Abstract
Using the culture independent TRACA system in conjunction with a comparative metagenomic approach, we have recently explored the pool of plasmids associated with the human gut mobile metagenome. This revealed that some plasmids or plasmid families are present in the gut microbiomes of geographically isolated human hosts with a broad global distribution (America, Japan and Europe), and are potentially unique to the human gut microbiome. Functions encoded by the most widely distributed plasmid (pTRACA22) were found to be enriched in the human gut microbiome when compared to microbial communities from other environments, and of particular interest was the increased prevalence of a putative RelBE toxin-antitoxin (TA) addiction module. Subsequent analysis revealed that this was most closely related to putative TA modules from gut associated bacteria belonging to the Firmicutes, but homologues of the RelE toxin were associated with all major bacterial divisions comprising the human gut microbiota. In this addendum, functions of the gut mobile metagenome are considered from the perspective of the human host, and within the context of the hologenome theory of human evolution. In doing so, our original analysis is also extended to include the gut metagenomes of a further 124 individuals comprising the METAHIT dataset. Differences in the incidence and relative abundance of pTRACA22 and associated TA modules between healthy individuals and those with inflammatory bowel diseases are explored, and potential functions of pTRACA22 type RelBE modules in the human gut microbiome are discussed.
Key words: mobile genetic elements, mobile metagenome, gut microbiota, horizontal gene transfer, toxin-antitoxin addiction module, metagenomics, hologenome, holobiont
Introduction
Humans share the planet with an estimated 1030 prokaryotic cells and interaction with these microbes has shaped the course of our development.1–8 Modern humans have co-evolved with microbial communities that have colonized various habitats offered by the human body, and now maintain commensal or symbiotic relationships with their metazoan hosts. Of the numerous microbial communities harbored by the human body, the gastrointestinal tract (GIT) is home to the largest. The microbiota resident in the distal colon of an individual adult consists of an estimated 1013–1014 individual prokaryotic cells belonging to an estimated 150–800 species, which are derived from ∼1,380–4,018 distinct operational taxonomic units observed in collective gut microbiomes analyzed.8–11 The numerous beneficial functions undertaken by this microbial community, and its capacity to direct development of host physiology, epitomises the co-evolution of the gut microbiota with its human host.3,7,11–17 This influence may even extend to aspects of human behavior, and the benefits gained by acquisition of beneficial microbes may have partly contributed to the evolution of social behavior and group living, which facilitates the transmission of microbial symbionts to new hosts.18
Investigation of host-microbe interaction in the GIT has begun to reveal the mechanisms underlying these beneficial functions, and emphasised the intricate and intimate relationship between eukaryotic host and prokaryotic symbiont.12–17 More recently, metagenomic approaches which permit access to the greater uncultivated fraction of the gut microbiota have revealed functions enriched in the gut microbiome, and highlighted gene families shared among the disparate microbial lineages in this ecosystem.10,14,19–22 In parallel to these studies, there is currently much effort focused on defining the bacterial and archaeal species comprising the common phylogenetic core of the human gut microbiome.10,11 Together, such studies will ultimately lead to the resolution of the fundamental structure and metabolic outputs of this community, which will allow us to properly define its impact on human health and development.
However, just as the human gut harbors a complex microbial ecosystem, bacteria comprising the gut microbiota in turn play host to their own “hitchhikers” in the form of mobile genetic elements (MGE). These include plasmids, transposons and integrons and collectively the different elements associated with the human gut microbiota may be referred to as its mobile metagenome.22–24 Bacteriophage also share many of the properties associated with these elements, and although are not MGE in the same sense as plasmids, transposons or integrons, they are capable of mediating horizontal gene transfer (HGT) between bacteria, and for the purposes of this article they are considered to be part of the gut mobile metagenome.
Investigation of MGE infecting key cultivatable species comprising the human gut microbiota have highlighted the role of elements such as plasmids and conjugative transposons (CTn) in the maintenance and spread of antibiotic resistance genes within this community.25–39 Dissemination of erythromycin resistance between Bacteriodes species in the human gut has been largely attributed to horizontal transfer of the relevant genes by conjugative transposons,26,28–31 and the role of MGE in the acquisition and spread of tetracycline resistance in the human gut microbiota is also well documented.25–27,32–34 Significant similarity has been identified between tetracycline resistance genes present in human and animal gut commensals, including regions of DNA flanking these genes,32 and evidence for an evolutionarily recent transfer of tetQ from animal to human gut bacterial species has been presented.25
Overall, the gut mobile metagenome is considered a reservoir for antibiotic resistance genes.31,38 This view has been strengthened by recent function-driven metagenomic studies highlighting the frequent association of resistance determinants with genes and sequences related to MGE in the mammalian gut microbiota,27,38 and the observation that MGE encoding resistance to antibiotics such as tetracycline were prevalent in gut isolates of Bacteriodes sp. collected prior to the widespread clinical use of these drugs.28,31 However, there is growing interest in other functions that may be encoded by MGE resident in the gut microbiota, and the role of the mobile metagenome in the development and functioning of this community.
