Abstract
Anti-cancer therapy faces major challenges, particularly in terms of specificity of treatment. The ideal therapy would eradicate tumor cells selectively with minimum side effects on normal tissue. Gene or cell therapies have emerged as realistic prospects for the treatment of cancer, and involve the delivery of genetic information to a tumor to facilitate the production of therapeutic proteins. However, there is still much to be done before an efficient and safe gene medicine is achieved, primarily developing the means of targeting genes to tumors safely and efficiently. An emerging family of vectors involves bacteria of various genera. It has been shown that bacteria are naturally capable of homing to tumors when systemically administered resulting in high levels of replication locally. Furthermore, invasive species can deliver heterologous genes intra-cellularly for tumor cell expression. Here, we review the use of bacteria as vehicles for gene therapy of cancer, detailing the mechanisms of action and successes at preclinical and clinical levels.
Key words: bactofection, tumor, gene delivery, cell therapy
Introduction
Cancer is the most common cause of death in developed countries.1 Many patients, by the time of presentation, have already developed secondary tumors (metastases), which are generally responsible for fatalities. Conventional therapies for cancer such as chemotherapy and radiotherapy are characterized by poor survival rates due to multiple factors including tumor development of drug-resistance and their lack of tumor specificity, resulting in undesirable side effects on healthy cells and therefore limitations on therapeutic dose.2 For patients with such metastatic cancers, the development of alternative therapies is essential. Gene therapy is a realistic prospect for the treatment of all cancers, and involves the delivery of genetic information to the patient to facilitate the production of therapeutic proteins. Gene therapy has evolved from conceptually focusing on the treatment of monogenetic disorders such as cystic fibrosis, severe combined immunodeficiency (SCID) and muscular dystrophy, to a broader potential including approaches to produce therapeutics in vivo or inducing cell death, and is being investigated and indeed employed clinically for a wider range of both inherited and acquired diseases.3 The basic principle lies in the delivery of a nucleic acid effecting the expression of a gene of interest (transgene) in the desired location of the patient's body.
Therapeutic agents utilized in gene therapy strategies are typically encoded by DNA and produced within target cells, such as cancer cells. The process generally involves introduction of DNA to a cell (transfection), which encodes for a protein and the necessary genetic elements required for expression of the gene of interest to effect successful protein production. Therapeutic molecules delivered are not confined to pieces of DNA, and can be RNA (e.g., to inhibit expression of a disease causing gene), or indeed the delivery agent itself may be engineered to be cytotoxic to the cancer cell (oncolytic therapy). Another expansion of the concept is cell therapy, where the therapeutic gene is not expressed by the tumor cell but instead produced within the target tissue from newly introduced vectors pre-transfected with genes (discussed below). Independent of the therapeutic strategy chosen for delivery, the target cells can include the tumor cells themselves, immune cells or tissue such as muscle to act as a source of protein production.
Efficient delivery of the therapeutic gene to the target tissue or cell is the most significant hurdle for successful gene therapy. Since naked DNA is rapidly cleared or degraded in vivo by phagocytic immune cells or extracellular nucleases, a means of protecting the transgene is required.4,5 Furthermore, a vehicle for effecting tissue or cell entry is also required, due to the poor efficiency of spontaneous DNA uptake. Thus, DNA is normally combined with a gene delivery vehicle of some type, commonly known as a vector, to protect and mediate effective tissue or cell entry of the gene of interest.6
Gene Delivery Vectors
Gene delivery systems can be grouped into non-biological (chemical and physical approaches of introducing plasmid DNA to mammalian cells) or biological (viruses and bacteria). Non-viral gene delivery systems normally involve the transfer genes carried on plasmid DNA. Plasmids employed do not generally replicate in mammalian cells. The use of chemical means to carry DNA into cells involves cationic polymers, cationic peptides and cationic lipids (liposomes).7 Mechanical or physical techniques include the application of energy waves to cells to create transient pores in the cell membrane, thereby permitting entry of plasmid without killing the cell. Mammalian cell ‘poration’ systems include electroporation8,9 and sonoporation.10 Overall, while the safety profile of non-viral vectors is attractive, the efficiency of current non-viral approaches compare poorly with viral vectors, thereby limiting clinical efficacy and restricting the range of applicable therapeutic approaches.11
By virtue of their natural life cycle, pathogenic viruses possess an innate ability to effectively invade human cells and express their genes within the cell. Gene therapists have harnessed the ability of viruses to package DNA, transfer it to a cell, and produce proteins within it. Viral vectors have an advantage in that their superb efficiency as delivery vehicles has evolved naturally.12 However, several problems exist with current viral vectors. In terms of practicality of usage, there are associated difficulties in production, size restrictions on transgenes in some viral vectors, anti-vector immunologic responses limit their usage to a single application, while several human cancers are devoid of viral receptors and thus not transducable by viral vectors.13–17 From a safety point of view, many viruses are toxic and elicit systemic inflammatory reactions and clinical trials have highlighted safety fears over viral vector integration (which has resulted in cases of leukemia) and fatal immune responses leading to an increased interest in non-viral systems.15,18
Bacteria as gene therapy vectors.
Like viruses, the innate biological properties of bacteria permit efficient DNA delivery to cells or tissues. The concept of exploiting bacterial species as biological gene vectors has existed for some time, and the use of bacteria to deliver therapeutics offers many advantages over other gene delivery approaches. Bacteria fall within the ‘non-viral’ class of delivery systems, investigated primarily for safety reasons, yet the biological nature of bacterial vectors means that many of the inherently beneficial traits of viral vectors are retained. Bacterial presence in excised patient tumors was first reported in the 1950s while the correlation between bacterial infection and tumor regression dates back to the nineteenth century when regression of certain tumors were noted in patients with streptococcal and clostridial infections.19
Today, there are two broad approaches for the use of bacteria as vectors and individual genera are inherently more suitable for different therapeutic strategies; (1) Tumor-specific bacterial replication or (2) intracellular plasmid transfer (bactofection).
Tumor targeting following systemic administration.
The main goal of cancer treatment is to focus therapy on tumors without harming healthy cells. When a patient's cancer has spread, then ideally, a treatment should be administered throughout the patient's body (e.g., intravenous administration) to target all potential tumors present, including secondary tumors at early stages of development. The ideal anti-cancer therapy would selectively eradicate tumor cells, whilst minimising side effects to normal tissue. To successfully address these issues, it is necessary to identify “unique” tumor attributes that separate them from normal surrounding tissue. Many conventional modalities utilize the accelerated cell turnover of tumors to achieve a therapeutic response; however, only 3–5% of tumor cells are in the growth fraction, limiting efficacy. Therefore, other tumor-specific factors may present more optimal targets, such as exploiting the knowledge that 95% of tumors are hypoxic to some degree.
The first bacteria observed to have an effect on cancer cells belong to the Clostridium genus. In 1813, regression was observed in tumors of patients who developed concomitant “gas gangrene” caused by C. perfringens.20 Clostridia are Grampositive, spore-forming, obligate anaerobes and many strains are pathogenic. It has previously been established for many species of Clostridium that when injected in spore form, they will only germinate once in an appropriate anaerobic environment. In more recent years, tumor-specific replication following IV administration has been demonstrated for numerous bacterial species, including Bifidobacterium, Salmonella, Escherichia coli, Vibrio cholerae and Listeria monocytogenes.21–25 This facet permits the use of even non-invasive strains expressing heterologous genes to achieve tumor localised expression and secretion of therapeutics (Fig. 1). In this ‘cell therapy’ approach, bacterial-mediated transgene expression occurs locally (outside tumor cells). Various preclinical trials have shown the ability of different bacterial strains to transport and amplify genes encoding factors such as prodrug-converting enzymes, toxins, angiogenesis inhibitors and cytokines (described later) specifically within tumors.26 Indeed, systemic delivery can be achieved not only through IV administration but for some species, also through oral administration of commensal non-pathogenic27 or pathogenic strains.28,29
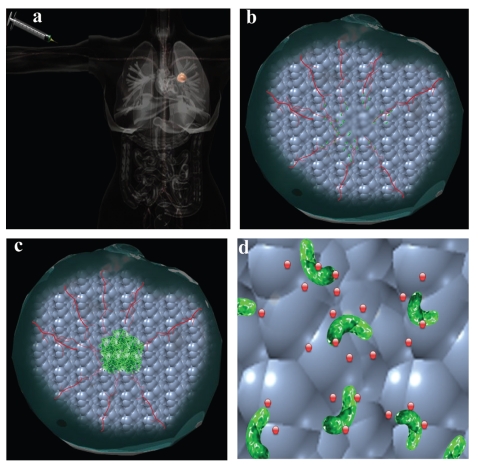
Figure 1.
