Organisms and Class of Organisms Associated with Anatomic Location. The organisms and respective class are listed in descending order based on frequency.
| Anatomic Location | Organism (n=70 distinct infections) |
Class of Organism |
|---|---|---|
| Head and Neck 13% (9) | Candida (4) Staph (4) Pseudomonas (1) |
Gram + (4) Fungus/Yeast (4) Gram − (1) |
| Anterior Torso 11% (8) | Candida (2) Serratia (2) Aspergillus (1) E. coli (1) Staph (1) Pseudomonas (1) |
Gram − (4) Fungus/Yeast (3) Gram + (1) |
| Posterior Torso 13% (9) | Pseudomonas (5) Aspergillus (2) Proteus (1) Candida (1) |
Gram − (6) Fungus/Yeast (3) Gram + (0) |
| Upper Extremity 17% (12) | Serratia (3) Staph (2) Enterococcus (2) Candida (2) Enterobacter (1) Aspergillus (1) Acinetobacter (1) |
Gram − (5) Gram + (4) Fungus/Yeast (3) |
| Lower Extremity 46% (32) | Pseudomonas (9) Candida (7) Acinetobacter (4) Aspergillus (4) Serratia (3) Proteus (2) Staph (1) Stenotrophomonas (1) Calcofluor (1) |
Gram− (19) Fungus/Yeast (12) Gram + (1) |
Eighteen of the SWIs contained two organisms. Of the 36 organisms in these infections, Pseudomonas aeruginosa (25%; 9/36), Candida species (22%; 8/36) and Aspergillus (14%;5/36) were most commonly identified. No specific anatomic location was associated with multiple organism SWIs.
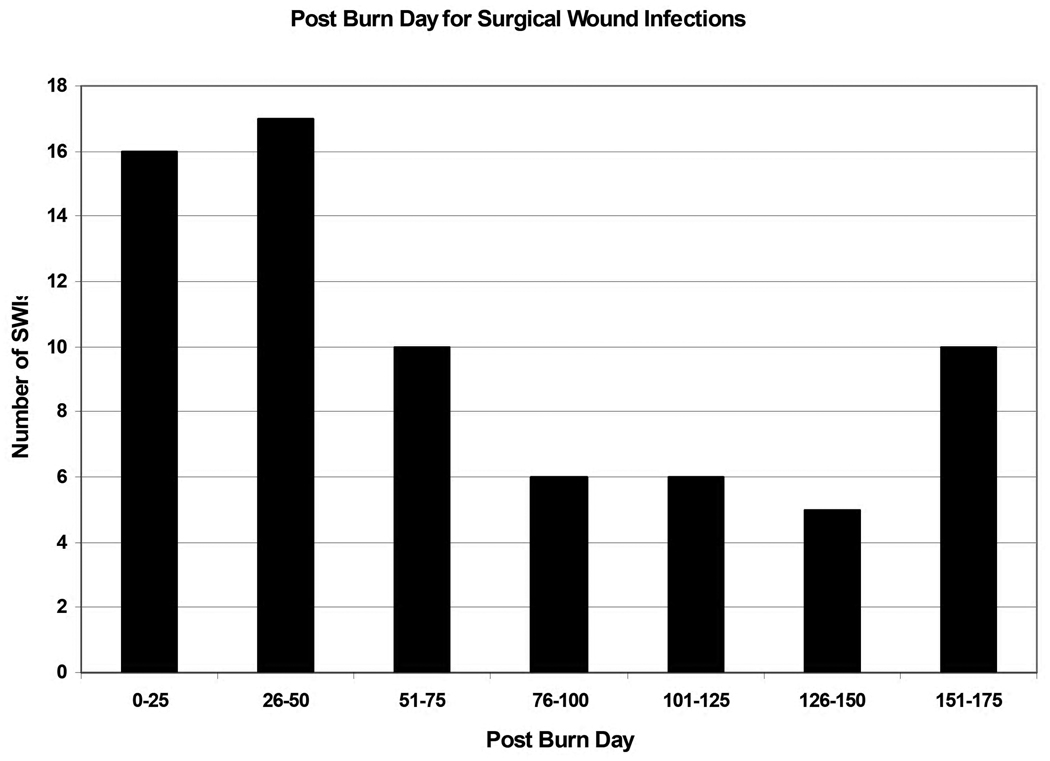
No SWIs occurred within the first 10 PBDs. The PBD on which SWIs occurred ranged from PBD# 11–174. Figure 2 shows the frequency of SWIs in relation to the PBD at which they occurred demonstrating the significant time post burn at which these infections occur.
