Abstract
In response to alkaline ambient pH, the Aspergillus nidulans PacC transcription factor mediating pH regulation of gene expression is activated by proteolytic removal of a negative-acting C–terminal domain. We demonstrate interactions involving the ∼150 C–terminal PacC residues and two regions located immediately downstream of the DNA binding domain. Our data indicate two full-length PacC conformations whose relative amounts depend upon ambient pH: one ‘open’ and accessible for processing, the other ‘closed’ and inaccessible. The location of essential determinants for proteolytic processing within the two more upstream interacting regions probably explains why the interactions prevent processing, whereas the direct involvement of the C–terminal region in processing-preventing interactions explains why C–terminal truncating mutations result in alkalinity mimicry and pH-independent processing. A mutant PacC deficient in pH signal response and consequent processing behaves as though locked in the ‘closed’ form. Single-residue substitutions, obtained as mutations bypassing the need for pH signal transduction, identify crucial residues in each of the three interactive regions and overcome the processing deficiency in the ‘permanently closed’ mutant.
Keywords: Aspergillus nidulans/PacC/pH regulation/protein interactions/proteolytic processing
Introduction
The PacC zinc finger transcription factor mediating regulation of gene expression by ambient pH in the fungus Aspergillus nidulans (Caddick et al., 1986; Tilburn et al., 1995; Espeso et al., 1997), in common with a number of other transcription factors, notably NF-κB (Thanos and Maniatis, 1995; Ghosh et al., 1998), Cubitus interruptus (Ruiz i Altaba, 1997; Ingham, 1998) and the sterol regulatory element-binding proteins (Brown and Goldstein, 1999), undergoes proteolytic processing activation (Orejas et al., 1995; Mingot et al., 1999). The ease with which A.nidulans can be manipulated genetically makes PacC ideally placed for understanding how a transcription factor prevents its proteolytic processing activation in the absence of appropriate signal transduction.
In response to a signal transduced at alkaline ambient pH by the products of the six pal genes (Denison et al., 1995, 1998; Maccheroni et al., 1997; Negrete-Urtasun et al., 1997, 1999) the 674 residue translation product of the A.nidulans pacC gene is processed to a functional form containing the ∼248–250 N–terminal residues (Orejas et al., 1995; Mingot et al., 1999), which activates expression of genes expressed preferentially under alkaline growth conditions (Espeso et al., 1993; Tilburn et al., 1995; Espeso and Peñalva, 1996) and represses genes expressed preferentially at acidic pH (Tilburn et al., 1995; Hutchings et al., 1999; E.A.Espeso and H.N.Arst, submitted). The PacC DNA binding domain (DBD), containing three Cys2His2 zinc fingers and binding to GCCARG promoter sites (Tilburn et al., 1995; Espeso et al., 1997), is located centrally within the processed form.
The alkaline pH-sensitive step in the regulatory cascade appears to be the accessibility of the PacC primary translation product to the processing protease, suggesting that PacC alternates between protease-resistant and protease-sensitive conformations in response to ambient pH (Mingot et al., 1999). Loss-of-function pal– mutations preventing ambient pH signal transduction and PacC proteolytic processing (Caddick et al., 1986; Denison et al., 1995; Tilburn et al., 1995; Negrete-Urtasun et al., 1999) lead to an acidity-mimicking phenotype, as do null (pacC–) or partial loss-of-function (pacC+/–) mutations in pacC. Gain-of-function pacCc mutations have an alkalinity-mimicking phenotype, obviating the need for ambient pH signalling and resulting in constitutive (i.e. pH-independent) PacC processing (Caddick et al., 1986; Orejas et al., 1995; Tilburn et al., 1995; Mingot et al., 1999). Most extant pacCc mutations result in truncation of 100–412 residues from the C–terminus of PacC, suggesting a crucial role for the carboxyl region of the protein in maintaining the inaccessibility of upstream processing determinants to the processing protease at inappropriate ambient pH. This suggests a simplified view of PacC in which a C–terminal domain would interact with sequences in the vicinity of the processing site to prevent proteolytic processing, a view that is further supported by the existence of several single-residue substitutions or deletions within the environs of the processing site, which also lead to alkalinity mimicry and ambient pH-independent processing (Mingot et al., 1999).
Here we provide compelling evidence, using one- and two-hybrid, co-immunoprecipitation, affinity column, gel mobility supershift and protease accessibility experiments, for the existence of interactions involving the C–terminal region of PacC and the environs of the processing site. The effects of various pacC mutations on these interactions fully support their physiological relevance. The proportion of wild-type full-length PacC available for external interactions increases with elevation of pH, indicating that it undergoes a conformational change in response to alkaline ambient pH. Therefore, there are three different forms of PacC: two conformationally different full-length versions and the processed form.
Results
To avoid confusion, readers are reminded that translation of pacC mRNA proceeds from methionine codon 5, but to maintain consistency with earlier publications residues are numbered as if translation proceeded from methionine codon 1 (Mingot et al., 1999).
Evidence from a two-hybrid system and in vitro experiments that the PacC C–terminus interacts with a region immediately downstream of the PacC DBD
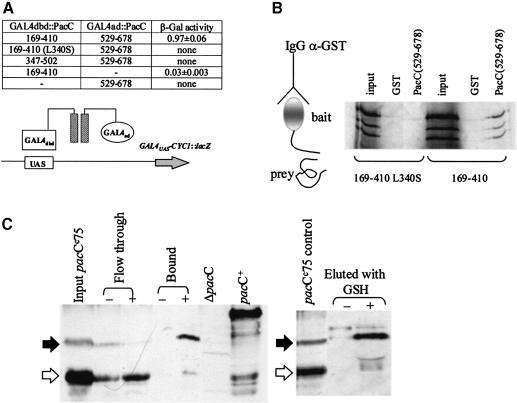
A two-hybrid experiment indicated that PacC residues 169–410 specifically interact with C–terminal residues 529–678 (Figure 1A). The alkalinity-mimicking pacCc69 (L340S) mutation, which results in constitutive PacC processing (Mingot et al., 1999), prevents the interaction, suggesting that it might have physiological significance.
Fig. 1. PacC residues 169–410 interact with C–terminal residues 529–678. (A) Yeast two-hybrid analysis of the interaction. Plasmid combinations in different recombinant yeast strains are shown together with their corresponding β–galactosidase activities. PacC regions (with L340S substitution where indicated) fused to the GAL4dbd (DNA binding domain) and the GAL4ad (activating domain) are indicated. A dash denotes that either the ‘bait’ or ‘prey’ plasmid is absent from the corresponding strain. (B) Co-immunoprecipitation of PacC(169–410) and PacC(529–678) peptides. Purified GST::PacC(529–678) fusion protein was incubated with in vitro synthesized, 35S-labelled PacC(169–410) (wild-type or L340S mutant) and immunoprecipitated with a rabbit polyclonal anti-GST antiserum. The immunoprecipitated material was analysed by SDS–PAGE and subsequent autoradiography. (C) The truncated PacC(5–430) (pacCc75) translation product (solid arrows), but not its processed 5 to ∼253 form (open arrows), is retained by a GST::PacC(529–678) affinity column. Equivalent pacCc75 extract samples (input) were each passed through glutathione–Sepharose columns loaded with either GST protein (–) or GST::PacC(529–678) (+) and the flow-through, bound (released as described in Materials and methods) or GSH-eluted materials for each column were analysed by Western blots with wild-type and null pacC control extracts, using an antibody recognizing the PacC DBD.