Tumor targeting with systemically administered bacteria. (A) Bacteria are administered systemically. (B) Bacterial cells enter solid tumors through leaky vasculature and (C) replicate to high levels. (D) Non-invasive species can be engineered to locally secrete therapeutic proteins.
Mechanism of tumor-specific replication. Bacterial colonisation of tumors was initially attributed to the hypoxic nature of solid tumors (low O2 levels).30 Hypoxia is caused by rapidly growing tumors with insufficient blood supply, and is a well established feature of solid tumors. It has been proposed that the anaerobic nature of hypoxic/necrotic regions within tumors promotes growth of anaerobic and facultatively anaerobic bacteria. Areas of necrosis may also provide nutrients such as purines to further promote the growth of bacteria.31 The involvement of bacterial chemotaxis towards chemo-attractant compounds present in necrotic regions (e.g., aspartate, serine, citrate, ribose or galactose) produced by quiescent cancer cells has also been suggested as a contributing factor.32 However, the hypoxia theory is evolving as it becomes apparent that other elements of the unique microenvironment within solid tumors may be involved, including aberrant neovasculature and local immune suppression.33 Furthermore, tumor selective bacterial colonisation now appears to be both bacterial species and tumor origin independent.
The irregular and leaky nature of tumor blood vessels may be an important factor in this phenomenon. As tumors develop, they promote the formation of new blood vessels (neo-angiogenesis). However, these newly formed vessels are highly disorganized with incomplete endothelial linings and blind ends, resulting in ‘leaky’ blood vessels and sluggish blood flow. This leaky tumor vasculature may allow circulating bacteria to enter tumor tissue and lodge locally. Indeed, Yu et al. demonstrated the ability of IV administered bacteria to home to and replicate within cutaneous wounds as they healed.34 The vasculature of healing wounds would closely resemble the neo-angiogenesis observed in tumors. Interestingly, bacteria were cleared from wounds in these experiments (presumably by the immune system) as they healed, unlike in tumors. It is also believed that tumors are an immunological sanctuary, where bacterial clearance mechanisms are inhibited.35,36 A variety of mechanisms are employed by cancerous cells to avoid detection by the immune system resulting in inadequate immune activity within tumors,9 potentially providing a sanctuary for bacteria to evade immune clearance.
Overall, understanding the exact mechanisms that account for the preferential bacterial tumor colonization would be of great value in terms of optimization, but as yet, the exact mechanism(s) remain unknown.
Exploitation of tumor-specific bacterial growth. The specific nature of bacterial colonisation of tumors, by taking advantage of their unique physiology, may be exploited to aid cancer treatment in several ways. Because of the high degree of specificity of bacterial accumulation within tumor masses, the activity of engineered bacteria is confined to cancer sites. In the case of non-invasive species, strains can be engineered to secrete therapeutic proteins locally within the tumor environment, external to tumor cells. This cell therapy approach is especially suitable for indirectly acting therapeutic strategies, such as anti-angiogenesis and immune therapy (described later). Invasive bacteria capable of delivering genes intracellularly to cancer cells may also be administered systemically with the aim of targeting bactofection to tumors (see below). However, many such pathogenic species also naturally invade healthy tissues (liver, spleen, etc.) and safety concerns need to be addressed before such an approach becomes applicable. Oncolytic vectors can also be administered systemically to achieve tumor-targeted activity (see below).
Given that a vital aspect of cancer treatment is early detection, the potential for use of tumor-specific bacteria in diagnostic applications is attractive. Bacteria which preferentially replicate in tumors can be engineered to express imaging agents, permitting not only detection of the bacteria but concurrently potential tumor sites. Real time representation of tumor tissues including metastasis can be achieved through the use of this method. As bacterial levels in tumors are amplifying over time, the necessity to deliver large amounts of probe or repeat administration for enhanced signal-to-noise ratio is removed. Genetically modified light-emitting bacteria could be visualised while colonising tumors. Both fluorescent (Green Fluorescent Protein and its variants) and luminescent (lux) genes are available for bacteria. Luminescence has yet to be utilized in the clinic, largely due to the fact that the substrate required for mammalian luminescence genes (e.g., firefly luciferase and its substrate luciferin) is not licensed for administration to humans. The advantage of using bacterial luciferase is that the lux cassette encodes the enzymes required for substrate biosynthesis, resulting in a directly imagable agent (Fig. 2).37,38 Other gene-based reporter systems have also been examined, including Positron Emission Topography (PET) scanning in combination with bacterial expression of thymidine kinase (tk), be it endogenous expression in E. coli39 or S. typhimurium engineered to express the tk gene from Herpes Simplex Virus (HSVtk).40,41
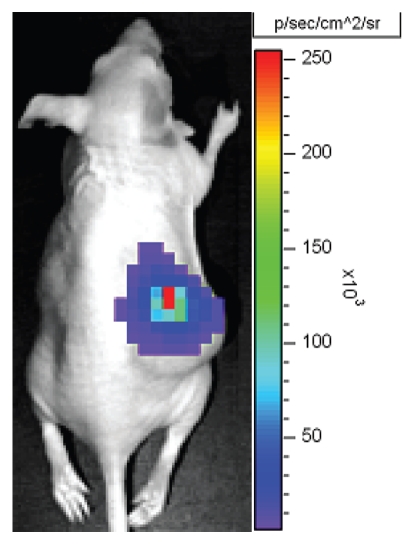
Figure 2.
Bioluminescent imaging of bacteria. Visualization of viable Bifidobacterium breve expressing the luxADCDE gene cassette in vivo growing specifically in Lewis Lung Carcinoma subcutaneous tumors in athymic mice. (Unpublished work from the authors' laboratory).
Bactofection.
The term ‘bactofection’ has been coined to describe bacterial-mediated transfer of plasmid DNA to mammalian cells, and has shown potential as a potent approach to express heterologous proteins in different mammalian cell types.25,42 In bactofection-based gene therapy, the bacterium is considered the ‘vector’, which mediates carriage of the plasmid-based gene to the new host cell. Delivery of genetic material is achieved through entry of the entire bacterium into target cells. Spontaneous or induced bacterial lysis leads to the release of plasmid for subsequent eukaryotic cell expression (Fig. 3). Various bacterial species including Salmonella spp., L. monocytogenes and E. coli have been examined as bactofection vectors. Bactofection of mammalian cells applies to both active invasion of non-phagocytic mammalian cells (e.g., tumor cells), and also ‘passive’ uptake by phagocytic immune cells (see DNA vaccination section below).
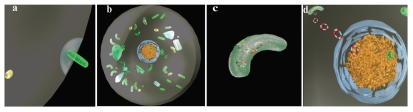
Figure 3.
Bactofection. (A) Invasive bacterium enters mammalian cell. (B) Replication competent bacterial vectors may escape from phagosome and replicate (e.g., L. monocytogenes). (C) Bacterial lysis occurs (spontaneous or induced via lysin or antibiotic) facilitating release of plasmid DNA. (D) Plasmid is randomly internalised in nucleus where transgene transcription occurs.
What is delivered? Most established gene delivery methods transfer DNA to mammalian cells for subsequent host cell transcription within the nucleus. DNA may be delivered directly to the nucleus with certain viruses, but with plasmid-based systems, the spontaneous trafficking of plasmid DNA into the nucleus is inefficient and is a major rate-limiting step of non-viral gene delivery. The nature of plasmids carried by bacterial vectors is the same as other non-viral strategies. Plasmids contain an appropriate bacterial origin of replication, an antibiotic resistance cassette and the gene of interest (therapeutic or mammalian reporter gene) which is transcribed from a eukaryotic promoter, frequently a constitutive, ubiquitous highly expressed promoter of viral origin such as the early promoter from Cytomegalovirus (CMV). Plasmids do not generally replicate in the host mammalian cells.
