Abstract
One of the major virulence factors of Helicobacter pylori is the vacuolating toxin vaca. It has been known for a long time that the toxin enters host cells by endocytosis. On the other hand there is ample evidence that vaca is able to trigger apoptosis and this effect has been attributed in part to interactions with mitochondria. However, for 10 years it was difficult to reconcile the obvious accumulation of vaca in endosomes with mitochondrial targeting. The accessibility of the mitochondria to the toxin was enigmatic. In our new study, we investigated the activities of p34, the toxic subunit of vaca, in more detail. We found that the p34 N-terminus carries a unique targeting sequence for import into mitochondria and for insertion into the mitochondrial inner membrane. By forming an anion channel in this membrane, the toxin has the ability to interfere directly with mitochondrial functions. Taking into account additional results from independent studies, we discuss the implications of our findings with respect to intracellular traffic, the remarkable possibility of a direct transfer of VacA from endosomes to mitochondria and vaca-dependent cell death.
Key words: Helicobacter pylori, VacA, endosome, mitochondria, protein targeting, protein transfer, protein import, Bax, Bak, apoptosis
Several important virulence factors of H. pylori were already identified many years ago.1 Most prominent are the urease of the bacteria and the toxins CagA and VacA: the urease helps the bacteria in keeping the environment at a neutral pH.2 CagA is a protein which is injected into host cells by a type IV secretion system and interferes with signalling pathways.3 The vacuolating toxin VacA is released by the bacteria using a C-terminal autotransporter. VacA binds to the outer surface of host cells and is internalized by endocytosis.4,5 Inside the cells, VacA can target mitochondria and at least in some cases, it triggers the release of cytochrome c and apoptosis.6,7 Until recently, it was completely unclear how and why VacA produces these effects.
p34 is the Toxic Subunit of VacA
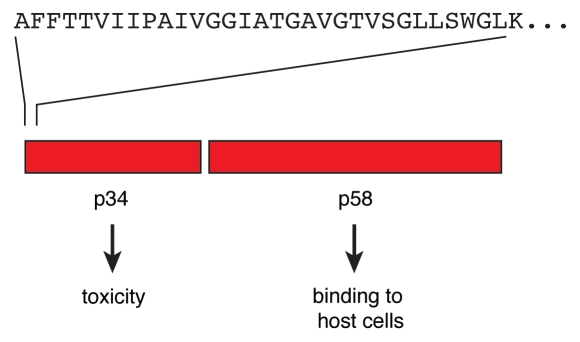
The VacA toxin is secreted by the bacteria as a single polypeptide of about 88 kDa. The polypeptide chain is easily cleaved at a site of a protease-sensitive loop to form a dimer of two subunits, p34 and p58, that remain non-covalently associated (Fig. 1).4,5,8 It is currently not clear to which degree the polypeptide is cleaved in vivo. Proteolytic processing is obviously not a prerequisite for toxicity. The p34 part is essential and sufficient to trigger cell death,6 while subunit p58 is essential for binding of the toxin to target cells.4,5 VacA is able to assemble in hexameric rosettes, p58 forming spikelike protrusions on the outside, p34 forming the internal core. Under appropriate conditions VacA can also form heptamers, dodecamers or tetradecames. In membranes, the complexes form anion channels of low conductivity, apparently with a preference for chloride ions.
Figure 1.
Subunits of VacA. The VacA toxin is initially secreted as a polypeptide of about 88 kDa. The toxin is easily cleaved to yield the two subunits p34 and p58. Both subunits stay connected by non-covalent interactions. Different values were reported for the precise size of the subunits. Reliable data obtained by mass spectrometry were published by Nguyen et al.8 The name VacA refers to the ability of the toxin to cause a formation of vacuoles in target cells.4,5 For entry into the cells, both the p58 part and a segment of p34 are required. Correspondingly, vacuolization is dependent on the p34 part and a portion of p58. Upon cytosolic expression, p34 alone is sufficient to trigger cell death.6 The upper part shows the N-terminal residues of p34.