Figure 2.
Post burn day for Surgical Wound Infections.
Clinical Characteristics of Patients Developing Surgical Wound Infections
The clinical characteristics associated with the development of a SWI were determined by comparing these characteristics between patients who did and did not develop an SWI (Table 4). Demographic characteristics including age, Baux score, sex and co-morbidities including diabetes, smoking history and BMI were not related to the development of a SWI. The total burn size including the percentage of 2nd and 3rd degree burn did not differ between those who did and did not develop a SWI. In terms of outcome, survival was not impacted by development of a SWI. However, patients with a SWI had higher total area of autograft (4437 v. 6369 cm2;p=0.03), almost three times longer lengths of hospitalization (31.68 v. 91.38 days;p <0.0001) and increased number of operative events (4.16 v. 11.96;p <0.001).
Table 4. Clinical Characteristics Associated with the Development of a Surgical Wound Infection.
Clinical Characteristics Associated with the Development of a Surgical Wound Infection. Data are reported as mean ± SE. For continuous groups, Mann-Whitney U test results are reported as p-values. For dependent groups, Fischer Exact test results are reported as p-value and odds ratio (OR). Characteristics with p-value ≤ 0.05 are in italics
| Parameter (n=62 total patients) |
Without SWI (38) | With SWI (24) | Fischer Exact or Mann-Whitney U |
|---|---|---|---|
| Demographics | |||
| Age | 44.50 ± 2.67 | 45.33 ± 3.40 | 0.88 |
| Baux Score | 81.11 ± 3.40 | 86.17 ± 4.06 | 0.25 |
| Sex (male) | 66% (25) | 63% (15) | 0.79 (0.398–3.345) |
| Burn Size and Time to OR | |||
| % TBSA Burn | 36.61 ± 2.39 | 40.83 ± 3.61 | 0.36 |
| % 2nd Degree | 5.85 ± 1.22 | 8.05 ± 1.83 | 0.30 |
| % 3rd Degree | 30.84 ± 2.59 | 32.83 ± 3.14 | 0.61 |
| Days to 1st OR | 6.90 ± 0.78 | 6.83 ± 1.09 | 0.81 |
| Comorbidities | |||
| Diabetes (yes) | 5% (2) | 21% (5) | 0.10 (0.037–1.193) |
| Smoker (yes) | 45% (17) | 38% (9) | 0.61 |
| BMI | 25.37 ± 0.95 | 27.01 ± 0.93 | 0.22 |
| Outcome | |||
| Survival (yes) | 82% (31) | 88% (21) | 0.73 (0.366–6.819) |
| Length of Stay (days) | 31.68 ± 3.49 | 91.38 ± 11.70 | <0.0001 |
| Area of autograft (cm2) | 4437 ± 365 | 6369 ± 693 | 0.03 |
| Number of OR Events | 4.16 ± 0.53 | 11.96 ± 1.51 | <0.0001 |
|
Development of a Nosocomial Infection (yes) |
63% (24) | 88% (21) | 0.04 |
|
Number of Nosocomial Infections |
1.55 ± 0.24 | 4.38 ± 0.96 | 0.012 |
Patients who developed a SWI were more likely to develop at least one nosocomial infection (63% without a SWI v. 88% with a SWI;p=0.04). The number of nosocomial infections was also significantly greater in those developing a SWI (1.55 v. 4.38 infections;p=0.012). Not only are the presence and number of nosocmial infections greater in SWI patients, but the pathogen which caused both the nosocomial infection and SWI is the same organism in 56% (22/39) of cases. The temporal relationship of the identification of the pathogenic organism does not follow a consistent pattern when the nosocomial infection and SWI shared the same pathogen. The nosocomial infection organism preceded the SWI 59% (13/22) of the time. The SWI organism preceded the nosocomial infection 46% (10/22) of the time. This total amounts to greater than 100% as 4/22 organisms were present in a noscomial infection both before and after the development of the SWI. In only 14% (3/22) of the cases was the nosocomial infection and SWI organism identical on the same post burn day.
