Abstract
Membranous (m) cells are specialized epithelial antigen-transporting cells scattered in the follicle-associated epithelium covering the gut lymphoid follicles such as Peyer's patches. Although the importance of M cells as a main portal for luminal antigens has long been recognized, molecular mechanisms for M-cell antigen uptake has remained largely elusive. We have recently found that glycoprotein 2 (GP2) is exclusively expressed on M cells among intestinal epithelial cells and serves as an uptake receptor for a subset of commensal and pathogenic bacteria. GP2 interacts with FimH, a major component of the type 1 pilus on the outer membrane of a subset of gram-negative enterobacilli such as E. coli and Salmonella enterica. Furthermore, GP2-FimH interaction is necessary for efficient uptake of FimH+ bacteria by M cells and subsequent bacteria-specific mucosal immune responses. Pancreatic GP2 may also be involved in innate immunity by ‘opsonization’ of FimH+ bacteria to facilitate their egestion in feces as well as translocation across the intestinal epithelium.
Key words: mucosal immunity, M cells, antigen uptake, bacteria, Escherichia coli, Salmonella typhimurium, type I pili, FimH, IgA
Membranous (M) cells are specialized epithelial antigen-transporting cells that constitute a minor proportion (5∼10% in humans and mice) of the follicle-associated epithelium (FAE) covering the lymphoid follicles of organized gut-associated lymphoid tissue (GALT) such as Peyer's patches (PPs).1–3 Some ninety years ago, a Japanese scientist, Dr. Kenzaburo Kumagai, discovered that Mycobacterium tuberculosis was taken up through the FAE and then appeared in GALT follicles.1,4 Fifty years later, Bockman and Cooper5 identified unique cells vigorously ingesting ferritin and india ink in the FAE of rabbit appendix and the chicken bursa of Fabricius, and Owen and Jones6 discovered the human counterparts in PP FAE, and named them M cells.
Since their discovery, M cells had been structurally analyzed by optical and electron microscopy.1–3 These studies have revealed that M cells can efficiently engulf particles as large as bacteria. They not only take up microorganisms, but also inert particles such as latex beads, suggesting that uptake of materials by M cells is rather nonspecific. By contrast, it has been reported that live, but not dead, Vibrio cholerae can be taken up by M cells.7,8 It has also been shown that the efficiency of uptake of Escherichia coli by M cells varies from strain to strain.7 These observations support the idea that uptake of antigens by M cells is, at least in part, receptor-mediated.3
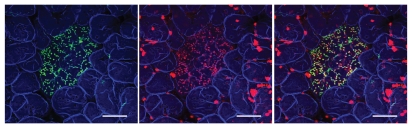
However, this hypothesis could not be directly tested until recently. During the 20th century, the study of M cells had been essentially restricted to morphological analyses, even three decades after their discovery, mainly because of the difficulty in purifying sufficient numbers of M cells for molecular or biochemical analysis. In the first place, there was no specific marker for M cells. Ulex europaeus agglutinin-1 (UEA-1), a plant lectin recognizing α(1–2)-fucose, has been the only reagent known that can react with M cells, but this reactivity is restricted to mouse M cells, among the well-studied animals including humans.2,3 Unfortunately, however, UEA-1 is not specific to M cells, in that it also recognizes goblet cells and Paneth cells among intestinal epithelial cells in mice (Fig. 1). This makes it difficult to purify M cells when using UEA-1 reactivity as the defining feature. Another problem is that the number of M cells is very limited: less than one in 107 of total intestinal epithelial cells, and 5–10% of FAE in humans and mice.2,3 These factors had long hampered the study of the molecular and cellular biology of M cells. The situation has changed with the emergence of new technologies; microarray analysis can be used to obtain gene expression profiles from a small amount of RNA of a given cell population or tissue. Thanks to this sophisticated technology, we and others have identified M-cell specific molecules in humans and mice.9,10
Figure 1.
Confocal laser microscopy image of whole-mount staining of mouse Peyer's patch FAE. GP2 (green, left) and UEA-1 (red, middle) largely colocalize (merge, right). Note that GP2 staining is restricted to the FAE (an oval area in the middle of the field), while UEA-1 is not specific to FAE but also reacts strongly to mucin-secreting goblet cells in the villi. F-actin staining (blue) depicts the shape of villi surrounding PP. Scale bars, 100 µm.