A property unique to bacterial vectors is the ability to deliver therapeutics in the form of RNA or protein, thus overcoming the rate limiting step of plasmid DNA nuclear entry. By placing a eukaryotic transgene sequence, including eukaryotic translation signals (ribosome binding site), under the transcriptional control of prokaryotic promoter, ‘pre-made’ translation-competent mRNA can be delivered to the cytoplasm of the target cell for immediate translation by the host cell machinery. Such a strategy has been shown to provide earlier and increased expression over plasmid delivery to tumor cells in vitro.43 Furthermore, the eukaryotic expression system can be bypassed by engineering the bacterial vector to generate the final protein product, which can be released following bacterial lysis or actively secreted within the cytoplasm of the target cell.44
Genera used in bactofection. Several invasive bacterial species are capable of transferring eukaryotic expression plasmids into host cells (Table 1). Salmonella, Shigella, L. monocytogenes and recombinant E. coli have been used successfully.21,45–47 Salmonella is the most widely studied genus for bactofection with numerous studies demonstrating therapeutic expression and anti-tumor efficacy.23,24,48,49 Preclinical trials utilizing various attenuated, replication incompetent strains of salmonellae delivered by direct intratumoral or systemic administration have achieved impressive anti-tumor responses using a range of therapeutic approaches. Vaccination strategies involving bactofection of antigen presenting cells (APC) using replication deficient mutants of S. typhimurium and other strains have also shown significant successes in the treatment of cancer and infectious diseases (see below).
Table 1.
Genera suitable for different therapeutic strategies
| Therapeutic approach | Suitable species | Therapeutic gene delivered | References |
| Oncolytic | Clostridium, Salmonella | None required | 58, 59, 127, 128 |
| Tumor cell transfection | Salmonella, Listeria, E. coli, Clostridium | Suitable for all | 21–25 |
| Cell therapy | Bifidobacterium | Anti-Angiogenic, Immune | 27, 129, 130 |
| Vaccination | Salmonella, Listeria | Tumor Antigen | 22, 120, 131 |
The mechanism of DNA transfer is at present poorly understood for many species, and may depend on properties inherent to the bacteria and the cell type involved.50 Entry into the cytosol is a crucial step in bacteria-mediated plasmid transfer. Salmonella and E. coli spp. remain trapped in the target cell phagosome post invasion, unlike L. monocytogenes which has the capacity to escape from the phagosome and access the cytoplasm enabling more efficient gene transfer.22 The efficiency of L. monocytogenes bactofection vectors for gene delivery to tumor cells in vitro and in vivo has been demonstrated by a number of groups.22,51 The use of a replication competent vector permits intra-tumoral spread over time.25 Furthermore, the efficiency of lysis and subsequent plasmid release can be improved through incorporation of phage lysin genes and/or use of antibiotics.51,52 Similar approaches would be applicable to other invasive genera to optimise bactofection efficiency. In addition, bacteria without innate invasive abilities have been engineered to act as bactofection vectors through addition of invasive genetic elements, e.g., recombinant E. coli expressing the invasin gene from Yersinia pseudotuberculosis53 or L. lactis expressing L. monocytogenes Internalin A (inlA) gene.54
Therapeutic Strategies
Numerous preclinical and clinical studies have investigated the potential for bacterial delivery of therapies for cancer (Table 2). A wide range of gene therapy strategies exists aimed at inducing malignant cell death, be it directly or indirectly.
Table 2.
Example therapeutic strategies utilizing bacterial vectors at preclinical and clinical levels
| Mode of action | Bacterium | Gene | Tumor model | Reference |
| Suicide gene | S. typhimurium (TAPET) | Cytosine Deaminase (TAPET-CD) gene delivery | Various murine models; Clinical trials | 61, 62, 132 |
| S. typhimurium VNP20009 | Carboxypeptidase G2 (CPG2) | Murine models of breast and colon cancer | 133 | |
| B. infantis, B. longum | Cytosine Deaminase | Murine melanoma model | 80, 81 | |
| Apoptosis induction | S. typhimurium | TRAIL gene delivery, under control of prokaryotic radiation-inducible RecA promoter | 4T1 mammary carcinoma model | 75 |
| Oncolytic Vector | C. butyricum M-55 | Clinical trial-malignant gliomas | 63 | |
| C. novyi-NT | Combination with radiation therapy | Murine B16; CT26 | 70 | |
| C. butyricum, Various non-pathogenic clostridia | Ehrlich Carcinoma Model. | 56 | ||
| S. typhimurium | Phase I clinical trial, metastatic melanoma | 125 | ||
| Auxotrophic/replication competent S. typhimurium A1 | PC3 prostate tumor model | 134 | ||
| Immunotherapy | S. typhimurium | Fas ligand | Murine D2F2 breast carcinoma; CT-26 colon carcinoma; B16 melanoma | 96 |
| S. typhimurium | IL-18 | Murine D2F2 breast carcinoma; CT-26 colon carcinoma | 97 | |
| S. typhimurium | CCL21 cytokine | Murine D2F2 breast carcinoma; CT-26 colon carcinoma | 98 | |
| DNA Vaccination | Various Salmonella spp. | Various tumor specific antigens | Various murine models | 131, 135 |
| L. monocytogenes | HPV 16 antigens, MAGE antigen. | Phase I clinical trial; various murine models | 22, 135 | |
| Anti-angiogenesis | B. longum | Endostatin | Murine model HepG2 liver cancer | 86 |
| S. choleraesuis | Endostatin gene delivery | Murine B1 melanoma | 48 |
Direct cell killing.
The most direct gene therapy strategy to treat tumors involves introducing a vector and gene to a malignant cell that directly induces death of that cell. There are a number of mechanisms by which this can be achieved, including the delivery of genes cytotoxic to the cell (pro-apoptotic genes or so-called ‘suicide genes’) or through oncolysis induced by the bacterial vector itself (as is observed with Clostridium and Salmonella).
Oncolytic vectors. The oncolytic approach uses replication competent bacteria that are capable of spreading through the tumor tissue to infect neighbouring cells, with cancer cells killed as a result of infection. Therapeutic trials employing clostridial species mainly rely on the natural oncolytic activity of the vector to achieve tumor therapeutic responses. Following IV administration, clostridial spores germinate within tumors, killing cancer cells as they replicate, and have been shown to produce significant oncolytic effects in preclinical and clinical studies.30,55–57 Replication-competent Salmonella vectors have also been shown to be oncolytic.58,59 Zhao and co-workers demonstrated that the S. typhimurium A1 strain grows in the cytoplasm of prostate cancer cells and causes nuclear destruction and cell death.60 The clinical safety of S. typhimurium VNP20009 has been shown in Phase I trials, as well as its derivative TAPET-CD (which expresses E. coli cytosine deaminase).61,62 Despite the pre-clinical successes with Clostridium and Salmonella, therapeutic efficacy has not always translated into human studies. With clostridia, despite evidence of oncolysis in human trials, rates of tumor recurrence were demonstrated to be unaffected by treatment.63 Although oncolysis and destruction of large parts of the tumors was generally observed, the outer rim of the solid tumor was unaffected by these bacteria, hypothesized to be due to a higher oxygen potential in this more vascular region unsuitable for clostridial survival, which eventually led to re-growth of tumors.30 It also appears that clostridia require a “threshold” size of 3 cm3 along with a minimum spore dose of 106 to exert their oncolytic effect, thereby limiting its applicability for the treatment of smaller metastases.64,65 Significant safety concerns regarding the severe pathogenicity and immunogenicity of these species also exist, as they are known to cause life-threatening infections in clinical practice. Efforts to refine the process have involved pre-treatment measures to make the tumor environment more hypoxic,66–68 combination therapies69,70 and more recently, genetic engineering.71–74 However, clostridia are typically difficult to manipulate genetically, which has hampered their development in terms of expression or delivery of heterologous genes. Only recently have strains been engineered to encode additional heterologous genes, aimed at enhancing the therapeutic effect (see below). Nonetheless, there is still cause for optimism with this treatment strategy in line with further improvements in genetic technologies for the species.
Cytotoxic genes. Bacterial vectors can mediate expression of agents that are cytotoxic to the host cell. The extracellular domain of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a potent apoptotic agent in tumor cells, with minimal toxicity to normal cells. Attenuated S. typhimurium has been used to express TRAIL under the control of the prokaryotic radiation-inducible RecA promoter, with systemic administration of this vector resulting in xenograft tumor reduction.75 B. longum has also been utilized to express this agent within murine tumors resulting in significant regression.76 Indirectly cytotoxic genes have also shown promise. Prodrug activating genes (suicide genes) encode a protein that is capable of directly or indirectly causing cell death. While some suicide genes express products that are directly toxic for the cell, e.g., Diphtheria toxin or Pseudomonas exotoxin, the best known agents encode enzymes that convert non-toxic pro-drugs into highly toxic metabolites.77 Gene-directed enzyme prodrug therapy (GDEPT) is a two-step approach. In the first step, the transgene is delivered into the tumor, while in the second step, a prodrug is administered which is selectively activated by the expressed enzyme. The most widely used system is the thymidine kinase gene of the Herpes Simplex Virus (HSVtk) in combination with the prodrug ganciclovir. HSVtk phosphorylates ganciclovir to produce a cytotoxic metabolite.78 Other systems include the cytosine deaminase (CD) gene in conjunction with 5-fluorocytosine. A number of bacterial vectors have been successfully utilized to deliver suicide genes as summarized in Table 2.62,72,73,79–81
Anti-angiogenic therapy.
