Abstract
Normal brain function requires constant adaptation, as an organism learns to associate important sensory stimuli with appropriate motor actions. Neurological disorders may disrupt these learned associations, and require the nervous system to reorganize itself. As a consequence, neural plasticity is a crucial component of normal brain function and a critical mechanism for recovery from injury. Associative, or Hebbian pairing of pre- and postsynaptic activity has been shown to alter stimulus-evoked responses in vivo, however, to date, such protocols have not been shown to affect the animal’s subsequent behavior. We paired stimulus trains separated by a brief time delay to two electrodes in rat sensorimotor cortex, which changed the statistical pattern of spikes during subsequent behavior. These changes were consistent with strengthened functional connections from the leading electrode to the lagging electrode. We then trained rats to respond to a microstimulation cue, and repeated the paradigm using the cue electrode as the leading electrode. This pairing lowered the rat’s ICMS detection threshold, with the same dependence on intra-electrode time lag that we found for the functional connectivity changes. The timecourse of the behavioral effects was very similar to that of the connectivity changes. We propose that the behavioral changes were a consequence of strengthened functional connections from the cue electrode to other regions of sensorimotor cortex. Such paradigms might be used to augment recovery from stroke, or to promote adaptation in a bidirectional brain machine interface.
1. Introduction
There is plentiful, although indirect, evidence that learning can cause long-lasting changes in synaptic efficacy. In the work we report here, we explore the other direction of this linkage: augmentation of behavioral performance brought about by artificially altered neural connections. Thoughts about changing connectivity in vivo extend back to the ideas of Donald Hebb, who postulated that if the activity of one neuron consistently preceded that of another, the connection from the first to the second neuron would strengthen (Hebb 1949). This idea of Hebbian, or associative, plasticity has now been demonstrated throughout the nervous system. Perhaps the most well known examples of Hebbian conditioning are long-term potentiation (LTP) and long-term depression (LTD). Repetitive pairing of pre- and post- synaptic activation has been used to drive changes in synaptic strength, in vitro (Artola and Singer 1987; Bliss and Lomo 1973; Feldman and Brecht 2005; Mulkey and Malenka 1992). A number of studies have shown that the relative timing of the pre- and post- synaptic spiking is one critical factor that determines the sign and magnitude of the synaptic changes (Bi and Poo 2001; Markram et al. 1997). More recently, similar studies have been done in vivo using electrical (Froc et al. 2000; Hodgson et al. 2005; Ivanco and Racine 2000; Trepel and Racine 1998; Werk and Chapman 2003), or sensory stimulation (Fu et al.; Meliza and Dan 2006; Yao and Dan 2001) to elicit changes in single-neuron responses.
The relation between learning and these Hebbian-like changes is a topic of considerable interest. Learning has been shown to cause significant changes in both sensory and motor cortical maps. In a number of studies, Hebbian pairing of natural sensory stimuli has been used to drive remapping of sensory representations within somatosensory (Jenkins et al. 1990; Wang et al. 1995), auditory (Recanzone et al. 1992), and barrel (Diamond et al. 1993) cortices. Similar paradigms have been shown to change the orientation selectivity of individual neurons in visual cortex (Yao and Dan 2001). In humans, several hours of daily piano practice (Pascual-Leone et al. 1994) or repeated thumb movements (Classen et al. 1998) altered the subsequent digit movements produced by transcranial magnetic stimulation.
The past decade has seen tremendous development of brain-machine interfaces (BMIs), including efferent systems that use signals recorded from the brain to the control an external device, and afferent systems that supply information about the external world directly to the brain via electrical stimulation. The activity of the small number of motor cortical cells used for an efferent BMI is at best, a crude representation of the natural output of M1. In an effort to improve the resulting performance, considerable effort has devoted to the development of complex, nonlinear control algorithms (Kemere et al. 2004; Magjarevic et al. 2009; Truccolo et al. 2005; Wu et al. 2006). In parallel, there has been increasing interest in the user’s adaptation to the novel motor task represented by the BMI. Changes in the tuning properties of the individual neurons used for control have been observed that are similar to the timecourse of behavioral adaptation (Ganguly and Carmena 2009; Jarosiewicz et al. 2008; Taylor et al. 2002).
There has also been work to develop an afferent BMI: intracortical microstimulation (ICMS) used to convey information directly to the central nervous system. ICMS has been shown to generate percepts in visual cortical areas (Bak et al. 1990; Brindley and Lewin 1968; Murphey and Maunsell 2007), auditory cortex (Deliano et al. 2009; Otto et al. 2005; Rousche and Normann 1999), barrel cortex (Butovas and Schwarz 2007) and somatosensory cortex (Fitzsimmons et al. 2007; London et al. 2008; Romo et al. 1998). The eventual conjunction of these efferent and afferent interfaces will raise many interesting questions about the resultant plastic changes that may take place within the cortex. At this point, implementation of a true bidirectional BMI remains an elusive goal, although some progress has been made (O’Doherty et al. 2009). Ultimately, under those conditions, untangling the relation between natural learning and stimulus driven cortical changes may become more tractable experimentally. These are the conditions we have attempted to mimic here.
In previous work, we demonstrated the ability to identify isolated changes in the inferred functional connectivity (IFC) among large networks of simulated neurons. A further series of experiments demonstrated that spike-triggered electrical stimulation could be used to change the IFC in rodent sensorimotor cortex in a predictable manner (Rebesco et al. 2010). In the current study, we trained rats to perform a simple motor task when cued by ICMS on a single electrode. We potentiated the functional connectivity from this electrode to a second electrode. The potentiation protocol increased the IFC as well as animal’s ability to detect the ICMS behavioral cue. The behavioral effect had the same latency dependence as did the changes in IFC, and similar timecourse of both onset and offset effects. These results may have important implications for the use of such Hebbian conditioning protocols in both rehabilitative and BMI applications.
2. Methods
2.1 Experimental design
We performed two types of experiments designed to measure the effects of the conditioning stimulation. In the IFC experiments, we measured changes in the inferred connectivity between recorded neurons. Our goal was to induce changes in the IFC using a stimulation paradigm that did not require that individual spikes be discriminated and used to trigger precisely timed stimulation. Instead, rats received electrical stimulation at two electrode sites, with a fixed latency between the two trains. The stimulation ran nearly continuously for 72 hours, interrupted only by brief periods used to record the activity of all observable neurons. These recordings were used to infer changes in functional connectivity as a consequence of the electrical stimulation.
In the behavioral experiments, we sought to use the conditioning stimulation to effect actual changes in the rat’s behavior. The rats were trained to respond to an ICMS cue by pressing a lever to receive a juice reward. The rat’s detection threshold was determined by randomly varying the stimulus current. After training, we implemented a conditioning paradigm identical to that used in the IFC experiments to test for changes in the rats’ detection thresholds. Adult Long-Evans rats were used for all experiments. All procedures were reviewed and approved by Northwestern University’s Institutional Animal Care and Use Committee.