Surgical Wound Infections and Regrafting
Only 18% (11/62) of patients overall required regrafting due to a SWI. Of the 24 patients with a SWI, 46% (11/24) required regrafting. The percentage of distinct infections requiring regrafting based on pathogen is listed in Table 5. Regrafting occurred in 45% (5/11) of infections with Gram positive organisms, 35% (12/34) with Gram negative organisms and 56% (14/25) with fungi/yeast. The anatomic location of the SWI did influence need for regrafting as SWIs in the posterior torso (67%) and lower extremities (63%) had high rates of regrafting (Table 6).
Table 5. % of infections requiring regrafting based on organism.
Percentage of infections requiring regrafting based on organism. Organisms causing SWIs and also requiring regrafting are displayed in 3 groups (Gram +, Gram − and Fungi).
| Organism (n=70 distinct infections) |
% of distinct infections requiring regrafting |
|---|---|
| Gram positive | |
| Staphylococcus aureus | 38% (3/8) |
| Enterococcus faecalis | 100% (2/2) |
| Enterobacter species | 0% (0/1) |
| Gram negative | |
| Pseudomonas aeruginosa | 44% (7/16) |
| Serratia marcescens | 38% (3/8) |
| Acinetobacter baumannii | 0% (0/5) |
| Proteus mirabilis | 66% (2/3) |
| Escherichia coli | 0% (0/1) |
| Stenotrophomonas maltophilia | 0% (0/1) |
| Fungi/Yeast | |
| Candida species | 41% (7/17) |
| Aspergillus species | 86% (6/7) |
| Calcofluor | 100% (1/1) |
Table 6. % of Regrafting Based on Anatomic Location.
Percentage of Regrafting Based on Anatomic Location. Percentage of wounds with a SWI that required regrafting sorted based on anatomic location.
| Anatomic Location | % Requiring Regrafting |
|---|---|
| Head and Neck | 22% (2/9) |
| Anterior Torso | 25% (2/8) |
| Posterior Torso | 67% (6/9) |
| Upper Extremity | 8% (1/12) |
| Lower Extremity | 63% (20/32) |
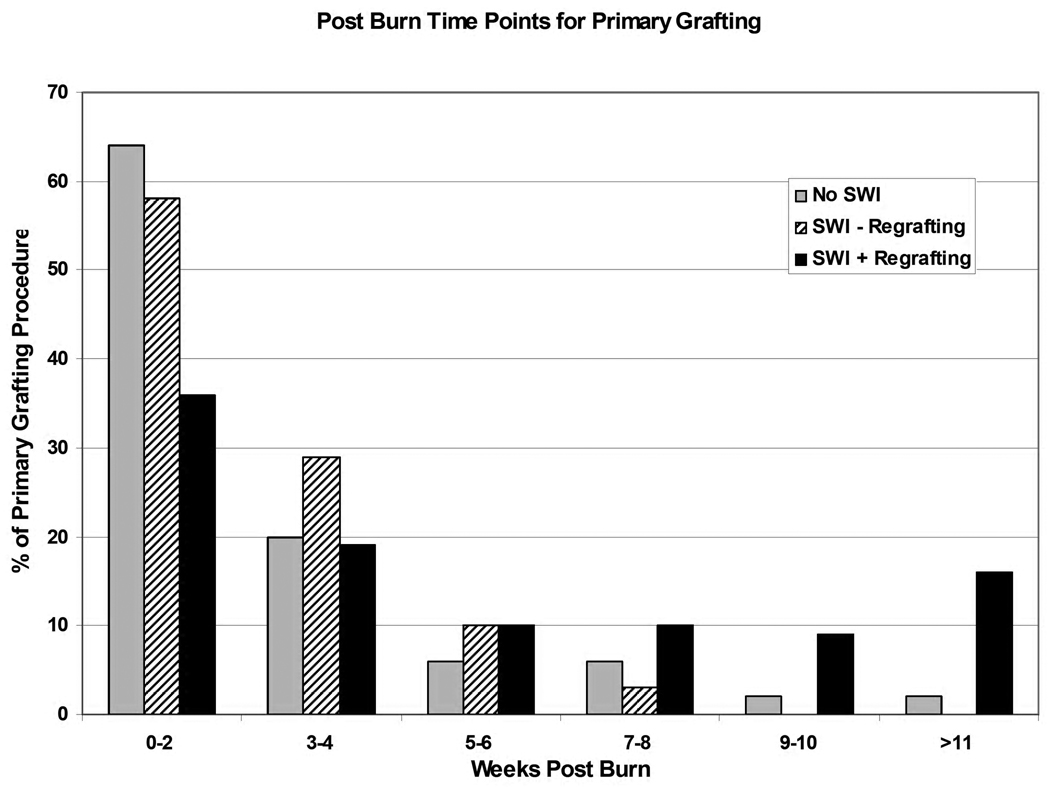
To determine the risk factors for and the clinical consequences of regrafting due to a SWI, the same clinical characteristics used to distinguish patients with and without SWIs were used to evaluate SWI patients based on need for regrafting (Table 7). Patients who develop a SWI and need regrafting had their primary grafting procedure at much later time points following the burn injury in comparison to those without a SWI and those with a SWI not requiring regrafting (Figure 3). Age, sex, time to 1st OR and comorbidities had no impact on regrafting. However, the Baux score (77 no regrafting v. 97 regrafting;p=0.003), % TBSA burn (30.20 v. 53.56;p=0.0003) and % 3rd degree burn (23.23 v. 44.18;p=0.0011) were all significantly increased in patients requiring regrafting. In terms of outcome, survival was not impacted by the need for regrafting. Length of stay (54.31 v. 135.18 days;p<0.0001), area of autograft (4380 v. 8720 cm2;p=0.003) and number of OR events (7.54 v. 17.18;p=0.0009) were significantly increased in those requiring regrafting. The percentage of patients developing a nosocomial infection did not differ, but there was a significant increase in the number of nosocomial infections in the regrafting group (2.62 v. 6.46;p=0.04).
Table 7. Clinical Characteristics Associated with Regrafting Following a Surgical Wound Infection.
Clinical Characteristics of Patients with a SWI Based on Need for Regrafting. Data are reported as mean ± SE. For continuous groups, Mann-Whitney U test results are reported as p-values. For dependent groups, Fischer Exact test results are reported as pvalue and odds ratio (OR). Characteristics with p-value ≤ 0.05 are in italics
| Parameter | No Regrafting (13) | Regrafting (11) | Fischer Exact or Mann-Whitney U |
|---|---|---|---|
| Demographics | |||
| Age | 46.77 ± 5.01 | 43.64 ± 4.66 | 0.61 |
| Baux Score | 77.0 ± 5.11 | 97.00 ± 4.91 | 0.03 |
| Sex (male) | 64% (23) | 65% (17) | 1.0 (0.33–2.69) |
| Burn Size and Time to OR | |||
| % TBSA Burn | 30.20 ± 2.6 | 53.36 ± 5.1 | 0.0003 |
| % 2nd Degree | 7.07 ± 1.7 | 9.21 ± 3.5 | 0.84 |
| % 3rd Degree | 23.23 ± 2.7 | 44.18 ± 4.0 | 0.0011 |
| Days to 1st OR | 7.39 ± 1.91 | 6.18 ± 0.81 | 0.56 |
| Comorbidities | |||
| Diabetes (yes) | 23% (3) | 18% (2) | 1.0 (0.099–5.49) |
| Smoker (yes) | 46% (6) | 27% (3) | 0.42 (0.079–2.44) |
| BMI | 27.92 ± 1.3 | 25.92 ± 1.3 | 0.15 |
| Outcome | |||
| Survival (yes) | 85% (11) | 91% (10) | 1.0 (0.27–7.53) |
| Length of Stay (days) | 54.31 ± 10.23 | 135.18 ± 13.57 | <0.0001 |
| Area of autograft (cm2) | 4380 ± 358 | 8720 ± 1100 | 0.0031 |
| Number of OR Events | 7.54 ± 1.49 | 17.18 ± 1.80 | 0.0009 |
| Development of a Nosocomial Infection (yes) |
77% (23) | 100% (11) | 0.22 (0.352–166.78) |
|
Number of Nosocomial Infections |
2.62 ± 0.84 | 6.46 ± 1.68 | 0.04 |
Figure 3.