Co-immunoprecipitation using glutathione S-transferase (GST)-tagged PacC residues 529–678 as ‘bait’ and 35S–labelled in vitro synthesized polypeptide ‘prey’ containing PacC residues 169–410 with and without the L340S substitution confirmed the two-hybrid result (Figure 1B). The mobilities of the largest and most pronounced of the three in vitro coupled transcription–translation bands correlated approximately with the predicted aggregate size. α-GST polyclonal antiserum precipitated the wild-type ‘prey’ polypeptides, but not those containing the L340S substitution.
A further test of this interaction is that C–terminal truncation pacCc translation products, predicted to be in a protease-accessible ‘open’ conformation, would be expected to interact with PacC residues 529–678 via their residues 169–410. The most suitable extant pacCc allele for this purpose is pacCc75 as it encodes only 20 PacC residues beyond 410 and no out-of-frame residues (Mingot et al., 1999). Confirming this prediction, an affinity column loaded with GST::PacC(529–678) (but not a control column loaded with GST) retains the full-length pacCc75 translation product, but at most only a trace of the processed form (Figure 1C). Thus, there is little or no interaction between PacC residues 5 to ∼253 and the C–terminal residues 529–678, and the interaction requires the presence of residues ∼254–430 (or 410 in view of data in Figure 1A and B).
One-hybrid analysis in yeast indicates the presence of interactions in PacC
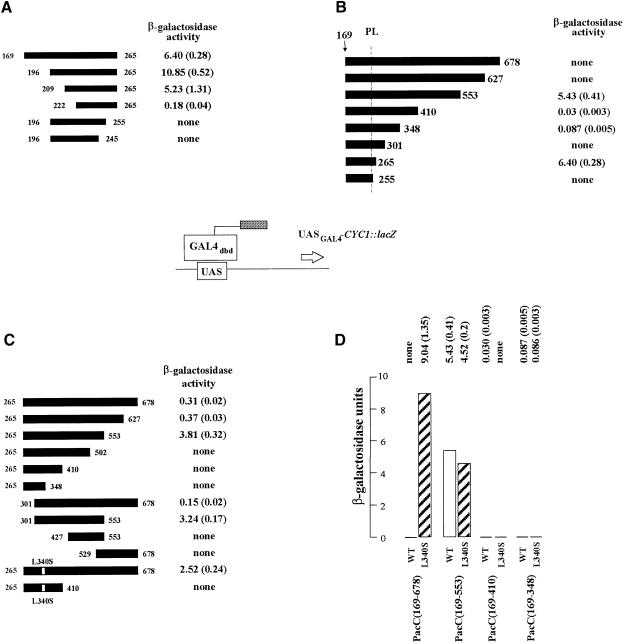
Two regions of PacC having transcription activation activity in yeast have been identified using a one-hybrid system in which different regions of PacC are tethered via the GAL4 DBD to a promoter driving lacZ expression. One is located largely within residues 209–265 (Figure 2A). The other lies between residues 301 and 553 (Figure 2B). As the processing limit of PacC is approximately residues 252–254 (Mingot et al., 1999), we have no evidence that either of these regions is physiologically involved in transcription activation by PacC in A.nidulans, but they are useful in the detection of interactions involving PacC molecules.
Fig. 2. Localization of PacC domains having activating or masking activity and the effect of the L340S substitution in a yeast one-hybrid system. (A–D) The indicated PacC coding regions (solid bars) were fused to the GAL4dbd and transformed into a yeast strain carrying a GAL4-dependent CYC1::lacZ reporter. For all panels β–galactosidase activities are the average of three independent experiments for each construct, with standard errors shown in parentheses. The L340S substitution is present where indicated. PL, processing limit.
These two activation domains are largely masked by residues between 554 and 627 or 678 (Figure 2B), which reveals functional interactions between the C–terminal region and these upstream domains. An indication that these interactions might be physiologically significant is provided by the effects of the alkalinity-mimicking pacCc69 (L340S) mutation. The L340S substitution largely prevents the masking of both activation domains by the C–terminal region, as shown by the much more dramatic unmasking effect of L340S observed when both activation domains are present as compared with that observed when only the domain between residues 301 and 573 is present (Figure 2C and D). However, there is no additivity between L340S and removal of the 125 C–terminal residues, suggesting that both the truncation and the substitution achieve the same end (Figure 2D). Appropriate controls in Figure 2C and D establish that L340S does not create an activation domain fortuitously. A second interaction, unaffected by the L340S substitution, would be suggested by the masking effect of residues between 266 and 410 on the activation domain between residues 209 and 265 (Figure 2B and D).
Single amino acid substitutions that restore the ability of the pacC+/–20205-encoded protein to undergo processing
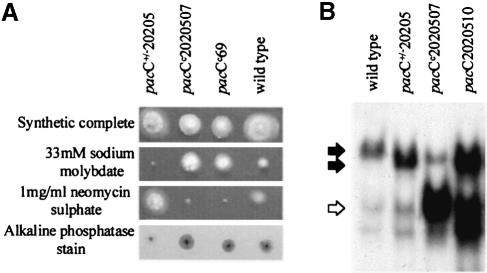
The pacC+/–20205 allele contains two compensating frameshift mutations (pacC20205 and pacCc202) resulting in replacement of PacC residues 465–540 by an out-of-frame octapeptide (Mingot et al., 1999). This mutant PacC is largely unable to respond to the pH signal and confers a strong acidity-mimicking phenotype through a lack of PacC processing, suggesting that it is permanently shifted towards the protease-resistant conformation (Mingot et al., 1999). To identify residues crucially involved in this protease resistance, we isolated second-site supressors of pacC+/–20205. One such suppressor mutation leading to strong alkalinity mimicry (Figure 3) is pacCc2020507. Remarkably, this mutation results in an L340S substitution. The ability of the L340S substitution to correct the processing defect of pacC+/–20205 (Figure 3B) indicates that this processing recalcitrance is likely to involve the interaction between residues 169–430 and 529–678. The importance of Leu340 in maintaining this interaction is underlined by the selection of pacCc234 (in an otherwise pacC+) background. pacCc234 results in L340F and a weaker than L340S alkalinity-mimicking phenotype and processing constitutivity (data not shown), suggesting that both the size and the hydrophobicity of Leu340 are pertinent.
Fig. 3. Single-residue substitutions suppressing the acidity-mimicking phenotype and PacC processing deficiency resulting from pacC+/–20205. The pacCc2020507 and pacC2020510 mutations result in L340S and R573W substitutions, respectively, and are in the presence of pacC+/–20205. pacCc69 results in L340S in an otherwise wild-type pacC background. (A) Phenotypes in plate tests. The pacC+/–20205 strain has an acidity-mimicking phenotype, whereas the pacCc2020507 and pacCc69 strains have alkalinity-mimicking phenotypes. (B) EMSA showing full-length and processed forms of PacC. Acidic growth conditions were used, resulting in minimal processing of the wild-type PacC but revealing constitutive processing due to the pacCc2020507 and pacC2020510 mutations. Solid arrows denote the full-length form complexes (which have a higher mobility in strains having the pacC+/–20205 deletion). An open arrow denotes the processed form complex.
