Abstract
Global concern over the depletion of fossil fuel reserves, and the detrimental impact that combustion of these materials has on the environment, is focusing attention on initiatives to create sustainable approaches for the production and use of biofuels from various biomass substrates. The development of a low-cost, safe and eco-friendly process for the utilization of renewable resources to generate value-added products with biotechnological potential as well as robust microorganisms capable of efficient fermentation of all types of sugars are essential to underpin the economic production of biofuels from biomass feedstocks. Saccharomyces cerevisiae, the most established fermentation yeast used in large scale bioconversion strategies, does not however metabolise the pentose sugars, xylose and arabinose and bioengineering is required for introduction of efficient pentose metabolic pathways and pentose sugar transport proteins for bioconversion of these substrates. Our approach provided a basis for future experiments that may ultimately lead to the development of industrial S. cerevisiae strains engineered to express pentose metabolising proteins from thermophilic fungi living on decaying plant material and here we expand our original article and discuss the strategies implemented to improve pentose fermentation.
Key words: pentose fermentation, cofactor imbalance, metabolic engineering
Introduction
The baker's yeast, Saccharomyces cerevisiae is the most well established fermentation yeast for large scale ethanolic fermentation of the hexose sugars glucose, mannose and galactose. However, unlike some other yeast species such as Pachysolen sp. and Pichia sp., S. cerevisiae does not metabolise the pentose sugars, xylose and arabinose, and it was not until the late 1970s that the first steps were taken to develop methods to engineer pentose metabolism in this yeast. The ability of S. cerevisiae in fermenting lignocellulose hydrolysates has been demonstrated repeatedly.1 S. cerevisiae produces ethanol with stoichiometric yields from hexose sugars and tolerates a wide spectrum of inhibitors and elevated osmotic pressure. For these reasons, it has been recognized that genetic engineering of naturally fermenting microorganisms such as S. cerevisiae is required for transport and efficient bioconversion of pentose sugars to bioethanol. Pathways for pentose sugar metabolism are essential for microorganisms living on decaying plant material and are of prime interest in biotechnology when low-cost plant hydrolysates are to be fermented to ethanol efficiently.
Pentose Metabolism
A common step in the catabolism of both xylose and arabinose in all microorganisms is that both sugars are converted to D-xylulose-5-phosphate. However, the pathways to convert L-arabinose and D-xylose to D-xylulose-5-phosphate are distinctly different in bacteria and fungi (Fig. 1). In bacteria, D-xylose is converted to D-xylulose by an isomerase (EC 5.3.1.5) and then phosphorylated by xylulokinase (EC 2.1.7.53) while L-arabinose is first converted to L-ribulose by an isomerase (EC 5.3.1.3), and then phosphorylated by ribulokinase (EC 2.1.7.47). L-Ribulose-5-phosphate is then converted to D-xylulose-5-phosphate by an epimerase (EC 5.3.1.3).
Figure 1.
