Abstract
Spectrin tetramer is the major structural member of the membrane-associated skeletal network of red cells. We show here that disruption of the spectrin-ankyrin-band 3 link to the membrane leads to dissociation of a large proportion of the tetramers into dimers. Non-covalent perturbation of the linkage was induced by a peptide containing the ankyrin-binding site of the spectrin β-chain, and covalent perturbation by treatment with the thiol reagent, N-ethylmaleimide (NEM). This reagent left the intrinsic self-association capacity of the spectrin dimers unaffected, and affected only the ankyrin-band 3 interaction. The dissociation of spectrin tetramers on the membrane into functional dimers was confirmed by the binding of a spectrin peptide directed against the self-association sites. Dissociation of the tetramers resulted, we infer, from detachment of the proximal ends of the constituent dimers from the membrane, thereby reducing their proximity to one another and thus their association. The measured affinity of the interaction of the peptides with the free dimer ends on the membrane permits an estimate of the equilibrium between intact and dissociated tetramers on the native membrane. This indicates that in the physiological state the equilibrium proportion of the dissociated tetramers may be as high as 5-10%. These findings enabled us to identify an additional important functional role for spectrin-ankyrin-band 3 link in regulating spectrin self-association in the red cell membrane.
The shear elasticity of the red cell membrane and its exceptional resistance to mechanical stress are independently regulated properties (1), yet both are wholly dependent on the proteins of the membrane skeletal network. This consists of long elastomeric spectrin molecules, predominantly in the form of tetramers, attached at their distal ends to nodes, or junctions, made up of actin, 4.1R and other proteins (see Mohandas and Gallagher for review (2)). Shear elasticity of the membrane is thought to be primarily regulated by extension of the spectrin tetramers and reversible unfolding of certain of their constituent triple helical repeats, while maintenance of normal membrane mechanical stability depends on the tetrameric state of spectrin (1, 3, 4). Decreased spectrin self-association due to mutations in α- or β-spectrin leads to reduced membrane mechanical stability and fragmentation of the cell (2). It appears paradoxical that the apparent dissociation of tetramers into their constituent dimers, with consequent interruption of the continuity of the network, as occurs when red cells are treated with the thiol-directed reagent, N-ethylmaleimide (NEM), not only reduces membrane mechanical stability but also increases the stiffness of the membrane (1, 5, 6). A further unexplained feature of the effect of NEM on the cells is that, regardless of the NEM concentration and time of reaction, the proportion of spectrin dissociated into dimers does not exceed 70-75% (4). We have investigated these phenomena, and the results appear to indicate the need for a re-examination of the structural basis of spectrin self-association and its relation to membrane elasticity.
Keywords: Spectrin, red cell membrane, membrane elasticity, membrane cohesion
MATERIALS ANS METHODS
Reagents and antibodies
Polyclonal antibodies against ankyrin and band 3 were generated in our laboratory. Unless otherwise indicated, all other reagents were purchased from Sigma Aldrich (St Louis, MO).
Cells
Fresh blood was taken with informed consent from healthy volunteers by venipuncture, with EDTA as anticoagulant. After discarding the buffy coat, the red cells were washed 3 times with PBS (137 mM NaCl, 10 mM Na phosphate, 2.7 mM KCl, pH 7.4) and resuspended in PBS.
Microscopy
Cells and resealed ghosts, sampled before and after manipulations involving reagents, were suspended at 10% hematocrit in PBS, supplemented with 0.1% w/v bovine serum albumin (BSA), and examined by phase-contrast microscopy.
Peptides
The preparation and properties of the peptides corresponding to the sites of interaction of α- and β-chains in forming the tetramer from two dimers of both spectrin I (erythroid) and II (non-erythroid) has been previously described (7, 8). A spectrin fragment corresponding to structural repeats 14 and 15 of the spectrin I β-chain, which contains the ankyrin-binding site was sub-cloned into pGEX-4T-2 vector using EcoR-I and Sal-I restriction enzymes upstream and downstream respectively. The fidelity of the construct was confirmed by DNA sequencing. All the recombinant fragments were purified on a glutathione-Sepharose 4B affinity column.
Reaction with NEM
Red cells at 10% hematocrit were treated with NEM at concentrations of up to 2mM for 15 min at 37°C. In other experiments the modification was carried out on ghosts, prepared by lysis of washed cells in 35 volumes of ice-cold hypotonic buffer A (5 mM Tris, 5 mM potassium chloride, pH 7.4), followed by resuspension in the same buffer and centrifugation for 10 min at 20,000g until white. Isotonicity was then restored by addition of 50 mM Tris, 1.5 M potassium chloride, pH 7.4, before treatment with up to 2 mM NEM for 1 h at 4°C. The reaction was terminated by incubation with 10 mM dithiothreitol (DTT) for 15 min at 37°C (intact cells) or 2 min at 4°C (ghosts).
Binding of peptides
The procedure followed was that of An et al. (7). Peptides αI(1-154), αII(1-149) and βI(15-C) and βIR(14-15) at a series of concentrations were each added to hypotonic ghosts on ice; these ghosts were prepared by lysis in buffer A, containing in addition 1 mM Mg-ATP. Resealing was effected by restoration of tonicity and incubation at 37°C. After washing to remove external peptide, the sealed ghosts were incubated at the desired temperature for 40 min. Bound peptide was determined by extraction of the total spectrin as described below, followed by electrophoresis under non-denaturing conditions in 5% polyacrylamide gel in a Tris-Bicine buffer system in the cold (9). The relative concentrations of spectrin dimer-peptide complex and spectrin tetramer were evaluated by staining and densitometry, using ImageJ software (available from NIH).
