We recently reported that intestinal concentrations of soluble stimulants of Toll-like receptor (TLR)-2 and TLR4 are markedly elevated in two murine models of inflammatory bowel disease (IBD); dextran-sodium-sulphate (DSS)-induced colitis and Toxoplasma gondii-induced ileitis. It was also shown that while enterobacterial species, such as Escherichia coli, released abundant soluble pro-inflammatory stimulants of macrophage TNFα secretion and TLR2-signalling into their growth environment, the release of such stimulants by Gram-positive organisms, including Bifidobacterium bifidum, Lactobacillus plantarum and Enterococcus fecalis, was approximately three orders of magnitude lower, leading to the proposal that Gram-positive commensals are unlikely to be major contributors of soluble pro-inflammatory TLR-stimulants during IBDs. In this addendum to the previous study, additional data are presented to address the question of whether elevated soluble TLR2-stimulants may derive from the action of intestinal lysozyme on the host micribiota via increased release of bacterial lipopeptides. It is shown that while lysozyme treatment of Gram-positive organisms, including Lactobacillus plantarum and Enterococcus fecalis, promotes the release of TLR2-stimulants from these organisms, lysozyme did not promote the release of soluble pro-inflammatory stimulants from two model intestinal Gram-negative organisms (Escherichia coli and Bacteroides fragilis), or from human fecal samples. The increase in TLR2-stimulants in murine colitis and ileitis therefore most likely reflects the overgrowth of enterobacterial species, rather than the action of lysozyme on Gram-positive bacteria.
Sequestration of TLR2-stimulants by the Gram-Positive Cell Wall is Reversed by Lysozyme
It is now widely accepted that inappropriate inflammatory responses directed against the host commensal microbiota play a key role in the initiation and propagation of IBDs.1 As the TLRs represent a major family of innate immune receptors involved in the induction of inflammation, it has been proposed that, depending on the nature of the TLR-ligand and the cell-types involved, the stimulation of TLR-signalling in the intestine may contribute to the development of IBD.2–4 Notably, the severity of colitis and markers of intestinal inflammation were shown to be reduced in mice deficient in TLR2 or TLR4 compared to wild-type animals in at least one model of DSS-colitis, suggesting that luminal stimulants of TLR2 or TLR4 could contribute to IBD pathology.5 The observation that intestinal concentrations of soluble TLR2- and TLR4-stimulants are up to ∼3,000-fold higher in murine IBDs therefore prompted two further questions. These were: (1) what is responsible for the increase in TLR-stimulants in the diseased gut and (2) could increased luminal concentrations of TLR-stimulants promote IBD pathology?
Our previous results suggested that the increase in luminal TLR4-stimulants is due to the overgrowth of enterobacterial species, which is a feature of both colitis and ileitis in mice,5,6 since the biological activity of intestinal TLR4-stimulants was blocked by the LPS-sequestering molecule polymyxin-B, and the LPS of various Bacteroides species did not stimulate TLR4-dependent signalling.7 Enterobacterial overgrowth was also considered to be the likely cause of the increase in TLR2-stimulants, since enterobacterial species released approximately 1,000-fold more TLR2-stimulants into their growth environment than did a selection of Gram-positive intestinal organisms or Bacteroides spp.7
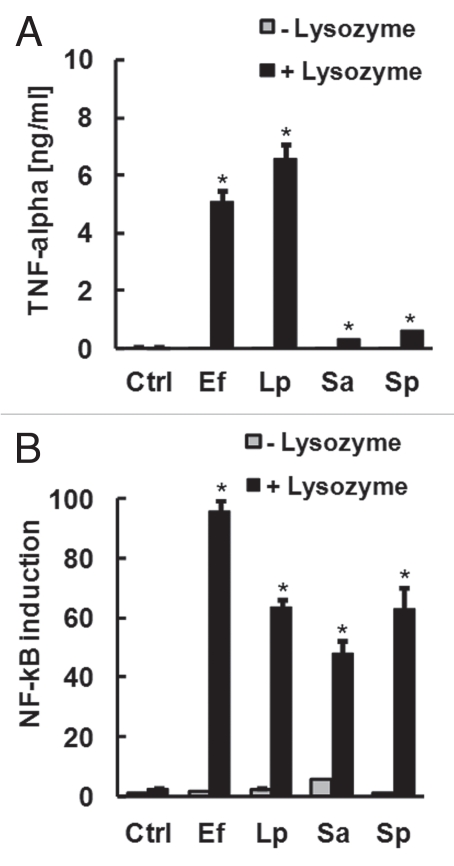
However, since several studies have shown that luminal concentrations of the peptidoglycan-degrading enzyme lysozyme can be up to 50-fold higher in subjects with Crohn disease or ulcerative colitis compared to healthy subjects,8–10 the question of whether elevated lysozyme could contribute to the release of TLR2 stimulants from otherwise non-inflammatory Gram-positive commensals remained to be answered. To address this, a panel of Gram-positive organisms were heat-killed, resuspended in PBS and treated with or without hen-egg lysozyme for 2 h. The filtered supernatant of each culture was then analyzed for capacity to stimulate TLR2-signalling or cytokine production in macrophages. Lysozyme treatment of Gram-positive bacteria significantly increased the capacity of conditioned medium to stimulate macrophage TNFα secretion (Fig. 1A), and TLR2-dependent signalling in transfected HEK-293 cells (Fig. 1B). Taken together, these results suggest that the thick peptidoglycan layer of the Gram-positive cell wall may play a key role in the observed sequestration of pro-inflammatory lipopeptides from detection via the growth medium.