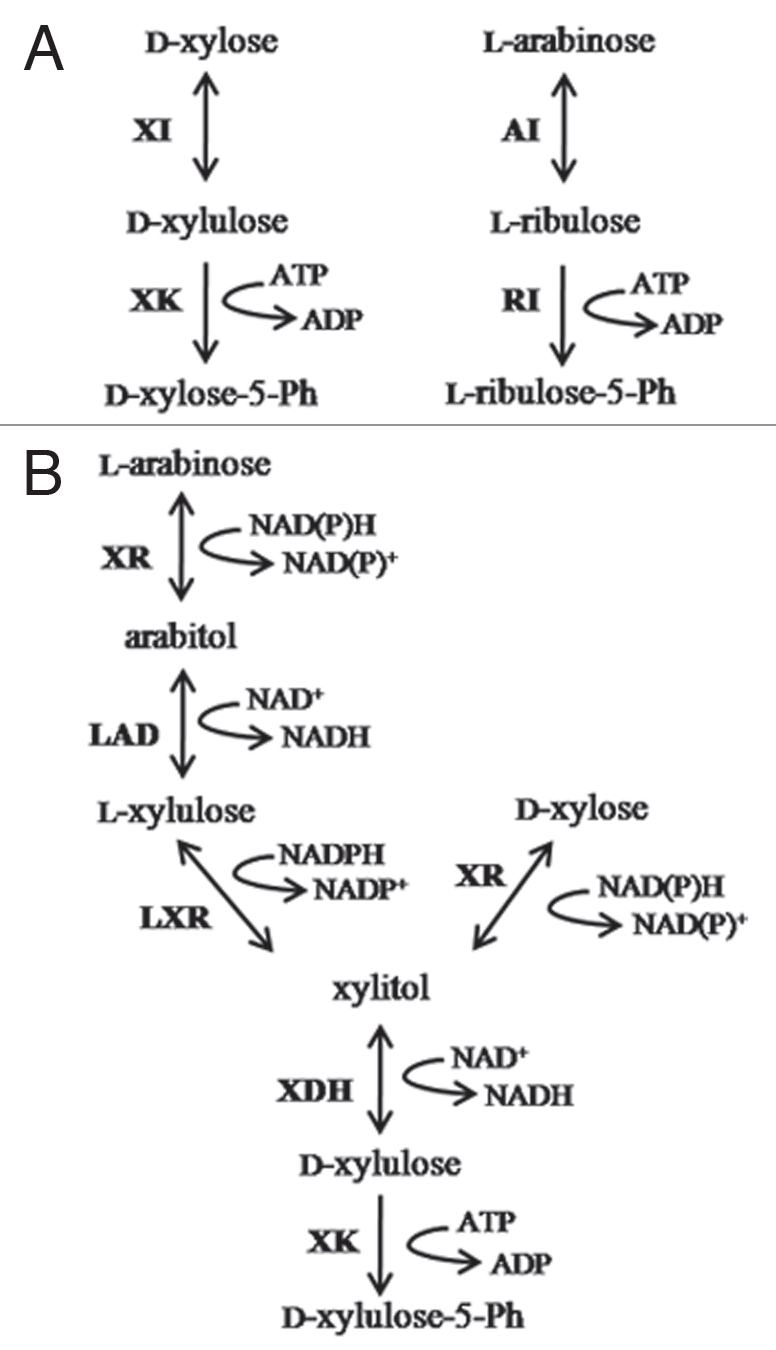
Bacterial (A) and fungal (B) pentose utilization pathways. Enzyme designations are: XI, xylose isomerase; XK, xylulose kinase; AI, arabinose isomerase; RI, ribulose kinase; XR, xylose reductase; LAD, L-arabitol dehydrogenase; LXR, L-xylulose reductase; XDH, xylitol dehydrogenase.
In fungi, both pentose sugars go through oxidation and reduction reactions before they are phosphorylated by xylulokinase. D-xylose is reduced to xylitol by a reduced nicotinamide adenine dinucleotide phosphate (NADPH)-consuming reaction and xylitol is then oxidised by an NAD+-consuming reaction to form D-xylulose. L-Arabinose goes through four redox reactions; two are NAD+-dependent oxidation reactions and two reductions are linked to NADPH consumption. All of the enzymes in the fungal D-xylose pathway can also be used in the L-arabinose pathway. D-xylulose then enters the Pentose Phosphate Pathway (PPP) after phosphorylation to D-xylulose-5-phosphate. The conversion of L-arabinose and D-xylose to D-xylulose is redox neutral, but different redox cofactors are used, which affects cellular demands for oxygen. Fermentation of D-xylose and L-arabinose to equimolar amounts of ethanol and CO2 under anaerobic conditions is possible in S. cerevisiae engineered for pentose metabolism but the fermentation requires careful aeration otherwise the fermentation product is mainly biomass or xylitol and CO2. Pentoses are therefore not efficiently fermented to ethanol because of the imbalance of these redox cofactors. Since NADPH is regenerated mainly in the oxidative phase of the PPP, where the reduction of NADP+ is coupled to the generation of CO2, it has an effect on the redox balance. When extra CO2 is produced in this pathway, the pentose fermentation to ethanol and CO2 is no longer redox neutral. To remove excess NADPH, either xylitol is produced or aeration is required, which leads to further unwanted CO2 production or a combination of both processes.2
Metabolic Engineering and the Redox Metabolism
Several metabolic engineering strategies have been developed to generate organisms (yeasts or bacteria) that can produce ethanol efficiently from biomass-derived hydrolysates. To date, no studies have been conducted to engineer filamentous fungi for ethanolic fermentation even though some species of anaerobic filamentous fungi were shown to produce ethanol and also ferment pentose sugars.1 For commercial fermentation, yeasts present a number of advantages over bacteria. Yeasts have superior resistance to hydrolysate inhibitors, better growth at low pH and less stringent nutritional requirements. Saccharomyces sp. have been traditionally used in industry for fermentation of sugar-based materials. However, the best hexose fermentor to produce ethanol, S. cerevisiae, is unable to metabolise the pentose sugar components of biomass. Metabolic engineering has been used to improve the fermentative capability of S. cerevisiae. Expression of a xylose reductase (XYL1) and a xylitol dehydrogenase (XYL2) from Pichia stipitis in S. cerevisiae