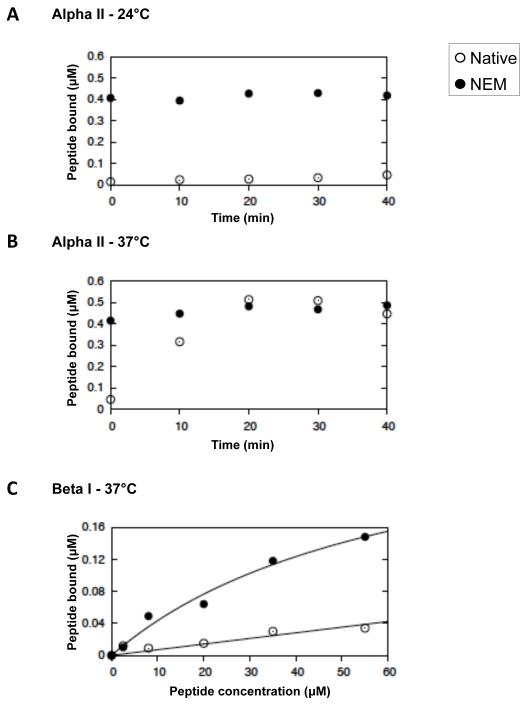
Fig 2. Equilibria and kinetics of binding of peptides to the self-association sites of spectrin in the red cell membrane.
Kinetics of binding to native (open circles) and NEM-reacted (filled circles) red cell ghosts at 24°C (A.) and at 37°C (B.) of αII spectrin peptide, directed at the spectrin dimer self-association site. C. Equilibrium binding at 37°C of βI spectrin peptide, directed at the dimer self-association sites. The curves are best-fits, giving apparent association constants, Kapp = 1.3 (± 0.1) × 104 and 1.5 (± 0.2) × 103 M−1, respectively, uncorrected for dimer-tetramer equilibrium (see text).