Another second-site suppressor of pacC+/–20205 is pacC2020510 resulting in a weaker (than pacCc2020507) alkalinity-mimicking phenotype and a lesser degree of pH-independent PacC processing (Figure 3B). pacC2020510 results in R573W, suggesting that whereas L340S disables the 169–430 interacting region, R573W impairs the 529–678 interacting region.
Supershifts in electrophoretic mobility shift assay (EMSA) constitute a convenient assay for interactions
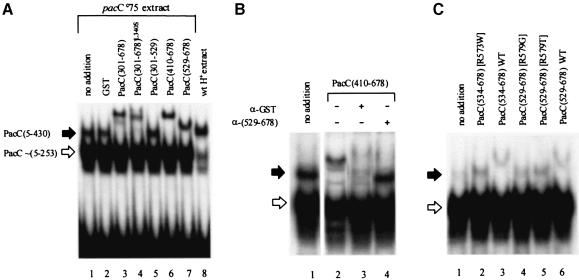
A particularly convenient and sensitive method for detecting and characterizing PacC forms containing the DBD is EMSA (Orejas et al., 1995; Espeso et al., 1997; Mingot et al., 1999). Provided that PacC interactions are reasonably strong, it should be possible to observe them by supershifts in EMSA. Indeed, the pacCc75 full-length translation product PacC(5–430) band is supershifted by GST fusion proteins with PacC residues 301–678, 410–678 and 529–678, but not 301–529 (Figure 4A), and the magnitude of the supershift is commensurate with the sizes of the interacting GST fusion proteins. As a further control, polyclonal antiserum against a GST::PacC(529–678) fusion protein prevented the supershift, whereas anti-GST antiserum largely removed the low-mobility complex, presumably because its size upon immunoglobulin binding prevented penetration into the gel (Figure 4B). No supershift occurred involving the above PacC C–terminal GST fusion proteins and the processed form of PacC (Figure 4A), a result confirmed by the inability of complexes formed by PacC(5–265) or full-length forms of the pacC+/–508 (5–224 + 13 out-of-frame residues), pacC+/–515 (5–227 + 22), pacCc/–20000 (5–251 + 5) or pacCc50 (5–266) products (Orejas et al., 1995; Tilburn et al., 1995; Mingot et al., 1999) to be supershifted by any of the GST::PacC proteins shown in Figure 4A (data not shown). These data demonstrate that only residues 529–678 are required for interaction with a wild-type 169–430 region, in agreement with previous results. As expected, the presence of the L340S substitution in GST::PacC(301–678) does not prevent the supershift, although it decreases its magnitude, possibly as a result of conformational differences (Figure 4A).
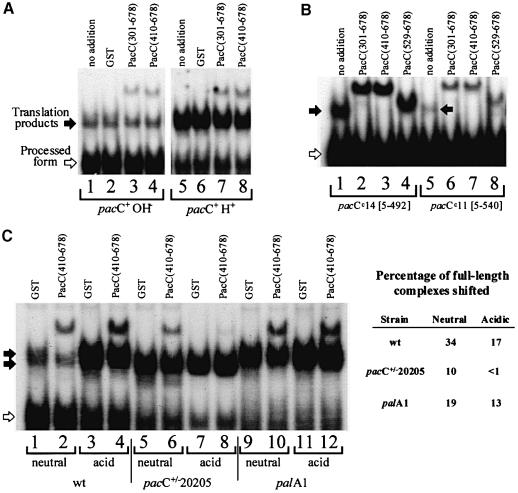
Fig. 4. Supershifting of the PacC(5–430)–DNA complex by purified GST::PacC fusion proteins containing the C–terminus of PacC. Acidic grown strains were used. (A) The indicated GST::PacC proteins were added to binding reactions containing a pacCc75 (truncating PacC after residue 430) extract and the 32P-labelled ipnA2 PacC binding site, and the resulting complexes were resolved by electrophoresis in an 8% (w/v) polyacrylamide gel. With no addition or upon addition of GST, a major complex corresponding to the processed PacC form (open arrow, whose predominance is favoured by the alkalinity-mimicking, truncating pacCc75 mutation) and a minor complex corresponding to the PacC(5–430) translation product (solid arrow) are resolved. The latter is supershifted by all GST fusion proteins containing PacC residues 529–678. A wild-type extract (acidic growth conditions) was used in lane 8 as a control. (B) Antiserum raised against a GST::PacC(529–678) fusion protein prevents supershifting, whereas anti-GST antiserum prevents penetration of the supershifted complex into the gel. Only the relevant portion of the gel is shown. (C) Certain alkalinity-mimicking single-residue substitutions within residues 529–678 prevent supershifting.
We tested in this supershift assay the above pacC2020510 R573W substitution and two additional substitutions, pacCc232 R579G and pacCc200 pacC20042 R579T, leading to alkalinity mimicry and constitutive PacC processing (data not shown), which were likely candidates to disable the above interactive C–terminal region. Indeed, their presence in the GST::PacC C–terminal fusion protein region prevented the supershift (Figure 4C).
Comparisons of wild-type and mutant PacC proteins using the supershift assay
If PacC accessibility to the protease were prevented by the interactions documented above, the full-length, wild-type PacC protein should be able to adopt a ‘closed’ conformation unavailable for interaction with a C–terminal polypeptide provided in trans, but should adopt a more ‘open’ conformation as the pH becomes more alkaline, triggering ambient pH signal transduction and PacC processing. Figure 5A supports the hypothesis of ‘closed’ and ‘open’ conformations whose relative amounts are influenced by ambient pH. Under conditions in which the full-length form complexes for pacCc75 (5–430) (Figure 4A), pacCc14 (5–492) and pacCc11 (5–540) (Figure 5B) are quantitatively supershifted, only a minor proportion of the full-length form complex from an acidic grown wild type is supershifted (Figure 5A). Supershifting is significantly more efficient if alkaline or neutral growth conditions are used (Figure 5A and C). In a palA1 background, in which pH signal transduction and consequently processing are blocked, the proportion of full–length PacC complex supershifted, irrespective of growth pH, is similar to that from an acidic grown wild-type strain. In contrast, the pacC+/–20205 complex from acidic growth conditions is fully recalcitrant to supershifting (Figure 5C), supporting the interpretation that this mutant PacC is largely unable to convert from an inaccessible to an accessible conformation. However, a small degree of supershift, less than that observed with acidic grown palA1 or wild-type strains, is seen using extracts of a neutral grown pacC+/–20205 strain (Figure 5C) and correlates with the small increase in processing seen under neutral growth conditions (Mingot et al., 1999; Figure 5C). This small but reproducible difference in behaviour of a pacC+/–20205 product according to whether growth was at acidic or neutral pH indicates that the pacC+/–20205 deletion allows a small but inadequate response to ambient pH signal transduction.