In our study,9 we isolated p34 and found that the purified subunit was able to form both hexamers and anion channels. The electrophysiological characterization was carried out by Michael Meinecke together with Anke Harsman in the laboratory of Richard Wagner at the Institute for Biophysics of the University of Osnabrück. Reconstituted in a planar lipid bilayer, p34 showed a conductivity of about 12 pS. The values closely resembled the conductivity of the holotoxin as published in previous studies.10 To our knowledge, there is currently no evidence that p34 ever separates from the p58 subunit under in vivo conditions. However, it is remarkable that the p34 part alone is able to form the ion channel independently of the p58 subunit. In our experiments, the p34 hexamers showed a surprising stability, suggesting that p34 may act as an independent unit. The p34 complexes were retained even in the presence of 4 M urea. Oligomers of other proteins easily dissociate under such conditions.11
Oligomerization and channel formation of p34 were not only independent of subunit p58 but also independent of the hydrophobic N-terminal part of p34. This observation was particularly puzzling because the N-terminal 32 residues of p34 had previously been thought to form the ion-conducting channel.4,5,12 Is the function of the p34 N-terminus dependent on interactions with the p58 part of the toxin? Remarkably, data of previous studies have already indicated that the p34 N-terminus of intact VacA might not be essential for pore formation: a mutant VacA protein lacking residues 6–27 oligomerized properly and showed a conductivity similar to the wild-type protein.13 Strikingly, the current was detectable only after a significant delay when compared with the wildtype VacA.13 Similar effects were also described for the S2 subtype of the VacA toxin which is produced by some strains of H. pylori. The s2 subtype carries an additional peptide of 12 hydrophilic residues at the N-terminus. It forms membrane channels at a reduced rate as compared to the common s1 type, but the channels exhibit similar anion selectivities.14 If the p34 N-Terminus is not essential for channel formation, what then is the function of this part of the toxin?
The N-terminal Residues of p34 Act as a Mitochondrial Targeting Sequence
p34 is essentially a hydrophilic protein, however the N-terminal 32 residues are different. Many of these residues are hydrophobic, and they are devoid of charged side chains, the lysine in position 33 being the first charged residue of the sequence (Fig. 1). In our study, we found that the p34 N-terminus is essential in targeting of p34 to the mitochondria.9 This observation was surprising because a similar targeting structure has to date not been identified in any endogenous mitochondrial protein.15 The p34 N-terminus appears to be both essential and sufficient for mitochondrial targeting. The primary receptor structure for p34 at the mitochondrial outer surface seems to be Tom20, a component of the outer membrane TOM complex that is usually required for import of endogenous mitochondrial proteins.15 Tom40, the central component of the TOM complex, mediates the import of p34 into the mitochondria. Our data indicate that p34 eventually accumulates in the mitochondrial inner membrane. Considering that holo-VacA can also be imported into mitochondria,6 it is conceivable that the holotoxin may similarly translocate to the inner membrane. This conclusion was recently supported by an independent study using isolated mouse liver mitochondria and the 35S-labeled toxin synthesized in reticulocyte in vitro.16 Many experiments have thus confirmed the capability of VacA to enter mitochondria, but how is it possible for a toxin to target mitochondria if it is trapped in endosomes?
The Fate of VacA Inside Target Cells
What exactly happens to VacA after endocytosis? The name of the toxin refers to the observation that in cell culture, the uptake of VacA causes a massive formation of small vacuoles containing protein markers of early and late endosomes.4,5 Unfortunately, the relevance of this striking phenomenon is not clear. Similarly unclear is the subsequent fate of the toxin. Initially it was assumed that VacA may stay in the membranes, however some data suggested that VacA or parts of VacA, may interact with cytosolic partner proteins.17 We found that upon expression in the cytosol, holo-VacA or p34 fused to a GFP moiety were rapidly targeted to mitochondria while p58-GFP stayed in the cytosol.6 From these observations it was clear that both p34 and the holotoxin contained a mitochondrial targeting sequence. However, it was not clear if in vivo, in the infected tissue, an independent p34 is indeed released with an opportunity to target mitochondria. In fact, our data did not reveal if only p34 or whether the entire VacA toxin targets the mitochondria in vivo.