Ektacytometry
Modified and control ghosts were suspended in 40% dextran (Mw 40,000 kDa), and fragmentation under shear as a function of time was measured in the ektacytometer (10). The membrane stability was expressed as the rate of decrease of deformability index (DI) at a constant applied shear stress of 750 dynes cm−2.
Extraction of spectrin
Ghosts prepared by lysis of red cells in ice-cold hypotonic buffer A were twice washed in the same buffer, and twice more with extraction medium (Buffer B: 0.25 mM sodium-phosphate, pH 7.4). Spectrin was extracted by overnight dialysis at 4°C in buffer B and recovered in the supernatant after centrifugation for 20 min at 20,000g. Protein concentration was determined spectrophotometrically, taking E(280 nm; 1 mg ml−1; 1 cm) = 1.07.
Analysis of dimer-tetramer equilibrium of modified spectrin in solution
Total extraction of spectrin was performed by incubating ghosts with 40 volumes of 0.5 mM EDTA for 30 min at 37°C, followed by centrifugation as before. The resulting spectrin dimer was made isotonic and allowed to react with NEM at a series of concentrations for 1 hr at 37°C. Excess reagent was quenched by addition of 10 mM DTT. The modified spectrin samples thus generated were incubated at 30°C for 4 hr to allow the system to reach self-association equilibrium. The relative concentrations of dimer and tetramer were determined by polyacrylamide gel electrophoresis in the cold as before.
Preparation and modification of membrane skeletons
Ghosts were incubated in 20 volumes of buffer C (625 mM sodium chloride, 6.25 mM sodium phosphate, 0.625 mM EGTA, 0.625 mM DTT, pH 7.5) with or without 6% v/v Triton X-100 for 30 min at 4°C. The resulting membrane skeletons were collected by centrifugation (20,000g for 20 min) and washed by resuspension in PBS. They were then incubated in Buffer A with or without NEM, resuspended after centrifugation in an equal volume of Tris-glycine gel electrophoresis buffer containing 10% SDS (11), heated, and applied to a 10% SDS-polyacrylamide gel in the same buffer.
Extraction of ankyrin from spectrin-depleted inside-out vesicles of modified ghosts
Inside-out vesicles (IOVs) from ghosts before and after NEM treatment were prepared by extraction of spectrin in buffer C at 37°C, as above. For extraction of ankyrin, the IOVs were incubated for 30 min at 37°C in 40 volumes of solutions containing potassium iodide at concentrations of up to 1 M in buffer D (10 mM sodium phosphate, 1 mM EDTA, 1 mM DTT, 1 mM diisopropylfluorophosphate (DFP), 0.02% v/v Tween 20). After centrifugation for 30 min at 20,000g, the IOVs were washed by suspension in buffer D, dissolved in electrophoresis sample buffer as above and subjected to electrophoresis in a 10% SDS-polyacrylamide gel. The separated proteins were electrophoretically transferred to nitrocellulose membrane (BioRad, Hercules, CA), which was blocked for 1 hr in PBS-T (137 mM NaCl, 10 mM Na phosphate, 2.7 mM KCl, 0.1% Tween 20, pH 7.4), containing 4% (w/v) nonfat milk powder and 1% (w/v) bovine serum albumin (BSA), followed by incubation for 1 hr at room temperature with anti-ankyrin or anti-band 3 antibody. Blots were then washed three times with PBS-T and incubated 1 hr at room temperature with a horseradish-peroxidase-conjugated anti-rabbit antibody. Immunoreactive bands were detected by the enhanced chemiluminescence (ECL) method (Pierce, Rockford, IL). Bands were quantified with the aid of ImageJ software.
Extraction of band 3
To eliminate the extracellular glycosyl chains from band 3, red cells were washed three times with PBS, resuspended at 50% hematocrit in PBS and incubated with 1 mg ml−1 chymotrypsin for 1 hr at 37°C. The enzyme was inactivated by addition of Protease Inhibitor Cocktail. NEM treatment was performed for 15 min at 37°C, as above, and the cells were then lysed in Buffer A and washed in the same buffer until white. Band 3 was extracted, following the protocol of Bennett et al. (12): the ghosts were resuspended in Buffer E (100 mM potassium chloride, 10 mM sodium phosphate, 1 mM EDTA, 0.2 mM DTT, 1 mM DFP, pH 7.5) with or without Triton X-100 for 15 min at 4°C. After centrifugation at 40,000g for 15 min, the samples were washed with Buffer D, resuspended in an equal volume of electrophoresis buffer (11), and applied to a 10% SDS-polyacrylamide gel. Western blots of these gels were analysed as before for relative concentrations of band 3 and ankyrin, normalized to that of actin.
Estimation of lipid asymmetry
To assay for exposure of phosphatidylserine in the outer leaflet of the intact cell membrane, cells were incubated with fluorescein-conjugated annexin V and 1 mM calcium chloride in PBS, and subjected to flow cytometry, following De Jong et al. (13).
RESULTS
We have examined the effects of disrupting the link between spectrin, ankyrin and band 3 close to the proximal ends of the dimers, which unite to form tetramers. We have established that the covalent and non-covalent perturbations we have employed to bring this about resulted in substantial dissociation of tetramers but had no effect on the interactions of the constituent spectrin dimers at their distal ends with the proteins that define the nodes of the intact membrane skeletal lattice.