Characterization of plasmids from cultivatable commensal and pathogenic species found in the human GI tract have illuminated some of the functions encoded by this sphere of the gut microbiome.24,39–59 These include functions relevant to survival in the GIT and interaction with the human host, such as utilization of carbohydrates and other nutrients, bacteriocine production, adhesion to host cells, resistance to bile acids and virulence factors.24,41–59 A particularly interesting example of MGE encoding such functions are the large megaplasmids that appear to be widespread in Lactobacillus salivarius, and in other Lactobacilli sp. resident in the human GIT.41,44
In the case of the probiotic L. salivarius UCC118, this organism harbors three plasmids, including a megaplasmid encoding genes related to bile tolerance, redox balance, cell wall biosynthesis and bacteriocine production.44 Bacteriocine production has also been identified as a plasmid encoded function in other gut associated species,44 while genes providing the ability to resist the antimicrobial effects of bile in the small intestine appear to be encoded by plasmids and MGE associated with a diverse range of gut bacterial species.24,44,51 Functions involved in gut colonization and survival have also been characterized on plasmids infecting the Enterobacterial population of the human gut microbiota. For example, the recent genome sequence of the human gut commensal E. coli strain SE11 included six plasmids, which carried genes involved in adherence to epithelial cells, tetracycline resistance and bacteriocine production.41
Conversely, plasmid encoded functions may also contribute to virulence in pathogenic bacteria, and gut associated pathogens harbor some of the best characterized examples.52–59 These virulence plasmids have been found to carry genes required for the production of specific virulence factors such as toxins, or factors that facilitate colonization and survival in this environment.52–59 In the latter case, there is potential for pathogens to acquire these functions from commensal or symbiotic members of this community through HGT, while conjugative plasmids in general may contribute to colonization of the human GI tract by both pathogens and commensals, since conjugation machinery has been found to facilitate adherence of bacteria to various surfaces including epithelial cells.40,53,60
On the whole, genes and functions encoded by MGE that are long term members of the gut mobile metagenome will also reflect the co-evolution of resident microbes and their human host.22,24,49 Recent studies indicate that plasmids harbored by gut bacteria also display the signatures of long-term association with the human host evident in the core genomes of their bacterial hosts, with the observed patterns of long-term gene convergence and conservation evident in chromosomes of gut bacteria, even more pronounced for plasmids.49 These observations support the hypothesis for a role of the mobile metagenome in broader aspects of community development and are likely to be reflected in the gut mobile metagenome in general.24
The functions encoded by this flexible gene pool, along with the capacity for MGE to mediate gene flow between distinct bacterial species, undoubtedly generates an additional sphere of complexity and variability in terms of how this community develops, functions, evolves and interacts with the human host. Despite this, the majority of the human gut mobile metagenome remains unexplored and undefined, both in terms of its significance to the human host and human evolution, as well as total genetic content. Overall, the mobile metagenome is a facet of the gut microbiota not readily captured by current efforts to characterize this community, but one that will be important in attaining a complete understanding of this “virtual organ”.
Utilizing a comparative metagenomic approach we have recently revealed the existence of plasmids potentially unique to the human gut microbiome, which appear to be present in the gut communities of geographically isolated hosts distributed across the globe.22 Of particular interest was the unexpected finding that some of these plasmids encoded genes which are enriched in the gut microbial community when compared to other microbial ecosystems,22 reinforcing the hypothesis of the human gut mobile metagenome as a reservoir of genetic information involved in key aspects of community function. This addendum attempts to place the gut mobile metagenome within the framework of recent theories of host-microbe co-evolution, considering functions of this malleable genetic resource from the perspective of the human host. In doing so, our original analysis of plasmids in the human gut mobile metagenome is extended to encompass an additional 124 human gut microbiomes,10 and a preliminary exploration of differences in plasmid encoded functions in health and disease is presented.
An Integrated View of the Human Genome: A Place for the Mobile Metagenome?
The understanding that our prokaryotic passengers are more than simply opportunistic hitchhikers, and play an intimate role in our development and wellbeing, has resulted in a revision to the view of humans (and many other complex organisms) as entities composed exclusively of eukaryotic cells. In light of our increasing knowledge of host-microbe (prokaryote-eukaryote) interaction in the gastrointestinal tract and other body sites, the concept of humans and other animals as organisms has evolved to encompass our symbiotic and commensal prokaryotes.2–8
Mammals, including humans, are now generally accepted to be amalgams of both prokaryotic and eukaryotic cells, and frequently referred to as superorganisms.2–8 In this context the gut microbiota has been described as a “virtual organ” that undertakes a wealth of functions from which we benefit, but which our eukaryotic cells have not evolved for themselves.3 These include a barrier function against colonization by intestinal pathogens, salvage of energy from dietary components exigent to host digestive mechanisms, development of the immune system, as well as aspects of intestinal physiology and control of epithelial cell proliferation in the GIT.7,12–16,24,61,62
If we accept the paradigm of humans as gestalt entities consisting of both prokaryotic and eukaryotic cells, with the gut microbiota as a “virtual organ”, then we must also acknowledge the human genome to be composed of both eukaryotic and prokaryotic components. This has resulted in the development of ecological and evolutionary models which integrate both components of the human genome to explain the co-evolution of host and microbe.4–6,8,18 Recently, Zilber-Rosenberg & Rosenberg (2008) described humans and other complex metazoans as holobionts and presented the hologenome theory of evolution, in which the overall unit of selection in evolution is composed of the total genetic content of the eukaryotic host organism, plus that of its symbiotic microbial partners.4 In this model, our eukaryotic cells carry the primary core of indispensible, human defining genetic information, while our prokaryotic symbionts collectively house a secondary, variable portion of the human genome, which directs beneficial accessory functions not encoded for by the primary eukaryotic segment.