Angiogenesis is the formation of new capillary blood vessels from existing microvessels.82 For cancer therapy, strategies based on the manipulation of angiogenesis are referred to as anti-angiogenic strategies and seek to prevent new vessel formation or to inactivate pre-existing vessels. Gene-based anti-angiogenic therapy holds the potential to provide long-term anti-angiogenic protein production, and can be readily used in conjunction with other strategies. Endostatin is an endogenous inhibitor of angiogenesis, first discovered in 1997.83 It suppresses endothelial cell proliferation and acts as a competitor of angiogenic inducers secreted by tumor cells, such as fibroblast growth factor and vascular endothelial growth factor. However, results with administration of recombinant endostatin protein in clinical trials have been disappointing, due to poor solubility of the protein, in addition to the requirement for long-term multiple administrations.84,85 Bifidobacterial expression of endostatin or similar genes presents a potential solution to these issues, and preclinical results in various tumor models have shown promise.76,86–89 Salmonella VPN20009 has also been used to mediate anti-angiogenic therapy successfully.90,91
Immune therapy.
Insights into the regulation of immune responses and malignant process have facilitated the emergence of new immune-based therapies for cancer. Tumor cells are known to secrete immune suppressive factors (various cytokines) that impair proliferation of cytotoxic lymphocytes and create an immune-suppressive environment.92–95
Upregulating the immune system. Cancer immunotherapy approaches concentrate on killing the tumor cells through direct or indirect intervention of various effector cells of the immune system, which include antibody-producing B cells, CD8+ CTL, CD4+ helper T cells, and NK cells. Gene therapy can be employed to induce tumor or other cells to produce immune upregulating cytokines that can attract and enhance anti-tumor activity of various lymphocytes. S. typhimurium has been used in several murine trials examining immunotherapies, with significant tumor reduction resulting from local bacterial expression or tumor cell expression of the immune-stimulating molecules IL-18, CCL21, LIGHT or Fas ligand.96–99 Preclinical studies have also used bifidobacteria in combination therapy with cytokines such as granulocyte colony-stimulating factor (GCSF), resulting in superior anti-tumor effects.89 Interestingly, the immune response was primarily directed against tumor cells rather than the bacterial vector cells.
DNA vaccination. The goal of cancer vaccines is to break tolerance of the immune system to specific antigens known to be expressed mainly or exclusively by particular tumor cells. DNA vaccines expressing a defined tumor antigen have shown significant promise both preclinically and in clinical trials.100,101 This strategy involves delivery of a vector that expresses the gene of interest, and functions to target immune activity in a similar manner to which traditional vaccines work. Vaccination strategies attempt to stimulate immune responses by generating cytotoxic T lymphocytes and/or antibodies from B cells to break the pre-existing tolerance to specific antigens. Bacteria that target inductive cells of the immune system are highly attractive candidates for vaccine delivery and have been developed as live vehicles for inducing protective responses to a wide variety of antigens.102–104 Members of the Salmonella genus have been widely used as antigen carriers and several well-characterized safety attenuated strains are available.105–108 Salmonella is capable of triggering both humoural and antigen-specific T-helper and cytotoxic responses.109 S. typhimurium vectors deliver transgenes to the body via the monocyte cell population. After oral intake, the bacterial vector cells are phagocytosed by monocytes in the intestine. The monocytes differentiate and migrate to lymph nodes and the spleen. Finally, the attenuated auxotrophic S. typhimurium (unable to replicate in mammals) lyse and release plasmid into the cytoplasm of monocytes, followed by expression of the desired antigen and presentation to the immune system. This delivery platform has shown success in several preclinical tumor models employing various tumor antigens.110–114
A number of live attenuated strains of Listeria have been developed expressing a broad range of tumor antigens, such as Her-2/neu (an oncoprotein associated with a wide variety of cancers115,116), Melanoma Associated Antigen (MAGE)117 and prostate specific antigen (PSA).118,119 The cytoplasmic location of L. monocytogenes is significant as this potentiates entry of the antigen into the Class I MHC antigen-processing pathway leading to priming of specific CD8+ T-cell responses. IV administered attenuated L. monocytogenes expressing HPV16 E7 was recently used in phase I clinical trial on patients with metastatic cervical cancer.120 Apart from some flu-like symptoms and fever-related hypertension in some patients, the vector was well tolerated. In addition, 30% tumor reduction was noted with an increase in overall survival, indicating the safety and efficacy of listerial vectors in patients and paving the way for clinical development of this vector strategy.
Conclusions
Despite the potential for live bacterial delivery systems, it is clear that in many cases further work is required to limit potential adverse side-effects and to optimise delivery. Since cancer is a multi-faceted disease, combining therapies with different therapeutic targets can achieve synergistic effects. Oncolytic bacterial therapies have been examined in combination with clinically applicable treatments such as radiation therapy,121,122 chemotherapy69 and thermal radiofrequency.66 Other combinations examined preclinically involve augmentation of tumor bacterial colonisation by attempting to make the tumor environment more hypoxic to “attract” bacteria.66–68 For any use of recombinant bacteria in humans, particular care must be taken to prevent lateral gene transfer to other bacteria and to limit environmental spread of the vector. ‘Biological containment’ strategies may aid in overcoming these issues, whereby the vector is engineered to survive in the host but not in the external environment where specific nutrients are limiting.123 A safety property unique to bacterial vectors is their sensitivity to clinically available antibiotic treatments, enabling their control post-administration, an invaluable property for safe gene therapy. It is significant that many of the nascent bacterial delivery platforms described have entered human clinical trials. Use of clostridial species for targeted tumor killing and attenuated S. typhimurium vectors for oral vaccination or tumor gene delivery, represent the most widely applied bacterial vectors at the clinical trial level.61–63,124–126 The clinical safety and efficacy observed to date with current generation vectors indicates that we are closer to an era in which recombinant live bacterial vectors will soon be acceptable for therapeutic use. When compared with viral vectors, bacterial vectors are practical and cheap to manufacture while viral vector generation is an extremely cumbersome, time consuming and expensive process. Traditional GMP-grade naked plasmid DNA isolation is significantly less expensive, but often requires combination with an expensive chemical vector (liposomes, cationic polymers, etc.) or delivery equipment (electroporation, etc.). The necessary infrastructure and expertise already exists for industrial scale manufacturing of bacterial vectors at a low cost as bacterial culture systems have long been in operation in biotechnology industries.
Overall, the development of live bacterial vectors with potential for delivery of therapeutic agents is an exciting area of research that is gaining acceptance by clinicians and regulatory authorities for its potential to deliver positive clinical outcomes. Whilst more needs to be done to improve the safety and efficacy of some systems, this is clearly a technological approach that will yield dividends in the coming years.
Acknowledgements
The authors wish to acknowledge funding from the Cork South Infirmary Victoria University Hospital Breast fund, the Health Research Board (RP/2007/75) and the Irish Cancer Society (CRI07TAN). We thank Xuefeng Gao, University College Cork for figure images.
