Abstract
Human embryonic stem cells (hES Cs) are an attractive alternative cell source for hematopoietic gene therapy applications as the cells are easily modified with lentiviral or other vectors and can be subsequently induced to differentiate into hematopoietic progenitor cells. However, demonstration of the full hematopoietic potential of hESC-derived progeny is challenging due to low marrow engraftment and the difficulty of detecting cells in the peripheral blood of human/mouse xenografts. Methotrexate (MTX) chemotherapy coupled with expression of a drug resistant dihydrofolate reductase such as Tyr22 (Tyr22DHFR) has the potential to selectively increase engraftment of gene-modified human hematopoietic cells in mice, which would allow for better phenotypic characterization of hESC-derived cells in vivo. We showed that hES Cs transduced with Tyr22DHFR-GFP encoding lentivirus vectors differentiate into MTX resistant (MTXr) hemato-endothelial cells. MTX treatment of immunodeficient mice infused with Tyr22DHFR hESC-derived hemato-endothelial cells increased the long-term engraftment of human cells in the bone marrow of MTX-treated mice. In contrast to previous studies, these results indicate that MTX administration has the potential to support in vivo selection that is maintained after cessation of treatment. The MTX/Tyr22DHFR system may therefore be useful for enrichment of gene-modified cell populations in human stem cell and gene therapy applications.
Key words: dihydrofolate reductase, methotrexate, human embryonic stem cells, in vivo selection, gene therapy, drug resistance
Hematopoietic stem cells (HSCs) are defined by their ability to self-renew and give rise to clonal progenitors that further differentiate to reconstitute the mature components of the blood system.1 While HSCs can be isolated from bone marrow based on phenotypic surface antigens, self-renewal and ex vivo expansion of HSCs has been a challenging goal as culture of HSCs typically results in the loss of self-renewal and repopulation ability in vivo.2 However, HSCs are maintained in the bone marrow as any losses due to normal turnover or injury is compensated by an increase in asymmetric cell division to reestablish equilibrium in the stem cell pool.3 Together, these characteristics make HSCs a compelling cell population for regenerative medicine and gene therapy.
Alternative cell populations, such as hematopoietic progenitors derived from hESCs or induced pluripotent stem cells (iPSCs), provide another option for gene therapy applications. Human ESCs are derived from the inner cell mass of the pre-implantation embryo. Unlike primary HSCs, hESCs maintain their pluripotency in vitro and may be expanded essentially indefinitely without undergoing differentiation or senescence.4,5 Multiple studies have now been done over the past decade to support differentiation of hESCs and iPSCs into diverse cell lineages, including hematopoietic cells.6
One way in which gene therapy has been applied to transplantation of HSCs is by the introduction and expression of drug resistance genes. In this strategy, when the engrafting donor HSCs (or other cell type) do not inherently possess a selective advantage compared to resident recipient HSC, expression of a drug resistance gene in donor cells, coupled with drug administration, has the potential to simultaneously protect the healthy donor cells from post-transplantation drug toxicity and support selective engraftment and expansion of the gene-modified donor cells. Therefore, drug resistance gene expression has the potential to facilitate reconstitution with donor HSCs for the purpose of hematopoietic recovery during chemotherapy or phenotype correction. This approach is conceptually applicable to reconstitution with HSCs derived from hESCs or iPSCs as well.