The content of the principal prokaryotic segment may be defined as the core genome content of the collective microbial species comprising communities such as the gut microbiota, and is itself a metagenome. Consequently this secondary prokaryotic metagenome has the potential to vary in terms of genetic content (and therefore its functional output and impact on host fitness) not only between individuals but also over the lifetime of the host.8–11,63,64 This is highlighted by the most in depth analysis of the human gut microbiota to date, which identified around 1,000–1,150 prevalent bacterial species present in the combined gut communities of the 124 individuals investigated.10 However, only 18 of these species were common to all individuals, and it was estimated that only around 38% of the bacterial genes present in an individual's gut metagenome are shared with other individuals.10 This is in stark contrast to our primary eukaryotic genome, which in comparison varies little between individuals (∼0.1% by single nucleotide polymorphisms, 0.3% by inversions, 1.2% by copy number variations/insertions or deletions),65 or even between Homo sapiens and its closest living relative, Pan troglodytes (∼4%).66
In addition, our primary eukaryotic genomes are essentially fixed in terms of content throughout the life time of the host, and lack the plasticity inherent in our secondary prokaryotic metagenomes. Prokaryotes frequently exchange genetic information, and the mobile metagenome associated with the human microbiota may represent an important fraction of the human hologenome. This highly flexible tertiary gene pool may benefit the human host indirectly via effects on constituent species, or encode functions that impact directly on fitness of the holobiont.24 Studies of MGE associated with microbial communities from plants and invertebrates demonstrate the potential for the gut mobile metagenome to encode genes involved in symbiosis of human and microbe, as well as specific functions of direct benefit to the human host.67–71
Therefore, while the primary “human” segment of our genome evolves relatively slowly due to a long generation time and its relatively static nature, our secondary prokaryotic segment has the capacity to rapidly adapt to new environmental conditions by virtue of short generation times and recruitment of new genetic material.4 A major advantage of this arrangement for the human host is the retention of a genetically flexible secondary genome which may facilitate adaptation to new environmental conditions such as changes in available food supply. This model also affords the possibility that horizontal gene transfer (HGT) among human-associated microbes is of direct benefit to the human host, and raises the possibility that HGT in the gut microbiota (as well as other human associated microbial communities) is in itself an important process in the evolution of the human holobiont as a whole.
The finding that certain MGE and the functions they encode are enriched and generally conserved in the human gut microbiome,10,20–24,72 underscores the potential contribution of mobile genetic elements to the development and perhaps overall functioning of this community, and therefore also to host-microbe co-evolution. For example, the genes underlying many of the core functions of the gut microbiota appear broadly distributed among members of this community, and HGT seems to have underwritten the expansion of these beneficial gene sets ensuring functional stability of the gut ecosystem.20,21,72–74 MGE are also capable of introducing new genetic material into an existing microbial community, facilitating the development of new functional pathways within an established ecosystem, which is almost certainly of significance to community function past, present and future.24,72–74 In broader terms, the ability of MGE to transfer genetic material between disparate bacterial species may in itself be an attribute of the mobile metagenome as a whole, that has, and continues to benefit both host and microbe, and HGT may constitute a key mechanism underlying the proposed adaptive role of the prokaryotic fraction of the human hologenome.
The Gut Mobile Metagenome: A Role for Microbial HGT in Human Evolution?
Activities of MGE are generally studied in terms of their impact on fitness of the bacterial host. However, activities of bacteria belonging to communities associated with higher host organisms, such as the gut microbiota, are ultimately selected for at the level of the metazoan host through effects on host fitness.2,8,24 It is therefore of note that the human gut microbiome is considered to be a hotspot for genetic exchange and there is increasing evidence to support this claim.20,71–73,75 If so, then it follows that a high level of HGT among members of this community may also be of benefit to the human host.
HGT is a driving force in bacterial evolution and facilitates rapid adaptation to new environments. This may benefit the metazoan host either directly by facilitating development of beneficial functions of the gut microbiota, or indirectly by facilitating the adaptation of community members to the gut environment, and development of a stable gut microbiota with associated benefits to the host.24 While the levels of HGT extant in the gut microbiota may be primarily a consequence of the environmental parameters in the mammalian gut, such as a high population density in close spatial proximity, it is possible that this facet of the community has been of benefit to the human host and is important in the evolution of the human holobiont as a whole. This theory is supported by advantages offered directly to the human host though HGT between autochthonous species of the gut microbiota, as well as between indigenous and transiently colonizing allochthonous species: (1) Introduction of new beneficial traits and rapid adaptation to environmental change, possibly within the life time of the host. (2) Generation of functional redundancy, increasing functional stability of the gut microbiota. Both proposed advantages of HGT to the human holobiont are supported by the hologenome theory of evolution.4
In contrast, there is also potential for such widespread HGT to introduce functions which may ultimately prove deleterious to the human host, such as antibiotic resistance.28–39 However, it would be expected that the introduction of overtly harmful traits into the human hologenome, including those that may destabilize important functions undertaken by our prokaryotic components (such as the gut microbiota), would be countered by holobiont level selection, and ultimately reduced in or eliminated from the gut microbiome. Traits such as antibiotic resistance may be exceptions, since carriage of antibiotic resistance genes does not appear to be directly harmful to the human holobiont, and results in detrimental effects only when harbored by community members able to cause disease, and then only when particular environmental stresses arise (exposure to relevant antibiotics).