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/13146
References
- 1.Russell RCG, Williams NS, Bulstrode CJK. Bailey & Love's short practice of surgery. Arnold. 2000 [Google Scholar]
- 2.Ryan RM, Green J, Lewis CE. Use of bacteria in anti-cancer therapies. Bioessays. 2006;28:84–94. doi: 10.1002/bies.20336. [DOI] [PubMed] [Google Scholar]
- 3.Buhles A, Collins SA, van Pijkeren JP, Rajendran S, Miles M, O'Sullivan GC, et al. Anti-metastatic effects of viral and non-viral mediated Nk 4 delivery to tumours. Genetic Vaccines and Therapy. 2009;7:5. doi: 10.1186/1479-0556-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawabata K, Takakura Y, Hashida M. The fate of plasmid DNA after intravenous injection in mice: involvement of scavenger receptors in its hepatic uptake. Pharm Res. 1995;12:825–830. doi: 10.1023/a:1016248701505. [DOI] [PubMed] [Google Scholar]
- 5.Lechardeur D, Sohn KJ, Haardt M, Joshi PB, Monck M, Graham RW, et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 1999;6:482–497. doi: 10.1038/sj.gt.3300867. [DOI] [PubMed] [Google Scholar]
- 6.Palffy R, Gardlik R, Hodosy J, Behuliak M, Reško P, Radvansky J, et al. Bacteria in gene therapy: bactofection versus alternative gene therapy. Gene Therapy. 2006;13:101–105. doi: 10.1038/sj.gt.3302635. [DOI] [PubMed] [Google Scholar]
- 7.Walsh M, Tangney M, O'Neill MJ, Larkin JO, Soden DM, McKenna SL, et al. Evaluation of cellular uptake and gene transfer efficiency of pegylated poly-L-lysine compacted DNA: implications for cancer gene therapy. Mol Pharm. 2006;3:644–653. doi: 10.1021/mp0600034. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad S, Casey G, Sweeney P, Tangney M, O'Sullivan GC. Optimised electroporation mediated DNA vaccination for treatment of prostate cancer. Genet Vaccines Ther. 2010;8:1. doi: 10.1186/1479-0556-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangney M, Casey G, Larkin JO, Collins CG, Soden D, Cashman J, et al. Non-viral in vivo immune gene therapy of cancer: combined strategies for treatment of systemic disease. Cancer Immunol Immunother. 2006;55:1443–1450. doi: 10.1007/s00262-006-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey G, Cashman JP, Morrissey D, Whelan MC, Larkin JO, Soden DM, et al. Sonoporation mediated immunogene therapy of solid tumours. Ultrasound Med Biol. 2010;36:430–440. doi: 10.1016/j.ultrasmedbio.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Alton EW, Middleton PG, Caplen NJ, Smith SN, Steel DM, Munkonge FM, et al. Non-invasive liposome-mediated gene delivery can correct the ion transport defect in cystic fibrosis mutant mice. Nat Genet. 1993;5:135–142. doi: 10.1038/ng1093-135. [DOI] [PubMed] [Google Scholar]
- 12.Collins SA, Guinn BA, Harrison PT, Scallan MF, O'Sullivan GC, Tangney M. Viral vectors in cancer immunotherapy: which vector for which strategy? Curr Gene Ther. 2008;8:66–78. doi: 10.2174/156652308784049345. [DOI] [PubMed] [Google Scholar]
- 13.Cusack JC, Jr, Tanabe KK. Introduction to cancer gene therapy. Surg Oncol Clin N Am. 2002;11:497–519. doi: 10.1016/s1055-3207(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 14.Lundstrom K. Latest development in viral vectors for gene therapy. Trends Biotechnol. 2003;21:117–122. doi: 10.1016/S0167-7799(02)00042-2. [DOI] [PubMed] [Google Scholar]
- 15.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 16.Emtage PC, Wan Y, Hitt M, Graham FL, Muller WJ, Zlotnik A, et al. Adenoviral vectors expressing lymphotactin and interleukin 2 or lymphotactin and interleukin 12 synergize to facilitate tumor regression in murine breast cancer models. Hum Gene Ther. 1999;10:697–709. doi: 10.1089/10430349950018463. [DOI] [PubMed] [Google Scholar]
- 17.Nasu Y, Bangma CH, Hull GW, Lee HM, Hu J, Wang J, et al. Adenovirus-mediated interleukin-12 gene therapy for prostate cancer: suppression of orthotopic tumor growth and pre-established lung metastases in an orthotopic model. Gene Ther. 1999;6:338–349. doi: 10.1038/sj.gt.3300834. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Curiel DT. Cancer gene therapy. Technol Cancer Res Treat. 2005;4:315–330. doi: 10.1177/153303460500400402. [DOI] [PubMed] [Google Scholar]
- 19.Svoboda MG, Rothman BK, Maps G. Culturing cancer in the american century. Bulletin of Science Technology and Society. 1999;19:219–230. [Google Scholar]
- 20.Hall SS. A commotion in the blood: life, death and the immune system. London: Little, Brown; 1998, 1997. [Google Scholar]
- 21.Dietrich G, Bubert A, Gentschev I, Sokolovic Z, Simm A, Catic A, et al. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat Biotechnol. 1998;16:181–185. doi: 10.1038/nbt0298-181. [DOI] [PubMed] [Google Scholar]
- 22.Tangney M, Gahan CGM. Listeria monocytogenes as a vector for anti-cancer therapies. Curr Gene Ther. 2010:10. doi: 10.2174/156652310790945539. [DOI] [PubMed] [Google Scholar]
- 23.Yang N, Zhu X, Chen L, Li S, Ren D. Oral administration of attenuated S. typhimurium carrying shRNA-expressing vectors as a cancer therapeutic. Cancer Biol Ther. 2008;7:145–151. doi: 10.4161/cbt.7.1.5195. [DOI] [PubMed] [Google Scholar]
- 24.Fu W, Lan H, Li S, Han X, Gao T, Ren D. Synergistic antitumor efficacy of suicide/ePNP gene and 6-methylpurine 2′-deoxyriboside via Salmonella against murine tumors. Cancer Gene Ther. 2008;15:474–484. doi: 10.1038/cgt.2008.19. [DOI] [PubMed] [Google Scholar]
- 25.van Pijkeren JP, Morrissey D, Monk IR, Cronin M, Rajendran S, O'Sullivan GC, et al. A novel Listeria monocytogenes-based DNA delivery system for cancer gene therapy. Hum Gene Ther. 2010;21:405–416. doi: 10.1089/hum.2009.022. [DOI] [PubMed] [Google Scholar]
- 26.Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncology. 2003;4:548–556. doi: 10.1016/s1470-2045(03)01194-x. [DOI] [PubMed] [Google Scholar]
- 27.Cronin M, Morrissey D, Rajendran S, El Mashad SM, van Sinderen D, O'Sullivan GC, et al. Orally administered bifidobacteria as vehicles for delivery of agents to systemic tumors. Mol Ther. 2010;18:1397–1407. doi: 10.1038/mt.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia LJ, Wei DP, Sun QM, Huang Y, Wu Q, Hua ZC. Oral delivery of tumor-targeting Salmonella for cancer therapy in murine tumor models. Cancer Sci. 2007;98:1107–1112. doi: 10.1111/j.1349-7006.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen G, Wei DP, Jia LJ, Tang B, Shu L, Zhang K, et al. Oral delivery of tumor-targeting Salmonella exhibits promising therapeutic efficacy and low toxicity. Cancer Sci. 2009;100:2437–2443. doi: 10.1111/j.1349-7006.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei MQ, Mengesha A, Good D, Anné J. Bacterial targeted tumour therapy-dawn of a new era. Cancer Letters. 2007;259:16–27. doi: 10.1016/j.canlet.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Al-Mariri A, Tibor A, Lestrate P, Mertens P, De Bolle X, Letesson JJ. Yersinia enterocolitica as a vehicle for a naked DNA vaccine encoding Brucella abortus bacterioferritin or p39 antigen. Infect Immun. 2002;70:1915–1923. doi: 10.1128/IAI.70.4.1915-1923.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasinskas RW, Forbes NS. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Research. 2007;67:3201. doi: 10.1158/0008-5472.CAN-06-2618. [DOI] [PubMed] [Google Scholar]
- 33.Samoszuk MK, Walter J, Mechetner E. Improved immunohistochemical method for detecting hypoxia gradients in mouse tissues and tumors. J Histochem Cytochem. 2004;52:837. doi: 10.1369/jhc.4B6248.2004. [DOI] [PubMed] [Google Scholar]