2.2 Array implantation
Arrays of 16 tungsten microwire electrodes (Tucker-Davis Technologies, Alachua FL) were implanted in the forelimb area of sensorimotor cortex. The arrays were arranged in two rows of eight electrodes, with 250 μm between electrodes and 500 μm between rows. The arrays were implanted stereotaxically, centered at 3.0 mm lateral and 0.5 mm anterior of bregma. A full description of the array implementation and surgical procedures has been published previously (Rebesco et al. 2010).
2.3 Data collection
Acquisition of spike waveforms and behavioral data, as well as stimulation control, was performed using a 16-channel recording and stimulating system (Tucker-Davis Technologies, Alachua FL) with custom software. Electrode signals were bandpass filtered (300–3000 Hz) and sampled at 25 kHz. When any electrode signal crossed a voltage threshold, a spike waveform of 1.5 ms duration (beginning 0.4 ms prior to threshold crossing) was saved for off-line sorting, and analysis.
2.4 Paired-pulse conditioning
The paired-pulse conditioning protocol we used in these experiments is similar to others that have been described previously (Werk et al. 2006) We used the identical protocol for both the functional connectivity and behavioral experiments. In each case, the leading electrode (which we will refer to as the “trigger” electrode to maintain consistency with earlier work) received bursts of electrical stimuli (consisting of 5, 20 μA, 200 μsec, biphasic pulses. The inter-pulse period within each burst was 1 ms, and the interval between bursts was 200 ms (5 Hz), roughly matching the natural spiking rates recorded under these conditions. After a fixed latency, the lagging, or “target” electrode was stimulated with the same train. Bursts of 1 and 3 biphasic pulses (data not presented here) failed to elicit significant potentiation. For short latency experiments, the target electrode pulse train was initiated 5 ms after the last pulse delivered to the trigger electrode. For long latency experiments, the delay was 100 ms. In previous work, we used either 5 or 500 ms between a recorded spike and a single stimulus pulse (Rebesco et al. 2010). However, because of the fixed, 200 ms interval between bursts, in the current study, it was impossible to achieve an effective 500 ms delay between the trigger and target trains. 100 ms latency provided the greatest separation between bursts in the two trains This latency lies outside the temporal window that has been shown to lead to spike-timing dependent plastic changes (Bi and Poo 1998).
2.5 Neuron discrimination and coregistration
Recorded spikes were sorted manually offline (Offline Sorter, Plexon Inc, Dallas TX). We used information from both the shape of the spike waveforms as well as the spiking statistics to identify the subset of neurons that were successfully recorded over multiple sessions. For any neuron putatively believed to be the same over multiple recording sessions, we computed difference measures based on the action potential waveforms and spiking statistics. Null distributions were computed using pairs of neurons recorded from different electrodes. Using these null distributions, a neuron was considered to be tracked across sessions if the empirical p-value was below 0.05. For a full description of the method, see (Rebesco et al. 2010; Tolias et al. 2007).
2.6 Altered functional connectivity experiment
Two electrodes with discriminated single neurons were selected from among all those recorded. One was designated as the “leading” and the other as the “target”. The selection was based on recording stability and high signal-to-noise ratio. The pair of neurons was stimulated at either short or long latency, as described above. Hour long recordings were taken of the entire network during the rat’s unconstrained behavior at 24 hours before and immediately before stimulation onset. During the stimulation period, which lasted for 48–72 hours, brief (30–60 minute) breaks were taken to record again from the entire network. After the conclusion of the stimulation period, continuous recordings were taken for 24–48 hours to assess the timecourse of washout of the induced potentiation.
2.7 Inferred functional connectivity algorithm
In order to determine the nature of the functional interactions among a group of recorded neurons, we described the network activity using a generalized linear model (GLM), similar to many in the literature (Okatan et al. 2005; Paninski 2004; Pillow et al. 2008; Rigat et al. 2006; Stevenson et al. 2009; Truccolo et al. 2005). In this IFC model, the firing rate of a neuron is a linear weighted sum of spiking inputs from all the other observed neurons, the neuron’s own spiking history, and a baseline firing rate. From this firing rate, spikes are stochastically generated. The time history of all spiking inputs are considered, so the free parameters in the model are time-varying kernels that describe the effect of the previous activity of the entre observed network on the given neuron. Specifically, for each pair of neurons i and j, a time-dependent kernel αij(m) exists that describes the functional connection between the two neurons. Summing this kernel αij(m) over time gives a measure of the net effect of neuron j’s firing on neuron i, termed Wij. The matrix W = {Wij} describes the entire network connectivity. We fit the kernels using a maximum a-posteriori approach, with a prior that favors sparse kernels. A complete description of these methods has been published previously (Rebesco et al. 2010).
2.8 Altered ICMS detection threshold experiment
We assayed changes in the rats’ detection threshold using a simple cue-detection task. The rat initiated trials in a self-paced manner by pressing a “cue lever” that caused a cue to be presented in a randomized interval ranging between 1 and 8 seconds later. Following presentation of the cue, the rat had two seconds to press a second lever, termed the “reward lever”. If the rat pressed the reward lever within the two second window, the trial was successful, and the rat was given a juice reward. Pressing the reward lever prior to the presentation of the cue aborted the trial. Failing to press the reward lever, or pressing it after the reward window ended, resulted in a failed trial.
The rat was initially trained with an auditory cue. After satisfactory performance was achieved (>70% of trials completed successfully), the animal was simultaneously presented with both an auditory cue and an ICMS cue. The ICMS cue was 100 biphasic pulses, cathode first, each of duration 200 μsec, delivered at 300 Hz. The volume of the auditory cue was gradually reduced over several sessions until the rat was able to perform the task using only the ICMS cue. The rats were then trained with a fixed, 25 μA ICMS cue until performance reached an asymptote. On subsequent sessions, the current of the ICMS cue was drawn randomly on each trial from the range 0–24 μA. The same electrode was used for behavioral training over the full length of the experiment. While the behavioral training was performed without continuous, single-electrode recordings, the cueing electrode was always chosen using criteria similar to those of the functional connectivity experiments: good apparent recording stability and high signal-to-noise ratio. We applied no other anatomical or physiological criteria to the choice of this electrode We computed the change in threshold by calculating the difference in threshold from the beginning to the end of the paired-pulse stimulation. With this convention, a reduction of threshold, equivalent to the animal becoming more sensitive to the ICMS cue, has a negative sign.
During periods of potentiation induction, the rats received paired-pulse stimulation except during the behavioral task, when the only stimulation they received was the ICMS cue. The daily behavioral sessions lasted approximately 30–45 minutes. Much like the recording periods in the functional connectivity experiments, these sessions were the only interruptions in the paired-pulse conditioning stimulation.