Covalent modification
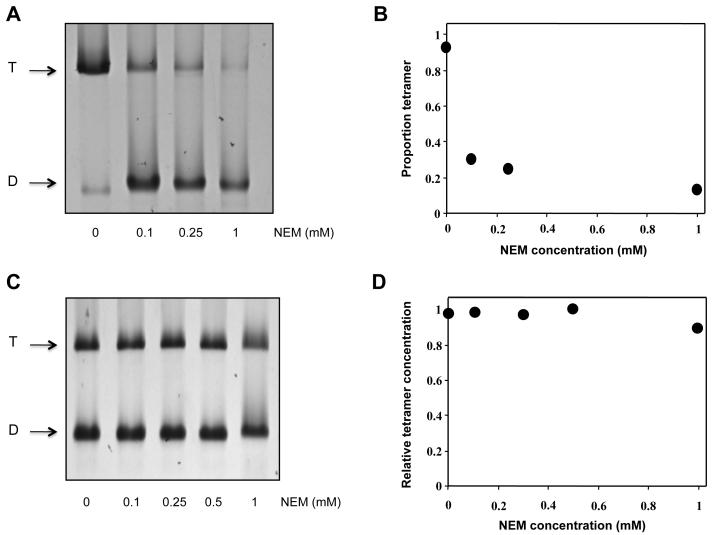
Intact red cells incubated with NEM under the conditions defined by Smith and Palek (4) showed some shape disturbances, with loss of the smooth contour, but no appreciable swelling or shrinkage. Deformation under shear in the ektacytometer was markedly reduced relative to untreated cells, as previously reported (1). As observed by Smith and Palek, the fraction of spectrin extracted from the membrane at 4°C and low ionic strength in the form of dimers reached a maximum of 70-75%, as revealed by polyacrylamide gel electrophoresis (Fig. 1A, B). We examined whether this reflects an inability of this fraction, but not the remaining 25-30% of the dimers, to form tetramers in the NEM-treated cells. We recovered the total spectrin in the form of dimers from the membranes of cells exposed to increasing concentrations of NEM (up to 1mM) and from untreated control cells by extraction in low ionic-strength medium at 37°C. The solution was brought to physiological ionic strength by addition of tenfold-concentrated PBS, and aliquots were incubated at 30°C for 4 hr, and analysed by gel electrophoresis in the cold (Fig. 1C, D). The association constant determined from the dimer-tetramer ratios was almost indistinguishable from that of native spectrin under the same conditions, namely 1.1 – 1.5 × 106 M−1 (9, 14, 15). Treatment of cells with higher concentrations of NEM gave some evidence of accretion of irreversible dissociation of tetramers. This observation was not pursued further.
Fig 1. Effect of N-ethylmaleimide on the association state of spectrin in the red cell membrane and in solution.
A. Native polyacrylamide gel showing separation of spectrin dimer (D) and tetramer (T), after extraction from cells treated with the indicated concentrations of NEM. B. Proportion of spectrin converted to dimer in situ, determined by densitometry of the gels. C. Polyacrylamide gel and D. densitometric evaluation of equilibrium mixture of spectrin dimer and tetramer after treatment in solution with the indicated concentrations of NEM.
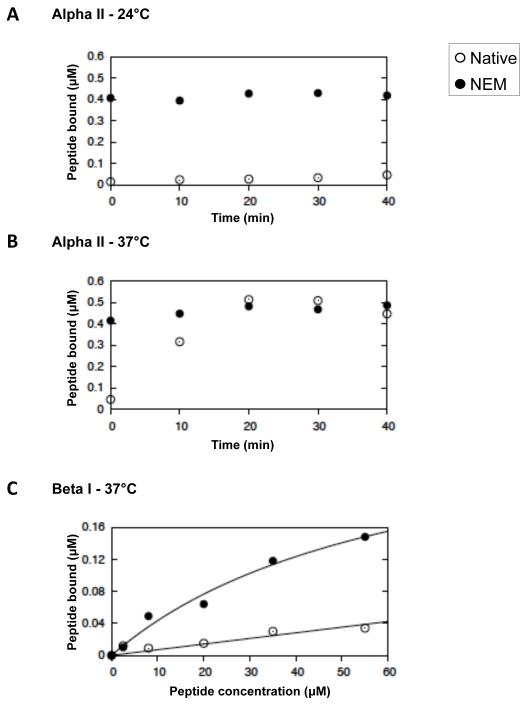
To establish whether the self-association sites on the spectrin dimers generated in the cell by NEM treatment were indeed functional in situ, we introduced peptides, corresponding to these sites (7) into ghosts derived from the treated cells. These peptides bind competitively to the self-association sites, thereby causing dissociation of the tetramers in situ and in solution (7). The peptide derived from αII spectrin (the isoform found in neural and many other cells) binds much more strongly and more rapidly than αI or βI (erythroid) peptides to the termini of the erythroid spectrin chains (8). The proportions of spectrin dimers and tetramers on the membranes and the amounts of peptide bound were determined by gel electrophoresis as before. Equilibrium and kinetic data for the binding of peptides to the membranes are shown in Fig. 2. The titre of αII peptide bound corresponds, within error, to the full complement of spectrin in both unreacted and NEM-modified membranes (Fig 2A and B). The weaker binding of the βI peptide (Fig. 2C) discriminates between the highly reactive free chain termini of the preformed dimers in the NEM-treated cells and the remainder, shielded in the residual tetramers. The apparent association constants for these two populations of sites, calculated from the binding profiles are 1.3 (± 0.1) × 104 M−1 and 1.5 (± 0.2) × 103 M−1 respectively.
Fig 2. Equilibria and kinetics of binding of peptides to the self-association sites of spectrin in the red cell membrane.
Kinetics of binding to native (open circles) and NEM-reacted (filled circles) red cell ghosts at 24°C (A.) and at 37°C (B.) of αII spectrin peptide, directed at the spectrin dimer self-association site. C. Equilibrium binding at 37°C of βI spectrin peptide, directed at the dimer self-association sites. The curves are best-fits, giving apparent association constants, Kapp = 1.3 (± 0.1) × 104 and 1.5 (± 0.2) × 103 M−1, respectively, uncorrected for dimer-tetramer equilibrium (see text).