For each patient, the percentage of all primary grafting procedures are reported during the week post-burn in which they occurred. Patients are divided to three groups based on presence of a SWI and need for regrafting.
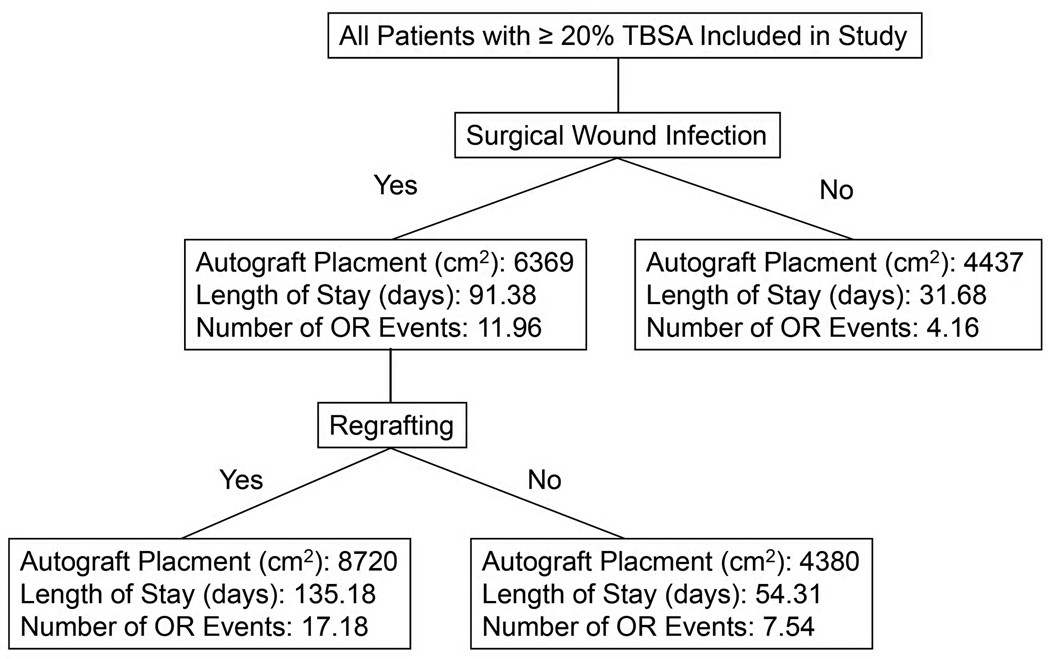
The subset analysis of SWI patients based on regrafting point toward the need for regrafting as having the most significant impact on outcome (Figure 4). For example, patients without a SWI required 4437 cm2 of autograft, those with a SWI but not needing regrafting required a similar amount (4380 cm2) while those with a SWI and requiring regrafting used 8720 cm2. A similar trend holds true for length of stay and number of OR events. Therefore, the poorer outcome characteristics between those with a SWI in comparison to those without a SWI do not necessarily lie in the development of a SWI but rather the development of a SWI that later requires regrafting.
Figure 4.
Flowchart demonstrating that the difference in autograft placement, length of stay and number of OR events for patients with and without SWIs rests in the significant increases in these characteristics for patients with an SWI requiring regrafting.
DISCUSSION
In our study, SWIs were found in 39% of patients with ≥ 20% TBSA burn. The organisms causing these SWIs were primarily Candida species, Pseudomonas aeruginosa, Serratia marcescens and Staphylococcus aureus (Table 2). SWIs occurred throughout the entire body with highest rates in the lower extremities (46% of all infections). Specific organism or class of organism were not identified in one particular anatomic location, but a trend toward more Gram negative organisms in the lower extremites was found (Table 3).