It is therefore noteworthy that essential features of the VacA holotoxin are retained in the purified p34 subunit:9 Both p34 and VacA show mitochondrial import upon expression in mammalian cells, both proteins carry the same mitochondrial targeting sequence (p34 corresponds to the N-terminal part of VacA), assemble in stable hexamers and form anion channels of similar conductivity. Eventually, both proteins—upon cytosolic expression—are able to trigger apoptosis. p34 is obviously the toxic subunit of VacA, irrespective of the presence or absence of p58.
VacA Transfer from Endosomes to Mitochondria
What then is the fate of the VacA toxin in the infected cells? What is the pathway of the toxin from the endosomal membranes to mitochondria? The diphtheria toxin is a famous example of an A/B-toxin that initially accumulates in endosomal membranes and subsequently releases its toxic A subunit into the cytosol.18 It is tempting to speculate that VacA may act in a similar manner. Alternatively, VacA or the toxic subunit p34, could be transferred from endosomal to mitochondrial membranes by direct contact. It is remarkable that currently there is a general tendency in cell biology, to acknowledge that there may be more relations between the different intracellular membrane systems than previously anticipated. It is well established that ER membranes can be directly connected to mitochondrial outer membranes through specific structures.19 It is therefore conceivable that membranes containing VacA molecules may similarly get in direct contact with mitochondria, thus facilitating a direct transfer of VacA or of a VacA subunit.
Direct support of this notion was recently provided by a spectacular study on the intracellular traffic of VacA, using a combination of fluorescence microscopy and cell fractionation.20 The authors added VacA to mouse embryonic fibroblasts and found that the toxin first accumulated in endosomes, but subsequently localized to mitochondria. Interestingly, they observed that in VacA-intoxicated cells, endosomes were juxtaposed to mitochondria. The association of the endosomal and mitochondrial membranes was dependent on the presence of VacA. Moreover, the authors found that an intact N-terminus of the toxin was essential for the transfer to the mitochondria. In their experiments, the complete VacA polypeptides made their way to the mitochondria. Proteolytic cleavage or processing of VacA was not observed.
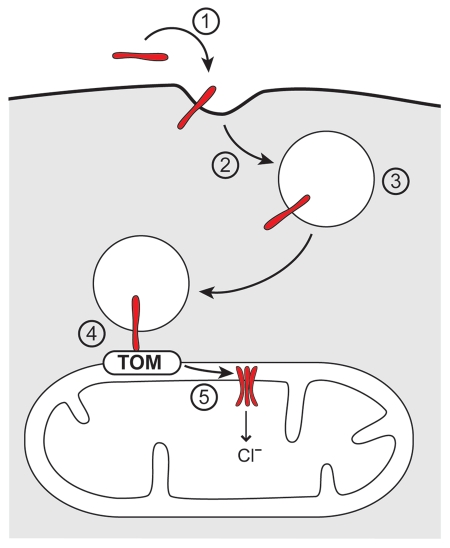
Taking the data from this study20 together with the results from our own investigations,9 a completely new scheme of an intracellular pathway of protein traffic is emerging. Considering that the p34 N-terminus is a targeting signal and not required for the formation of the p34 ion channel,9 it seems reasonable to assume that the N-terminus mediates the decisive step in the transfer of the toxin from the endosomal compartment to the mitochondria. The pathway of VacA to the mitochondria that we propose is illustrated in Figure 2: (1) Binding of VacA to target cells is dependent on subunit p58. (2) VacA enters the target cells by endocytosis. (3) It accumulates in early and late endosomes. (4) It mediates a specific and direct interaction of the endosomal membranes with mitochondrial outer membranes. (5) VacA is eventually imported into the mitochondria. The transfer of VacA from the endosomal membranes to the mitochondria is mediated by the VacA N-terminus. The import pore for translocation across the mitochondrial outer membrane is provided by the TOM complex. In this step, VacA thereby uses the same import pore as endogenous mitochondrial proteins. Inside the mitochondria, VacA forms anion channels of about 12 pS in the mitochondrial inner membrane.