It is thus evident that the dissociation by NEM of spectrin into its constituent dimers on the membrane is not caused by modification of the self-association site on either the α- or the β-chain. It is in fact only the terminal part of the β-chain that contains cysteine residues (two in number). (Reaction of NEM with amino groups, which is a known, though rare occurrence, could be excluded (5)). Because of the weak self-association of spectrin at physiological temperature (14, 15), it can be inferred that the predominance of tetramers on the membrane is due to the close proximity of the association sites of the constituent dimers. This is imposed by the tight attachment to the membrane of one of the spectrin dimers of each tetramer at a point near its proximal end, where the ankyrin binds to the β-chain in making a bridge to band 3. One way in which the apposition of the dimer-dimer binding sites could be disturbed would be by dissociation of the distal ends of the tetramers from the network junctions, since only one of the dimers in each tetramer is attached to the membrane through an ankyrin bridge (16). Such an effect would reveal itself by the disintegration of the isolated skeletal network on treatment with NEM. Membrane skeletons prepared from cells by extraction with Triton X-100 (17) showed no loss of spectrin nor discernible loss of any of the junction proteins when examined by SDS-gel electrophoresis (data not shown). There is thus no disruption of the lattice junctions by NEM, and the spectrin tetramers evidently re-form when the membrane skeletons are liberated (in greatly condensed state (18, 19)) from the lipid bilayer.
This leaves as the likeliest explanation for the dissociation of the spectrin tetramers in the NEM-treated cells the loss of spectrin attachments to the membrane near the dimer self-association sites. This could result from breakage of either the spectrin-ankyrin or the ankyrin-band 3 link, since the ankyrin binding site in spectrin is located on the β-chain, near its C-terminus, where it binds to the α-chain of the partner dimer. Binding of spectrin to inner-leaflet lipids, phosphatidylserine (20-22) and phosphatidylethanolamine (23, 24), ought also to be considered, in view of the evidence that such interactions contribute to the stability of the membrane (25), and because NEM is known to inactivate the translocase, partly responsible for maintaining the asymmetric distribution of these lipids (26). If, for whichever of these reasons, the proximal ends of the spectrin dimers detach from the membrane surface, and so acquire an additional degree of freedom of motion, the effective local two-dimensional concentration of association sites would diminish. Reduced association between the dimers would then be expected to ensue.
We have eliminated loss of spectrin-lipid interactions as a factor in the dissociation of tetramers by assaying for exposed phosphatidylserine at the outer cell surface before and after NEM treatment. Flow cytometry of cells labelled with fluoresent annexin V (13) revealed no significant difference (data not shown). To determine whether NEM treatment had impaired the spectrin-ankyrin interaction or that of ankyrin with band 3 we examined the binding of spectrin dimers to inside-out vesicles prepared by extraction of spectrin from the membranes of NEM-modified cells, and of spectrin from such cells to vesicles from untreated cells (Fig. 3). In both cases binding was essentially unchanged from that of native spectrin dimers to inside-out vesicles from untreated cells.
Fig 3. Binding of native spectrin to inside-out membrane vesicles from cells treated with N-ethylmaleimide, and of spectrin from cells thus treated to vesicles of untreated cells.
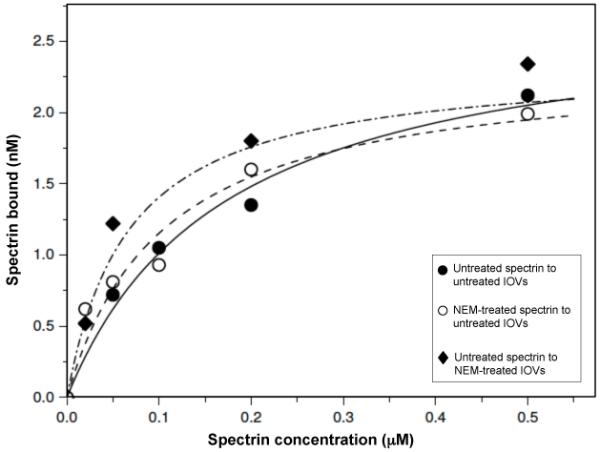
Spectrins bound to vesicles as a function of spectrin concentration: vesicles and spectrin from untreated cells (dashed circles). Spectrin from NEM-treated cells and vesicles from untreated cells (open circles), spectrin from untreated cells and vesicles from NEM-treated cells (filled circles). The curves are best fits for binding to a population of identical and independent sites, giving saturation binding titres of 2.8 + 0.3, 2.6 + 0.2 and 2.4 + 0.3 nM respectively, with corresponding apparent association constants of 0.6 + (0.13) × 106, 1.5 (+ 0.3) × 106, and 1.0 (+ 0.3) × 106 M−1.