- 34.Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 35.Bermudes D, Low B, Pawelek J. Tumor-targeted Salmonella. Highly selective delivery vectors. Adv Exp Med Biol. 2000;465:57. doi: 10.1007/0-306-46817-4_6. [DOI] [PubMed] [Google Scholar]
- 36.Sznol M, Lin SL, Bermudes D, Zheng LM, King I. Use of preferentially replicating bacteria for the treatment of cancer. J Clin Invest. 2000;105:1027–1030. doi: 10.1172/JCI9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riedel CU, Monk IR, Casey PG, Morrissey D, O'Sullivan GC, Tangney M, et al. Improved luciferase tagging system for Listeria monocytogenes allows real-time monitoring in vivo and in vitro. Appl Environ Microbiol. 2007;73:3091–3094. doi: 10.1128/AEM.02940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cronin M, Sleator RD, Hill C, Fitzgerald GF, van Sinderen D. Development of a luciferase-based reporter system to monitor Bifidobacterium breve UCC2003 persistence in mice. BMC Microbiol. 2008;8:161. doi: 10.1186/1471-2180-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brader P, Stritzker J, Riedl CC, Zanzonico P, Cai S, Burnazi EM, et al. Escherichia coli Nissle 1917 facilitates tumor detection by positron emission tomography and optical imaging. Clin Cancer Res. 2008;14:2295–2302. doi: 10.1158/1078-0432.CCR-07-4254. [DOI] [PubMed] [Google Scholar]
- 40.Soghomonyan SA, Doubrovin M, Pike J, Luo X, Ittensohn M, Runyan JD, et al. Positron emission tomography (PET) imaging of tumor-localized Salmonella expressing HSV1-TK. Cancer Gene Ther. 2005;12:101–108. doi: 10.1038/sj.cgt.7700779. [DOI] [PubMed] [Google Scholar]
- 41.Tjuvajev J, Blasberg R, Luo X, Zheng LM, King I, Bermudes D. Salmonella-based tumor-targeted cancer therapy: tumor amplified protein expression therapy (TAPET) for diagnostic imaging. J Control Release. 2001;74:313–315. doi: 10.1016/s0168-3659(01)00340-6. [DOI] [PubMed] [Google Scholar]
- 42.Vassaux G, Nitcheu J, Jezzard S, Lemoine NR. Bacterial gene therapy strategies. J Pathol. 2006;208:290–298. doi: 10.1002/path.1865. [DOI] [PubMed] [Google Scholar]
- 43.Schoen C, Kolb-Maurer A, Geginat G, Loffler D, Bergmann B, Stritzker J, et al. Bacterial delivery of functional messenger RNA to mammalian cells. Cell Microbiol. 2005;7:709–724. doi: 10.1111/j.1462-5822.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 44.Loeffler DI, Schoen CU, Goebel W, Pilgrim S. Comparison of different live vaccine strategies in vivo for delivery of protein antigen or antigen-encoding DNA and mRNA by virulence-attenuated Listeria monocytogenes. Infect Immun. 2006;74:3946–3957. doi: 10.1128/IAI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sizemore DR, Branstrom AA, Sadoff JC. Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization. Science. 1995;270:299–302. doi: 10.1126/science.270.5234.299. [DOI] [PubMed] [Google Scholar]
- 46.Courvalin P, Goussard S, Grillot-Courvalin C. Gene transfer from bacteria to mammalian cells. C R Acad Sci III. 1995;318:1207–1212. [PubMed] [Google Scholar]
- 47.Buttaro C, Fruehauf JH. Engineered E. coli as vehicles for targeted therapeutics. Curr Gene Ther. 2010;10:27–33. doi: 10.2174/156652310790945593. [DOI] [PubMed] [Google Scholar]
- 48.Lee CH, Wu CL, Shiau AL. Systemic administration of attenuated Salmonella choleraesuis carrying thrombos-pondin-1 gene leads to tumor-specific transgene expression, delayed tumor growth and prolonged survival in the murine melanoma model. Cancer Gene Ther. 2005;12:175–184. doi: 10.1038/sj.cgt.7700777. [DOI] [PubMed] [Google Scholar]
- 49.Xu DQ, Zhang L, Kopecko DJ, Gao L, Shao Y, Guo B, et al. Bacterial delivery of siRNAs: a new approach to solid tumor therapy. Methods Mol Biol. 2009;487:161–187. doi: 10.1007/978-1-60327-547-7_8. [DOI] [PubMed] [Google Scholar]
- 50.Loessner H, Weiss S. Bacteria-mediated DNA transfer in gene therapy and vaccination. Expert Opin Biol Ther. 2004;4:157–168. doi: 10.1517/14712598.4.2.157. [DOI] [PubMed] [Google Scholar]
- 51.Pilgrim S, Stritzker J, Schoen C, Kolb-Maurer A, Geginat G, Loessner MJ, et al. Bactofection of mammalian cells by Listeria monocytogenes: improvement and mechanism of DNA delivery. Gene Therapy. 2003;10:2036–2045. doi: 10.1038/sj.gt.3302105. [DOI] [PubMed] [Google Scholar]
- 52.Tangney M, Gahan CGM. The use of Listeria monocytogenes as a DNA delivery vector for cancer gene therapy. Bioengineered Bugs. 2010;1:1–4. doi: 10.4161/bbug.1.4.11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Critchley-Thorne RJ, Stagg AJ, Vassaux G. Recombinant Escherichia coli expressing invasin targets the Peyer's patches: the basis for a bacterial formulation for oral vaccination. Mol Ther. 2006;14:183–191. doi: 10.1016/j.ymthe.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Guimaraes VD, Gabriel JE, Lefevre F, Cabanes D, Gruss A, Cossart P, et al. Internalin-expressing Lactococcus lactis is able to invade small intestine of guinea pigs and deliver DNA into mammalian epithelial cells. Microbes Infect. 2005;7:836–844. doi: 10.1016/j.micinf.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Parker RC, Plummer HC. Effect of histolyticus infection and toxin on transplantable mouse tumors. 1947:461. doi: 10.3181/00379727-66-16124. [DOI] [PubMed] [Google Scholar]
- 56.Moese JR, Moese G. Oncolysis by Clostridia I. Activity of Clostridium Butyricum (M-55) and other nonpathogenic clostridia against the ehrlich carcinoma. Cancer Res. 1964;24:212–216. [PubMed] [Google Scholar]
- 57.Mengesha A, Wei JZ, Zhou SF, Wei MQ. Clostridial spores to treat solid tumours—potential for a new therapeutic modality. Curr Gene Ther. 10:15–26. doi: 10.2174/156652310790945548. [DOI] [PubMed] [Google Scholar]
- 58.Luo X, Li Z, Lin S, Le T, Ittensohn M, Bermudes D, et al. Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models. Oncol Res. 2001;12:501–508. doi: 10.3727/096504001108747512. [DOI] [PubMed] [Google Scholar]
- 59.Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proceedings of the National Academy of Sciences. 2005;102:755. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong Z, Kazmierczak RA, Dino A, Khreis R, Eisenstark A, Schatten H. Salmonella-host cell interactions, changes in host cell architecture, and destruction of prostate tumor cells with genetically altered Salmonella. Microsc Microanal. 2007;13:372–383. doi: 10.1017/S1431927607070833. [DOI] [PubMed] [Google Scholar]
- 61.Nemunaitis J, Cunningham C, Senzer N, Kuhn J, Cramm J, Litz C, et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003;10:737–744. doi: 10.1038/sj.cgt.7700634. [DOI] [PubMed] [Google Scholar]
- 62.King I, Itterson M, Bermudes D. Tumor-targeted Salmonella typhimurium overexpressing cytosine deaminase: a novel, tumor-selective therapy. Methods Mol Biol. 2009;542:649–659. doi: 10.1007/978-1-59745-561-9_33. [DOI] [PubMed] [Google Scholar]
- 63.Heppner F, Mose JR. The liquefaction (oncolysis) of malignant gliomas by a non pathogenic Clostridium. Acta Neurochir (Wien) 1978;42:123–125. doi: 10.1007/BF01406639. [DOI] [PubMed] [Google Scholar]
- 64.Engelbart K, Gericke D. Oncolysis by Clostridia V. Transplanted tumors of the hamster. Cancer Res. 1964;24:239–242. [PubMed] [Google Scholar]
- 65.Gericke D, Engelbart K. Oncolysis by Clostridia. Ii. experiments on a tumor spectrum with a variety of clostridia in combination with heavy metal. Cancer Res. 1964;24:217–221. [PubMed] [Google Scholar]
- 66.Dietzel F, Gericke D. Intensification of the oncolysis by clostridia by means of radio-frequency hyperthermy in experiments on animals—dependence on dosage and on intervals (author's transl) Strahlentherapie. 1977;153:263–266. [PubMed] [Google Scholar]