resulted in growth on xylose but low levels of ethanol production.3 In addition, the overexpression of the endogenous xylulose kinase (XYL3) together with XYL1 and XYL2 was undertaken4 and the effect and optimization of expression levels of these genes has been studied.5 The use of NAD(P)H-dependent XR and NAD(+)-dependent XDH from P. stipitis creates a cofactor imbalance resulting in xylitol accumulation. The effect of replacing the native P. stipitis Xr with a K270M-mutated Xr6 which has reduced affinity for NADPH was investigated, resulting in enhanced ethanol yields and decreased xylitol formation. However, when the Km for NADPH was enhanced, the Km for xylose also increased concomitantly.
NADH-specific xylose reductase enzymes would enable efficient recycling of the co-enzyme in the next step in xylose and arabinose metabolism, which involves conversion of xylitol to xylulose and arabitol to L-xylulose by NAD+-specific dehydrogenases. Furthermore NADH is more stable and intracellular concentrations are naturally much higher than NADPH. Recombinant S. cerevisiae strain carrying a single copy of the Candida tenuis xylose reductase K274R − N276D double mutant which was shown to have undergone almost complete reversal of co-enzyme preference from NADPH to NADH and displayed improved fermentative capabilities in terms of ethanol when compared to S. cerevisiae harboring the wild-type C. tenuis xylose reductase.7
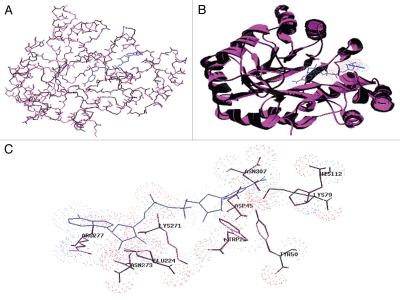
The gene encoding Xylose reductase (TeXR) was also isolated from the thermophilic fungus Talaromyces emersonii,8 a fungus known by its thermostable lignocellulolytic enzyme systems.9 Amino acid residues identified in C. tenuis xylose reductase critical in substrate recognition and co-factor preference were conserved in TeXr (Fig. 2). The coenzyme selectivity of TeXr was altered by site-directed mutagenesis and the ability of TeXrK271R + N273D double mutant to use NADH preferentially to NADPH as a coenzyme in the first step of pentose metabolism could have dramatic effects on improving the xylose conversion process by reducing the redox imbalance showing that T. emersonii may be a novel and highly efficient ‘toolbox’ for biotechnological conversion of lignocellulose to bioethanol. Comparable changes in altered coenzyme preference from NADPH to NADH were obtained with a CtXrK274R+N276D double mutant10 PsXrK270R+N272D double mutant11,12 and a PsXrK270S+S271G+N272P+R276F quadruple mutant.11 The next logical steps in our approach would be to perform a detailed investigation of TeXrK271R + N273D double mutant effects in engineered strains in order to improve xylose fermentation rates and establish a comparison between other xylose reductase mutants. The S. cerevisiae strain engineered with the C. tenuis double mutant showed 42% enhanced ethanol yield and decreased xylitol and glycerol production compared to the reference strain harbouring wild-type XR.13 Similar tendencies were observed with S. cerevisiae strains engineered with the P. stipitis PsXrK270R + N272D double mutant.12
Figure 2.