Figure 1.
Lysozyme promotes the release of soluble TLR2 stimulants from Gram-positive bacteria. Gram-positive organisms (Ef, E. fecalis; Lp, L. plantarum; Sa, S. aureus; Sp, S. pyogenes) were heat-killed, resuspended in PBS at 109 bacteria/ml and treated with or without 1 mg/ml lysozyme for 2 h. Conditioned medium was then filter-sterilized, diluted 1:100 in tissue culture medium and applied to RAW macrophages for the measurement of secreted TNFα (A) or HEK-293 cells transfected with TLR2 and NFκB reporter for the measurement of TLR2-dependent NFκB signalling (B). *p < 0.05 vs. cells cultured in medium alone (Ctrl), ANOVA with Dunnet's test.
Effect of Lysozyme on Release of TLR-Stimulants by Gram-Negative Bacteria and Human Fecal Samples
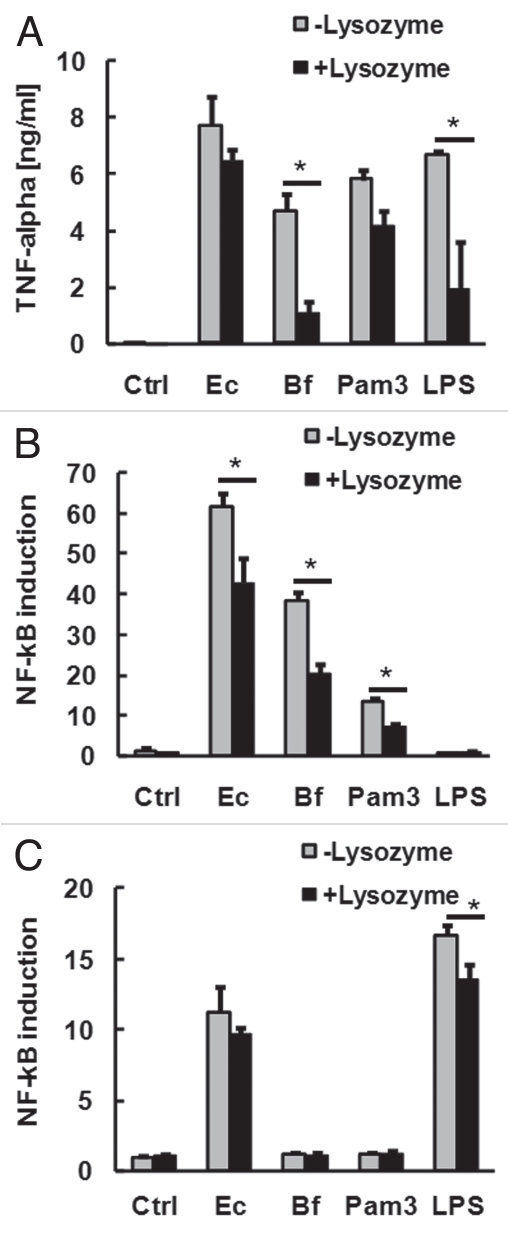
We next aimed to establish if lysozyme also promoted the release of soluble pro-inflammatory stimulants from the Gram-negative organisms E. coli and B. fragilis. Surprisingly, lysozyme tended to reduce the capacity of supernatant of these organisms to stimulate macrophage TNFα production (Fig. 2A). This was accompanied by a reduction in the biological activity of TLR2-stimulants in the supernatant of these organisms (Fig. 2B). Pre-treatment with lysozyme also significantly reduced the capacity of LPS or the synthetic bacterial lipopeptide Pam3CSK4 to stimulate TLR2- or TLR4-signalling (Fig. 2B and C). This was not due to non-specific inhibition of TLR-signalling or NFκB activation, as concurrent treatment of cells with lysozyme and Pam3CSK4 or LPS without pre-incubation did not result in reduced TNFα production or NFκB activation (data not shown). Overnight incubation with lysozyme also reduced further the capacity of extracts to stimulate TLR-signalling compared to treatment for 2 h.