We first developed a method to recover the intestinal epithelial cell layer as a sheet with minimal contamination by the lamina propria (LP), and to further separate the FAE and the villous region from the recovered epithelial sheet. Using these as the starting materials, we compared the gene expression profiles of FAE and villous epithelium (VE). The genes showing higher expression in FAE compared to VE were candidates for FAE- or M cell-specific genes (the data are available on Reference Database of Immune Cells [RefDIC], http://refdic.rcai.riken.jp). The candidates FAE/M cell-specific genes were further subjected to quantitative RT-PCR to confirm their FAE-specific expression, and subsequent in situ hybridization and/or immunohistochemistry determined the localization of the candidate molecules.9
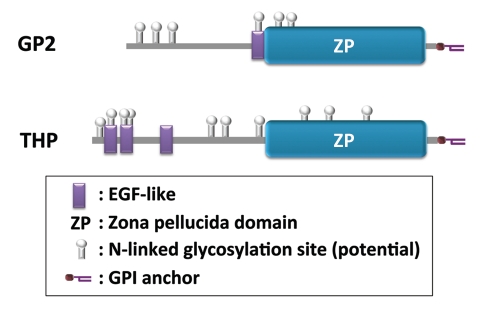
Glycoprotein 2 (GP2) was one of the M cell-specific molecules identified (Fig. 1).11 We showed the M cell-specific expression of GP2 among intestinal epithelium in humans and mice. Considering the conservation of GP2 gene among mammals besides humans and mice, including rats, apes, monkeys, dogs, cattle, horses and pigs (NCBI database; http://www.ncbi.nlm.nih.gov/gene), it would not be surprising that these species also express GP2 on their M-cell surface. GP2 was originally identified as a glycosylphosphatidylinositol (GPI)-anchored protein abundantly expressed in the exocrine granules of the pancreatic acinar cells (Fig. 2).12,13 However, a score of years after its discovery and even after establishment and analyses of GP2-deficient mice, the function of GP2 has remained elusive.14,15
Figure 2.
Schematic domain structure of GP2 and THP. Domain structure of mouse GP2 and THP is drawn based on the information obtained through UniProt (http://www.uniprot.org/). FimH-binding region of GP2 is predicted based on our unpublished observation that a recombinant GP2 protein lacking the ZP domain still bound to E. coli in our in vitro binding assay.
We have now discovered that the function of GP2 expressed on M cells is as a bacterial uptake receptor.11 GP2 recognizes FimH, a major component of the type 1 pilus on the outer membrane of a subset of gram-negative enterobacilli such as E. coli and Salmonella enterica.11,16 FimH has a lectin-like capacity to bind to certain glycoproteins on mammalian cells in a mannose-dependent manner.17 This is also the case for FimH-GP2 binding.11,16 However, it seems possible that FimH recognizes the protein structure of GP2 in addition to the mannose moiety considering the almost total abrogation of E. coli uptake by M cells in GP2-deficient mice, since it is difficult to imagine that GP2 is the sole apical membrane protein with mannose residues on the apical surface of M cells.
M cells are thought to be the predominant entry site for Salmonella to initiate infection in mice.2,3,18 It has been reported that mutants of S. enterica serovar Typhimurium (S. typhimurium) deficient in inv genes, which render them severely defective for invasion, retain the ability to invade mouse PP M cells,19 although the efficiency seems far less compared to the invasion-competent S. typhimurium.20 Our data appended a new aspect to the M-cell-targeted invasion of S. typhimurium, in that the FimH-deficient S. typhimurium could not efficiently enter PP even though they are invasion-competent. Reciprocally, FimH+ S. typhimurium can enter PP in wild-type (i.e., GP2-sufficient) mice but not in GP2-deficient mice.11 These data clearly demonstrate that the interaction of the bacterial FimH and GP2 on the host M cells is crucial for the entry of FimH+ bacteria. Taken together, we propose that recognition of FimH by GP2 is prerequisite for the active uptake of FimH+ bacteria by M cells, a process in which the bacterial invasion machinery could be a facilitator but not necessarily be an absolute requirement. Considering the more efficient delivery of invasive S. typhimurium than non-invasive E. coli (our unpublished observation), bacterial invasion could act additively with phagocytic uptake by M cells for bacterial translocation from the intestinal lumen to the PP. Although we have shown that GP2 is required for efficient uptake of FimH+ bacteria, we do not know whether GP2 is directly involved in the actual uptake of these bacteria; it is also possible that GP2 is primarily involved in the binding/capture of these bacteria, and additional molecule(s) are required for the uptake itself. Further studies are required to clarify the question.