- 67.Dietzel F, Gericke D, Konig W. Tumor hyperthermia using high frequency for increase of oncolysis by Clostridium butyricum (M 55) Strahlentherapie. 1976;152:537–541. [PubMed] [Google Scholar]
- 68.Dietzel F. Basic principles in hyperthermic tumor therapy. Recent Results Cancer Res. 1983;86:177–190. doi: 10.1007/978-3-642-82025-0_31. [DOI] [PubMed] [Google Scholar]
- 69.Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci USA. 2001;98:15155–15160. doi: 10.1073/pnas.251543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bettegowda C, Dang LH, Abrams R, Huso DL, Dillehay L, Cheong I, et al. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. Proc Natl Acad Sci USA. 2003;100:15083–15088. doi: 10.1073/pnas.2036598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu SC, Minton NP, Giaccia AJ, Brown JM. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther. 2002;9:291–296. doi: 10.1038/sj.gt.3301659. [DOI] [PubMed] [Google Scholar]
- 72.Theys J, Pennington O, Dubois L, Anlezark G, Vaughan T, Mengesha A, et al. Repeated cycles of Clostridium-directed enzyme prodrug therapy result in sustained antitumour effects in vivo. Br J Cancer. 2006;95:1212–1219. doi: 10.1038/sj.bjc.6603367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Theys J, Landuyt W, Nuyts S, Van Mellaert L, van Oosterom A, Lambin P, et al. Specific targeting of cytosine deaminase to solid tumors by engineered Clostridium acetobutylicum. Cancer Gene Ther. 2001;8:294–297. doi: 10.1038/sj.cgt.7700303. [DOI] [PubMed] [Google Scholar]
- 74.Fox ME, Lemmon MJ, Mauchline ML, Davis TO, Giaccia AJ, Minton NP, et al. Anaerobic bacteria as a delivery system for cancer gene therapy: in vitro activation of 5-fluorocytosine by genetically engineered clostridia. Gene Ther. 1996;3:173–178. [PubMed] [Google Scholar]
- 75.Ganai S, Arenas RB, Forbes NS. Tumour-targeted delivery of TRAIL using Salmonella typhimurium enhances breast cancer survival in mice. Br J Cancer. 2009;101:1683–1691. doi: 10.1038/sj.bjc.6605403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu B, Kou L, Li C, Zhu LP, Fan YR, Wu ZW, et al. Bifidobacterium longum as a delivery system of TRAIL and endostatin cooperates with chemotherapeutic drugs to inhibit hypoxic tumor growth. Cancer Gene Ther. 2009;16:655–663. doi: 10.1038/cgt.2009.7. [DOI] [PubMed] [Google Scholar]
- 77.Zheng JY, Chen D, Chan J, Yu D, Ko E, Pang S. Regression of prostate cancer xenografts by a lentiviral vector specifically expressing diphtheria toxin A. Cancer Gene Ther. 2003;10:764–770. doi: 10.1038/sj.cgt.7700629. [DOI] [PubMed] [Google Scholar]
- 78.Freeman SM. Suicide gene therapy. Adv Exp Med Biol. 2000;465:411–422. doi: 10.1007/0-306-46817-4_36. [DOI] [PubMed] [Google Scholar]
- 79.Cunningham C, Nemunaitis J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version: April 9, 2001. Hum Gene Ther. 2001;12:1594–1596. [PubMed] [Google Scholar]
- 80.Yi C, Huang Y, Guo ZY, Wang SR. Antitumor effect of cytosine deaminase/5-fluorocytosine suicide gene therapy system mediated by Bifidobacterium infantis on melanoma. Acta Pharmacol Sin. 2005;26:629–634. doi: 10.1111/j.1745-7254.2005.00094.x. [DOI] [PubMed] [Google Scholar]
- 81.Nakamura T, Sasaki T, Fujimori M, Yazawa K, Kano Y, Amano J, et al. Cloned cytosine deaminase gene expression of Bifidobacterium longum and application to enzyme/pro-drug therapy of hypoxic solid tumors. Biosci Biotechnol Biochem. 2002;66:2362–2366. doi: 10.1271/bbb.66.2362. [DOI] [PubMed] [Google Scholar]
- 82.Malecki M, Kolsut P, Proczka R. Angiogenic and antiangiogenic gene therapy. Gene Ther. 2005;12:159–169. doi: 10.1038/sj.gt.3302621. [DOI] [PubMed] [Google Scholar]
- 83.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 84.Eder JP, Jr, Supko JG, Clark JW, Puchalski TA, Garcia-Carbonero R, Ryan DP, et al. Phase I clinical trial of recombinant human endostatin administered as a short intravenous infusion repeated daily. J Clin Oncol. 2002;20:3772–3784. doi: 10.1200/JCO.2002.02.082. [DOI] [PubMed] [Google Scholar]
- 85.Herbst RS, Hess KR, Tran HT, Tseng JE, Mullani NA, Charnsangavej C, et al. Phase I study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol. 2002;20:3792–3803. doi: 10.1200/JCO.2002.11.061. [DOI] [PubMed] [Google Scholar]
- 86.Fu GF, Li X, Hou YY, Fan YR, Liu WH, Xu GX. Bifidobacterium longum as an oral delivery system of endostatin for gene therapy on solid liver cancer. Cancer Gene Ther. 2005;12:133–140. doi: 10.1038/sj.cgt.7700758. [DOI] [PubMed] [Google Scholar]
- 87.Li X, Fu GF, Fan YR, Liu WH, Liu XJ, Wang JJ, et al. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther. 2003;10:105–111. doi: 10.1038/sj.cgt.7700530. [DOI] [PubMed] [Google Scholar]
- 88.Zhu LP, Yin Y, Xing J, Li C, Kou L, Hu B, et al. Therapeutic efficacy of Bifidobacterium longum -mediated human granulocyte colony-stimulating factor and/or endostatin combined with cyclophosphamide in mouse-transplanted tumors. Cancer Sci. 2009;100:1986–1990. doi: 10.1111/j.1349-7006.2009.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu YF, Zhu LP, Hu B, Fu GF, Zhang HY, Wang JJ, et al. A new expression plasmid in Bifidobacterium longum as a delivery system of endostatin for cancer gene therapy. Cancer Gene Ther. 2007;14:151–157. doi: 10.1038/sj.cgt.7701003. [DOI] [PubMed] [Google Scholar]
- 90.Zhou S. Combination therapy with bacteria and angiogenesis inhibitors: strangling cancer without mercy. Cancer Biol Ther. 2005;4:846–847. doi: 10.4161/cbt.4.8.2021. [DOI] [PubMed] [Google Scholar]
- 91.Jia LJ, Xu HM, Ma DY, Hu QG, Huang XF, Jiang WH, et al. Enhanced therapeutic effect by combination of tumor-targeting Salmonella and endostatin in murine melanoma model. Cancer Biol Ther. 2005;4:840–845. doi: 10.4161/cbt.4.8.1891. [DOI] [PubMed] [Google Scholar]
- 92.Borrello I, Pardoll D. GM-CSF-based cellular vaccines: a review of the clinical experience. Cytokine Growth Factor Rev. 2002;13:185–193. doi: 10.1016/s1359-6101(01)00034-x. [DOI] [PubMed] [Google Scholar]
- 93.O'Connell J, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 95.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 96.Loeffler M, Le'Negrate G, Krajewska M, Reed JC. Inhibition of tumor growth using salmonella expressing Fas ligand. J Natl Cancer Inst. 2008;100:1113–1116. doi: 10.1093/jnci/djn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loeffler M, Le'Negrate G, Krajewska M, Reed JC. IL-18-producing Salmonella inhibit tumor growth. Cancer Gene Ther. 2008;15:787–794. doi: 10.1038/cgt.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Loeffler M, Le'Negrate G, Krajewska M, Reed JC. Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunol Immunother. 2009;58:769–775. doi: 10.1007/s00262-008-0555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loeffler M, Le'Negrate G, Krajewska M, Reed JC. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci USA. 2007;104:12879–12883. doi: 10.1073/pnas.0701959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 101.Lai WC, Bennett M. DNA vaccines. Crit Rev Immunol. 1998;18:449–484. doi: 10.1615/critrevimmunol.v18.i5.30. [DOI] [PubMed] [Google Scholar]
- 102.Hormaeche CE. Live attenuated Salmonella vaccines and their potential as oral combined vaccines carrying heterologous antigens. J Immunol Methods. 1991;142:113–120. doi: 10.1016/0022-1759(91)90298-t. [DOI] [PubMed] [Google Scholar]
- 103.Fairweather NF, Chatfield SN, Charles IG, Roberts M, Lipscombe M, Li LJ, et al. Use of live attenuated bacteria to stimulate immunity. Res Microbiol. 1990;141:769–773. doi: 10.1016/0923-2508(90)90109-4. [DOI] [PubMed] [Google Scholar]