2.9 Threshold fitting
Each trial in the cuing experiment gave a success or failure and reaction time for a particular current level. The successes and failures were binned by delivered current in increments of 4 μA, and success probabilities as a function of current were computed. The normalized success probability was then fit to a sigmoid function:
To account for variations in the animal’s motivational level, the data were scaled by the minimum (pmin) and maximum (pmax) success rates. From this, a slope, β, as well as a detection threshold, I0, were fit. The threshold, I0, is the primary metric of performance we tracked across sessions. A Laplace approximation was used to estimate error bounds on the fit of the threshold.
3. Results
The primary objective of this work was to demonstrate changes in behavior arising from changes in functional connectivity that are driven by the paired-pulse stimulus protocol. To this end, we have established the means for inferring changes in functional connectivity, a method for altering functional connectivity, and metrics for quantifying the animal’s behavior.
3.1 Alteration of inferred connectivity with paired-pulse stimulation
We implemented a paired-pulse stimulation paradigm that ran nearly continuously for 72 hours, to create a Hebbian-like association between trigger and target neuron activity. This stimulation paradigm was interrupted at various time points to record 30–60 minutes of activity of all the neurons. These recordings were used to infer the functional connectivity throughout the experiment. When the latency of the paired-pulse stimulation was short (5 ms), significant changes in the functional connectivity between trigger and target neurons occurred (Figure 1). A baseline measure of connectivity was established with measurements 24 hours before, 6 hours before, and immediately prior to the onset of stimulation. No change in IFC was observed in this period (Figure 1, solid black lines). However, during the subsequent 72 hours of stimulation, IFC from trigger to target increased significantly. All changes in IFC are plotted with respect to their value at time zero. Following the end of stimulation, IFC returned nearly to baseline within 24 hours.
Figure 1.
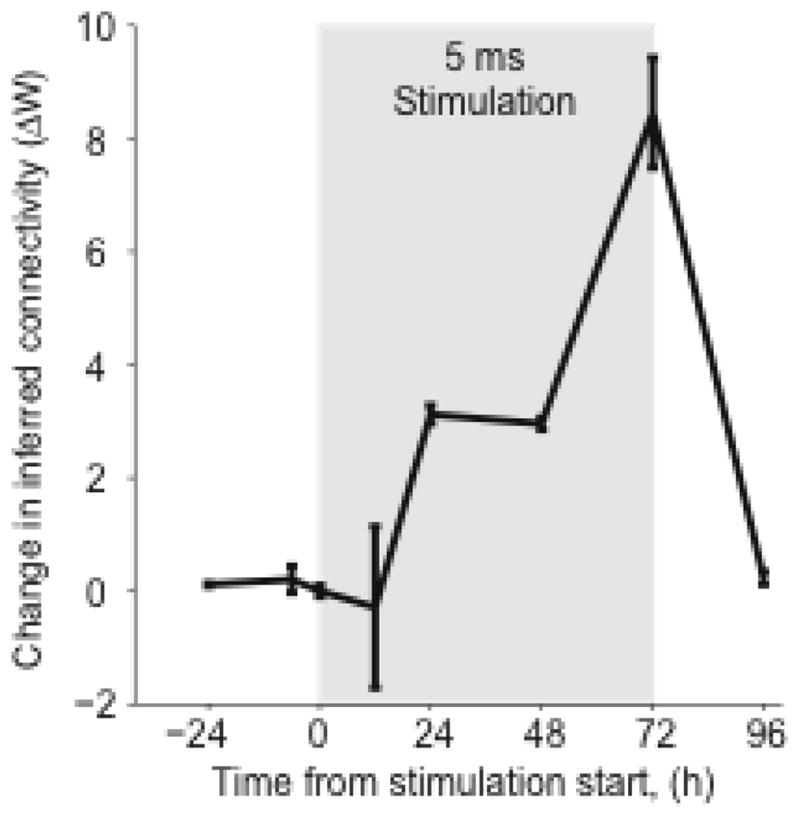
Timecourse of the change in inferred functional connectivity strength from trigger to target (ΔW). Stimulation took place during the 72-hour window highlighted in gray; latency between trigger and target stimulation was 5 ms.
3.2 Network effects of paired-pulse stimulation
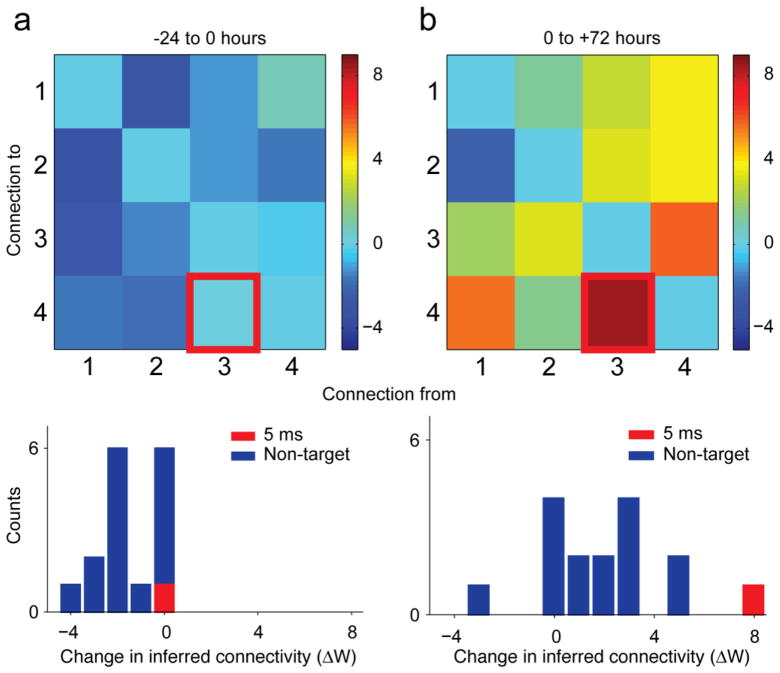
When delivered at short latency, the paired-pulse stimulation effected changes in the trigger to target IFC. However, the stimulation protocol changed IFC for other connections in the network as well. This non-targeted potentiation was a consistent finding in these experiments. Prior to stimulation, the network IFC was stable (Figure 2a). However, following the 72 hours of stimulation, while the targeted connection was potentiated (Figure 2b), a number of other connections within the network were strengthened as well. No significant targeted or non-targeted potentiation was observed at a latency of 100 ms (not shown).
Figure 2.
Network-wide potentiation effects. Matrix of IFC changes ΔW for the stimulation experiment of Figure 1. In all plots, the trigger-to-target connection is highlighted (red). (a) ΔW matrix and histogram for the period from −24 to 0 hours for the 5 ms latency paired stimulation. Color scale indicates size of IFC changes, ΔW. (b) Corresponding results for the period from 0 to 72 hours after the onset of stimulation.