Perhaps of greater importance is the potential for pathogens to acquire new functions from well adapted gut symbionts, which may include traits that facilitate colonization of the gut or inhibit treatment of infections. In this context the transfer of antibiotic resistance genes from members of the normal gut microbiota to transiently colonizing pathogens is cause for concern. Species naturally present in the human gut that survive in the external environment and cause disease at other body sites, such as uropathogenic E. coli, may also serve to disseminate antibiotic resistance and other genes from the gut microbiome.
Host fitness and rapid adaptation.
On the scale of the human holobiont, a major function of the mobile metagenome may be as a conduit for gene flow between the secondary prokaryotic portion of the human genome (in this case the gut metagenome) and the wider, external pool of prokaryotic genetic information. The mobile metagenome facilitates adaptation and evolution of the secondary prokaryotic fraction of the human genome by mediating the introduction of new traits into this community.71–74 These directly or indirectly affect the fitness of the human host, and in so doing, the human holobiont as a whole.
Adding functional capacity to the gut microbiota through HGT should be a faster route to acquiring new activities and adapting to new environmental factors,4,6 while entailing far less risk than recruiting new member species. Although attaining new traits by recruitment of new species is undoubtedly an important mechanism underpinning the evolution of the gut microbiome, and the development of this community in new hosts, in the case of an established community this entails the adaptation of an entire organism to the gut environment and its integration into a complex and pre-existing metabolic network, without compromising host fitness. In contrast the acquisition of new genetic material, and ultimately new functional capacity through HGT is more likely to maintain the status quo and essentially permits the upgrade of an already functioning and well adapted ecosystem.
Examples of holobiont adaptation mediated by microbial HGT, and the mobile metagenome of host associated microbial communities have been described previously.67–70,74,76–79 For instance, the symbiotic relationship between Rhizobium sp. and the roots of leguminous plants is heavily influenced by MGE encoded genes, with the majority of functions essential for symbiosis carried by plasmids, and frequently requiring the acquisition of several distinct MGE for successful host-microbe interaction.67,68 Here, Rhizobial plasmids have been found to encode essential functions which facilitate adaptation not only to plant-microbe symbiosis, but also to a free living lifestyle, such as tolerance of low pH, heat, drought or starvation.67,68,77–79 This strategy provides Rhizobium with a highly mobile gene pool that facilitates rapid adaptation to diverse and often transient ecological niches, and subsequently allows modulation of core community functions undertaken by the symbiotic microbiota to be adapted to the host legume.24,69 As such the Rhizobial mobile metagenome likely facilitates rapid adaptation of the plant-microbe holobiont as a whole.
A further example of how the mobile metagenome may encompass functions of direct benefit to the metazoan host comes from recent studies of the aphid symbiont Hamiltonia defensa.70 H. defensa protects its aphid host against attack from parasitoid wasps by killing wasp larvae before they can fully develop and bring about the demise of the aphid.70 Notably, the production of toxins necessary to kill wasp larvae is not directed by the principal genome of H. defensa, but acquired through infection of the microbial symbiont with a lysogenic bacteriophage carrying the necessary genes.70 In this instance, the phage encodes functions which essentially ensure that the habitat of its bacterial host (the aphid) is protected and therefore that suitable host bacteria will be available for replication. However, since aphids with a microbiota lacking this ability will be vulnerable to attack from parasitoid wasps, the benefit to the metazoan host will also result in selection for this attribute from the level of the aphid host, and therefore for the phage providing these functions. The lysogenic life cycle of this bacteriophage is notable in this context, and may in part result from selective pressure exerted by the aphid-bacteria-phage holobiont to provide a functionally stable gut microbiota, and minimize the disruption to key prokaryotic components of the holobiont that could arise from infection by lytic phage. Overall, this example demonstrates how the mobile metagenome can add functional capacity to the prokaryotic fraction of an organisms hologenome, and in so doing facilitate adaptation and influence evolution of the holobiont as a whole.
Recent comparisons of functional differences between the gut microbiomes of American and Japanese individuals have also provided evidence for HGT mediated adaptation of the human holobiont, and identified a role of the human gut mobile metagenome in this process. In this case a gain in function of direct benefit to the human host was identified in the Japanese gut microbiome, and its acquisition through HGT revealed.71 This functional gain relates to a primary activity of the human gut microbiome: the salvage of energy from the diet.2,14,80 It has been estimated that up to 10% of our daily calories are derived from microbial fermentation of plant polysaccharides resistant to host digestive mechanisms,80 which recovers energy from the diet that is otherwise inaccessible to the human host.