Superimposition using SWISS-MODEL of 2–319 amino acid backbone atoms (1,260) between the template structure 1mi3A (pink) TeXR (black) model (A) Cα trace, (B) ribbon trace and (C) amino acids relevant to catalysis, substrate binding and co-enzyme (blue) interaction.
Other strategies to introduce xylose metabolic pathways into S. cerevisiae in an attempt to decrease xylitol formation included the expression of the Thermus thermophilus xylose (glucose) isomerase14 and the XYLA gene encoding the Piromyces sp. xylose isomerase.15 However, the latter enzyme is strongly inhibited by xylitol and because S. cerevisiae produces an aldose reductase (Gre3) capable of reducing xylose to xylitol, a major by product in the process was xylitol. Therefore GRE3 deletion strains are essential for xylose fermentation when a xylose isomerase is introduced into S. cerevisiae.16 Comparison of xylose-fermenting ability by S. cerevisiae starins engineered with xylose reductase and xylitol dehydrogenase with strains engineered with xylose isomerase revealed that the Xr-Xdh xylose utilization pathway is much better than the xylose isomerase pathway due to the insufficient in vivo activity of xylose isomerase. This low xylose isomerase activity limits xylose utilization in recombinant S. cerevisiae strains and therefore it would be useful to exploit further the Xr-Xdh pathway using T. emersonii XrK271R + N273D double mutant to potentially construct a new pathway to reduce the redox imbalance achieving more effective ethanol production from xylose by recombinant S. cerevisiae.
NADPH is mainly regenerated in the oxidative part of PPP and is coupled to the generation of CO2. In this pathway, D-glucose-6-phosphate dehydrogenase (G6PDH), encoded by ZWF1 and 6-phosphogluconate dehydrogenase, encoded by GND1 and GND2 are responsible for the oxidation of D-glucose-6-phosphate and release of 2 moles of NADPH and 1 mole of CO2 per mole of D-glucose-6-phosphate.17 Ethanol yield from xylose can be increased by lowering the oxidative PPP flux. Modifications in the pathway such as deletion of ZWF1 and GND1 were proven to block PPP giving an ethanol yield of 0.41 g g−1 and a xylitol yield of only 0.05 g g−1,18 and a reduced phosphoglucose isomerase activity, an enzyme that converts glucose-6-phosphate into fructose-6-phosphate, was shown to increase ethanol yield.19 Insertion of a NADP+-dependent D-glyceraldehyde-3-phosphate dehydrogenase (NADP-GAPDH) from Kluyveromyces lactis facilitated NADPH regeneration with no production of CO2 in S. cerevisiae strains with a ZWF1 deletion.17 In S. cerevisiae strains exibiting a range of production levels of G6PDH, overexpression of a transhydrogenase from Azotobacter vinelandii, reduced the xylitol yield but enhanced the yield of glycerol during xylose fermentation.20
A different approach to modifying the redox metabolism would require modification of ammonia assimilation in recombinant S. cerevisiae. For ammonia incorporation, ATP-dependent synthesis of glutamine from glutamate and ammonia is catalysed by glutamine synthetase, while a NADPH-dependent glutamate dehydrogenase, encoded by GDH1 is responsible for 2-ketoglutarate amination. Another glutamate dehydrogenase, encoded by GDH2 is NADH-dependent and converts glutamate to 2-ketoglutarate and ammonium. Virtually all microorganisms that fix atmospheric nitrogen assimilate ammonia through the GS-GOGAT complex which converts ammonia to glutamate using NADH and ATP and consists of two enzymes: glutamate synthetase encoded by GLT1 and glutamine synthetase encoded by GLN1. Deletion of NADPH-dependent GDH1 and overexpression of NADH-dependent GDH2 or GS-GOGAT complex was shown to improve cofactor utilization in xylosefermenting S. cerevisiae and improved the ethanol yield.21