The folate analog MTX is a reliable cancer chemotherapeutic and is also widely used for GvHD prophylaxis after allogeneic hematopoietic cell transplantation.7,8 This extensive clinical experience provides the basis for achieving bona fide chemoprotection and in vivo selection using MTX/DHFR through strategic development and the incorporation of new scientific advances that will drive progress to effective clinical trials. Given that MTX acts on highly proliferative cells, blocking nucleotide synthesis and therefore DNA synthesis through competitive inhibition of DHFR,9 it is unlikely that a MTX-based in vivo selection strategy would support expansion of relatively quiescent HSCs. Indeed, previous studies by our group and others have shown that MTX-related in vivo selective effects on DHFR-expressing hematopoietic cells are only transient and are dependent upon continued drug administration.10–12 Historically, long-term selection has not been achieved by MTX administration alone, because the inhibitory activity of MTX affects primarily highly proliferative cells, such as myeloid and lymphoid progeny. In vivo selection has been achieved using the anti-folate trimetrexate when administered along with the nucleoside transport inhibitor nitrobenzylmercaptopurine ribose phosphate.11–13
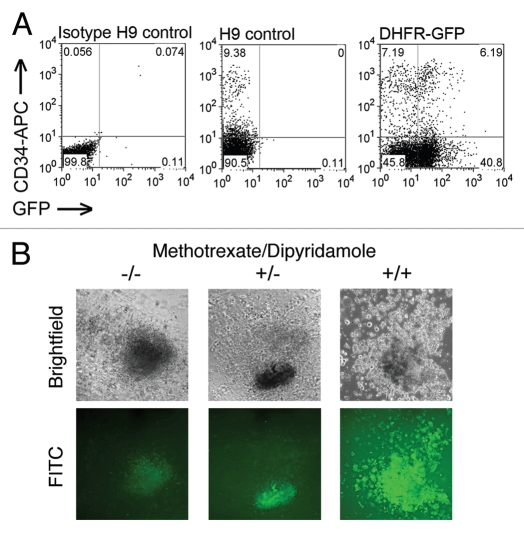
Our study is the first to demonstrate long-term expression of a drug resistance gene in hESCs and differentiated progeny without in vitro selection.14 In addition, we are the first to show that short-term MTX treatment is sufficient to support selective long-term engraftment of Tyr22-DHFR-expressing human hematopoietic cells in the bone marrow. The Tyr22DHFR-GFP+ hESCs gave rise to hematopoietic progenitors and MTXr-hematopoietic colony forming cells (CFC) in vitro under conditions with or without MTX (Fig. 1). The nucleoside transport inhibitor dipyridamole (DP) was included with MTX to provide more stringent selective conditions.15 As previously demonstrated for other hESC populations, the gene-modified hESCs routinely produced hematopoietic progenitor cells, as quantified in this CFC assay. Incubation with MTX alone did not inhibit colony formation by control GFP-transduced cells. However, in the presence of both MTX and DP, CFCs were maintained for all Tyr22DHFR-transduced cells and significantly reduced for GFP-transduced populations. Hematopoietic cells within the colonies retained GFP expression. These data demonstrate that Tyr22DHFR+ CFC have a survival advantage over control GFP-only cells when both folate metabolism and nucleoside transport are inhibited.
Figure 1.
Hematopoietic differentiation of DHFR-GFP hESC. (A) Co-expression of CD34 and GFP in gene-modified hESC after 21 days in M2-10B4 co-culture. Cell populations as indicated: GFP-negative isotype control, CD34-APC-stained H9, and DHFR-GFP H9 hESC-derived hematopoietic progeny. For flow cytometric analysis, gates were determined by isotype controls for each population. (B) MTXr-hematopoietic CFC from hESC-derived hematopoietic progenitors. At day 18 of co-culture with M2-10B4 stromal cells, populations were evaluated for CFC using no drug (−/−), 30 nM MTX (+/−), or both MTX and 5 µM dipyridimole (+/+). Drug-resistant GFP+ colonies differentiated from gene-modified hESC formed in the presence and absence of selective conditions. DHFR, dihydrofolate reductase; GFP, green fluorescent protein; CFC, colony-forming cells; DP, dipyridimole; hESC, human embryonic stem cell; MTX, methotrexate; MTXr, methotrexate-resistant.
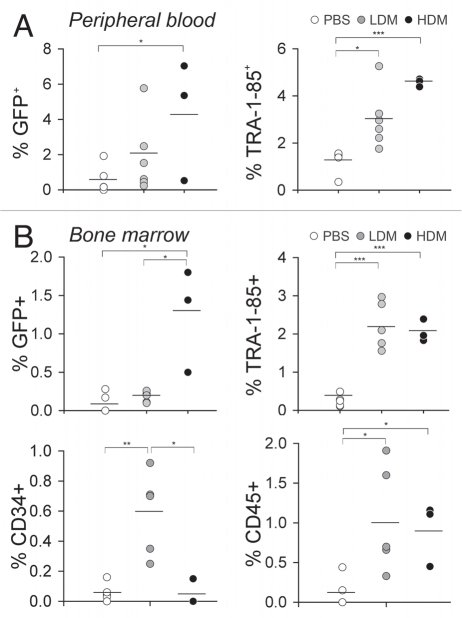
For in vivo studies, we also demonstrate MTX treatment significantly increased long-term engraftment of hESC-derived gene-modified hematopoietic cells in the bone marrow of nonobese diabetic/severe combined immunodeficient/IL-2Rγcnull (NSG) mice. Higher percentages of CD34+ and CD45+ populations were detected in the blood and bone marrow of treated mice to show that MTX selection does occur at the level of CD34+ progenitor cells, as well as more mature CD45+ hematopoietic cells (Fig. 2). If selection is achieved using the MTX/DHFR system, it is likely that other drug resistance genes may also support selective engraftment of hESC-derived hematopoietic progenitors. Reprogramming of human somatic cells into iPSCs that possess phenotypic and functional characteristics of ESCs presents another alternative cell source for gene therapy applications.5,16,17 In the event that hESC- or iPSC-derived hematopoietic cells are considered for clinical trials in humans, MTXr-DHFR expression during post transplantation immunosuppressive prophylaxis may prevent graft rejection of these cells.
Figure 2.