Seaweeds, and in particular Nori, are major components of the Japanese diet and marine bacteria colonizing these algae produce a range of porphyranse and agarase enzymes in order to utilize the polysaccharides they generate.71 Hehemann and co workers demonstrated the acquisition of porphyranse and agarase degrading ability by the gut microbiota of Japanese individuals, through horizontal transfer of these genes from marine bacteria naturally colonizing dietary seaweed (which are consumed without cooking), to members of the Japanese gut microbiota.71 In this case genes associated with conjugal transfer machinery were identified in regions of DNA surrounding the acquired porphyranase genes in the gut symbiont Bacteriodes plebius.71 These showed homology to plasmid gene sequences which encode the putative ancestral porphyranase genes in the candidate donor species of marine bacteria.71
Thus, it appears that this activity was acquired by members of the Japanese gut microbiota through conjugal transfer of plasmids encoding this ability from marine bacteria to gut symbionts. Subsequently its utility resulted in integration and fixation in the genomes of human gut symbionts, enhancing functionality of the Japanese gut microbiome. Notably, this appears to be an evolutionarily recent gain in function, and this activity could not be detected in any of the gut metagenomic datasets derived from American individuals analyzed in this study,71 indicating that the Japanese gut microbiota has evolved this trait as a specific adaptation to host diet. These findings reinforce the proposed adaptive role of the human prokaryotic genome segment in the human holobiont, and illustrate how the gut mobile metagenome can facilitate this process.
Evidence for a role of HGT in the evolution of other key functions of the gut microbiota relating to dietary energy salvage and maintenance of gut health, have also recently emerged. Carbohydrates that escape host digestive processes are fermented by the colonic microbiota resulting in the production of short chain fatty acids (SCFA).81,82 This process not only liberates additional energy from the diet that would not normally be accessible to the host, but certain SCFA are considered pivotal to gut health.81–83 Butyrate in particular has been associated with a range of beneficial effects,81–83 and not only is this SCFA a primary energy source for colonic epithelial cells, but has also been implicated in the regulation of epithelial cell proliferation.81–83 Recent characterization of butyrate synthesizing bacteria from the human colon has implicated HGT in the development of this pathway in intestinal bacteria.74 It seems likely that this originally arose as a general adaptation to the gut environment,74 and subsequently, this pathway may have been selected for at host-level due to its beneficial effects.
Overall, these studies demonstrate the capacity for the mobile metagenome to facilitate adaptation of the gut microbiota to new environmental conditions, such as composition of the host diet or environmental conditions in the GIT itself, and in doing so facilitate adaptation of the human holobiont as a whole. In particular, the capacity to rapidly adapt to new dietary components would serve the human holobiont by allowing new food sources to be exploited as they become available or when access to the normal food supply is restricted. It is easy to see how such dietary flexibility, and the ability to obtain the maximum possible energy yield from a wide range of food sources would benefit the ancestors of modern humans, for whom the source of each meal would almost certainly vary significantly.84,85
This may be reflected in the division of the genetic information encoding these important pathways of dietary energy salvage, which despite the obvious impact on host fitness, are not present in the primary eukaryotic portion of our genomes, but remain in the secondary, flexible prokaryotic segment. The reason for this, at least in part, may be the advantage of rapid adaptation offered by the prokaryotic metagenome, which through the mobile metagenome has access to a vast genetic resource. In the examples discussed here, HGT rather than changes in species composition, has facilitated the adaptation of the holobiont,67,69–71,74,76–79 supporting the hypothesis that HGT in the gut microbiota is of direct benefit to the human host.
This also affords the intriguing possibility that the observed degree of gene exchange extant in the gut microbial community may not only be due to the physical characteristics of the gut environment and intrinsic properties of species comprising this ecosystem, but also the result of host-level selection for activities of the gut microbiota which impact favorably on host fitness. However, more in depth studies of the relative levels of HGT between various host associated microbial communities and those not subject to host level selection are required to validate this theory.
The mobile metagenome and functional stability of the gut microbiota.
Core functions of the gut microbiota that impact on host health are ultimately selected for at the level of the human host, and there is pressure to ensure they are stable over time and consistently delivered by this community.8 However, in light of the plasticity of bacterial genomes and the potential for the species composition of the gut microbiota to fluctuate throughout the lifetime of the host, there is scope for considerable variation in the prokaryotic section of the human genome, and therefore the functional output of this community.2,3,8,63, 64 Despite this, in the adult human holobiont, the human gut microbiota is considered to exhibit a high level of stability in terms of the major core functions of this community, such as energy salvage.2,8
How this functional output is stabilized and maintained in spite of the variable nature of the prokaryotic metagenome has not yet been fully elucidated, but the generation of functional redundancy within this community is considered an important mechanism.8,20,21,72 Redundancy may be achieved through the wide spread distribution of genes underlying community level functions to diverse and disparate members of the gut community.8,20,21,72 This should guard against the loss of key activities and pathways from the community and allow the gut microbiota to maintain its overall functional output despite shifts in community composition.
Although redundancy of core functions may be achieved via the recruitment of diverse species with overlapping metabolic capabilities, evidence for a key role of HGT in generating functional redundancy in this community has also emerged.20,21,72–74 The movement of MGE between disparate species belonging to the mammalian gut microbiota has been demonstrated both in vitro, in vivo, and in silico,21,22,24–27,86–88 and HGT has already been linked with development of several primary activities of the gut microbiota which exhibit a high level of redundancy in this community.21,72,73,88,89 Therefore, the mobile metagenome has likely facilitated the dissemination of key traits involved in core community functions to a wide range of community members, playing a major role in developing a functionally stable ecosystem.21,24,72
In particular, it would be expected that challenges faced by most or all of the microbes colonizing a particular environment, would promote the acquisition of genes providing solutions from other species colonizing the same habitat.8,21,72 For bacteria comprising the human gut microbiota, the barriers to survival encountered in the gut environment must be overcome without adverse effects on host fitness, and the resulting host-level selection could drive the dissemination of relevant genes to many diverse members of this community, generating functional redundancy in the process.