Analysis of the outcomes of patients without SWI, with SWI without regrafting and with SWI requiring regrafting (Table 4, G and Figure 4) clearly indicate that it is the need for regrafting rather than SWI alone that increases length of stay, area of autograft and number of OR events. The increased length of stay and number of OR events in these patients are associated with the need for regrafting. Also, the increased area of autograft is most likely due to a combination of increased % TBSA and % 3rd degree burn requiring primary grafting and SWI sites requiring regrafting. The retrospective nature of this study does not allow for the determination of how much burn size or regrafting contribute to overall autograft size in those with SWI requiring regrafting. However, the relationship between the increased % TBSA burn, increased area of autograft and also, the delayed time point at which primary grafting (Figure 3) occur in these patients may reveal a relationship between quality of donor sites and SWI with regrafting. In patients with greater % TBSA burn, there is less available donor site. As a result, primary grafting occurs later (Figure 3), and there is re-use of previous donor sites. As many of the SWIs occur late in the patient’s hospitalization (Figure 2), it may be these re-used donor sites that predispose to SWI with regrafting. Further investigation into the cellularity and composition of re-used donor site may reveal a tissue that is not the ideal wound coverage.
SWIs and the need for regrafting has a significant effect on outcome with increased length of hospitalization, number of OR events and number of nosocomial infections. As a result, techniques to prevent a SWI will considerably benefit patient care. What, then, are the surgical and wound management techniques that prevent SWIs and also the need for regrafting? As this is a single center study with uniform wound care and surgical techniques, it is impossible to compare wound management styles. Most likely, available donor sites and post operative dressings dictate the development of a SWI. It is our practice to dress donor sites with Glucan II (Brennen Medical, St. Paul, Minnesota). A variety of donor site dressings are available with no consensus on which dressing may provide the best tissue for grafting (20–25). In addition, the choice and site of homograft, if needed, in preparation for autograft placement may play a role in SWI and regrafting susceptibility. Both donor site dressings and homograft placement for large wounds and their relationship to SWIs should be evaluated prospectively in order to determine the best method of reducing overall SWIs.
This study does not identify clinical characteristics that predict the development of a SWI. As shown in Table 4, age, Baux score, sex, size of burn, days to first operative event, presence of diabetes, smoking and BMI, all factors present before any surgical management of the wound, did not differ between those who did and did not develop a SWI. However, this study does reveal an interesting relationship between nosocomial infections and SWIs. Patients who developed a SWI were more likely to acquire one or multiple nosocomial infections. Whether the nosocomial infection is a risk factor for a SWI or vice versa, the high concentration of the infectious organism in either type of infection may predispose to developing further infectious sequelae.
Other studies on burn wound infections have not investigated the relationship between organism in the wound and either invasive infection or nosocomial infection (8, 11–14). Given the high frequency of reinfection with SWI organisms at other anatomic locations, at later times and in nosocomial infections, identifying patient specific organisms may allow for better anticipation and directed antimicrobial therapy. In addition, the number of nosocomial infections were increased with both the development of a SWI and development of a SWI requiring regrafting. This may be related to an increased opportunity for infection given the increased length of stay, a worsened critical illness and subsequent increased susceptibility to nosocomial infections or more invasive wound infection leading to regrafting. Close review of prior nosocomial infection and SWI pathogens may allow for more appropriate and beneficial surgical prophylaxis. This would have to be studied in a prospective, randomized fashion before being implemented.
This analysis specifically focuses on wound infections in surgically treated wounds. By focusing on SWIs, the significant clinical consequences and morbidity associated with these infections becomes apparent. Given the limited clinical relevance, surveillance cultures may be an expensive endeavor with little patient benefit.
Fungal/yeast infections are commonly found in SWIs. Candida species, Aspergillus species and Calcofluor accounted for 35% of all SWIs. Other studies on burn wound colonization (14, 26, 27) have shown an increase in fungal/Candida rates in burn units in recent years. Interestingly, these are not benign infections as about 60% of all SWIs due to fungi/yeast required regrafting, and other studies have shown that Candida colonization and candidemia are associated with high mortality rates in burn patients (28–30).