Fig. 5. Supershifting of PacC–DNA complexes from various mutants and ambient pH conditions analysed in a 4% (w/v) polyacrylamide gel. Only the relevant portions of autoradiograms are shown. Supershifting PacC peptides containing C–terminal residues were added to binding reaction mixtures as purified GST fusion proteins, as in Figure 4. Control reactions with no addition or with GST addition are included. In all panels, solid arrows indicate wild-type and mutant full-length complexes, whereas open arrows indicate processed form complexes. (A) Supershifting of the acidic or alkaline grown, wild-type, full-length form complex. The full-length form complex predominates under acidic growth conditions. (B) Supershifting of the translation product complexes of two strong alkalinity-mimicking alleles, with encoded PacC residues indicated in square brackets. Cells were grown under acidic conditions, but processing is favoured by the alkalinity-mimicking mutations. (C) Supershift assay of the wild-type or mutant PacC full-length complex from wild-type, palA1 and pacC+/–20205 strains cultured under acidic and neutral growth conditions (the palA1 and pacC+/–20205 mutations prevent growth under alkaline conditions), showing the relative inability of GST::PacC fusions containing the C–terminus to supershift a pacC+/–20205 product complex. The proportion of wild-type or mutant translation product complex that is supershifted (estimated by phosphoimaging) is shown.
A second protein–protein interaction involving Leu340 is essential for the interaction involving the PacC C–terminus
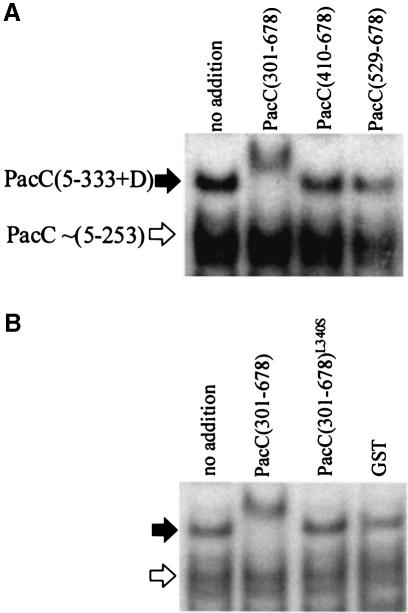
In contrast to the behaviour of PacC forms truncated after residues 430, 492 and 540, and wild-type PacC (Figures 4A, 5A and B), the complex formed by the full-length form of PacC from a pacCc2020503 strain, truncated after residue 333 followed by a single out-of-frame Asp residue (Mingot et al., 1999), was supershifted by GST::PacC(301–678) but not by shorter C–terminal fusion proteins (Figure 6A), suggesting that in the absence of residues 334–430 these residues can be provided in trans attached to the C–terminus. The interaction does not occur if the GST::PacC(301–678) fusion protein contains an L340S substitution (Figure 6B). In contrast, the complex formed by PacC(5–430) (i.e. the pacCc75 product) is still supershifted in the presence of the L340S substitution in the C–terminal fusion protein (Figure 4A, lane 4). An interpretation of this contrasting behaviour is that the interactive region within residues 169–430 actually consists of two independent interactive regions, one within residues 169–333 and the other within residues 334–430. The acceptor structure for the C–terminal interactive region within residues 529–678 would then interact with the structure formed through interaction of the two more N–terminal interactive regions with each other, the latter interaction being affected by L340S. For convenience we designate the region within residues 169–333 as A, that within residues 334–430 as B, and the C–terminal interactive region within residues 529–678 as C. In view of the evidence that interactive regions A and B can be provided by residues 169–410 (Figures 1A, B and 7) and that a transcriptional activation activity residing between residues 209 and 265 can be masked by residues between 265 and 410 (Figure 2A and C), the maximum limits for region B can probably be considered as residues 334 and 410.
Fig. 6. Supershifting of the PacC(5–333 + D)–DNA complex requires C–terminal proteins containing PacC residues upstream of residue 410 and is prevented by L340S. (A and B) An extract of a pacCc2020503 strain was used for complex formation. This mutation creates a frameshift truncating the normal sequence after residue 333 with a C–terminal out-of-frame Asp, a protein which is inefficiently and somewhat aberrantly processed (Mingot et al., 1999). Supershifting reactions using purified GST fusion proteins were carried out as described for Figure 4. Arrows in (B) are as in (A).
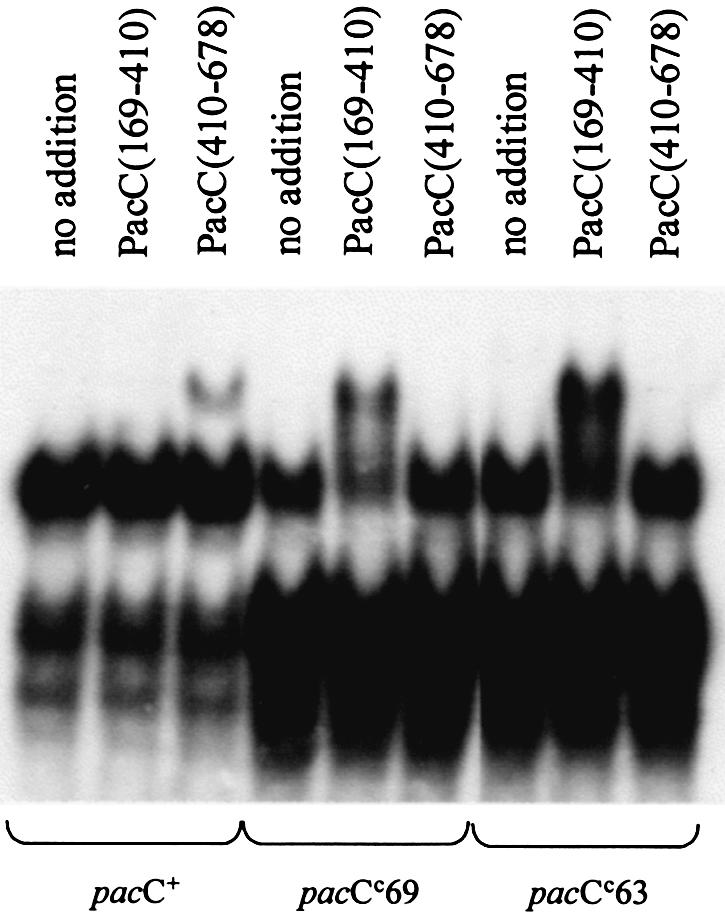
Fig. 7. Supershifting of PacC full-length form complexes with and without the L340S or L259R substitutions. Extracts of acidic grown wild-type, pacCc69 (L340S) and pacCc63 (L259R) strains were mixed with the indicated purified GST fusion proteins and analysed using the supershift assay in a 4% (w/v) polyacrylamide gel.
Wild-type PacC obtained using acidic growth conditions, although unable to interact with a GST::PacC(169–410) fusion protein containing regions A and B, is competent to a small extent to interact with region C from GST::PacC(410–678) (Figure 7). A reverse pattern of interaction would be predicted for full-length proteins disabled for region A or B as their ‘open’ conformation should make their region C available for interaction. This prediction is fulfilled for two mutant full-length proteins in which single-residue substitutions leading to alkalinity mimicry disable region A or B (Figure 7), and that are largely supershifted by GST::PacC(169–410) but not by GST::PacC(410–678). The L340S (pacCc69) substitution would debilitate region B, whereas L259R (pacCc63) would affect region A. The experiment in Figure 7 also shows that interactions involving separate PacC molecules can be observed regardless of whether functional regions A and B or region C are/is in cis to the PacC DBD.