The salvage of energy from the host diet is a prime example of a core function of the gut microbiota that exhibits a high level of redundancy and stability in this community.2,9,20,61,71–74 The ability of gut microbes to utilize carbohydrates and release energy to the host in the form of SCFA, appears to be distributed among a diverse array of species spanning the main bacterial divisions of the human gut microbiota (Bacteroidetes, Firmicutes, Actinobacteria and Proteobacteria).2,9,20,61,71–74 While it is likely that some of this redundancy is the result of early events in the development of the gut microbiota, reflecting the recruitment of species capable of colonizing and utilizing available nutrients without harming the host, recent studies have also highlighted the pivotal role of the mobile metagenome in the convergence and expansion of the required gene sets.71–74
Comparative genomic and metagenomic analyses of Bacteroides species from the normal human gut microbiota with non-gut Bacteroides sp. have highlighted the importance of HGT in the adaptation of gut-associated species to the human GI tract.73 This included the acquisition of genes related to the interaction of these organisms with the host immune system, as well as utilization of carbohydrates indigestible to the human host.73 This feature of the analyzed genomes also demonstrates that in these species MGE mediate access to a diverse genetic resource, through which adaption to new ecological niches and environmental conditions may be rapidly achieved. In a more global analysis, Lozupone et al. (2008) investigated the distribution of glycoside hydrolase and glycoside transferase encoding genes among 36 genomes representing the dominant bacterial divisions in the gut microbiota, as well as archaeal species.72 This illuminated the convergence of key carbohydrate active gene families among diverse members of the gut microbiota, and again indicated HGT as a major process facilitating the dissemination and expansion of gene sets involved in core community functions.72
Microbes inhabiting the human gut also face a number of direct barriers to colonization which must be overcome without reducing host fitness.21 Among these are the toxic effects of conjugated bile acids (CBA) encountered in the small intestine. HGT has also been implicated in the dissemination of genes which facilitate resistance to CBA (bile salt hydrolase: BSH) among a broad range of microbes in the gut community.21,24,88,89 The realization that bile acids also function as key signalling molecules regulating important aspects of host metabolism and immune function, means that bile acid modification by gut microbes also has the potential to greatly influence host physiology and development.21
Although our understanding of how this aspect of the gut microbiota relates to host health is currently limited, the very broad distribution of BSH activity and the resulting stability of this function in the community indicates host-level selection for this activity, and therefore a benefit to the human holobiont as a whole.21 In keeping with this, the general enrichment and excellent functional redundancy of BSH activity means that although overall capacity for this activity may fluctuate over the lifetime of the host in response to changes in population structure of the gut microbiota, it is highly unlikely that this function will be lost from an established gut community.21 It seems likely that much of the functional redundancy for BSH activity in this community has been achieved through HGT, perhaps even transcending barriers between distinct domains of life (Bacteria and Archaea).21,24,51,88,89
In our recent comparative metagenomic study of plasmids from the gut mobile metagenome, we also provided evidence for a high level of redundancy and general enrichment of certain toxin-antitoxin (TA) modules in this ecosystem.22 This was indicated by the broad phylogenetic distribution of the pTRACA22 type RelE toxin sequences among all major bacterial divisions in the gut microbiota. However, the role of these TA modules, if any, in the overall functioning of the gut microbiota remains to be established, and is discussed in subsequent sections of this article.
MGE Enriched in the Gut Mobile Metagenome
The proposed integration of the prokaryotic mobile metagenome into the human hologenome proper, leads to certain predictions about the composition of the pool of MGE comprising the gut mobile metagenome that may be investigated in more detail. In particular, if the gut mobile metagenome constitutes a component of the human hologenome as a whole, then the co-evolution of host and microbe should also be reflected in the MGE associated with the gut microbiota, and the functions encoded by constituent MGE will also be subject to selective pressure at the level of the human host.22–24
This hypothesis is supported by recent studies of gene convergence in gut associated bacterial species compared to non-gut species, in which plasmids carried by gut bacteria were found to reflect the overall signatures of increased specialization at short-phylogenetic distances and long-term gene convergence at greater phylogenetic distances, that were observed for bacterial chromosomes.49 In addition, the long-term convergence of gene content identified in gut associated plasmids also suggest the general functional conservation exhibited by the human gut microbiome of individual human hosts, may also extend to aspects of the gut mobile metagenome.49
As such it would be expected that the most successful and well adapted MGE in this community, which satisfy the demands of both bacterial and human hosts, will exhibit the greatest prevalence in the gut mobile metagenome, and be common in the gut microbiomes of a board range of individuals. These gut “specific” elements would be more likely to encode factors that provide bacterial hosts with an advantage in this environment, such as colonization of and survival in the gut, as well as to carry genes involved in host-microbe interaction and functions of the community influencing host health. MGE mediated host-microbe interactions have already been described in bacterial communities associated with plant and insect hosts, and in particular the example of bacteriophage encoded protection of aphid hosts from parasitoid wasps would appear to support this theory.67–70,76,79
Therefore, the identification and characterization of MGE enriched in the gut has the potential to greatly increase our understanding of the gut microbiota, and its interaction with the human host. However, cataloguing the MGE comprising the human gut mobile metagenome, and identification of gut enriched elements is a challenging task. In light of the enormous and predominantly uncharacterized diversity of the microbial world, the inherent promiscuity of MGE, and the methodological challenges associated with studying these elements, identification of MGE potentially unique to or enriched in the gut will not be straightforward.24 Furthermore, membership of the gut mobile metagenome, in accordance with the nature of MGE, is likely to be much less exclusive or committed than for the core commensal or symbiotic bacterial species comprising this community. As such this section of the human hologenome is likely to be a much less clearly definable gene-space than the principal prokaryotic gut metagenome. Nevertheless, several recent studies including our own, have provided evidence supporting the existence of gut specific MGE, and offered good candidate elements.