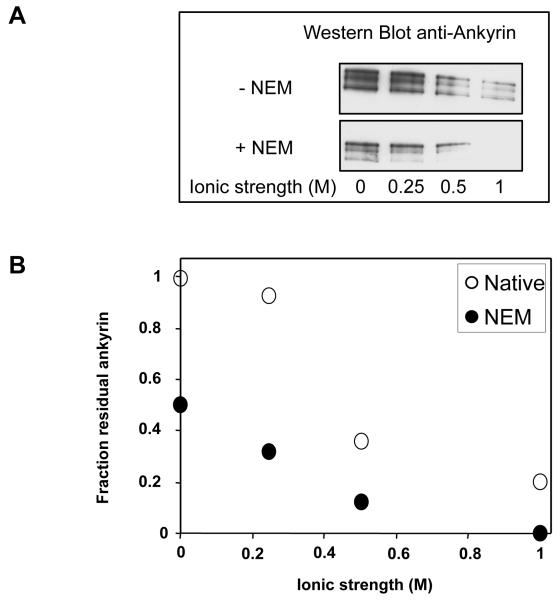
We explored lastly the alternative explanation, that attachment of spectrin to the membrane is weakened by dissociation of the ankyrin-band 3 bridge. Fig. 4 shows the extraction of ankyrin from spectrin-depleted IOVs as a function of salt concentration. Whereas we saw no perceptible extraction from untreated cells at ionic strengths of up to at least 0.25 M, about half of their complement of ankyrin was lost from NEM-treated cells during the low-salt extraction of spectrin. At the extraction volume in our experiments nearly all the remainder was liberated at physiological ionic strength. Similarly, band 3 was much more readily extracted by detergent-containing solutions (17) from the membrane skeletons of NEM-treated than untreated cells (data not shown). Ankyrin, accordingly, is barely retained in the membranes of the modified cells. We conclude that NEM does indeed sever the attachment of spectrin to the membrane by eliminating or greatly weakening the ankyrin-band 3 interaction.
Fig 4. Extraction of ankyrin from inside-out vesicles of cells before and after treatment with N-ethylmaleimide as a function of ionic strength of the extracting medium.
A. Residual ankyrin on the membranes as a function of ionic strength of the extraction buffer in spectrin-depleted inside-out vesicles before and after NEM treatment as documented by western blot analysis. B. Fractions of ankyrin remaining in the vesicles after extraction: filled circles refer to NEM-treated, and open circles to control cells. Note that in the former, the proportion of residual protein refers to the ankyrin present in the vesicles as prepared, which have already lost nearly half their ankyrin in the course of preparation.
Non-covalent modification
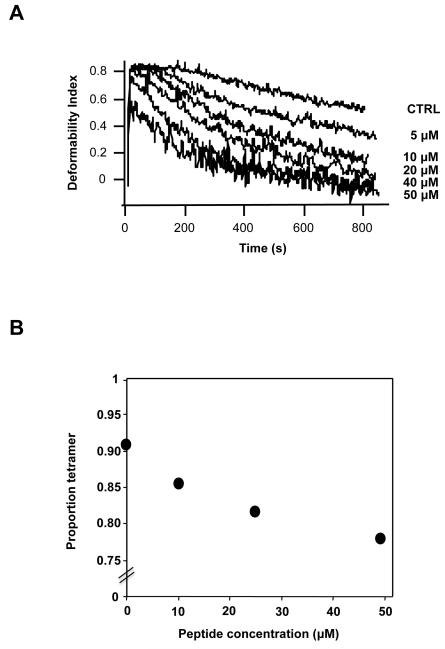
To determine whether the spectrin-membrane link can also be disrupted without chemical modification we prepared a spectrin β-chain fragment, βIR(14-15) containing the ankyrin binding site (27). Introduction of increasing concentrations (up to 50 μM) of this peptide into ghosts led to a substantial loss of stability of the membrane under shear in the ektacytometer (Fig. 5A). Moreover, spectrin extracted from the membrane by dialysis in the cold revealed the presence of 15-20% of dimers (Fig. 5B). We are unable to determine what proportion of the spectrin-ankyrin links were dissociated in these conditions (the maximum attainable peptide concentration), but it is clear that, as in the NEM-treated cells, the partial dissociation of the spectrin from its primary site of attachment to the membrane is accompanied by partial dissociation of the spectrin tetramers.
Fig 5. Effect of incorporation of β-spectrin peptide that binds to ankyrin (βIR(14-15) on membrane mechanical stability and spectrin dimer- tetramer equilibrium.
A. membrane mechanical stability of the resealed ghosts was measured by ektacytometry. Membrane stability, expressed as the rate of decline in deformability index, DI, diminishes (decay curve displaced toward shorter times) with increasing concentrations of peptide incorporated into cells. B. Densitometric evaluation of native electrophoretic gels of spectrin extracted from cells treated with peptide, showing partial dissociation of spectrin tetramers.
DISCUSSION
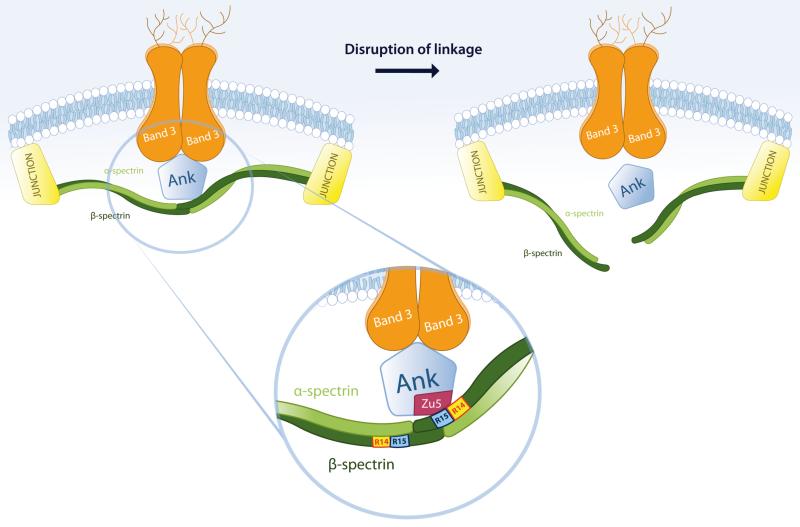
Our results raise some questions regarding the generally accepted schemes, elaborated over the last decades, to explain the remarkable elastic properties and the exceptional resistance to mechanical stress of the red cell membrane. Our observations confirm that the predominant self-association of spectrin dimers at physiological temperature in situ, despite their weak intrinsic affinity for one another in free solution, depends on their close apposition on the membrane. This depends in turn on the band 3-ankyrin bridge to the lipid bilayer. Our model for the relation between the disruption of the spectrin-membrane association and spectrin tetramer dissociation is schematically depicted in Fig. 6.