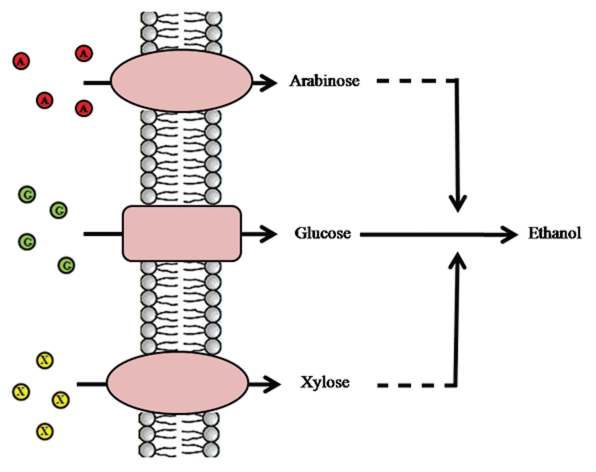
In further metabolic engineering of xylose metabolism, the xylose transport step should receive special attention. The rather high Km values of xylose reductase and xylose isomerise enzymes implies that achieving high rates of xylose fermentation may require the introduction of heterologous high-affinity xylose transporters that catalyses xylose uptake. S. cerevisiae also lacks an efficient transport system for pentose sugars, although the transport of these sugars occurs through hexose transporters with very low affinity and competition with glucose restricts xylose assimilation. Simultaneous and effective transport of both hexose and pentose sugars by pentose utilizing strains of S. cerevisiae would therefore be a significant improvement for bioconversion of biomass feedstocks to bioethanol (Fig. 3). A gene encoding a glucose/xylose facilitated diffusion transporter and a gene encoding a glucose/xylose symporter from C. intermedia36 were successfully expressed in S. cerevisiae. However the C. intermedia symporter was not able to support vigorous growth of the recombinant S. cerevisiae strain on xylose or glucose when used as sole carbon sources. Among filamentous fungi relatively few sugar transporters have been identified and characterized.
Figure 3.
Ideal simultaneous transport and fermentation of hexose and pentose sugars.
T. emersonii, the thermophilic fungus used in our studies, inhabits the soil, decaying masses of plant material piles of agricultural and forestry products, and other accumulations of organic matter wherein the warm, humid and aerobic environment with accessible carbon source provides the basic conditions for survival.23 T. emersonii also grows rapidly on hexoses and pentoses as a sole carbon source and thermostable enzyme systems required for hemicellulose degradation have been described and characterized previously.24,25 So we therefore anticipated the presence of high affinity sugar transporter genes in T. emersonii genome.
We searched the T. emersonii chromosomal DNA for genes with strong sequence and predicted structural similarities with transporter genes from the microorganisms whose function has been demonstrated and therefore the isolation of two putative transporters from the filamentous fungus T. emersonii, TeHTX (GenBank FJ985745) and TeXYLT (GenBank Accession No.: FJ985746) has been achieved. The transporters were shown to have 12 transmembrane domains which is a characteristic feature of the Major Facilitator Superfamily. Detailed sequence analysis of both transporters suggests that TeHXT is potentially a hexose transporter similarly to Gal2 from S. cerevisiae while TeXYLT has the potential to be a glucose/xylose transporter (unpublished data).
Despite the high similarity of TeHXT to fungal high-affinity transporters and the similarity of TeXYLT to other transporters previously shown to transport xylose, Talaromyces genes did not restore growth on different sugars of an engineered S. cerevisiae strain, in which all hexose transporters were deleted (unpublished data). We hypothesized that T. emersonii transporter proteins may not have been correctly directed to the plasma membrane, were folded incorrectly or a different composition of phospholipid sterols in S. cerevisiae may have caused changes in T. emersonii transporter conformation. Further expansion of these results of our research can open an alternative route to the development of industrial xylose-utilizing strains of S. cerevisiae.