3.3 Aggregate changes in functional connectivity
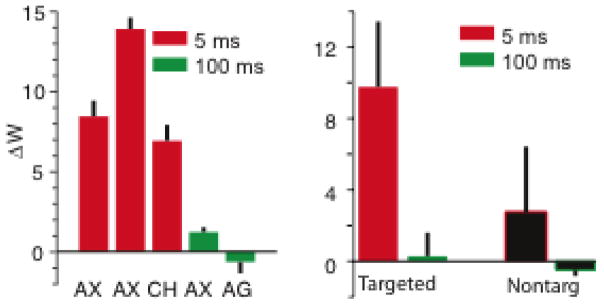
All experiments in which the animals received short latency stimulation showed clear potentiation while those at long latency did not (Figure 3a). For targeted connections, the mean ΔW was 9.8±3.7. Targeted connections in the 100 ms experiments did not change; the targeted ΔW was 0.3±1.3 (Figure 3b). In the 5 ms latency experiments, the average non-targeted potentiation was 2.7±3.7. This was significantly greater than zero (p < 0.01, n = 74, Wilcoxson sign-rank test), but smaller than that of the targeted potentiation (p = 0.02, n = 3, Wilcoxson sign-rank test).
Figure 3.
Potentiation of targeted and non-targeted connections at short and long latencies. In all panels, red refers to 5 ms latency between trigger and target stimulation, and green refers to 100 ms latency. (a) Weight change of targeted connections in 5 separate experiments conducted with 3 different rats. (b) Average weight change for targeted and non-targeted connections.
3.4 Short-latency potentiation and detection thresholds
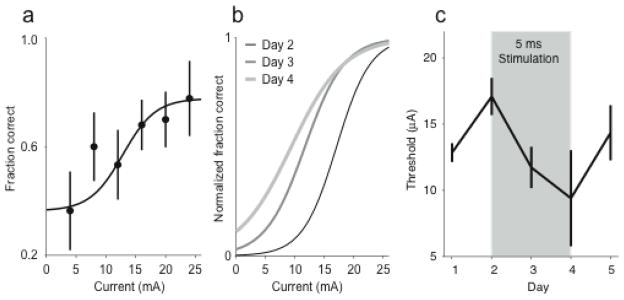
We used paired-pulse stimulation in an effort to alter the rats’ detection threshold to the ICMS cue. By varying the current of the ICMS on a trial-by-trial basis, we assessed the detection threshold for the animal within a given session. Between behavioral sessions, we delivered paired-pulse stimulation to the animal to drive changes in functional connectivity between the site used for the ICMS cue and another electrode that had recorded neurons. If the changes in IFC demonstrated in the previous experiment have actual behavioral consequences, these changes should be reflected in an altered ICMS detection threshold. A single example is shown in Figure 4. In this experiment, the animal received paired-pulse stimulation at 5 ms latency immediately subsequent to the Day 2 session until immediately before the Day 4 session. Within any one session, the animal was cued with currents ranging up to 24 μA. The fraction of correct responses as a function of current was fit to a sigmoid which was used to quantify the animal’s detection threshold (Figure 4a). We also tested the effect of stimulus current on reaction time for this and all other experiments and found no consistent effect. This process was repeated for each session before, during and after paired stimulation (Figure 4b). In the example shown here the threshold decreased by 7.7 μA, from the beginning to the end of the paired-pulse stimulation period. It returned nearly to baseline following the end of the stimulation (Figure 4c).
Figure 4.
Change in detection threshold from Hebbian conditioning. (a) Behavioral data from one session are fit to a sigmoid to create a psychometric curve. (b) Psychometric curves for a five day experiment, including three days of stimulation. Hebbian conditioning started immediately after the day 2 data were taken, and ended immediately prior to the day 4 session. (c). Change in detection threshold during 5-day experiment preceding and following stimulation (gray shaded region)
3.5 Summary of induced changes in ICMS-detection threshold
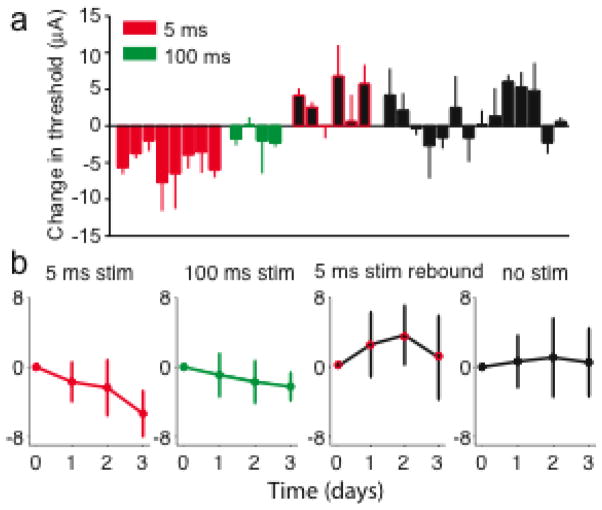
These experiments included five different experimental states. The 5 and 100 ms stimulation states describe days in which the animal received paired-pulse stimulation at either latency. The “5 ms rebound” state includes the period of three days following short-latency stimulation, while the “100 ms rebound” state is the corresponding period following long-latency stimulation. The “null” state includes periods in which the animal was not currently receiving stimulation, and had not been stimulated within the preceding 3 days.
There was a reduction of the detection threshold in nearly all 5 ms stimulation experimental days (Figure 5a, red bars). The mean change in threshold across all experiments was significant (−4.9±1.9 μA; p <0.01, n = 8, Wilcoxson signed rank test). The threshold did not change significantly in the 100-ms stimulation state (Figure 5a, green bars; −1.5±1.1 μA; p = 0.25, n = 4, Wilcoxson signed rank test). The difference between short and long latency stimulation was also significant (p<0.01, n=4, Wilcoxson rank-sum test). The change during the 5 ms rebound state was larger (3.3 ±2.7 μA) than the change that occurred in all other states without stimulation (1.3±2.9 μA), but this difference was not significant (p = 0.17, n = 6, Wilcoxson rank-sum test).
Figure 5.
Summary of daily detection threshold changes for four of the different experimental states defined in text. (a) Individual daily threshold changes for all experiments. Each bar represents the results of a single, three-day experiment. Data came from five individual rats tested in multiple experiments. (b) Average timecourse of changes for each of the experimental states.
The timecourses of the different stimulus states, averaged across sessions and animals, are shown in Figure 5b. The change in threshold for the 5-ms latency experiments was nearly linear, suggesting that 72 hours may not have been sufficient to reach an asymptotic effect. Over the first 48 hours, the average rate of change for the 5 ms rebound state was nearly twice that of the short latency experiments. This may suggest a more rapid timecourse for the return to baseline.
3.6 Yoked-animal experiments
In order to control for environmental factors that may have added noise and affected both the animals’ ICMS-detection thresholds in parallel, we did a series of experiments with a pair of behaviorally yoked animals (Figure 6). In this experiment, the animals alternated three-day sessions in which they received paired-pulse stimulation at either 5 or 100 ms latency, while their detection thresholds were tracked. The 5 ms latency sessions consistently lowered the threshold. For rat PR there was a decrease of −6.6 and −3.9 μA for the two 5 ms sessions. For HC, the changes were −3.5 and −6.0 μA. During the subsequent 5 ms rebound state, the threshold increased (PR: 3.9 and 2.0 μA, HC: 5.6 μA). A slight, but significant change in threshold (−2.3 μA) occurred during one of the 100 ms latency sessions for rat HC. The corresponding change for rat PR was not significantly different from zero.