This study was limited in several aspects. The sample size was small preventing further and more robust conclusions to be drawn from our results. A larger sample size may also make the correlation between nosocomial infection and SWI pathogen stronger. Also, we did not collect data on antibiograms of the isolates. This data would have helped to identify trends for specific organisms like methicillin-resistant Staphylococcus aureus or vancomycin-resistant Enterococcus which would be important for the treatment of nosocomial infections. Wound biopsies were not obtained and histologic invasion of the wound was not documented. However, the main clinical correlate was the need for regrafting which may be completely independent of the histologic invasion of the organism.
A larger, prospective study in which the relationship between SWIs, nosocomial infection development, the need for regrafting and repeat donor site use may help to better uncover the major factors contributing to SWI and their associated poor outcomes. In fact, small trends like increased regrafting and SWI rates along the posterior torso and lower extremities may reveal how management of these grafts should be different than other locations. In addition, further study on the depth of excision and adequacy of blood supply may show how wound preparation may influence graft outcome.
This study describes the characteristics and clinical correlates of SWIs. We examined the basic characteristics of open-burn related surgical wound infections and their association with clinical variables, nosocomial infections and need for regrafting. We find that SWIs are common and often lead to regrafting. In particular, the need for regrafting is associated with increased length of stay, area of autograft and number of operative events with the main risk factor for regrafting being the size and depth of the burn wound.
Acknowledgments
We acknowledge the contribution of the Inflammation and the Host Response to Injury Large-Scale Collaborative Project Award # 2-U54-GM062119 from the National Institute of General Medical Sciences.
Funding
NIH T32 GM008750, Dr. Ralph and Marian C. Falk Medical Research Trust
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The Inflammation and the Host Response to Injury “Glue Grant” program is supported by the National Institute of General Medical Sciences. This manuscript was prepared using a dataset obtained from the Glue Grant program and does not necessarily reflect the opinions or views of the Inflammation and the Host Response to Injury Investigators or the NIGMS.
References
- 1.Mosier MJ, Gibran NS. Surgical excision of the burn wound. Clin Plast Surg. 2009;36:617–625. doi: 10.1016/j.cps.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Heimbach DM. Early burn excision and grafting. Surg Clin North Am. 1987;67:93–107. doi: 10.1016/s0039-6109(16)44135-6. [DOI] [PubMed] [Google Scholar]
- 3.Sheridan RL, Szyfelbein SK. Trends in blood conservation in burn care. Burns. 2001;27:272–276. doi: 10.1016/s0305-4179(00)00110-8. [DOI] [PubMed] [Google Scholar]
- 4.Desai MH, Herndon DN, Broemeling L, et al. Early burn wound excision significantly reduces blood loss. Ann Surg. 1990;211:753–759. doi: 10.1097/00000658-199006000-00015. discussion 759–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tompkins RG, Remensnyder JP, Burke JF, et al. Significant reductions in mortality for children with burn injuries through the use of prompt eschar excision. Ann Surg. 1988;208:577–585. doi: 10.1097/00000658-198811000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong YS, Samuel M, Song C. Meta-analysis of early excision of burns. Burns. 2006;32:145–150. doi: 10.1016/j.burns.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Mayhall CG. The epidemiology of burn wound infections: then and now. Clin Infect Dis. 2003;37:543–550. doi: 10.1086/376993. [DOI] [PubMed] [Google Scholar]
- 8.Husain MT, Karim QN, Tajuri S. Analysis of infection in a burn ward. Burns. 1989;15:299–302. doi: 10.1016/0305-4179(89)90006-5. [DOI] [PubMed] [Google Scholar]
- 9.Santucci SG, Gobara S, Santos CR, et al. Infections in a burn intensive care unit: experience of seven years. J Hosp Infect. 2003;53:6–13. doi: 10.1053/jhin.2002.1340. [DOI] [PubMed] [Google Scholar]