The L340S substitution and a C–terminal truncation increase accessibility of PacC to a protease present in A.nidulans extracts
Whereas in vitro synthesized, 35S-labelled, wild-type PacC(5–678) remained largely intact upon incubation with a crude A.nidulans extract, a full-length protein containing the L340S substitution or a protein truncated after residue 529 was cleaved extensively by a protease (Figure 8). Immunoprecipitation of the L340S protein proteolysis products using antiserum against the PacC C–terminus or DBD, as well as nested deletion experiments, showed that the lower mobility (doublet) band (indicated by an open arrow in Figure 8) contains the C–terminus, whereas the more diffuse higher mobility band (solid arrow) contains the DBD (data not shown). The size of one of the C–terminal doublet bands plus that of the DBD-containing band approximates that of the full-length L340S PacC substrate. Although this reaction resembles somewhat the processing proteolysis, there is no definitive evidence that the protease responsible actually mediates processing in vivo. In fact the DBD-containing band is slightly larger than the physiologically processed form of PacC. Nevertheless, this experiment shows that the L340S substitution and a C–terminal truncation, both shown above to disrupt interactions involving PacC, dramatically increase the accessibility of PacC to a protease, consistent with a major conformational change.
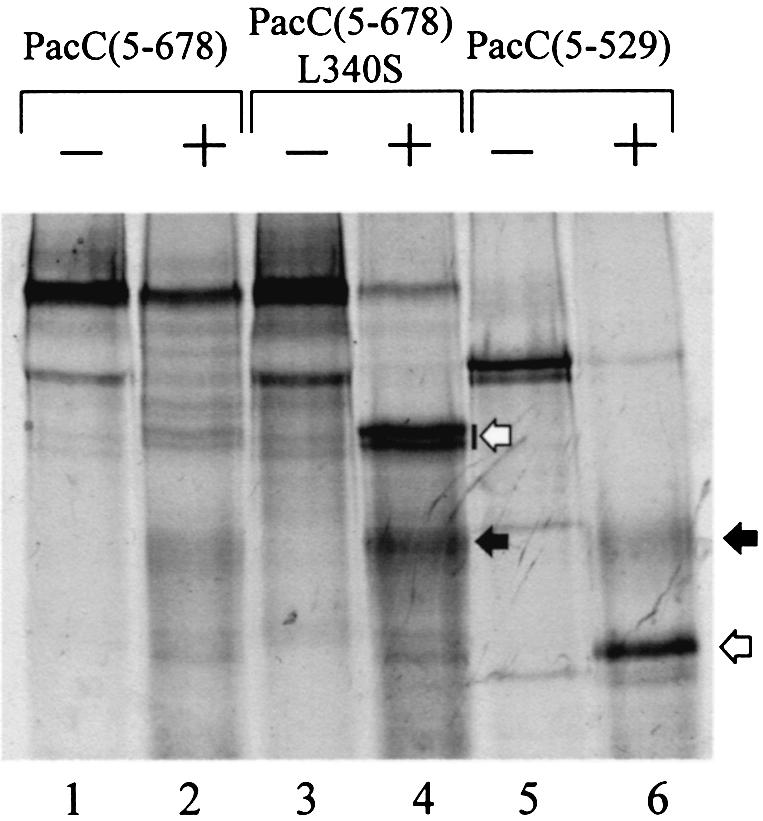
Fig. 8. Accessibility of in vitro synthesized PacC proteins to a protease present in an A.nidulans extract. The substrate proteins indicated were labelled with [35S]methionine using a coupled transcription–translation system and incubated in the presence (+) or absence (–) of an S-100 extract of an A.nidulans ΔpacC strain. The reaction mixtures were resolved by SDS–PAGE and autoradiographed. Solid arrows indicate proteolysis products containing the DBD but 30–50 residues larger than the processed form. Open arrows indicate C–terminal proteolysis products.
Discussion
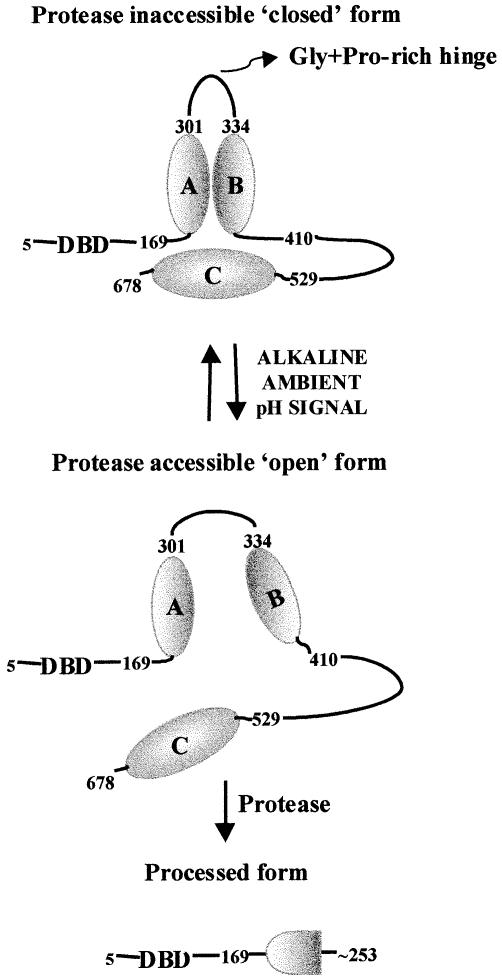
Proteolytic processing of PacC converting the 674 residue translation product to an activated form containing the ∼248–250 N–terminal residues is a crucial step in pH regulation. The ambient pH signal is required to sensitize full–length PacC to proteolytic processing (Orejas et al., 1995; Mingot et al., 1999). We show here that sensitization of PacC to the protease occurs through an ambient pH-mediated conformational change. Interaction between two regions (A and B) of PacC downstream of the DBD creates a structure that interacts with region C located near the C–terminus. These interactions are required to maintain a ‘closed’ conformation (see Figure 9) in which the presence of the processing limit and essential determinants for processing efficiency and fidelity within regions A and B would render them inaccessible unless the alkaline ambient pH signal disrupts the interactions, leading to an ‘open’ conformation and accessibility. The presence of a Gly– plus Pro-rich region at the approximate boundary between regions A and B (314-GPYGGGPHPAPAYHLPP-330; Tilburn et al., 1995) might serve as a ‘hinge’, particularly as the corresponding residues of the Aspergillus niger (307-GGGGYSPGGAPSAPAYHLPP-326; MacCabe et al., 1996) and Penicillium chrysogenum (290-GGGGGGYGGGAPQPPGYHLPP-321; Suárez and Peñalva, 1996) isofunctional homologues are also Gly plus Pro rich.
Fig. 9. A model showing interactions involving three regions of PacC and their role in preventing PacC processing. The model assumes that PacC exists as a monomer. There is no convincing evidence for oligomerization of PacC, but the possibility cannot be ruled out.