Persistence of hESC-derived hematopoietic cells in the peripheral blood and long-term engraftment in the bone marrow of recipient mice. After 10 days in co-culture with M2-10B4 stromal cells, DHFR-GFP+ hESC-derived hematopoietic cells were infused into DHFR-BMT mice (HDM group) or non-irradiated mice (LDM, PBS groups). One week after transplantation, mice were treated daily with PBS, 0.5 mg kg−1 MTX (LDM), or 2 mg kg−1 MTX (HDM) for 4 weeks. (A) GFP marking and human cell content (as detected by staining with TRA -1-85 antibody) in the peripheral blood was evaluated at 12 weeks post cell transplantation. Mean percentages for each group are shown (bars). (B) Long-term engraftment of hESC-derived hematopoietic cells in mouse BM. Animals were evaluated 12–16 weeks after DHFR-GFP hESC-derived hematopoietic cell transplantation and 8 weeks after withdrawal of MTX chemotherapy. Total GFP marking and engrafted cell phenotypes were evaluated by flow cytometry. Mean percentages are shown. Levels of statistical significance: *p < 0.05. **p < 0.005 and ***p < 0.0005. BMT, bone marrow transplanted; DHFR, dihydrofolate reductase; GFP, green fluorescent protein; HDM, high-dose methotrexate; LDM, low-dose methotrexate; MTX, methotrexate; PBS, phosphate-buffered saline.
In our studies of hESCs, we demonstrate that MTX supports long-term selective expansion of Tyr22DHFR-hematopoietic cells in vivo. One important clinical application is to assess the feasibility of synergizing chemotherapeutic and immunotherapeutic approaches to cancer treatment. Given the studies described by Woll and colleagues showing that hESC-derived natural killer (NK) cells possess potent anti-tumor activity,18,19 one potential application would be to generate NK cells from MTXr-DHFR-hESCs and then compare the persistence and ability of MTXr-NK cells to kill both MTXr and MTX-sensitive tumor cells in vivo. As long as graft rejection presents a risk following transplantation of gene-modified HSCs, regardless of the cell source, MTX/DHFR may be incorporated as an important part of a gene therapy strategy for inherited, acquired and malignant diseases.
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/12390
References
- 1.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112:3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glimm H, Oh IH, Eaves CJ. Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G(2)/M transit and do not reenter G(0) Blood. 2000;96:4185–4193. [PubMed] [Google Scholar]
- 3.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 4.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman DS. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood. 2009;114:3513–3523. doi: 10.1182/blood-2009-03-191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertino JR. Karnofsky memorial lecture. Ode to methotrexate. J Clin Oncol. 1993;11:5–14. doi: 10.1200/JCO.1993.11.1.5. [DOI] [PubMed] [Google Scholar]
- 8.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 9.Crom WR, Evans WE. Methotrexate. ed third. Vancouver: Applied Therapeutics, Inc; 1992. [Google Scholar]
- 10.Gori JL, Podetz-Pedersen K, Swanson D, Karlen AD, Gunther R, Somia NV, et al. Protection of mice from methotrexate toxicity by ex vivo transduction using lentivirus vectors expressing drug-resistant dihydrofolate reductase. J Pharmacol Exp Ther. 2007;322:989–997. doi: 10.1124/jpet.107.123414. [DOI] [PubMed] [Google Scholar]
- 11.Allay JA, Persons DA, Galipeau J, Riberdy JM, Ashmun RA, Blakley RL, et al. In vivo selection of retrovirally transduced hematopoietic stem cells. Nature Med. 1998;4:1136–1143. doi: 10.1038/2632. [DOI] [PubMed] [Google Scholar]
- 12.Persons DA, Allay JA, Bonifacino A, Lu T, Agricola B, Metzger ME, et al. Transient in vivo selection of transduced peripheral blood cells using antifolate drug selection in rhesus macaques that received transplants with hematopoietic stem cells expressing dihydrofolate reductase vectors. Blood. 2004;103:796–803. doi: 10.1182/blood-2003-05-1572. [DOI] [PubMed] [Google Scholar]
- 13.Allay JA, Spencer HT, Wilkinson SL, Belt JA, Blakley RL, Sorrentino BP. Sensitization of hematopoietic stem and progenitor cells to trimetrexate using nucleoside transport inhibitors. Blood. 1997;90:3546–3554. [PubMed] [Google Scholar]
- 14.Gori JL, Tian X, Swanson D, Gunther R, Shultz LD, McIvor RS, et al. In vivo selection of human embryonic stem cell-derived cells expressing methotrexate-resistant dihydrofolate reductase. Gene Ther. 2010;17:238–249. doi: 10.1038/gt.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warlick CA, Sweeney CL, McIvor RS. Maintenance of differential methotrexate toxicity between cells expressing drug-resistant and wild-type dihydrofolate reductase activities in the presence of nucleosides through nucleoside transport inhibition. Biochem Pharmacol. 2000;59:141–151. doi: 10.1016/s0006-2952(99)00311-1. [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 18.Woll PS, Marcus RK, Kaufman DS. Human embryonic stem cells differentiate into a unique population of natural killer cells with highly potent anti-tumor activity. Blood. 2007;110:806. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]