Fig 6. Schematic model showing suggested consequences of disruption of the spectrin ankyrin-band 3 bridge on spectrin self-association.
The spectrin α-chains are shown in light green, the β-chains in dark green. Inset: The interactions between ankyrin and β–spectrin are highlighted. Zu5, a subdomain of ankyrin, binds to β–spectrin through the terminal part of R14 and most of the R15, and has been identified as the minimal binding domain for β–spectrin (27, 39).
Our finding that partial dissociation of spectrin tetramers can be induced by a non-covalent disturbance of the spectrin-membrane nexus is consistent with the similar effects exerted by genetic anomalies. Thus, Agre et al. (28) found a large deficit of ankyrin-binding sites on spectrin- and ankyrin-denuded inside-out vesicles, isolated from cells of patients with an elliptocytic haemolytic anaemia, characterized by the presence of spectrin dimers. Another spectrin variant with defective self-association, leading to severe haemolytic anaemia, was found to engender impaired ankyrin binding (29, 30). The consequence of rupture of the spectrin-ankyrin-band 3 complex may be somewhat mitigated by the interaction between spectrin and the phospholipids of the inner membrane leaflet (25); But this is clearly insufficient to compensate for the suppression of the spectrin-ankyrin interaction by the ankyrin-binding spectrin peptide. At the same time, the attachment of ankyrin to spectrin dimers has been found to cause a tenfold increase in the self-association equilibrium constant (31). The reaction with NEM leaves the ankyrin-spectrin association undisturbed, and therefore this consideration does not presumably enter into the NEM-induced dissociation of the spectrin tetramers. We cannot, however, exclude its participation in the non-covalent intervention.
We have established that NEM causes dissociation of spectrin tetramers without perturbing the dimer self-association sites, that the spectrin-ankyrin link is unaffected, and that the site of disruption of the spectrin-membrane interaction lies at the ankyrin-band 3 interface. The results explain the observation (4) that NEM causes dissociation of no more than ~75% of the spectrin tetramers (and higher oligomers) into dimers in the native membrane. We cannot determine whether the capacity of band 3 to bind to ankyrin is eliminated or merely weakened, for there is evidence that band 3 contains two binding sites for ankyrin (32) (which may account for the tetrameric state of the ankyrin-bound band 3, while the remainder is dimeric). In addition, the extraction of band 3 from the preparations of membrane skeletons from NEM-treated cells is incomplete at physiological ionic strength; this implies that an interaction with some membrane component survives NEM treatment. Proteins known to interact directly or indirectly with band 3 include 4.1R (33) and adducin (34) and, among transmembrane constituents, glycophorin A and a number of blood-group proteins (35).
We also established that the spectrin β-chain peptide, which contains the binding site for the α-chain, binds much more avidly to NEM-treated than to native cells. This bears out the conclusion that dimer self-association sites become available on treatment of the cell with NEM. Analysis of the interaction of the peptide with the spectrin in the NEM-treated cells now permits an estimate of the intrinsic affinity of the univalent peptide for free dimer ends on the membrane, and therefore of the strength of the dimer-dimer interaction in the unperturbed cell (7). Binding of the peptide to the native membrane (Fig. 2C) is governed by an apparent association constant, Kapp = (PS)/(P)(S), where (PS) represents the concentration of bound peptide, (P) that of free peptide and (S) that of free binding sites, and since in these experiments the total peptide concentration, Po >> (PS), Kapp ~ (PS)/Po(S). But the peptide binds only to free α-chain ends, and thus the true association constant is K = (PS)/Po(O), where (O) represents the concentration of ‘open’ dimers (twice the number of dissociated tetramers). Here (O) = So - (PS) – (C), where So is the total concentration of membrane-associated spectrin in terms of dimers, and (C) that of self-associated (‘closed’) dimers. The dimer-tetramer balance can be expressed in terms of a (dimensionless) pseudo-equilibrium constant K’ = (O)/(C). In the NEM-treated cells, with ca. 75% dimers at equilibrium, K’NEM ~ 3, whence the true association constant, K, between a univalent β-chain and the α-chain of an open dimer in situ can be estimated. This emerges as about 1.7 × 104 M−1 (Fig. 2B). In the native, unreacted cell, the open-closed equilibrium is defined by a constant K’o, analogous to K’NEM. Binding of the peptide to the unreacted membrane yields an apparent association constant, K°app, of ca. 1.3 × 103 M−1 (Fig. 2C). From this, given the value of K, we can make an estimate of K’o, the ratio of free to self-associated dimers on the unperturbed membrane. Ko’, as here determined, refers to binding of a univalent peptide to a divalent dimer, but in solution the association constants between two univalent α- and β-fragments and between two divalent αβ-fragments differ by no more than a factor of about 2 (14), and we may reasonably assume that a similar relation prevails on the membrane. This leads to an estimate of 0.08 for K’o, or in other words, some 8% of the dimers in the unperturbed membrane are in the dissociated (‘open’) state. This conforms to the small proportion of spectrin dimers, which appears always to be extracted from the membrane at low temperature, when the dimer-tetramer equilibrium is effectively frozen (14, 15). We stress that, given the experimental error, considerations of temperature and solvent conditions, possible molecular crowding by hemoglobin, uncertainty about whether the dissociated components of a dimer can both bind a peptide molecule, and other assumptions implicit in the calculation, the accuracy of the estimate of Ko’ is limited. Nevertheless, the implication stands that the association of the dimers on the membrane is quite weak (though of course much stronger than in solution).