Figure 2.
Intracellular traffic of VacA. (1) Binding of VacA (red) to target cells. (2) Endocytosis. (3) Accumulation in endosomes. (4) Endosome-mitochondria juxtaposition. (5) Import into mitochondria, assembly in hexamers and formation of anion channels in the mitochondrial inner membrane. Transfer of VacA from the endosomal membranes to mitochondria is essentially dependent on an intact VacA N-terminus (provided by subunit p34 as shown in Fig. 1). The pore for import into the mitochondria is formed by the TOM complex (translocase of the mitochondrial outer membrane).
Apoptosis.
What happens to a eukaryotic cell if the inner mitochondrial membranes receive a conductance for chloride ions? Little is known about the endogenous ion channels in mitochondria. The only mitochondrial inner membrane chloride channel that was characterized at the molecular level is mtCLIC/CLIC4.21 A study on the molecular basis of Myc-induced apoptosis identified the gene encoding this channel as a target.22 Is this of relevance with respect to VacA? It was repeatedly shown that administration of VacA to cells causes damage to mitochondria and leads to apoptosis.6,23–26 VacA-dependent apoptosis can be prevented by preincubation of the cells with the channel blocker NPPB (5-nitro-2-[3-phenylpropylamino] benzoic acid).25,26 Strikingly, this reagent also blocks the conductivity of holo-VacA27,28 and p34.9 This is currently the best evidence of a direct link between the channel activity of VacA/p34 and apoptosis.
Is anything known about the mechanisms of cell death that are triggered by VacA? Several observations indicated a participation of Bax and Bak in VacAdependent reactions.20,29 However, the precise function of these factors in cooperation with VacA is not clear. Insertion of VacA into the mitochondrial inner membrane should cause a rapid hyperpolarization, followed by an inhibition of mitochondrial functions and eventually a dissipation of the membrane potential. Loss of the mitochondrial membrane potential can easily initiate a translocation of Bax to the mitochondrial membranes and subsequent release of proapoptotic factors.30,31 However, the data reported by Calore et al.20 indicate that Bax and Bak may play a more specific role. Surprisingly, in Bax- and Bak-deficient cells, VacA is no longer observed in endosome-mitochondria juxtaposition, and VacA transport to mitochondria is inhibited. Additional investigations will be required to determine the molecular interactions and mechanisms that are responsible for these effects.
In any case it is remarkable that in elucidating the pathogenesis of H. pylori infections, for the first time a direct transfer of a protein from endosomes to mitochondria seems to have been identified. For 10 years the field of research on VacA had the problem of a missing link between the accumulation of VacA in endosomes and the obvious effects of the toxin on mitochondria. The concept of a direct transfer of VacA from endosomes to mitochondria offers a means to close the gap. It is tempting to speculate that other proteins, including other pore-forming toxins may take similar pathways.32–37 Moreover, the new data establish the mitochondrial inner membrane as an attractive intracellular target for toxins to interfere with the regulation of cell death and survival. Bacterial toxins are again providing a fascinating tool box.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/13894
References
- 1.Montecucco C, Rappuoli R. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol. 2001;2:457–466. doi: 10.1038/35073084. [DOI] [PubMed] [Google Scholar]
- 2.Sachs G, Weeks DL, Wen Y, Marcus EA, Scott DR, Melchers K. Acid acclimation by Helicobacter pylori. Physiology. 2005;20:429–438. doi: 10.1152/physiol.00032.2005. [DOI] [PubMed] [Google Scholar]