- 104.Dougan G, Chatfield S, Roberts M, Charles I, Comerford S, Li LJ, et al. Bacterial pathogens—a route to oral drug delivery. Biochem Soc Trans. 1990;18:746–748. doi: 10.1042/bst0180746. [DOI] [PubMed] [Google Scholar]
- 105.Darji A, Guzman CA, Gerstel B, Wachholz P, Timmis KN, Wehland J, et al. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell. 1997;91:765–775. doi: 10.1016/s0092-8674(00)80465-1. [DOI] [PubMed] [Google Scholar]
- 106.Moreno M, Kramer MG, Yim L, Chabalgoity JA. Salmonella as live trojan horse for vaccine development and cancer gene therapy. Curr Gene Ther. 2009;10:56–76. doi: 10.2174/156652310790945566. [DOI] [PubMed] [Google Scholar]
- 107.Dunstan SJ, Simmons CP, Strugnell RA. Comparison of the abilities of different attenuated Salmonella typhimurium strains to elicit humoral immune responses against a heterologous antigen. Infect Immun. 1998;66:732–740. doi: 10.1128/iai.66.2.732-740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Valentine PJ, Devore BP, Heffron F. Identification of three highly attenuated Salmonella typhimurium mutants that are more immunogenic and protective in mice than a prototypical aroA mutant. Infect Immun. 1998;66:3378–3383. doi: 10.1128/iai.66.7.3378-3383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aggarwal A, Kumar S, Jaffe R, Hone D, Gross M, Sadoff J. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J Exp Med. 1990;172:1083–1090. doi: 10.1084/jem.172.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paglia P, Medina E, Arioli I, Guzman CA, Colombo MP. Gene transfer in dendritic cells, induced by oral DNA vaccination with Salmonella typhimurium, results in protective immunity against a murine fibrosarcoma. Blood. 1998;92:3172–3176. [PubMed] [Google Scholar]
- 111.Chou CK, Hung JY, Liu JC, Chen CT, Hung MC. An attenuated Salmonella oral DNA vaccine prevents the growth of hepatocellular carcinoma and colon cancer that express alpha-fetoprotein. Cancer Gene Ther. 2006;13:746–752. doi: 10.1038/sj.cgt.7700927. [DOI] [PubMed] [Google Scholar]
- 112.Huebener N, Fest S, Strandsby A, Michalsky E, Preissner R, Zeng Y, et al. A rationally designed tyrosine hydroxylase DNA vaccine induces specific antineuroblastoma immunity. Mol Cancer Ther. 2008;7:2241–2251. doi: 10.1158/1535-7163.MCT-08-0109. [DOI] [PubMed] [Google Scholar]
- 113.Fest S, Huebener N, Bleeke M, Durmus T, Stermann A, Woehler A, et al. Survivin minigene DNA vaccination is effective against neuroblastoma. Int J Cancer. 2009;125:104–114. doi: 10.1002/ijc.24291. [DOI] [PubMed] [Google Scholar]
- 114.Fest S, Huebener N, Weixler S, Bleeke M, Zeng Y, Strandsby A, et al. Characterization of GD2 peptide mimotope DNA vaccines effective against spontaneous neuroblastoma metastases. Cancer Res. 2006;66:10567–10575. doi: 10.1158/0008-5472.CAN-06-1158. [DOI] [PubMed] [Google Scholar]
- 115.Seavey MM, Pan ZK, Maciag PC, Wallecha A, Rivera S, Paterson Y, et al. A novel human Her-2/neu chimeric molecule expressed by Listeria monocytogenes can elicit potent HLA-A2 restricted CD8-positive T cell responses and impact the growth and spread of Her-2/neu-positive breast tumors. Clin Cancer Res. 2009;15:924–932. doi: 10.1158/1078-0432.CCR-08-2283. [DOI] [PubMed] [Google Scholar]
- 116.Singh R, Dominiecki ME, Jaffee EM, Paterson Y. Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol. 2005;175:3663–3673. doi: 10.4049/jimmunol.175.6.3663. [DOI] [PubMed] [Google Scholar]
- 117.Kim SH, Castro F, Gonzalez D, Maciag PC, Paterson Y, Gravekamp C. Mage-b vaccine delivered by recombinant Listeria monocytogenes is highly effective against breast cancer metastases. Br J Cancer. 2008;99:741–749. doi: 10.1038/sj.bjc.6604526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shahabi V, Reyes-Reyes M, Wallecha A, Rivera S, Paterson Y, Maciag P. Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol Immunother. 2008;57:1301–1313. doi: 10.1007/s00262-008-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wallecha A, Maciag PC, Rivera S, Paterson Y, Shahabi V. Construction and characterization of an attenuated Listeria monocytogenes strain for clinical use in cancer immunotherapy. Clin Vaccine Immunol. 2009;16:96–103. doi: 10.1128/CVI.00274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: A Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 121.Dietzel F, Seibert G, Klobe G. The influence of highfrequency hyperthermia on Ehrlich-ascites carcinoma in the mouse. Strahlentherapie. 1975;149:105–117. [PubMed] [Google Scholar]
- 122.Gericke D, Dietzel F, Konig W, Ruster I, Schumacher L. Further progress with oncolysis due to apathogenic clostridia. Zentralbl Bakteriol Orig A. 1979;243:102–112. [PubMed] [Google Scholar]
- 123.Lee P. Biocontainment strategies for live lactic acid bacteria vaccine vectors. Bioengineered Bugs. 2010:1. doi: 10.4161/bbug.1.1.10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tangney M, Gahan CG. Editorial hot topic: bacterial vectors for gene & cell therapy. Curr Gene Ther. 2010;10:1–2. doi: 10.2174/156652310790945584. [DOI] [PubMed] [Google Scholar]
- 125.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kotton CN, Lankowski AJ, Scott N, Sisul D, Chen LM, Raschke K, et al. Safety and immunogenicity of attenuated Salmonella enterica serovar Typhimurium delivering an HIV-1 Gag antigen via the Salmonella Type III secretion system. Vaccine. 2006;24:6216–6224. doi: 10.1016/j.vaccine.2006.05.094. [DOI] [PubMed] [Google Scholar]
- 127.Mengesha A, Wei JZ, Zhou SF, Wei MQ. Clostridial spores to treat solid tumours—potential for a new therapeutic modality. Curr Gene Ther. 2009;10:15–26. doi: 10.2174/156652310790945548. [DOI] [PubMed] [Google Scholar]
- 128.Li Z, Fallon J, Mandeli J, Wetmur J, Woo SLC. A genetically enhanced anaerobic bacterium for oncopathic therapy of pancreatic cancer. J Natl Cancer Inst. 2008;100:1389–1400. doi: 10.1093/jnci/djn308. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Morrissey D, O'Sullivan GC, Tangney M. Tumour targeting with systemically administered bacteria. Curr Gene Ther. 2010;10:3–14. doi: 10.2174/156652310790945575. [DOI] [PubMed] [Google Scholar]
- 130.Hu B, Kou L, Li C, Zhu LP, Fan YR, Wu ZW, et al. Bifidobacterium longum as a delivery system of TRAIL and endostatin cooperates with chemotherapeutic drugs to inhibit hypoxic tumor growth. Cancer Gene Ther. 2009;16:655–663. doi: 10.1038/cgt.2009.7. [DOI] [PubMed] [Google Scholar]
- 131.Moreno M, Kramer MG, Yim L, Chabalgoity JA. Salmonella as live trojan horse for vaccine development and cancer gene therapy. Curr Gene Ther. 2010;10:56–76. doi: 10.2174/156652310790945566. [DOI] [PubMed] [Google Scholar]
- 132.King I, Bermudes D, Lin S, Belcourt M, Pike J, Troy K, et al. Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum Gene Ther. 2002;13:1225–1233. doi: 10.1089/104303402320139005. [DOI] [PubMed] [Google Scholar]
- 133.Friedlos F, Lehouritis P, Ogilvie L, Hedley D, Davies L, Bermudes D, et al. Attenuated Salmonella targets prodrug activating enzyme carboxypeptidase G2 to mouse melanoma and human breast and colon carcinomas for effective suicide gene therapy. Clin Cancer Res. 2008;14:4259–4266. doi: 10.1158/1078-0432.CCR-07-4800. [DOI] [PubMed] [Google Scholar]
- 134.Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paterson Y, Guirnalda PD, Wood LM. Listeria and Salmonella bacterial vectors of tumor-associated antigens for cancer immunotherapy. Semin. mmunol 2010;22:183–189. doi: 10.1016/j.smim.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]