Figure 2.
Effect of lysozyme on release of soluble TLR-stimulants from Gram-negative bacteria. E. coli (109/ml), B. fragilis (109/ml), Pam3CSK4 (1 µg/ml) and LPS (1 µg/ml) were resuspended in PBS with or without 1 mg/ml lysozyme for 2 h at 37°C. Filtered supernatants were then diluted 1:100 in tissue culture medium and applied to RAW macrophages for the measurement of secreted TNFα (A) or HEK-293 cells transfected with TLR2 (B) or TLR4/MD2 (C) for the measurement of TLR-dependent NFκB signalling. *p < 0.05 (ANOVA with Tukey's test).
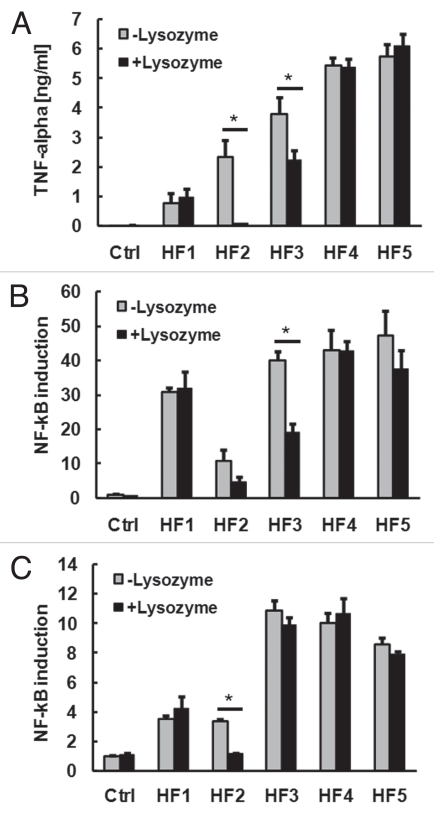
As lysozyme appeared to exert both pro- and anti-inflammatory effects on Gram-positive and Gram-negative bacterial suspensions, respectively, we next aimed to determine if lysozyme may modulate the capacity of the normal human fecal microbiota to release soluble pro-inflammatory TLR-stimulants. Lysozyme treatment of 5 human fecal samples for 2 h had little impact on soluble TLR2 and TLR4 stimulants in 3 of the samples, while two of the samples showed a significant reduction in soluble TLR2- or TLR4-stimulant activity, which was accompanied by a reduced capacity to promote macrophage TNFα production (Fig. 3).
Figure 3.
Effect of lysozyme on release of soluble TLR-stimulants from human fecal samples. Human fecal (HF) samples of 5 healthy subjects were resuspended in PBS (1:50 wt/vol) and treated with or without 1 mg/ml lysozyme for 2 h at 37°C. Filtered supernatants were then diluted 1:10 in tissue culture medium and applied to RAW macrophages for the measurement of TNFα production (A) or HEK-293 cells transfected with TLR2 (B) or TLR4 (C) for the measurement of TLR-dependent NFκB signalling. *p < 0.05 (ANOVA with Tukey's test).
Is Lysozyme Relevant to IBD Pathology?
The present findings suggest that lysozyme may play both pro- and anti-inflammatory roles in the context of the host microbiota and their products. Specifically, although lysozyme enhances the potential of Gram-positive bacteria to release TLR2-stimulants, other functions of lysozyme appear to reduce the biological activity of soluble lipopeptides and LPS once they are released. Although to our knowledge the capacity of lysozyme to reduce the activity of bacterial lipopeptides has not been reported previously, these findings are consistent with earlier reports that lysozyme binds to and reduces the biological activity of LPS in vitro and in vivo.11,12 Further studies will be required to establish how lysozyme reduces the biological activity of lipopeptides.