- 3.Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: the master key hypothesis. Helicobacter. 2010;15:163–176. doi: 10.1111/j.1523-5378.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 4.de Bernard M, Cappon A, Del Guidice R, Rappuoli R, Montecucco C. The multiple cellular activities of the VacA cytotoxin of Helicobacter pylori. Int J Med Microbiol. 2004;293:589–597. doi: 10.1078/1438-4221-00299. [DOI] [PubMed] [Google Scholar]
- 5.Cover L, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3:320–332. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 6.Galmiche A, Rassow J, Doye A, Cagnol S, Contamin S, de Thillot V, et al. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000;19:6361–6370. doi: 10.1093/emboj/19.23.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boquet P, Ricci V, Galmiche A, Gauthier NC. Gastric cell apoptosis and H. pylori: has the main function of VacA finally been identified? Trends Microbiol. 2003;11:410–413. doi: 10.1016/s0966-842x(03)00211-7. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen VQ, Caprioli RM, Cover TL. Carboxy-terminal proteolytic processing of Helicobacter pylori vacuolating toxin. Infect Immun. 2001;69:543–546. doi: 10.1128/IAI.69.1.543-546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domanska G, Motz C, Meinecke M, Harsman A, Papatheodorou P, Reljic B, et al. Helicobacter pylori VacA toxin/subunit p34: targeting of an anion channel to the inner mitochondrial membrane. PLoS Pathog. 2010;6:1000878. doi: 10.1371/journal.ppat.1000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwamoto H, Czajkowsky DM, Cover TL, Szabò G, Shao Z. VacA from Helicobacter pylori: a hexameric chloride channel. FEBS Lett. 1999;450:101–104. doi: 10.1016/s0014-5793(99)00474-3. [DOI] [PubMed] [Google Scholar]
- 11.Rausell C, Pardo-López L, Sánchez J, Muñoz-Garay C, Morera C, Soberón M, et al. J Biol Chem. 2004;279:55168–55175. doi: 10.1074/jbc.M406279200. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Chamberlain AK, Bowie JU. Membrane channel structure of Helicobacter pylori vacuolating toxin: role of multiple GXXXG motifs in cylindrical channels. Proc Natl Acad Sci USA. 2004;101:5988–5991. doi: 10.1073/pnas.0308694101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinion-Dubiel AD, McClain MS, Czajkowsky DM, Iwamoto H, Ye D, Cao P, et al. A dominant negative mutant of Helicobacter pylori vacuolating toxin (VacA) inhibits VacA-induced cell vacuolation. J Biol Chem. 1999;274:37736–37742. doi: 10.1074/jbc.274.53.37736. [DOI] [PubMed] [Google Scholar]
- 14.McClain MS, Cao P, Iwamoto H, Vinion-Dubiel AD, Szabò G, Shao Z, et al. A 12-amino-acid segment, present in type s2 but not type s1 Helicobacter pylori VacA proteins, abolishes cytotoxin activity and alters membrane channel formation. J Bacteriol. 2001;183:6499–6508. doi: 10.1128/JB.183.22.6499-6508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chacinska A, Koehler CM, Milencovic D, Lithgow T, Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138:628–644. doi: 10.1016/j.cell.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foo JH, Culvenor JG, Ferrero RL, Kwok T, Lithgow T, Gabriel K. Both p33 and p55 subunits of Helicobacter pylori VacA are targeted to mammalian mitochondria. J Mol Biol. 2010;401:792–798. doi: 10.1016/j.jmb.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 17.de Bernard M, Moschioni M, Napolitani G, Rappuoli R, Montecucco C. The VacA toxin of Helicobacter pylori identifies a new intermediate filament-interacting protein. EMBO J. 2000;19:48–56. doi: 10.1093/emboj/19.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falnes PØ, Sandvig K. Penetration of protein toxins into cells. Curr Opin Cell Biol. 2000;12:407–413. doi: 10.1016/s0955-0674(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calore F, Genisset C, Casellato A, Rossato M, Codolo G, Esposti MD, et al. Endosome-mitochondria juxtaposition during apoptosis induced by H. pylori VacA. Cell Death Differ. 2010;17:1707–1716. doi: 10.1038/cdd.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomaskova Z, Ondrias K. Mitochondrial chloride channels—what are they for? FEBS Lett. 2010;584:2085–2092. doi: 10.1016/j.febslet.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 22.Shiio Y, Suh KS, Lee H, Yuspa SH, Eisenman RN, Aebersold R. Quantitative proteomic analysis of Myc-induced apoptosis—A direct role for Myc induction of the mitochondrial chloride ion channel mtCLIC/CLIC4. J Biol Chem. 2006;281:2750–2756. doi: 10.1074/jbc.M509349200. [DOI] [PubMed] [Google Scholar]