In our recent study, plasmids were isolated from the gut microbiome using the culture-independent TRACA system, which is capable of capturing plasmids from a wide range of bacterial species comprising the gut microbiota, and facilitating their maintenance in surrogate host species.22–24 The complete nucleotide sequences of six plasmids captured from the human gut microbiome were used to search a range of metagenomic data sets derived from the human gut,19,20 murine gut,14 marine90 and terrestrial environments.91 In this comparative metagenomic analysis, sequences homologous to two of our plasmids (pTRACA10 and pTRACA22) were detected in multiple human gut metagenomes, indicating a broad distribution.22 In contrast no sequences with homology to any of the six plasmids analyzed were identified in any of the non-human metagenomes studied.22
While none of the metagenomic data sets currently available afford complete coverage of their representative communities (particularly for environmental ecosystems), this nonetheless indicates the capture of human gut specific MGE. The presence of sequences homologous to pTRACA22 in particular, in multiple human gut metagenomes derived from individuals with broad geographic origins (America, Japan and Europe), suggests a gut specific family of mobile elements which has a deep co-evolutionary relationship with this community and global distribution.22
A similar broad global distribution and general enrichment for a family of conjugative transposons in the human gut microbiome has also been described in reference 20. Designated CTnRINT (Conjugative transposons rich in intestine), these elements are related to the Tn-1549 family CTns found in a range of gut associated pathogenic microbes including Enterococcus faecalis and Clostridium difficile.20 CTnRINT elements display similar gene architecture to Tn-1549 elements from other bacteria, but differ in an accessory gene region. It is possible that the genes encoded by this region of the CTnRINT elements relate to activities advantageous to life as a human gut commensal or symbiont.20 Alternatively, the current enrichment of CTnRINT, as well as other MGE in the human gut mobile metagenome, may be a manifestation of community adaptation to modern environmental stresses such as the use of antibiotics.20
Metagenomic studies have also revealed the high abundance of bacteriophage associated with all microbial communities including the human gut microbiome, where it is estimated a minimum of ∼1,000 distinct viruses exist (although this is likely to be a substantial underestimate).92 Because the host range for bacteriophage typically varies from a few closely related species down to individual strains, the existence of gut specific bacteriophage seems a likely prospect, and candidate phage apparently unique to the human gut microbiome have already been described in reference 93. Phage infecting a common gut commensal, Bacteroides sp. GB124 which has been tentatively identified as a strain of Bacteroides ovatus (Ebdon J, personal communication), were isolated from human feces.92 However, this phage was found to be absent from fecal samples derived from a wide range of common domestic and wild animals, and was not present in the general environment.93 Furthermore, widespread carriage among the human population was also indicated by this study, but the actual level of inter-individual variation in carriage of phage GB124 remains to be established.93 Currently, our understanding of how bacteriophage may influence human health is limited, but phage not only have the potential to alter microbial community dynamics and metabolic output by selective elimination of species within the gut microbiota, but may themselves interact directly with the host via the immune system.94
However, coverage of the gut community offered by the datasets utilized in our original analysis of plasmids resident in the human gut mobile metagenome is generally low, and it was not possible to retrieve the complete sequence of the most widely distributed plasmid, pTRACA22, from either individual or combined data sets.22 In total we found that 81.9% of the pTRACA22 nucleotide sequence was represented in these combined datasets at an identity of 90% or greater, and likely indicates the general enrichment and conservation of a closely related family of plasmids of which pTRACA22 is a member.22
This suggests that metagenomic data sets with greatly increased coverage of the gut microbiota will afford a concordant increase in the degree of coverage of the plasmids analyzed in our study. Furthermore, since the general conservation of plasmid pTRACA22 in the gut mobile metagenomes of geographically isolated individuals suggests their association with the human gut microbiota is ancient, some divergence in plasmids resident in the gut microbiomes of various ethnic groups is to be expected. As such, use of larger metagenomic data sets that are also derived from the same broad ethnic group (European) as the original pTRACA22 plasmid should also increase coverage of these plasmids. Therefore, the recently released METAHIT data set, which affords the greatest coverage of the gut metagenome to date, was utilized to extend our original analysis to a further 124 European individuals.10 As predicted, using this data set permitted a much greater proportion of the pTRACA22 nucleotide sequence to be retrieved, and in general homologous sequences with much higher identity were recovered for all plasmids analyzed in our original study (Fig. 1C). In total we were able to recover 99.4% of the pTRACA22 nucleotide sequence at an average identity of 97.7% or greater using the METAHIT data set (Fig. 1C).