The strength of the interactions involving PacC regions allows their monitoring by supershift assays, which have served to detect the availability of regions A plus B or region C for interaction under different mutant and ambient pH conditions. These experiments have provided compelling evidence for two full-length conformations of PacC and their physiological relevance. For example, whereas the ‘closed’ form would not be available for supershift by a region C located in trans, the ‘open’ form of PacC is available. This implies that the interactions preventing protease accessibility are disrupted in the ‘open’ form and therefore that PacC is committed for proteolytic processing. As predicted, in the presence of a functional pH signal transduction system the ‘open’ conformation is favoured by alkaline growth conditions.
The role of the C–terminal region in maintaining the ‘closed’ conformation explains the commitment of the products of pacCc alkalinity-mimicking C–terminally truncating alleles to the ‘open’ conformation and consequently to pH-independent processing. pacCc alkalinity-mimicking missense mutations leading to L259R, L340S, L340F, R573W, R579G and R579T substitutions, also obviating the requirement for ambient pH signal transduction in processing and shown here to disrupt PacC interactions, identify residues crucial for each of the interacting regions. The importance of Arg579 is also underlined by the strongly alkalinity-mimicking phenotype of pacCc200 (Tilburn et al., 1995), which truncates PacC after residue 578.
The mutant product of the strong acidity-mimicking pacC+/–20205 allele, almost unable to respond to the ambient pH signal and undergo processing (Mingot et al., 1999), is abnormally displaced towards the ‘closed’ conformation, as determined by supershift assay. The characterization of second-site suppressor mutations of pacC+/–20205 leading to L340S or R573W substitution provides convincing evidence that the ‘closed’ conformation is mediated by PacC interactions described here. The L340S substitution had been independently identified previously (Mingot et al., 1999) as the change by which a pacCc69 mutation leads to alkalinity mimicry and pH-independent processing. In common with L340S, R573W, disabling interacting region C, increases the sensitivity of the pacC+/–20205 product to the processing protease.
We anticipate that this work will be helpful to others studying signal-dependent proteolytic processing of transcription factors. Of no lesser importance is its contribution to an understanding of how ambient pH controls gene expression in fungi, a critical determinant of, inter alia, pathogenicity (Saporito-Irwin et al., 1995; Muhlschlegel and Fonzi, 1997; De Bernardis et al., 1998; Wilson et al., 1999), entry into meiosis (Li and Mitchell, 1997), extracellular enzyme production (Lambert et al., 1997), penicillin production (Espeso et al., 1993; Suárez and Peñalva, 1996) and aflatoxin synthesis (Keller et al., 1997).
Materials and methods
Aspergillus nidulans strains, phenotype testing, culture conditions and genetic analysis
Aspergillus nidulans strains carried markers in standard use (Clutterbuck, 1993). Phenotype testing of pH regulatory mutations followed Tilburn et al. (1995). Mycelia for protein extraction were grown in PPB (penicillin production broth; Orejas et al., 1995; Mingot et al., 1999) or complete medium (Cove, 1966) with essentially the same results. Media were adjusted to acidic, neutral or alkaline initial pH values as in Orejas et al. (1995).
Aspergillus nidulans mutations affecting pH regulation have been described previously (Caddick et al., 1986; Tilburn et al., 1995; Mingot et al., 1999) with the following exceptions: pacCc2020507 and pacC2020510 were selected after UV mutagenesis of a strain of genotype pabaA1 pacC+/–20205 as allowing growth on pH 8 medium. pacCc232 and -234 were selected after 4-nitroquinoline-N-oxide mutagenesis of a strain of genotype pabaA1 yA2 palI30 pantoC3 as allowing growth in the presence of 18 mM molybdate. pacC20042 was obtained spontaneously in a strain of genotype biA1 pabaA1 areAr49 pacCc200 chaA1 palB7 as allowing utilization of 10 mM γ-aminobutyrate as nitrogen source.
Plasmids and fusion proteins
Constructs encoding PacC fusion proteins to GAL4dbd and GAL4ad were derived from pGBT9 and pGAD424 (obtained from Clontech), respectively, by inserting appropriate restriction or PCR-amplified DNA fragments in the polylinker site. Additional residues at the fusion borders and C–termini encoded by the fusion proteins are shown in Table I. GST::PacC fusion proteins (Table I), encoded by pGEX-2T-derived plasmids, were synthesized and purified from lysates of isopropyl-β-d-thiogalactopyranoside-induced bacteria essentially as described in Tilburn et al. (1995). N–terminally His-tagged (as shown in Table I) PacC proteins were expressed under the control of a T7 polymerase-dependent promoter using pET19b (Novagen)-derived expression plasmids pDT5 or pD1.
Table I. Constructs encoding PacC fusion proteins.
| Construct | Relevant PacC residues |
|---|---|
| GAL4dbd::PacC(169–265) | -PEFPGINSP-PacC(169–265)-GDRSVDLQPS |
| GAL4dbd::PacC(196–265) | -PEFP-PacC(196–265)-GDRSVDLQPS |
| GAL4dbd::PacC(196–245) | -PEF-PacC(196–245)-GSVDLQPS |
| GAL4dbd::PacC(209–265) | -PEF-PacC(209–265)-GSVDLQPS |
| GAL4dbd::PacC(196–255) | -PEF-PacC(196–255)-GSVDLQPS |
| GAL4dbd::PacC(222–265) | -PEF-PacC(222–265)-GSVDLQPS |
| GAL4dbd::PacC(169–678) | -PEFPA-PacC(169–678) |
| GAL4dbd::PacC(169–678)L340S | -PEFPA-PacC(169–678)L340S |
| GAL4dbd::PacC(169–627) | -PEFPA-PacC(169–627)-DPSTCSQANSGRISYDL |
| GAL4dbd::PacC(169–553) | -PEFPA-PacC(169–553)-DPSTCSQANSGRISYDL |
| GAL4dbd::PacC(169–553)L340S | -PEFPA-PacC(169–553)L340S-DPSTCSQANSGRISYDL |
| GAL4dbd::PacC(169–410) | -PEFPA-PacC(169–410)-GIRRPAAKLIPGEFLMIYDFYY |
| GAL4dbd::PacC(169–410)L340S | -PEFPA-PacC(169–410)L340S-GIRRPAAKLIPGEFLMIYDFYY |
| GAL4dbd::PacC(169–348) | -PEFPA-PacC(169–348)-PS |