Figure 6.
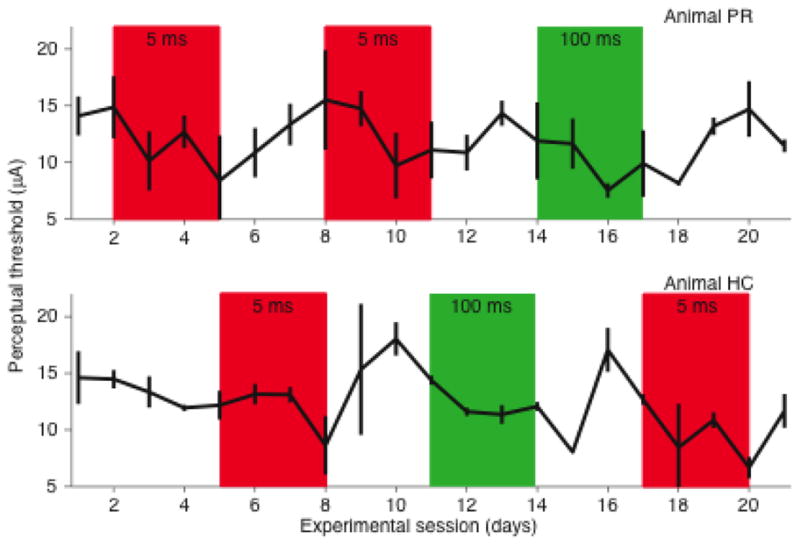
Paired-animal experiments. Change in detection threshold for two animals run simultaneously, with alternating periods of conditioning stimulation. Conditioning periods at short latency (5 ms) are shown in red, periods at long latency (100 ms) are shown in green.
We looked for evidence of correlated changes across these sessions as an indication of general, environmental effects. We first calculated the average change in threshold for each of the five stimulus states defined above. We then computed the residuals (the observed change minus the average change in the corresponding state) for each day. This gave a measure of how much a given day’s change in threshold deviated from the average change for that experimental state. Finally, we computed the correlation of these residuals between the yoked animals. Presumably, external factors affecting the experimental rat’s motivation or learning would have also affected the yoked animal, thereby yielding a positive correlation. For example, if there were unusual noise on a given day, we would expect that it would adversely effect both animals’ performance, causing both thresholds to increase relative to their expected values. There was, however, no such correlation (Figure 7; r = −0.15, p = 0.41), suggesting that there was little or no nonspecific effect of the environment on the rats’ performance.
Figure 7.
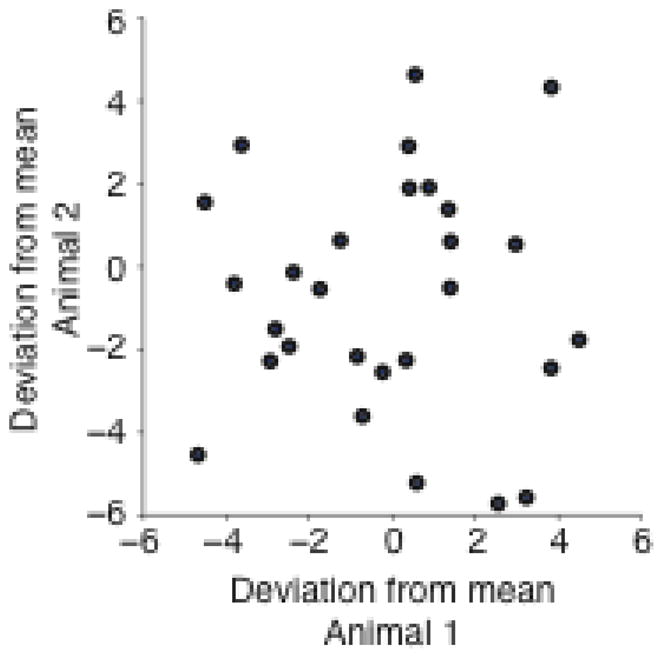
Correlation between threshold changes for paired-animal experiments. Each point compares the change in threshold on a particular day for a given rat and its yoked partner. The daily change in threshold is shown after subtracting the average change for the corresponding stimulus state.
4. Discussion
4.1 Summary of results
In this study, we have used a Hebbian paired-stimulation paradigm to effect changes in the inferred functional connectivity among a small network of neurons. The timing dependence of the effects of this conditioning paradigm were essentially like those of a spike-triggered stimulus paradigm that we have used previously (Rebesco et al. 2010). Potentiation of functional connections occurred only when the stimulation was paired at short (5 ms) latency. At long latency (100 ms), the effect disappeared. Furthermore, changes in IFC were present within 24 hours of the onset of conditioning, and largely disappeared within 24 hours of the end of the conditioning. We developed the paired stimulus conditioning paradigm with the goal of using it to effect changes to the animal’s actual behavior. To this end, we used a simple cue-detection behavioral task that measured the ability of rats to perceive an sensorimotor ICMS stimulus. Stimulation of the cue ICMS site prior to a second site caused a decrease in the detection threshold of the ICMS. We hypothesize that this increased sensitivity was caused by a larger network of neurons activated by the cue stimulus after the strength of its functional connections was increased by the Hebbian paradigm. This larger network might include connections from any neuron activated by the cue stimulus, to the targeted neuron and any other non-targeted neurons with increased IFC from the trigger electrode.
Like the IFC changes, this decrease in detection threshold was dependent on the latency of stimulation: no significant effect was observed when the stimulation was delivered at 100 ms latency. This latency dependence of both the IFC and cue threshold changes is consistent with the classic paired-exponential model of STDP (Bi and Poo 1998), and other studies of in vivo Hebbian conditioning (Fu et al. 2002; Jackson et al. 2006). Also like the effect on IFC, the change in detection threshold returned nearly to baseline levels within 24 hours of the end of conditioning stimulation. It should be noted that the behavior experiments had to be performed without stable single-neuron recordings over the length of the experiment, precluding a direct comparison of the timecourse of the two effects. This behavioral result demonstrates that appropriately crafted Hebbian conditioning paradigms, operating on small populations of neurons, may be able to speed or enhance the benefits of task training, either under normal conditions, or perhaps in the context of rehabilitation following neurological injury.
4.2 Timecourse of effect
In our study, we found significant decreases in cue detection threshold to be detectable within 24 hours of stimulation onset; after the stimulation ceased, the changes in threshold attenuated significantly within 24 hours as well. Related studies have shown significant variability in both the length of induction period as well as the persistence of effect. Fu and colleagues induced changes within minutes, and observed washout on a similar timescale (Fu et al. 2002). Other studies that presented Hebbian conditioning over many days found effects that persisted for days (Froc et al. 2000; Froc and Racine 2005; Ivanco and Racine 2000) to weeks (Jackson et al. 2006). We found some small residual effect of the stimulation but much less than in the previous studies.