Conclusion
More than a decade of research has been devoted to the development of strains for efficient pentose fermentation. The majority of the studies conducted used metabolic engineering through a rational selection of genes to be manipulated for the development of novel pentose-fermenting strains of S. cerevisiae with varying levels of success. The increased knowledge about pentose metabolism, substrate binding and cofactor specificity of pentose assimilating enzymes and sugar transport has contributed to the improvement of S. cerevisiae strains for bioconversion of pentose sugars to bioethanol. However, the mechanisms by which pentose-fermenting yeasts can accomplish an increased rate of ethanol production are still not fully understood and the simultaneous co-fermentation of hexose and pentose sugars still constitutes a major strain engineering challenge.
There is an increasing need to identify new sources of more stable biomass converting enzymes and more efficient systems to utilize all carbohydrate components of lignocellulose. Also better understanding of the sugar and oxygen regulatory system, sugar transport and the development of further transformation and expression systems with genes capable to overcome the cofactor imbalance will enable the construction of robust industrial yeast strains for pentose fermentation. The thermophilic fungus T. emersonii proved to be a potential source organism for extracellular and intracellular proteins with applications in biomass bioconversion strategies and future experiments using T. emersonii genes may further improve the desired traits or fermentative properties of the industrial organisms leading to a more efficient biotechnological conversion of lignocellulose to bioethanol.
Acknowledgements
This work was supported by an Irish government Department of Agriculture, Food and Fisheries award (DAFF RSF-05-225) under the research stimulus fund program 2007-13.
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/12724
References
- 1.Hahn-Hägerdal B, Jeppsson H, Olsson L, Mohagheghi A. An inter-laboratory comparison of the performance of ethanol-producing microorganisms in a xylose rich acid hydrolysate. Appl Environ Microbiol. 1994;41:62–72. [Google Scholar]
- 2.Hahn-Hägerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF. Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol. 2007;74:937–953. doi: 10.1007/s00253-006-0827-2. [DOI] [PubMed] [Google Scholar]
- 3.Kotter P, Ciriacy M. Xylose fermentation by S. cerevisiae. App Microb Biotechnol. 1993;38:776–783. [Google Scholar]
- 4.Ho NW, Chen Z, Brainard AP. Genetically engineered Saccharomyces yeast capable of effective cofermentation of glucose and xylose. Appl Environ Microbiol. 1998;64:1852–1859. doi: 10.1128/aem.64.5.1852-1859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao X, Gao D, Qu Y, Wang Z, Walfridssion M, Hahn-Hägerbal B. Effect on product formation in recombinant S. cerevisiae strains expressing different levels of xylose metabolic genes. Chin J Biotechnol. 1997;13:225–231. [PubMed] [Google Scholar]
- 6.Kostrzynska M, Sopher CR, Lee H. Mutational analysis of the role of the conserved lysine-270 in the P. stipitis xylose reductase. FEMS Microbiol Lett. 1998;159:107–112. doi: 10.1111/j.1574-6968.1998.tb12848.x. [DOI] [PubMed] [Google Scholar]
- 7.Petschacher B, Nidetzky B. Altering the coenzyme preference of xylose reductase to favor utilization of NADH enhances ethanol yield from xylose in a metabolically engineered strain of Saccharomyces cerevisiae. Microb Cell Fact. 2008;7:1–12. doi: 10.1186/1475-2859-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes S, Tuohy MG, Murray PG. Xylose reductase from the thermophilic fungus Talaromyces emersonii: cloning and heterologous expression of the native gene (Texr) and a double mutant (TexrK271R + N273D) with altered coenzyme specificity. J Biosci. 2009;34:881–890. doi: 10.1007/s12038-009-0102-7. [DOI] [PubMed] [Google Scholar]