These preliminary experiments also suggest that lysozyme does not necessarily increase the pro-inflammatory potential of the normal human fecal microbiota, but rather can in some instances reduce the capacity of such extracts to stimulate TLR-signalling or macrophage cytokine production. This suggests that under certain circumstances, intestinal lysozyme could exert an overall anti-inflammatory effect, as supported by the observation that oral supplementation with lysozyme reduces the inflammation and tissue damage associated with DSS-induced colitis in pigs.13
Are Soluble TLR-stimulant Concentrations Mediators or Markers of Murine IBD?
As the TLRs play key roles in the detection of bacterial products and the induction of inflammatory signalling, the roles played by TLRs in IBD have become the subject of intensive research. To date, the results of these studies have been largely conflicting, and have therefore become the subject of much debate. In essence, the point of discussion is that although in some models of murine IBD, TLR-signalling appears to play a key role in the maintenance of gut barrier integrity and protection against the development of IBDs,14–16 in other models, TLR-signalling, particularly via TLR4, was shown to exacerbate existing IBD by promoting inflammation.2–6,17
Only recently have new data emerged to provide a tenable explanation for the conflicting results observed in earlier studies of TLR function in IBD. These results suggest that while TLR-signalling in intestinal epithelial cells promotes the enhancement of barrier function and therefore protects against disease,14–16,18–20 TLR-signalling in cells of haematopoietic origin, particularly macrophages, may promote disease activity by responding to TLR-ligands with a proinflammatory response.2,4,6,21,22 These differences in the ways that certain cell-types respond to PAMPs are evident in the demonstration that TLR-signalling in intestinal epithelial cells promotes the upregulation of barrier-enhancing tight junction proteins and antimicrobial peptides, rather than overt inflammatory responses.14–16,18–20 By contrast, the stimulation of TLR-signalling in macrophages and other PAMP-sensitive cell types that reside beneath the intestinal epithelial layer can lead to the expression of pro-inflammatory cytokines, chemokines and other mediators that ultimately exacerbate IBD.2,4,6,21,22
These opposing and cell-type specific contributions of TLR-signalling to gut health are perhaps best exemplified by two very recent studies. Gong et al. showed that epithelial cell-specific inhibition of TLR-signalling via blockade of MyD88 results in chronic inflammation of the small intestine due to increased penetration of the microflora into the mucosa, where myeloid cells may then be exposed to bacterial products resulting in inflammation.23 Taking the opposite approach, Asquith et al. showed that MyD88-dependent activation of myeloid cells, but not epithelial cells, was required for the development of chronic intestinal inflammation.24 The emerging evidence therefore supports the notion that while TLR-signalling in epithelial cells is protective against IBD, TLR-signalling in myeloid cells can promote disease progression.
These recent findings therefore add complexity to the question of whether or not elevated luminal concentrations of TLR-stimulants could contribute to the development of IBDs. Current evidence suggests that elevated concentrations of PAMPs in the intestine are not in themselves sufficient to initiate inflammatory disease, as the healthy, intact colon is largely unresponsive to exogenously applied flagellin, LPS or lipoteichoic acid,21,22,25,26 and we showed that mice with high PAMP concentrations in the ileum did not develop inflammation in the colon.7 However, when the epithelial barrier is disrupted by agents such as DSS or infection, it has been shown that experimental administration of diverse PAMPs to the gut lumen can trigger inflammation2,4,6,21,22 and increase severity of disease.2–4 Taken together, these findings suggest that elevated concentrations of luminal TLR-stimulants could contribute to the inflammatory processes of IBDs if existing disease has caused damage to the epithelial layer.
The next question that requires to be addressed is whether or not intestinal concentrations of TLR2, TLR4 and TLR5 stimulants may be altered in human IBDs, such as Crohn disease and ulcerative colitis. If it turns out that intestinal TLR-stimulants are elevated in human disease, further studies may be warranted to investigate the therapeutic potential of inhibiting TLR-signalling for the remission of IBD. However, the emerging duality of TLR-function in the gut suggests that such approaches would have to balance carefully the requirement for epithelial stimulation by luminal PAMPs to maintain barrier integrity with the desired result of dampening mucosal inflammation.