- 23.Kuck D, Kolmerer B, Iking-Konert C, Krammer PH, Stremmel W, Rudi J. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect Immun. 2001;69:5080–5087. doi: 10.1128/IAI.69.8.5080-5087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cover T, Krishna US, Israel DE, Peek RM., Jr Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63:951–957. [PubMed] [Google Scholar]
- 25.Willhite DC, Cover TL, Blanke SR. Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of Helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J Biol Chem. 2003;278:48204–48209. doi: 10.1074/jbc.M304131200. [DOI] [PubMed] [Google Scholar]
- 26.Willhite DC, Blanke SR. Helicobacter pylori vacuolating cytotoxin enters cells, localizes to the mitochondria and induces mitochondrial membrane permeability changes correlated to toxin channel activity. Cell Microbiol. 2004;6:143–154. doi: 10.1046/j.1462-5822.2003.00347.x. [DOI] [PubMed] [Google Scholar]
- 27.Szabò I, Brutsche S, Tombola F, Moschioni M, Satin B, Telford JL, et al. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 1999;18:5517–5527. doi: 10.1093/emboj/18.20.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tombola F, Del Giudice G, Papini E, Zoratti M. Blockers of VacA provide insights into the structure of the pore. Biophys J. 2000;79:863–873. doi: 10.1016/S0006-3495(00)76342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamasaki E, Wada A, Kumatori A, Nakagawa I, Funao J, Nakayama M, et al. Helicobacter pylori vacuolating cytotoxin induces activation of the proapoptotic proteins Bax and Bak, leading to cytochrome C release and cell death, independent of vacuolation. J Biol Chem. 2006;281:11250–11259. doi: 10.1074/jbc.M509404200. [DOI] [PubMed] [Google Scholar]
- 30.Smaili SS, Hsu YT, Sanders KM, Russell JT, Youle RJ. Bax translocation to mitochondria subsequent to a rapid loss of mitochondrial membrane potential. Cell Death Differ. 2001;8:909–920. doi: 10.1038/sj.cdd.4400889. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bantel H, Sinha B, Domschke W, Peters G, Schulze-Osthoff K, Janicke RU. α-toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signalling. J Cell Biol. 2001;155:637–647. doi: 10.1083/jcb.200105081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genestier AL, Michallet MC, Prévost G, Bellot G, Chalabreysse L, Peyrol S, et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J Clin Invest. 2005;115:3117–3127. doi: 10.1172/JCI22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi CH, Lee EY, Lee YC, Park TI, Kim HJ, Hyun SH, et al. Outer membrane protein 38 of Acinetobacter baumannii localizes to mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005;7:1127–1138. doi: 10.1111/j.1462-5822.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- 35.Braun JS, Hoffmann O, Schickhaus M, Freyer D, Dagand E, Bermpohl D, et al. Pneumolysin causes neuronal cell death through mitochondrial damage. Infect Immun. 2007;75:4245–4254. doi: 10.1128/IAI.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozjak-Pavlovic V, Dian-Lothrup EA, Meinecke M, Kepp O, Ross K, Harsman A, et al. Bacterial porin disrupts mitochondrial membrane potential and sensitizes host cells to apoptosis. PLoS Pathog. 2009;5:1000629. doi: 10.1371/journal.ppat.1000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudel T, Kepp O, Kozjak-Pavlovic V. Interactions between bacterial pathogens and mitochondrial cell death pathways. Nat Rev Microbiol. 2010;8:693–705. doi: 10.1038/nrmicro2421. [DOI] [PubMed] [Google Scholar]