- 9.Moloney AP, McCrae SI, Wood TM, Coughlan MP. Isolation and characterization of the endoglucanases of T. emersonii. Biochem J. 1985;225:365–374. doi: 10.1042/bj2250365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petschacher B, Leitgeb S, Kavanagh KL, Wilson DK, Nidetzky B. The coenzyme specificity of Candida tenuis xylose reductase (AKR2B5) explored by site-directed mutagenesis and X-ray crystallography. Biochem J. 2005;385:75–83. doi: 10.1042/BJ20040363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang L, Zhang J, Lin Z. Altering coenzyme specificity of Pichia stipitis xylose reductase by the semi-rational approach CASTing. Microb Cell Fact. 2007;6:1–11. doi: 10.1186/1475-2859-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe S, Saleh AA, Pack SP, Annaluru N, Kodaki T, Makino K. Ethanol production from xylose by recombinant Saccharomyces cerevisiae expressing protein-engineered NADH-preferring xylose reductase from Pichia stipitis. Microbiology. 2007;153:3044–3054. doi: 10.1099/mic.0.2007/007856-0. [DOI] [PubMed] [Google Scholar]
- 13.Petschacher B, Nidetzky B. Altering the coenzyme preference of xylose reductase to favor utilization of NADH enhances ethanol yield from xylose in a metabolically engineered strain of Saccharomyces cerevisiae. Microb Cell Fact. 2008;7:1–12. doi: 10.1186/1475-2859-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walfridsson M, Bao X, Anderlund M, Lilius G, Bulow L, Hahn-Hagerdal B. Ethanolic fermentation of xylose with S. cerevisiae harboring the Thermus thermophilus xylA gene, which expresses an active xylose (glucose) isomerase. Appl Environ Microbiol. 1996;62:4648–4651. doi: 10.1128/aem.62.12.4648-4651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuyper M, Harhangi HR, Stave AK, Winkler AA, Jetten MS, de Laat WT, et al. High-level functional expression of a fungal xylose isomerase: the key to efficient ethanolic fermentation of xylose by S. cerevisiae. FEMS Yeast Res. 2003;4:69–78. doi: 10.1016/S1567-1356(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 16.Träff KL, Cordero Otero RR, van Zyl WH, Hahn-Hägerdal B. Deletion of the GRE3 aldose reductase gene and its influence on xylose metabolism in recombinant strains of S. cerevisiae expressing the xylA and XKS1 genes. Appl Environ Microbiol. 2001;67:5668–5674. doi: 10.1128/AEM.67.12.5668-5674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verho R, Londesborough J, Penttilä M, Richard P. Engineering redox cofactor regeneration for improved pentose fermentation in S. cerevisiae. Appl Environ Microbiol. 2003;69:5892–5897. doi: 10.1128/AEM.69.10.5892-5897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeppsson M, Johansson B, Hahn-Hägerdal B, Gorwa-Grauslund MF. Reduced oxidative pentose phosphate pathway flux in recombinant xylose-utilizing S. cerevisiae strains improves the ethanol yield from xylose. Appl Environ Microbiol. 2002;68:1604–1609. doi: 10.1128/AEM.68.4.1604-1609.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliasson A, Boles E, Johansson B, Österberg M, Thevelein JM, Spencer-Martins I, et al. Xylulose fermentation by mutant and wild-type strains of Zygosaccharomyces and Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2000;53:376–382. doi: 10.1007/s002530051629. [DOI] [PubMed] [Google Scholar]
- 20.Jeppsson M, Johansson B, Jensen PR, Hahn-Hägerdal B, Gorwa-Grauslund MF. The level of glucose-6-phosphate dehydrogenase activity strongly influences xylose fermentation and inhibitor sensitivity in recombinant S. cerevisiae strains. Yeast. 2003;20:1263–1272. doi: 10.1002/yea.1043. [DOI] [PubMed] [Google Scholar]
- 21.Roca C, Nielsen J, Olsson L. Metabolic engineering of ammonium assimilation in xylose-fermenting S. cerevisiae improves ethanol production. Appl Environ Microbiol. 2003;69:4732–4736. doi: 10.1128/AEM.69.8.4732-4736.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stolk A. The genus Talaromyces. Studies in Mycology. 1972:2. [Google Scholar]
- 23.Tuohy MG, Coughlan MP. Production of thermostable xylan degrading enzymes by T. emersonii CBS 814.70. Bioresource Technol. 1992;39:131–137. [Google Scholar]
- 24.Tuohy MG, Puls J, Claeyssens M, Vrsanská M, Coughlan MP. The xylan-degrading enzyme system of T. emersonii: novel enzymes with activity against aryl beta-D-xylosides and unsubstituted xylans. Biochem J. 1993;290:515–523. doi: 10.1042/bj2900515. [DOI] [PMC free article] [PubMed] [Google Scholar]