| GAL4dbd::PacC(169–348)L340S | -PEFPA-PacC(169–348)L340S-PS |
| GAL4dbd::PacC(169–301) | -PEFPA-PacC(169–301)-GIRRPAAKLIPGEFLMIYDFYY |
| GAL4dbd::PacC(169–255) | -PEFPGINSP-PacC(169–255)-GSVDLQPS |
| GAL4dbd::PacC(265–678) | -PEFELGTR-PacC(265–678) |
| GAL4dbd::PacC(265–627) | -PEFELGTR-PacC(265–627)-DPSTCSQANSGRISYDL |
| GAL4dbd::PacC(265–553) | -PEFELGTR-PacC(265–553)-DPSTCSQANSGRISYDL |
| GAL4dbd::PacC(265–502) | -PEFELGTR-PacC(265–502)-IRRPAAKLIPGEFLMIYDFYY |
| GAL4dbd::PacC(265–410) | -PEFELGTR-PacC(265–410)-GIRRPAAKLIPGEFLMIYDFYY |
| GAL4dbd::PacC(265–348) | -PEFELGTR-PacC(265–348)-PS |
| GAL4dbd::PacC(301–678) | -PEFPGIPHA-PacC(301–678) |
| GAL4dbd::PacC(301–553) | -PEFPGIPHA-PacC(301–553)-DPSTCSQANSGRISYDL |
| GAL4dbd::PacC(427–553) | -PEFP-PacC(427–553)-DPSTCSQANSGRISYDL |
| GAL4dbd::PacC(529–678) | -PEFP-PacC(529–678) |
| GAL4dbd::PacC(265–678)L340S | -PEFELGTR-PacC(265–678)L340S |
| GAL4dbd::PacC(265–410)L340S | -PEFELGTR-PacC(265–410)L340S-GIRRPAAKLIPGEFLMIYDFYY |
| GAL4dbd::PacC(347–502) | -PEFPIPWNQ-PacC(347–502)-IRRPAAKLIPGEFLMIYDFYY |
| GAL4ad::PacC(529–678) | -IEFP-PacC(529–678) |
| GST::PacC(301–678) | -PA-PacC(301–678) |
| GST::PacC(301–678)L340S | -PA-PacC(301–678)L340S |
| GST::PacC(301–529) | -PA-PacC(301–529)-NSS |
| GST::PacC(410–678) | -PacC(410–678) |
| GST::PacC(169–410) | -PacC(169–410)-GFIVTD |
| GST::PacC(529–678) | -P-PacC(529–678) |
| GST::PacC(529–678)R579G | -P-PacC(529–678)R579G |
| GST::PacC(529–678)R579T | -P-PacC(529–678)R579T |
| GST::PacC(534–678) | -PacC(534–678) |
| GST::PacC(534–678)R573W | -PacC(534–678)R573W |
| pDT5-His::PacC(169–410) | -MGHHHHHHHHHHSSGHIDDDDKHMEF-PacC(169–410)-GILTD |
| pDT5-His::PacC(169–410)L340S | -MGHHHHHHHHHHSSGHIDDDDKHMEF-PacC(169–410)L340S-GILTD |
| pD1-His::PacC(5–678) | -MGHHHHHHHHHHSSGHIDDDDKHMGS-PacC(5–678) |
| pD1- His::PacC(5–678)L340S | -MGHHHHHHHHHHSSGHIDDDDKHMGS-PacC(5–678)L340S |
| pD1-His::PacC(5–529) | -MGHHHHHHHHHHSSGHIDDDDKHMGS-PacC(5–529)-GKSD |
Yeast techniques
HF7c [Clontech Matchmaker kit, MATa, ura3-52, his3-200, lys2-801, ade2-101, trp1-901, leu2-3, 112, gal4-542, gal80-538, LYS2::GAL1-HIS3, URA3::(GAL4 17-mers)3-CYC1-lacZ] was used as recipient for transformation. For β–galactosidase assays, clones for each relevant construct were grown in appropriately supplemented synthetic-dextrose minimal medium until cultures reached mid-logarithmic phase. Cells were used to determine β–galactosidase activity with o-nitrophenyl-β-d–galactopyranoside as substrate following the protocol of the Clontech Matchmaker kit. Activities are given in Miller units (OD420 units × 103/min × ml × OD600 units).
In vitro synthesis of PacC polypeptides
PacC mutant and wild-type proteins and peptides were synthesized in vitro in the presence of [35S]methionine (1000 Ci/mmol) using the Promega TNT coupled transcription–translation system and 0.5–1 μg of the appropriate plasmid as template.
Aspergillus nidulans S-100 extract preparation
The A.nidulans S-100 extract was prepared from mycelia of a biA1 pabaAl argB2 ΔpacC::pyr4+ strain (Tilburn et al., 1995) after growth for 24 h at 37°C in neutral PPB of a 2 × 106 conidiospores/ml inoculum. Cells were lysed with a Braun MSK cell disintegrator and the resulting lysates were centrifuged at 100 000 g for 1 h at 4°C.
In vitro proteolysis assays
One-twenty-fifth of the TNT reaction mixture was incubated in a 50 μl volume with or without 25 μg of S-100 protein extract in 25 mM HEPES pH 7.5, 50 mM KCl and 10% (v/v) glycerol with 1 mM ATP and 3.5 mM MgCl2. Samples were incubated for 30 min at 30°C, trichloroacetic acid precipitated and resolved by 10% SDS–PAGE. Gels were dried and exposed to Kodak Biomax-MS film with a Biomax Transcreen LE.
Co-immunoprecipitation experiments
One microgram of either GST or GST::PacC(529–678) was incubated with 4 μl of in vitro synthesized 35S-labelled wild-type or L340S PacC(169–410) for 10 min at 0°C before the addition of 2 μl of anti-GST::PacC(529–678) rabbit antiserum (Orejas et al., 1995) and incubation for a further 10 min at 0°C. Then, 20 μl of protein A–Sepharose beads in 25 mM HEPES pH 7.5, 50 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, 2 mM dithiothreitol (DTT) and 50 mM NaCl (washing buffer) were added. Beads were washed with the same buffer, recovered in a pipette tip, resuspended and boiled in loading buffer before analysing appropriate aliquots by 10% SDS–PAGE and autoradiography of the dried gel.
DNA binding reactions and gel mobility supershift assays
Protein extraction for DNA binding reactions and EMSAs was as described (Orejas et al., 1995), using 4 or 8% (w/v) polyacrylamide gels as indicated. The probe was a 32P-labelled 31mer oligonucleotide containing the ipnA2 PacC binding site (Tilburn et al., 1995). For supershift analysis, 5 μg of protein extract and an excess (1–2 μg) of GST::PacC fusion protein were pre-incubated for 30 min at 0°C before addition of the probe and competitor poly(dI–dC):(dI–dC), followed by incubation for a further 30 min at 0°C. When the effects of anti-GST or anti-GST::PacC(529–678) antibodies in the supershift assay were determined, the corresponding antiserum (2 μl) or an equal volume of buffer was pre-incubated with 0.25 μg of GST::PacC(401–678) before its addition to the supershift mixture.
Affinity chromatography
Glutathione–Sepharose (Pharmacia) columns (0.3 ml) were loaded with extracts from Escherichia coli cells expressing either GST or GST::PacC(529–678) to give 1 mg protein/ml packed beads. After washing with several volumes of buffer BC-50 (20 mM HEPES pH 7.5, 20% glycerol, 1 mM DTT, 1 mM EDTA, 50 mM NaCl), 3.8 mg of a pacCc75 strain extract were loaded onto each column. After extensive washing in the same buffer, proteins retained in the columns were eluted with 10 mM glutathione. Samples of the input, the flow through and the Sepharose beads before glutathione elution were resolved by 10% SDS–PAGE and analysed by Western blotting using a rat anti-PacC(5–265) antiserum, as described in Mingot et al. (1999).