The dependence of the ability of the membrane to resist shear stresses on maintenance of spectrin in its tetrameric state is well established and understood (1,3). Why an increase in shear modulus should accompany the dissociation of a large proportion of the elastomeric members of the membrane skeletal network, as seen in NEM-treated cells and cells of HE patients with membranes containing a large proportion of spectrin dimers (36) remains unexplained. The increased freedom of the pendant spectrin dimers could perhaps allow them to form interactions with other proteins, and so impede the deformation of the membrane; or their association with phospholipids of the inner membrane leaflet could become similarly favoured. Three structural perturbations by which the cell can adapt to shear-induced deformations have been recognized: (1) the spectrin tetramers can increase, by perhaps threefold, their end-to-end separation, which in the unperturbed cell is constrained at well below its equilibrium value (see e.g. Vertessy and Steck (19)); (2) the spectrin tetramers can dissociate transiently into dimers (7, 8); (3) certain of the repeating units, of which the spectrin chains are composed, can unfold (3, 37). In the case that the spectrin dimer-tetramer equilibrium strongly favours the dimer, all these responses would presumably be restricted to the minority of spectrin molecules in the tetrameric state at any instant. The dissociation of the spectrin tetramers leads to relatively unimpeded translational diffusion of transmembrane proteins (38), and may therefore be expected to allow them to cluster, and possibly also bind to membrane skeletal constituents. Such interactions could conceivably render the membrane more rigid. A definitive explanation of the relation between the continuity of the membrane skeletal network and the elastic characteristics of the membrane demands more extensive investigation.
ACKNOWLEDGEMENTS
We thank Dr John Sleep and Dr Patrick Gallagher for valuable discussion and advice.
ABBREVIATIONS
- NEM
N-ethylmaleimide
- PBS
phosphate buffer saline
- BSA
bovine serum albumin
- D.I
deformability index
- DTT
dithiothreitol
- SDS
sodium dodecyl sulfate
- IOVs
inside-out vesicles
Footnotes
This work was supported by National Institute of Health (grants DK26263, DK32094, and HL31579)
REFERENCES
- 1.Chasis JA, Mohandas N. Erythrocyte membrane deformability and stability: two distinct membrane properties that are independently regulated by skeletal protein associations. J. Cell. Biol. 1986;103:343–350. doi: 10.1083/jcb.103.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112:3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson CP, Tang HY, Carag C, Speicher DW, Discher DE. Forced unfolding of proteins within cells. Science. 2007;317:663–666. doi: 10.1126/science.1139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DK, Palek J. Sulfhydryl reagents induce altered spectrin self-association, skeletal instability, and increased thermal sensitivity of red cells. Blood. 1983;62:1190–1196. [PubMed] [Google Scholar]
- 5.Fischer TM, Haest CW, Stöhr M, Kamp D, Deuticke B. Selective alteration of erythrocyte deformabiliby by SH-reagents: evidence for an involvement of spectrin in membrane shear elasticity. Biochim. Biophys. Acta. 1978;510:270–282. doi: 10.1016/0005-2736(78)90027-5. [DOI] [PubMed] [Google Scholar]
- 6.Rangachari K, Beaven GH, Nash GB, Clough B, Dluzewski AR, Myint-Oo, Wilson RJ, Gratzer WB. A study of red cell membrane properties in relation to malarial invasion. Mol. Biochem. Parasitol. 1989;34:63–74. doi: 10.1016/0166-6851(89)90020-0. [DOI] [PubMed] [Google Scholar]
- 7.An X, Lecomte MC, Chasis JA, Mohandas N, Gratzer W. Shear-response of the spectrin dimer-tetramer equilibrium in the red blood cell membrane. J. Biol. Chem. 2002;277:31796–31800. doi: 10.1074/jbc.M204567200. [DOI] [PubMed] [Google Scholar]
- 8.Salomao M, An X, Guo X, Gratzer WB, Mohandas N, Baines AJ. Mammalian alpha I-spectrin is a neofunctionalized polypeptide adapted to small highly deformable erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 2006;103:643–648. doi: 10.1073/pnas.0507661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahbakhti F, Gratzer WB. Analysis of the self-association of human red cell spectrin. Biochemistry. 1986;25:5969–5975. doi: 10.1021/bi00368a020. [DOI] [PubMed] [Google Scholar]
- 10.Mohandas N, Clark MR, Health BP, Rossi M, Wolfe LC, Lux SE, Shohet SB. A technique to detect reduced mechanical stability of red cell membranes: relevance to elliptocytic disorders. Blood. 1982;59:768–774. [PubMed] [Google Scholar]
- 11.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Bennett V, Stenbuck PJ. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979;280:468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- 13.de Jong K, Geldwerth D, Kuypers FA. Oxidative damage does not alter membrane phospholipid asymmetry in human erythrocytes. Biochemistry. 1997;36:6768–6776. doi: 10.1021/bi962973a. [DOI] [PubMed] [Google Scholar]
- 14.DeSilva TM, Peng KC, Speicher KD, Speicher DW. Analysis of human red cell spectrin tetramer (head-to-head) assembly using complementary univalent peptides. Biochemistry. 1992;31:10872–10878. doi: 10.1021/bi00159a030. [DOI] [PubMed] [Google Scholar]