4.3 Variability of threshold measurements
Even under normal conditions without the conditioning stimulation paradigm, the inferred detection threshold in our experiment was quite variable, with a variance of approximately 8 μA2. We were concerned that some of the variation may have been due to uncontrolled environmental factors in the lab setting. However, if this was the case, there would have been some correlation in the threshold changes across the paired animals, that received similar training and housing. We found no such correlation, suggesting that extrinsic environmental factors were not the primary source of variability. We conclude that the largest source of variability was intrinsic to the animals, possibly their individual motivational state. However, despite this intrinsic variability, the Hebbian conditioning drove repeatable, statistically significant changes in the threshold.
4.4 Mechanisms of plastic changes
The nervous system constantly exhibits plasticity on a variety of scales. From the individual synapse to entire brain areas, the nervous system is not static, but exists in a state of homeostatic flux. Associative (Hebbian) pairing of stimuli has been shown to drive plasticity at both extremes of this scale. At one end, numerous studies have shown that the mechanisms of LTP/LTD adjust strength of individual synapses based on the relative timing of pre- and post-synaptic activity (Bi and Poo 1998; Markram et al. 1997; Song et al. 2000). At the other, repetitive presentations of stimuli have been shown to cause substantial remapping of cortical areas (Buonomano and Merzenich 1998). There is also massive functional reorganization of the brain as a consequence of neurologic injury, often as the results of stroke (Nudo and Milliken 1996). Further, such reorganization has a dramatic manifestation in the phantom limb sensations often suffered by patients with amputated limbs or even spinal cord injury (Ramachandran et al. 1992). While these systems-level changes presumably reduce to many individual synaptic changes, it is nonetheless remarkable that similar associative mechanisms can describe the organized plastic changes across the nervous system.
4.5 Application to the Brain Machine Interface
Within the last 10 years, an increasing number of BMIs developed by various research groups, explicitly link the activity of mesoscale networks like those we have accessed here, to the control of external devices (Hochberg et al. 2006; Pohlmeyer et al. 2009; Serruya et al. 2002; Taylor et al. 2002; Wessberg et al. 2000). Work with these motor BMIs has demonstrated the ability subjects to adapt fairly quickly to artificial mappings from neural activity to the control of an external device (Ganguly and Carmena 2009).
However, the performance and control in these motor BMIs remain far removed from that enjoyed by intact subjects. Part of the limitation must certainly be due to the small number of neurons available for control. However, it has also very likely that the loss of short-latency proprioceptive feedback is important. Human patients who have lost somatosensation make very impoverished movements that can be only partially compensated for through vision (Ghez et al. 1990; Gordon et al. 1995; Sainburg et al. 1993). It is reasonable to expect that performance might be significantly enhanced by providing short-latency feedback to the user of a BMI about the state of the external device, that would imitate the lost somatosensory feedback (Mussa-Ivaldi and Miller 2003).
Sensory substitution is one means by which an intact sensory modality is used to replace lost function. Tactile stimulation in a grid-like pattern on the back, fingertip or tongue has been tested for blind patients (Bach-y-Rita et al. 1969; Bach-y-Rita and Kercel 2003), and tactile stimulation of reinervated skin on the chest has been used to provide a sense of grip force to patients with upper limb amputation wearing a prosthesis (Marasco et al. 2009; Schultz et al. 2009). For patients without intact peripheral input, direct stimulation of the central nervous system would be necessary in order to provide such feedback. For these patients, a bidirectional BMI that provides short-latency feedback through direct cortical activation, may be necessary.
Efforts to stimulate various sensory cortical areas to provide perception related to vision (Troyk et al. 2005), or hearing (Rauschecker and Shannon 2002) have been attempted for several decades. Similar efforts directed at somatosensation are more recent (Fitzsimmons et al. 2007; London et al. 2008; Romo et al. 1998). For this feedback to be successfully integrated into BMI control, the subject must perceive the cortical stimulation and learn to interpret the information it carries about the state of the device. If the device is being driven by recordings from motor areas of cortex, there will be short latency associations between the recorded signals and subsequent stimulation not unlike those we have reported here. Therefore, demonstrating the ability to manipulate an operant response to ICMS, as we have shown, is an important translational step for the use of Hebbian conditioning in bidirectional BMIs.
4.6 Application to Neurorehabilitation
A number of studies have demonstrated in human subjects, that it may be possible to enhance rehabilitation from stroke and neurologic disease through Hebbian conditioning protocols. Several studies have shown potentially important effects on afferent and efferent transmission as a result of stimulus induced, paired activity centrally and in the periphery. Paired stimulation of the median nerve and the contralateral hand area of M1, in sessions lasting about an hour, induces a potentiation of motor evoked potentials (Stefan et al. 2000). The effect was NMDA-receptor dependent, suggesting that LTP and related mechanisms may be the basis for the changes (Stefan et al. 2002). Likewise, motor evoked potentials were also shown to be enhanced by pairing electrical stimulation of the peroneal nerve with voluntary muscle activation (Khaslavskaia and Sinkjaer 2005) as well as timing-dependent paired cortical and peripheral nerve stimulation (Taylor and Martin 2009). The mechanisms underlying these effects may be similar to those responsible for the observation that functional electrical stimulation of muscles during attempted movement promotes enhanced recovery of function following stroke (Cauraugh et al. 2000; Popovic et al. 2002; Thrasher et al. 2008). In addition to Hebbian mechanisms, recovery from stroke engages more general homeostatic mechanisms in the brain as well. Shortly after stroke, a series of genetic pathways are activated that appear to open a window of increased cortical plasticity in around the injured area (Murphy and Corbett 2009; Nudo 2006). Targeted, intracortical Hebbian conditioning to drive cortical plasticity during this critical period of plasticity in the weeks following stroke may substantially extend traditional therapy (Harvey and Nudo 2007; Plow et al. 2009).
Acknowledgments
The authors gratefully acknowledge the contributions of Matt Bauman, and Rebecca Friesen, who assisted with the care and training of the animal subjects. Conversations with Drs. Sandro Mussa-Ivaldi and Konrad Kording were invaluable for the concept and experimental design, as well as the interpretation of data. Drs. Mark Laubach, Nandakumar Narayanan, Robert Rennaker and Andrew Sloan provided valuable expertise in electrophysiological recording from the rats. This work was supported by a grant from NINDS (R01 NS048845), and an NIH/NINDS fellowship (F31NS062552).