Acknowledgements
These studies were supported by a University of Leicester Department of Cardiovascular Sciences Research Fellowship.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/13726
References
- 1.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heimesaat MM, Fischer A, Jahn HK, Niebergall J, Freudenberg M, Blaut M, et al. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007;56:941–948. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotta T, Yoshida N, Yoshikawa T, Sugino S, Kondo M. Lipopolysaccharide-induced colitis in rabbits. Res Exp Med (Berl) 1986;186:61–69. doi: 10.1007/BF01851834. [DOI] [PubMed] [Google Scholar]
- 4.Obermeier F, Dunger N, Deml L, Herfarth H, Schölmerich J, Falk W. CpG motifs of bacterial DNA exacerbate colitis of dextran sulfate sodium-treated mice. Eur J Immunol. 2002;32:2084–2092. doi: 10.1002/1521-4141(200207)32:7<2084::AID-IMMU2084>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 5.Heimesaat MM, Fischer A, Siegmund B, Kupz A, Niebergall J, Fuchs D, et al. Shift towards proinflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS One. 2007;2:662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 7.Erridge C, Duncan SH, Bereswill S, Heimesaat MM. The induction of colitis and ileitis in mice is associated with marked increases in intestinal concentrations of stimulants of TLRs 2, 4 and 5. PLoS One. 2010;5:9125. doi: 10.1371/journal.pone.0009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klass HJ, Neale G. Serum and fecal lysozyme in inflammatory bowel disease. Gut. 1978;19:233–239. doi: 10.1136/gut.19.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Sluys Veer A, Brouwer J, Biemond I, Bohbouth GE, Verspaget HW, Lamers CB. Fecal lysozyme in assessment of disease activity in inflammatory bowel disease. Dig Dis Sci. 1998;43:590–595. doi: 10.1023/a:1018823426917. [DOI] [PubMed] [Google Scholar]
- 10.Hemrika MH, Costongs GM, Engels LG, Bos LP, Janson PC, Flendrig JA. Clinical relevance of lysozyme in the faeces. Neth J Med. 1989;34:174–181. [PubMed] [Google Scholar]
- 11.Takada K, Ohno N, Yadomae T. Detoxification of lipopolysaccharide (LPS) by egg white lysozyme. FEMS Immunol Med Microbiol. 1994;9:255–263. doi: 10.1111/j.1574-695X.1994.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 12.Takada K, Ohno N, Yadomae T. Lysozyme regulates LPS-induced interleukin-6 release in mice. Circ Shock. 1994;44:169–174. [PubMed] [Google Scholar]
- 13.Lee M, Kovacs-Nolan J, Yang C, Archbold T, Fan MZ, Mine Y. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Agric Food Chem. 2009;57:2233–2240. doi: 10.1021/jf803133b. [DOI] [PubMed] [Google Scholar]
- 14.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Araki A, Kanai T, Ishikura T, Makita S, Uraushihara K, Iiyama R, et al. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 16.Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijay-Kumar M, Wu H, Jones R, Grant G, Babbin B, King TP, et al. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am J Pathol. 2006;169:1686–1700. doi: 10.2353/ajpath.2006.060345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee SH, Im E, Riegler M, Kokkotou E, O'Brien M, Pothoulakis C. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA. 2005;102:13610–13615. doi: 10.1073/pnas.0502174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dionne S, Laberge S, Deslandres C, Seidman EG. Modulation of cytokine release from colonic explants by bacterial antigens in inflammatory bowel disease. Clin Exp Immunol. 2003;133:108–114. doi: 10.1046/j.1365-2249.2003.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong J, Xu J, Zhu W, Gao X, Li N, Li J. Epithelialspecific blockade of MyD88-dependent pathway causes spontaneous small intestinal inflammation. Clin Immunol. 2010;136:245–256. doi: 10.1016/j.clim.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Asquith MJ, Boulard O, Powrie F, Maloy KJ. Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology. 2010;139:519–529. doi: 10.1053/j.gastro.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders CJ, Yu Y, Moore DA, 3rd, Williams IR, Gewirtz AT. Humoral immune response to flagellin requires T cells and activation of innate immunity. J Immunol. 2006;177:2810–2818. doi: 10.4049/jimmunol.177.5.2810. [DOI] [PubMed] [Google Scholar]
- 26.Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, Michelsen KS, et al. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406–1415. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]