- 15.Ungewickell E, Gratzer W. Self-association of human spectrin. A thermodynamic and kinetic study. Eur. J. Biochem. 1978;88:379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- 16.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 17.Sheetz MP, Sawyer D. Triton shells of intact erythrocytes. J. Supramol. Struct. 1978;8:399–412. doi: 10.1002/jss.400080403. [DOI] [PubMed] [Google Scholar]
- 18.Svoboda K, Schmidt CF, Branton D, Block SM. Conformation and elasticity of the isolated red blood cell membrane skeleton. Biophys. J. 1992;63:784–793. doi: 10.1016/S0006-3495(92)81644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vertessy BG, Steck TL. Elasticity of the human red cell membrane skeleton. Effects of temperature and denaturants. Biophys. J. 1989;55:255–262. doi: 10.1016/S0006-3495(89)82800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen AM, Liu SC, Derick LH, Palek J. Ultrastructural studies of the interaction of spectrin with phosphatidylserine liposomes. Blood. 1986;68:920–926. [PubMed] [Google Scholar]
- 21.MacDonald RI. Temperature and ionic effects on the interaction of erythroid spectrin with phosphatidylserine membranes. Biochemistry. 1993;32:6957–6964. doi: 10.1021/bi00078a021. [DOI] [PubMed] [Google Scholar]
- 22.Maksymiw R, Sui SF, Gaub H, Sackmann E. Electrostatic coupling of spectrin dimers to phosphatidylserine containing lipid lamellae. Biochemistry. 1987;26:2983–2990. doi: 10.1021/bi00385a005. [DOI] [PubMed] [Google Scholar]
- 23.Hryniewicz-Jankowska A, Bok E, Dubielecka P, Chorzalska A, Diakowski W, Jezierski A, Lisowski M, Sikorski AF. Mapping of an ankyrin-sensitive, phosphatidylethanolamine/phosphatidylcholine mono- and bi-layer binding site in erythroid beta-spectrin. Biochem. J. 2004;382:677–685. doi: 10.1042/BJ20040358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sikorski AF, Michalak K, Bobrowska M. Interaction of spectrin with phospholipids. Quenching of spectrin intrinsic fluorescence by phospholipid suspensions. Biochim. Biophys. Acta. 1987;904:55–60. doi: 10.1016/0005-2736(87)90086-1. [DOI] [PubMed] [Google Scholar]
- 25.Manno S, Takakuwa Y, Mohandas N. Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1943–1948. doi: 10.1073/pnas.042688399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrot G, Hervé P, Zachowski A, Fellmann P, Devaux PF. Aminophospholipid translocase of human erythrocytes: phospholipid substrate specificity and effect of cholesterol. Biochemistry. 1989;28:3456–3462. doi: 10.1021/bi00434a046. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy SP, Warren SL, Forget BG, Morrow JS. Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin. J. Cell. Biol. 1991;115:267–277. doi: 10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agre P, Orringer EP, Chui DH, Bennett V. A molecular defect in two families with hemolytic poikilocytic anemia: reduction of high affinity membrane binding sites for ankyrin. J. Clin. Invest. 1981;68:1566–1576. doi: 10.1172/JCI110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burke JP, Van Zyl D, Zail SS, Coetzer TL. Reduced spectrin-ankyrin binding in a South African hereditary elliptocytosis kindred homozygous for spectrin St Claude. Blood. 1998;92:2591–2592. [PubMed] [Google Scholar]
- 30.Zail SS, Coetzer TL. Defective binding of spectrin to ankyrin in a kindred with recessively inherited hereditary elliptocytosis. J. Clin. Invest. 1984;74:753–762. doi: 10.1172/JCI111491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giorgi M, Cianci CD, Gallagher PG, Morrow JS. Spectrin oligomerization is cooperatively coupled to membrane assembly: a linkage targeted by many hereditary hemolytic anemias? Exp. Mol. Pathol. 2001;70:215–230. doi: 10.1006/exmp.2001.2377. [DOI] [PubMed] [Google Scholar]
- 32.Van Dort HM, Moriyama R, Low PS. Effect of band 3 subunit equilibrium on the kinetics and affinity of ankyrin binding to erythrocyte membrane vesicles. J. Biol. Chem. 1998;273:14819–14826. doi: 10.1074/jbc.273.24.14819. [DOI] [PubMed] [Google Scholar]
- 33.Pasternack GR, Anderson RA, Leto TL, Marchesi VT. Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J. Biol. Chem. 1985;260:3676–3683. [PubMed] [Google Scholar]
- 34.Anong WA, Franco T, Chu H, Weis TL, Devlin EE, Bodine DM, An X, Mohandas N, Low PS. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood. 2009;114:1904–1912. doi: 10.1182/blood-2009-02-203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruce LJ, Beckmann R, Ribeiro ML, Peters LL, Chasis JA, Delaunay J, Mohandas N, Anstee DJ, Tanner MJ. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood. 2003;101:4180–4188. doi: 10.1182/blood-2002-09-2824. [DOI] [PubMed] [Google Scholar]
- 36.Chabanel A, Sung KL, Rapiejko J, Prchal JT, Palek J, Liu SC, Chien S. Viscoelastic properties of red cell membrane in hereditary elliptocytosis. Blood. 1989;73:592–595. [PubMed] [Google Scholar]
- 37.Johnson CP, Gaetani M, Ortiz V, Bhasin N, Harper S, Gallagher PG, Speicher DW, Discher DE. Pathogenic proline mutation in the linker between spectrin repeats: disease caused by spectrin unfolding. Blood. 2007;109:3538–3543. doi: 10.1182/blood-2006-07-038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuji A, Ohnishi S. Restriction of the lateral motion of band 3 in the erythrocyte membrane by the cytoskeletal network: dependence on spectrin association state. Biochemistry. 1986;25:6133–6139. doi: 10.1021/bi00368a045. [DOI] [PubMed] [Google Scholar]
- 39.Ipsaro JJ, Huang L, Gutierrez L, MacDonald RI. Molecular epitopes of the ankyrin-spectrin interaction. Biochemistry. 2008;47:7452–7464. doi: 10.1021/bi702525z. [DOI] [PMC free article] [PubMed] [Google Scholar]