References
- Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Bach-y-Rita P, Collins CC, Saunders FA, White B, Scadden L. Vision substitution by tactile image projection. Nature. 1969;221:963–964. doi: 10.1038/221963a0. [DOI] [PubMed] [Google Scholar]
- Bach-y-Rita P, Kercel SW. Sensory substitution and the human-machine interface. Trends Cogn Sci. 2003;7:541–546. doi: 10.1016/j.tics.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Bak M, Girvin JP, Hambrecht FT, Kufta CV, Loeb GE, Schmidt EM. Visual sensations produced by intracortical microstimulation of the human occipital cortex. Medical & Biological Engineering and Computing. 1990;28:257–259. doi: 10.1007/BF02442682. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb’s postulate revisited. Annual review of neuroscience. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type. Journal of Neuroscience. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of physiology. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. The Journal of physiology. 1968;196:479–493. doi: 10.1113/jphysiol.1968.sp008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annual review of neuroscience. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Butovas S, Schwarz C. Detection psychophysics of intracortical microstimulation in rat primary somatosensory cortex. The European journal of neuroscience. 2007;25:2161–2169. doi: 10.1111/j.1460-9568.2007.05449.x. [DOI] [PubMed] [Google Scholar]
- Cauraugh J, Light K, Kim S, Thigpen M, Behrman A. Chronic Motor Dysfunction After Stroke: Recovering Wrist and Finger Extension by Electromyography-Triggered Neuromuscular Stimulation. Stroke. 2000;31:1360–1364. doi: 10.1161/01.str.31.6.1360. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. Journal of neurophysiology. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Deliano M, Scheich H, Ohl FW. Auditory cortical activity after intracortical microstimulation and its role for sensory processing and learning. J Neurosci. 2009;29:15898–15909. doi: 10.1523/JNEUROSCI.1949-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Experience-dependent plasticity in adult rat barrel cortex. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2082–2086. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science (New York, NY) 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons NA, Drake W, Hanson TL, Lebedev MA, Nicolelis MA. Primate Reaching Cued by Multichannel Spatiotemporal Cortical Microstimulation. J Neurosci. 2007;27:5593–5602. doi: 10.1523/JNEUROSCI.5297-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froc DJ, Chapman CA, Trepel C, Racine RJ. Long-term depression and depotentiation in the sensorimotor cortex of the freely moving rat. Journal of Neuroscience. 2000;20:438–445. doi: 10.1523/JNEUROSCI.20-01-00438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froc DJ, Racine RJ. Interactions between LTP- and LTD-inducing stimulation in the sensorimotor cortex of the awake freely moving rat. Journal of neurophysiology. 2005;93:548–556. doi: 10.1152/jn.00253.2004. [DOI] [PubMed] [Google Scholar]
- Fu YX, Djupsund K, Gao H, Hayden B, Shen K, Dan Y. Temporal specificity in the cortical plasticity of visual space representation. Science (New York, NY) 2002;296:1999–2003. doi: 10.1126/science.1070521. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Carmena JM. Emergence of a stable cortical map for neuroprosthetic control. PLoS biology. 2009;7:e1000153. doi: 10.1371/journal.pbio.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Gordon J, Ghilardi MF, Christakos CN, Cooper SE. Cold spring harbor symposia on quantitative biology. Cold spring harbor lab press; 1990. Roles of proproceptive input in the programming of arm trajectories; pp. 837–847. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Ghez C. Impairments of reaching movements in patients without proprioception. I. Spatial Errors. Journal of neurophysiology. 1995;73:347–360. doi: 10.1152/jn.1995.73.1.347. [DOI] [PubMed] [Google Scholar]
- Harvey RL, Nudo RJ. Cortical brain stimulation: a potential therapeutic agent for upper limb motor recovery following stroke. Topics in Stroke Rehab. 2007;14:54–67. doi: 10.1310/tsr1406-54. [DOI] [PubMed] [Google Scholar]
- Hebb D. The organization of behavior. New York: Wiley; 1949. [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Ji Z, Standish S, Boyd-Hodgson TE, Henderson AK, Racine RJ. Training-induced and electrically induced potentiation in the neocortex. Neurobiol Learn Mem. 2005;83:22–32. doi: 10.1016/j.nlm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Ivanco TL, Racine RJ. Long-term potentiation in the reciprocal corticohippocampal and corticocortico pathways in the chronically implanted, freely moving rat. Hippocampus. 2000;10:143–152. doi: 10.1002/(SICI)1098-1063(2000)10:2<143::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- Jarosiewicz B, Chase SM, Fraser GW, Velliste M, Kass RE, Schwartz AB. Functional network reorganization during learning in a brain-computer interface paradigm. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19486–19491. doi: 10.1073/pnas.0808113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. Journal of neurophysiology. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- Kemere C, Shenoy KV, Meng TH. Model-based neural decoding of reaching movements: a maximum likelihood approach. IEEE transactions on bio-medical engineering. 2004;51:925–932. doi: 10.1109/TBME.2004.826675. [DOI] [PubMed] [Google Scholar]
- Khaslavskaia S, Sinkjaer T. Motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve depends on the voluntary drive. Experimental brain research Experimentelle Hirnforschung. 2005;162:497–502. doi: 10.1007/s00221-004-2153-1. [DOI] [PubMed] [Google Scholar]
- London BM, Jordan LR, Jackson CR, Miller LE. Electrical stimulation of the proprioceptive cortex (area 3a) used to instruct a behaving monkey. IEEE Trans Neural Syst Rehabil Eng. 2008;16:32–36. doi: 10.1109/TNSRE.2007.907544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magjarevic R, Shin HC, Schieber M, Thakor N. Neural Decoding of Single and Multi-finger Movements Based on ML. In: Lim CT, Goh JCH, editors. 13th International Conference on Biomedical Engineering. Springer; Berlin Heidelberg: 2009. pp. 448–451. [Google Scholar]
- Marasco PD, Schultz AE, Kuiken TA. Sensory capacity of reinnervated skin after redirection of amputated upper limb nerves to the chest. Brain. 2009;132:1441–1448. doi: 10.1093/brain/awp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science (New York, NY) 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Meliza CD, Dan Y. Receptive-field modification in rat visual cortex induced by paired visual stimulation and single-cell spiking. Neuron. 2006;49:183–189. doi: 10.1016/j.neuron.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Murphey DK, Maunsell JH. Behavioral detection of electrical microstimulation in different cortical visual areas. Curr Biol. 2007;17:862–867. doi: 10.1016/j.cub.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nature reviews. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA, Miller LE. Brain-machine interfaces: computational demands and clinical needs meet basic neuroscience. Trends Neurosci. 2003;26:329–334. doi: 10.1016/S0166-2236(03)00121-8. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Mechanisms for recovery of motor function following cortical damage. Current Opinion in Neurobiology. 2006;16:638–644. doi: 10.1016/j.conb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. JNeurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- O’Doherty JE, Lebedev MA, Hanson TL, Fitzsimmons NA, Nicolelis MA. A brain-machine interface instructed by direct intracortical microstimulation. Front Integr Neurosci. 2009;3:20. doi: 10.3389/neuro.07.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okatan M, Wilson MA, Brown EN. Analyzing functional connectivity using a network likelihood model of ensemble neural spiking activity. Neural Computation. 2005;17:1927–1961. doi: 10.1162/0899766054322973. [DOI] [PubMed] [Google Scholar]