We have also observed that GP2 deficiency in the host, as well as lack of FimH by the bacteria, markedly reduces translocation of S. typhimurium into the mesenteric lymph node (MLN) as well as production of S. typhimurium-specific serum IgG upon oral administration of the bacteria.11 It has been shown that PP are dispensable for translocation of S. typhimurium into MLN and the subsequent S. typhimurium-specific systemic IgG response, because the bacteria can be taken up directly by dendritic cells (DCs) in the LP and delivered to MLN.20 An apparent discrepancy between these data20 and our data is that S. typhimurium did not seem to translocate into the LP in the absence of GP2 in our experimental setting.11 This raises the interesting possibility that GP2, most likely that secreted from the pancreas, may also contribute to the translocation of FimH+ bacteria through the LP. Collectively, these and our observations indicate that S. typhimurium could reach and/or be delivered to MLN both in PP-mediated and -bypassing pathways.
As mentioned above, GP2 is abundantly produced by pancreatic acinar cells and secreted in the pancreatic juice, due to post-translational cleavage of the membrane bound form of GP2.12,13 Since ingestion of food in stomach stimulates the excretion of pancreatic juice, we postulate that GP2 secreted in pancreatic juice likely participates in innate immunity against FimH+ bacterial contaminants in food by coating them and facilitating their egestion in the feces, in a manner analogous to uromodulin/Tamm-Horsfall protein (THP). THP is a GPI-anchored protein with a similar domain structure and 52% overall amino acid sequence identity with GP2 (Fig. 2).21 The expression of THP is restricted to the apical plasma membrane of renal tubular epithelium. THP specifically binds to uropathogenic E. coli and promotes bacterial clearance from the urinary tract.22 Another potential role for pancreas-derived GP2 is that ‘opsonization’ by GP2 may facilitate the translocation of FimH+ bacteria across the intestinal epithelium. Since GP2 self-polymerizes via its zona pellucida (ZP) domain (Fig. 2),21,23 it could be hypothesized that the interaction between ZP domains of bacteria-bound GP2 and M-cell surface GP2 facilitates attachment of FimH+ bacteria onto M cells. In addition, pancreatic GP2 might also be involved in the translocation of FimH+ bacteria to the LP as described above, although the underlying mechanism is unknown. In this regard, GP2 is also expressed in a subset of DCs (our unpublished observation), and this may promote the efficient uptake of GP2-opsonized bacteria by DCs. Further studies are required to clarify these hypotheses, including the establishment and analyses of M-cell- and pancreas-specific GP2 knockout mice.
We have recently found that cellular prion protein (PrPC), another GPI-anchored protein, is predominantly expressed on the apical surface of M cells among intestinal epithelial cells.24 Given that PrPC acts as the internalization receptor on macrophages for a zoonotic pathogen Brucella abortus,25 which is suspected to infect orally, one could postulate that PrPC on M cells serves as an uptake receptor for this microbe. Future studies of bacterial uptake receptors on M cells, including GP2 and PrPC will greatly increase our understanding of mucosal immunity and provide a new approach for ‘M-cell-targeted’ vaccination protocols.
Acknowledgements
We thank Dr. P.D. Burrows for reviewing the manuscript and English editing. This study was supported in part by Grants-in-Aid for Scientific research (B) (H.O.), Young Scientists (A) (K.H.), Scientific Research in Priority Areas (H.O. and K.H.), and Scientific Research on Innovative Areas (H.O.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/14078
References
- 1.Owen RL. Uptake and transport of intestinal macromolecules and microorganisms by M cells in Peyer's patches—a personal and historical perspective. Semin Immunol. 1999;11:157–163. doi: 10.1006/smim.1999.0171. [DOI] [PubMed] [Google Scholar]
- 2.Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000;16:301–332. doi: 10.1146/annurev.cellbio.16.1.301. [DOI] [PubMed] [Google Scholar]
- 3.Kato T, Owen RL. Structure and function of intestinal mucosal epithelium. In: Mestecky J, Lamm ME, McGhee JR, Bienenstock J, Mayer L, Strober W, editors. Mucosal Immunology. Elsevier Academic Press; 2005. pp. 131–151. [Google Scholar]
- 4.Kumagai K. Über den Resorptionsvergang der corpusculären Bestandteile im Darm. Kekkaku-Zassi. 1922;4:429–431. (Ger). [Google Scholar]