- 10.Ramakrishnan MK, Sankar J, Venkatraman J, et al. Infections in burn patients--experience in a tertiary care hospital. Burns. 2006;32:594–596. doi: 10.1016/j.burns.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Erol S, Altoparlak U, Akcay MN, et al. Changes of microbial flora and wound colonization in burned patients. Burns. 2004;30:357–361. doi: 10.1016/j.burns.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Pruitt BA, Jr, McManus AT, Kim SH, et al. Burn wound infections: current status. World J Surg. 1998;22:135–145. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 13.Vindenes H, Bjerknes R. Microbial colonization of large wounds. Burns. 1995;21:575–579. doi: 10.1016/0305-4179(95)00047-f. [DOI] [PubMed] [Google Scholar]
- 14.Pruitt BA, Jr, McManus AT. The changing epidemiology of infection in burn patients. World J Surg. 1992;16:57–67. doi: 10.1007/BF02067116. [DOI] [PubMed] [Google Scholar]
- 15.Peck MD, Weber J, McManus A, et al. Surveillance of burn wound infections: a proposal for definitions. J Burn Care Rehabil. 1998;19:386–389. doi: 10.1097/00004630-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein MB, Silver G, Gamelli RL, et al. Inflammation and the host response to injury: an overview of the multicenter study of the genomic and proteomic response to burn injury. J Burn Care Res. 2006;27:448–451. doi: 10.1097/01.BCR.0000227477.33877.E6. [DOI] [PubMed] [Google Scholar]
- 18.Greenhalgh DG, Saffle JR, Holmes JHt, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28:776–790. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 19.Injury IatHRt. Infectious and Non-Infectious Complications- Definitions from the Trauma-Related Database (TRDB) Available. [Google Scholar]
- 20.Kilinc H, Sensoz O, Ozdemir R, et al. Which dressing for split-thickness skin graft donor sites? Ann Plast Surg. 2001;46:409–414. doi: 10.1097/00000637-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Blome-Eberwein S, Johnson RM, Miller SF, et al. Hydrofiber dressing with silver for the management of split-thickness donor sites: A randomized evaluation of two protocols of care. Burns. 2009 doi: 10.1016/j.burns.2009.06.193. [DOI] [PubMed] [Google Scholar]
- 22.Innes ME, Umraw N, Fish JS, et al. The use of silver coated dressings on donor site wounds: a prospective, controlled matched pair study. Burns. 2001;27:621–627. doi: 10.1016/s0305-4179(01)00015-8. [DOI] [PubMed] [Google Scholar]
- 23.Voineskos SH, Ayeni OA, McKnight L, et al. Systematic review of skin graft donor-site dressings. Plast Reconstr Surg. 2009;124:298–306. doi: 10.1097/PRS.0b013e3181a8072f. [DOI] [PubMed] [Google Scholar]
- 24.Rakel BA, Bermel MA, Abbott LI, et al. Split-thickness skin graft donor site care: a quantitative synthesis of the research. Appl Nurs Res. 1998;11:174–182. doi: 10.1016/s0897-1897(98)80296-6. [DOI] [PubMed] [Google Scholar]
- 25.Smith DJ, Jr, Thomson PD, Garner WL, et al. Donor site repair. Am J Surg. 1994;167 doi: 10.1016/0002-9610(94)90012-4. 49S–51S. [DOI] [PubMed] [Google Scholar]
- 26.Branski LK, Al-Mousawi A, Rivero H, et al. Emerging infections in burns. Surg Infect (Larchmt) 2009;10:389–397. doi: 10.1089/sur.2009.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballard J, Edelman L, Saffle J, et al. Positive fungal cultures in burn patients: a multicenter review. J Burn Care Res. 2008;29:213–221. doi: 10.1097/BCR.0b013e31815f6ecb. [DOI] [PubMed] [Google Scholar]
- 28.Moore EC, Padiglione AA, Wasiak J, et al. Candida in burns: risk factors and outcomes. J Burn Care Res. 31:257–263. doi: 10.1097/BCR.0b013e3181d0f536. [DOI] [PubMed] [Google Scholar]
- 29.Struck MF, Stiller D, Corterier CC, et al. Fulminant, undetected Candida sepsis after an apparently survivable burn injury. J Burn Care Res. 2009;30:894–897. doi: 10.1097/BCR.0b013e3181b48794. [DOI] [PubMed] [Google Scholar]
- 30.Horvath EE, Murray CK, Vaughan GM, et al. Fungal wound infection (not colonization) is independently associated with mortality in burn patients. Ann Surg. 2007;245:978–985. doi: 10.1097/01.sla.0000256914.16754.80. [DOI] [PMC free article] [PubMed] [Google Scholar]