Acknowledgments
Acknowledgements
We thank E.Reoyo for technical assistance and the EU, CICYT and BBSRC (through grants BIO4-CT96-9535, BIO97-0348 and 60/PO5893 plus 60/PO11494, respectively) for support. E.A.E. held an EMBO long–term fellowship and an MEC postdoctoral contract. T.R. and E.D. held postdoctoral and predoctoral fellowships, respectively, of the Basque Government. J.A. held a PFPI-MEC fellowship and L.R. held a BBSRC studentship.
References
- Brown M.S. and Goldstein, J.L. (1999) A proteolytic pathway that controls the cholesterol content of membranes, cells and blood. Proc. Natl Acad. Sci. USA, 96, 11041–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddick M.X., Brownlee, A.G. and Arst, H.N.,Jr (1986) Regulation of gene expression by pH of the growth medium in Aspergillus nidulans. Mol. Gen. Genet., 203, 346–353. [DOI] [PubMed] [Google Scholar]
- Clutterbuck A.J. (1993) Aspergillus nidulans. In O'Brien,S.J. (ed.), Genetic Maps. Locus Maps of Complex Genomes. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 3.71–3.84. [Google Scholar]
- Cove D.J. (1966) The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta, 113, 51–56. [DOI] [PubMed] [Google Scholar]
- De Bernardis F., Muhlschlegel, F.A., Cassone, A. and Fonzi, W.A. (1998) The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect. Immun., 66, 3317–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison S.H., Orejas, M. and Arst, H.N.,Jr (1995) Signalling of ambient pH in Aspergillus involves a cysteine protease. J. Biol. Chem., 270, 28519–28522. [DOI] [PubMed] [Google Scholar]
- Denison S.H., Negrete-Urtasun, S., Mingot, J.M., Tilburn, J., Mayer, W.A., Goel, A., Espeso, E.A., Peñalva, M.A. and Arst, H.N.,Jr (1998) Putative membrane components of signal transduction pathways for ambient pH regulation in Aspergillus and meiosis in Saccharomyces are homologous. Mol. Microbiol., 30, 259–264. [DOI] [PubMed] [Google Scholar]
- Espeso E.A. and Peñalva, M.A. (1996) Three binding sites for the Aspergillus nidulans PacC zinc-finger transcription factor are necessary and sufficient for regulation by ambient pH of the isopenicillin N synthase gene promoter. J Biol. Chem., 271, 28825–28830. [DOI] [PubMed] [Google Scholar]
- Espeso E.A., Tilburn, J., Arst, H.N.,Jr and Peñalva, M.A. (1993) pH regulation is a major determinant in expression of a fungal penicillin biosynthetic gene. EMBO J., 12, 3947–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeso E.A., Tilburn, J., Sánchez-Pulido, L., Brown, C.V., Valencia, A., Arst, H.N.,Jr and Peñalva, M.A. (1997) Specific DNA recognition by the Aspergillus nidulans three zinc finger transcription factor PacC. J. Mol. Biol., 274, 466–480. [DOI] [PubMed] [Google Scholar]
- Ghosh S., May, M.J. and Kopp, E.B. (1998) NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol., 16, 225–260. [DOI] [PubMed] [Google Scholar]
- Hutchings H., Stahmann, K., Roels, S., Espeso, E.A., Timberlake, W.E., Arst, H.N.,Jr and Tilburn, J. (1999) The multiply-regulated gabA gene encoding the GABA permease of Aspergillus nidulans: a score of exons. Mol. Microbiol., 32, 557–568. [DOI] [PubMed] [Google Scholar]
- Ingham P.W. (1998) Transducing Hedgehog: the story so far. EMBO J., 17, 3505–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller N.P., Nesbitt, C., Sarr, B., Phillips, T.D. and Burow, G.B. (1997) pH regulation of sterigmatocystin and aflatoxin biosynthesis in Aspergillus spp. Phytopathology, 87, 643–648. [DOI] [PubMed] [Google Scholar]
- Lambert M., Blanchin-Roland, S., Le Louedec, F., Lépingle, A. and Gaillardin, C. (1997) Genetic analysis of regulatory mutants affecting synthesis of extracellular proteinases in the yeast Yarrowia lipolytica: identification of a RIM101/pacC homolog. Mol. Cell. Biol., 17, 3966–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. and Mitchell, A.P. (1997) Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics, 145, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCabe A.P., Van den Hombergh, J.P.T.W., Tilburn, J., Arst, H.N.,Jr and Visser, J. (1996) Identification, cloning and analysis of the Aspergillus niger gene pacC, a wide domain regulatory gene responsive to ambient pH. Mol. Gen. Genet., 250, 367–374. [DOI] [PubMed] [Google Scholar]
- Maccheroni W., May, G.S., Martínez-Rossi, N.M. and Rossi, A. (1997) The sequence of palF, an environmental pH response gene in Aspergillus nidulans. Gene, 194, 163–167. [DOI] [PubMed] [Google Scholar]
- Mingot J.M., et al. (1999)Specificity determinants of proteolytic processing of Aspergillus PacC transcription factor are remote from the processing site and processing occurs in yeast if pH signalling is bypassed. Mol. Cell. Biol., 19, 1390–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlschlegel F.A. and Fonzi, W.A. (1997) PHR2 of Candida albicans encodes a functional homolog of the pH-regulated gene PHR1 with an inverted pattern of pH-dependent expression. Mol. Cell. Biol., 17, 5960–5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete-Urtasun S., Denison, S.N., Arst and H.N.,Jr (1997) Characterization of the pH signal transduction pathway gene palA of Aspergillus nidulans and identification of possible homologs. J. Bacteriol., 179, 1832–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete-Urtasun S., Reiter, W., Díez, E., Denison, S.H., Tilburn, J., Espeso, E.A., Peñalva, M.A., Arst and H.N.,Jr (1999) Ambient pH signal transduction in Aspergillus: completion of gene characterization. Mol. Microbiol., 33, 994–1003. [DOI] [PubMed] [Google Scholar]
- Orejas M., Espeso, E.A., Tilburn, J., Sarkar, S., Arst, H.N.,Jr and Peñalva, M.A. (1995) Activation of the Aspergillus PacC transcription factor in response to alkaline ambient pH requires proteolysis of the carboxy–terminal moiety. Genes Dev., 9, 1622–1632. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. (1997) Catching a Gli-mpse of Hedgehog. Cell, 90, 193–196. [DOI] [PubMed] [Google Scholar]
- Saporito-Irwin S.M., Birse, C.E., Sypherd, P.S. and Fonzi, W.A. (1995) PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol. Cell. Biol., 15, 601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez T. and Peñalva, M.A. (1996) Characterization of a Penicillium chrysogenum gene encoding a PacC transcription factor and its binding sites in the divergent pcbAB-pcbC promoter of the penicillin biosynthetic cluster. Mol. Microbiol., 20, 529–540. [DOI] [PubMed] [Google Scholar]
- Thanos D. and Maniatis, T. (1995) NF-κB: a lesson of family values. Cell, 24, 529–532. [DOI] [PubMed] [Google Scholar]
- Tilburn J., Sarkar, S., Widdick, D.A., Espeso, E.A., Orejas, M., Mungroo, J., Peñalva, M.A. and Arst, H.N.,Jr (1995) The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J., 14, 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.B., Davis, D. and Mitchell, A.P. (1999) Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol., 181, 1868–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]