- 5.Bockman DE, Cooper MD. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix and Peyer's patches. An electron microscopic study. Am J Anat. 1973;136:455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- 6.Owen RL, Jones AL. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974;66:189–203. [PubMed] [Google Scholar]
- 7.Kato T, Owen RL. Structure and functon of intestinal mucosal eptihelium. In: Mestecky J, Lamm ME, McGhee JR, Bienenstock J, Mayer L, Strober W, editors. Mucosal Immunology. Amsterdam: Elsevier Inc; 2005. pp. 131–151. [Google Scholar]
- 8.Owen RL, Pierce NF, Cray WCJ. Efffects of bacterial inactivation methods, toxin production, and oral immunization on uptake of Vibrio cholerae by Peyer's patch lymphoid folicles. In: Kuwahara S, Pierce NF, editors. Advances in Research on Cholera and Related Diarrheas. Tokyo: KTK Scientific Publishers; 1988. pp. 189–197. [Google Scholar]
- 9.Hase K, Ohshima S, Kawano K, Hashimoto N, Matsumoto K, Saito H, et al. Distinct gene expression profiles characterize cellular phenotypes of follicle-associated epithelium and M cells. DNA Res. 2005;12:127–137. doi: 10.1093/dnares/12.2.127. [DOI] [PubMed] [Google Scholar]
- 10.Verbrugghe P, Waelput W, Dieriks B, Waeytens A, Vandesompele J, Cuvelier CA. Murine M cells express annexin V specifically. J Pathol. 2006;209:240–249. doi: 10.1002/path.1970. [DOI] [PubMed] [Google Scholar]
- 11.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 12.Hoops TC, Rindler MJ. Isolation of the cDNA encoding glycoprotein-2 (GP-2), the major zymogen granule membrane protein. Homology to uromodulin/Tamm-Horsfall protein. J Biol Chem. 1991;266:4257–4263. [PubMed] [Google Scholar]
- 13.Fukuoka S, Freedman SD, Scheele GA. A single gene encodes membrane-bound and free forms of GP-2, the major glycoprotein in pancreatic secretory (zymogen) granule membranes. Proc Natl Acad Sci USA. 1991;88:2898–2902. doi: 10.1073/pnas.88.7.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi K, Yanagihara K, Ishiguro K, Fukuoka S. GP2/THP gene family of self-binding, GPI-anchored proteins forms a cluster at chromosome 7F1 region in mouse genome. Biochem Biophys Res Commun. 2004;322:659–664. doi: 10.1016/j.bbrc.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 15.Yu S, Michie SA, Lowe AW. Absence of the major zymogen granule membrane protein, GP2, does not affect pancreatic morphology or secretion. The J Biol Chem. 2004;279:50274–50279. doi: 10.1074/jbc.M410599200. [DOI] [PubMed] [Google Scholar]
- 16.Yu S, Lowe AW. The pancreatic zymogen granule membrane protein, GP2, binds Escherichia coli Type 1 fimbriae. BMC Gastroenterol. 2009;9:58. doi: 10.1186/1471-230X-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pizarro-Cerda J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124:715–727. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exper Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark MA, Reed KA, Lodge J, Stephen J, Hirst BH, Jepson MA. Invasion of murine intestinal M cells by Salmonella typhimurium inv mutants severely deficient for invasion of cultured cells. Infect Immun. 1996;64:4363–4368. doi: 10.1128/iai.64.10.4363-4368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinoli C, Chiavelli A, Rescigno M. Entry route of Salmonella typhimurium directs the type of induced immune response. Immunity. 2007;27:975–984. doi: 10.1016/j.immuni.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Fukuoka S, Freedman SD, Yu H, Sukhatme VP, Scheele GA. GP-2/THP gene family encodes self-binding glycosylphosphatidylinositol-anchored proteins in apical secretory compartments of pancreas and kidney. Proc Natl Acad Sci USA. 1992;89:1189–1193. doi: 10.1073/pnas.89.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol. 2004;286:795–802. doi: 10.1152/ajprenal.00357.2003. [DOI] [PubMed] [Google Scholar]
- 23.Jovine L, Qi H, Williams Z, Litscher E, Wassarman PM. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat Cell Biol. 2002;4:457–461. doi: 10.1038/ncb802. [DOI] [PubMed] [Google Scholar]
- 24.Nakato G, Fukuda S, Hase K, Goitsuka R, Cooper MD, Ohno H. New approach for m-cell-specific molecules screening by comprehensive transcriptome analysis. DNA Res. 2009;16:227–235. doi: 10.1093/dnares/dsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watarai M, Kim S, Erdenebaatar J, Makino S, Horiuchi M, Shirahata T, et al. Cellular prion protein promotes Brucella infection into macrophages. J Exper Med. 2003;198:5–17. doi: 10.1084/jem.20021980. [DOI] [PMC free article] [PubMed] [Google Scholar]