- Otto KJ, Rousche PJ, Kipke DR. Cortical microstimulation in auditory cortex of rat elicits best-frequency dependent behaviors. Journal of neural engineering. 2005;2:42–51. doi: 10.1088/1741-2560/2/2/005. [DOI] [PubMed] [Google Scholar]
- Paninski L. Maximum likelihood estimation of cascade point-process neural encoding models. Network: Computation in Neural Systems. 2004;15:243 – 262. [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge [see comments] Science (New York, NY) 1994;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- Pillow JW, Shlens J, Paninski L, Sher A, Litke AM, Chichilnisky EJ, Simoncelli EP. Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature. 2008;454:995–999. doi: 10.1038/nature07140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive Cortical Stimulation to Promote Recovery of Function After Stroke: A Critical Appraisal. Stroke. 2009;40:1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmeyer EA, Oby ER, Perreault EJ, Solla SA, Kilgore KL, Kirsch RF, Miller LE. Toward the Restoration of Hand Use to a Paralyzed Monkey: Brain-Controlled Functional Electrical Stimulation of Forearm Muscles. PLoS ONE. 2009;4:e5924. doi: 10.1371/journal.pone.0005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic MB, Popovic DB, Sinkjaer T, Stefanovic A, Schwirtlich L. Restitution of reaching and grasping promoted by functional electrical therapy. Artificial organs. 2002;26:271–275. doi: 10.1046/j.1525-1594.2002.06950.x. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D, Stewart M. Perceptual correlates of massive cortical reorganization. Science (New York, NY) 1992;258:1159–1160. doi: 10.1126/science.1439826. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Shannon RV. Sending sound to the brain. Science (New York, NY) 2002;295:1025–1029. doi: 10.1126/science.1067796. [DOI] [PubMed] [Google Scholar]
- Rebesco JM, Stevenson IH, Koerding K, Solla SA, Miller LE. Rewiring neural interactions by micro-stimulation. Frontiers in Systems Neuroscience. 2010;4:12. doi: 10.3389/fnsys.2010.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone G, Jenkins W, Hradek G, Merzenich M. Progressive improvement in disciminative abilites in adult owl monkeys performing a tactile frequency discrimination task. J Neurophysiology. 1992;67:1015–1030. doi: 10.1152/jn.1992.67.5.1015. [DOI] [PubMed] [Google Scholar]
- Rigat F, de Gunst M, van Pelt J. Bayesian modeling and analysis of spatio-temporal neuronal networks. Bayesian Analysis. 2006;1:733–764. [Google Scholar]
- Romo R, Hernandez A, Zainos A, Salinas E. Somatosensory discrimination based on cortical microstimulation. Nature. 1998;392:387–390. doi: 10.1038/32891. [DOI] [PubMed] [Google Scholar]
- Rousche PJ, Normann RA. Chronic intracortical microstimulation (ICMS) of cat sensory cortex using the Utah Intracortical Electrode Array. IEEE Trans Rehabil Eng. 1999;7:56–68. doi: 10.1109/86.750552. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Poizner H, Ghez C. Loss of proprioception produces deficits in interjoint coordination. Journal of neurophysiology. 1993;70:2136–2147. doi: 10.1152/jn.1993.70.5.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz AE, Marasco PD, Kuiken TA. Vibrotactile detection thresholds for chest skin of amputees following targeted reinnervation surgery. Brain Res. 2009;1251:121–129. doi: 10.1016/j.brainres.2008.11.039. [DOI] [PubMed] [Google Scholar]
- Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002;416:141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- Song S, Miller KD, Abbott LF. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nature neuroscience. 2000;3:919–926. doi: 10.1038/78829. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. The Journal of physiology. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stevenson IH, Rebesco JM, Hatsopoulos NG, Haga Z, Miller LE, Kording KP. Bayesian inference of functional connectivity and network structure from spikes. IEEE Trans Neural Syst Rehabil Eng. 2009;17:203–213. doi: 10.1109/TNSRE.2008.2010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science (New York, NY) 2002;296:1829–1832. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Martin PG. Voluntary Motor Output Is Altered by Spike-Timing-Dependent Changes in the Human Corticospinal Pathway. J Neurosci. 2009;29:11708–11716. doi: 10.1523/JNEUROSCI.2217-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher TA, Zivanovic V, McIlroy W, Popovic MR. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehabil Neural Repair. 2008;22:706–714. doi: 10.1177/1545968308317436. [DOI] [PubMed] [Google Scholar]
- Tolias AS, Ecker AS, Siapas AG, Hoenselaar A, Keliris GA, Logothetis NK. Recording chronically from the same neurons in awake, behaving primates. Journal of neurophysiology. 2007;98:3780–3790. doi: 10.1152/jn.00260.2007. [DOI] [PubMed] [Google Scholar]
- Trepel C, Racine RJ. Long-term potentiation in the neocortex of the adult, freely moving rat. Cerebral Cortex. 1998;8:719–729. doi: 10.1093/cercor/8.8.719. [DOI] [PubMed] [Google Scholar]
- Troyk PR, Bradley D, Bak M, Cogan S, Erickson R, Hu Z, Kufta C, McCreery D, Schmidt E, Sung S, Towle V. Intracortical visual prosthesis research - approach and progress. Conf Proc IEEE Eng Med Biol Soc. 2005;7:7376–7379. doi: 10.1109/IEMBS.2005.1616216. [DOI] [PubMed] [Google Scholar]
- Truccolo W, Eden UT, Fellows MR, Donoghue JP, Brown EN. A point process framework for relating neural spiking activity to spiking history, neural ensemble, and extrinsic covariate effects. Journal of neurophysiology. 2005;93:1074–1089. doi: 10.1152/jn.00697.2004. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich M, Sameshima K, Jenkins W. Remodelling hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378:71–76. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- Werk CM, Chapman CA. Long-term potentiation of polysynaptic responses in layer V of the sensorimotor cortex induced by theta-patterned tetanization in the awake rat. Cerebral Cortex. 2003;13:500–507. doi: 10.1093/cercor/13.5.500. [DOI] [PubMed] [Google Scholar]
- Werk CM, Klein HS, Nesbitt CE, Chapman CA. Long-term depression in the sensorimotor cortex induced by repeated delivery of 10 Hz trains in vivo. Neuroscience. 2006;140:13–20. doi: 10.1016/j.neuroscience.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, Kim J, Biggs SJ, Srinivasan MA, Nicolelis MA. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000;408:361–365. doi: 10.1038/35042582. [DOI] [PubMed] [Google Scholar]
- Wu W, Gao Y, Bienenstock E, Donoghue JP, Black MJ. Bayesian population decoding of motor cortical activity using a Kalman filter. Neural Comput. 2006;18:80–118. doi: 10.1162/089976606774841585. [DOI] [PubMed] [Google Scholar]
- Yao H, Dan Y. Stimulus timing-dependent plasticity in cortical processing of orientation. Neuron. 2001;32:315–323. doi: 10.1016/s0896-6273(01)00460-3. [DOI] [PubMed] [Google Scholar]