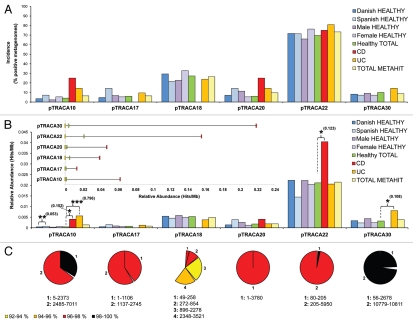
Figure 1.
Incidence and relative abundance of plasmids pTRACA10, pTRACA17, pTRACA18, pTRACA20, pTRACA22 and pTRACA30 in the METAHIT gut metagenome data set. The complete nucleotide sequences of each plasmid were used to search the METAHIT10 dataset using Blastn, and calculate incidence and relative abundance according to methods described previously in reference 21 and 22. For this analysis, only hits matching the following criteria were considered significant: An identity of 90% or greater over 100 nucleotides or more, and an e-value of 1 e−10 or lower. In addition to the complete METAHIT data set, incidence and relative abundance of plasmids in sub sets of individuals represented in the METAHIT dataset was also explored based on nationality (Danish or Spanish), gender, and disease status (healthy, Crohn disease or ulcerative colitis). For the purpose of this analysis individuals were designated as healthy unless indicated as being diagnosed with ulcerative colitis (UC) or Crohn disease (CD), regardless of age or body mass index. The statistical significance of observed incidence and relative abundance data was explored using the χ2 distribution.22 Where significant differences in relative abundance of plasmids between metagenomic datasets were identified, the statistical power afforded by the available sample sizes in each group was calculated using the Piface program.111 This indicates the probability that the observed differences are truly reflective of the wider population, with a probability of 0.8 considered to indicate adequate power. Symbols above bars indicate the level of significance in χ2 analysis, *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. Statistical power is shown in adjacent parentheses, and in all cases has been computed to p = 0.05 using variation (standard deviation) observed within the total METAHIT dataset. Danish HEALTHY, Spanish HEALTHY = Combined gut metagenomes from individuals of each nationality in the METAHIT data set, excluding those diagnosed with IBD; Male HEALTHY, Female HEALTHY = Combined gut metagenomes from individuals of each gender in the METAHIT data set, excluding those diagnosed with IBD; Healthy TOTAL = Combined gut metagenomes from all healthy individuals in the METAHIT dataset; CD, UC = Combined gut metagenomes from individuals diagnosed with respective IBDs; TOTAL METAHIT = Combined gut metagenomes of all 124 individuals regardless of nationality, gender or disease status. (A) Shows the incidence of each plasmid (and closely related elements) among the gut metagenomes comprising the METAHIT data set, when grouped by nationality, gender and disease statues, as well in the METAHIT cohort as a whole. Incidence is expressed as the percentage of metagenomes in which at least one significant hit to each plasmid was detected, (according to criteria outlined above). In this analysis, only differences found to be supported by adequate statistical power (0.8 at p = 0.05) were considered significant overall. (B) Shows the relative abundance of each plasmid, expressed as hits/Mb, among the gut metagenomes comprising the METAHIT data set, as grouped in (A).21,22 For this analysis relative abundance was calculated based on the total number of significant hits (according to criteria outlined above) in each group (gender, nationality, disease status), and the average relative abundance in each cohort displayed. INSET: Shows the overall inter-individual variation for each plasmid among all 124 individuals in the METHIT dataset, with yellow bars representing lowest observed abundance, red bars highest observed abundance, and green bars showing the average abundance over all 124 individuals. (C) Shows the percentage of each plasmid nucleotide sequence that could be recovered from the total METHIT dataset. Colors of segments represent % identity of recovered METAHIT sequences corresponding to respective regions of each plasmid, and numbers below pie charts provide the coordinates in the complete plasmid sequence for each segment. Yellow segments = 92–94% identity; orange segments = 94–96% identity; red segments = 96–98% identity; black segments = 98–100% identity.
The METAHIT data set also permitted the retrieval of sequences homologous to several other plasmids characterized in our recent study, which were either poorly represented in the American and Japanese data sets originally utilized (pTRACA18), or for which no sequences with significant homology could be retrieved (pTRACA17, pTRACA20, pTRACA30; Fig. 1).22 Notably pTRACA22 still exhibited significantly higher relative abundance (p = 2–25 or lower) in these human gut microbiomes compared to the other plasmids, and the greatest incidence of the six plasmids with sequences homologous to pTRACA22 detected in 73.3% of the 124 individual metagenomes comprising the METAHIT data set (Fig. 1). However, it is not clear if this is solely due to the greater coverage afforded by the METAHIT data set, or is a result of its European origin and therefore a reflection of variation in the gut mobile metagenomes of different ethnic groups, but is likely a combination of both factors.
Furthermore, the plasmids isolated in our original analysis range in size from 3.7 to 10.8 kb. It is possible that this size range reflects a restriction of the TRACA system used to isolate them, the dominance of smaller plasmids in the gut microbiome, or a combination of these factors.22–24 While the smaller size of these plasmids do not alter the observation that some plasmid families are generally enriched and broadly distributed in the human gut microbiome,22 similar studies of larger plasmids would be useful in understanding how plasmid size may relate to coverage in metagenomic datasets.
Overall, MGE potentially enriched in the human gut mobile metagenome such as pTRACA22 family plasmids, CTnRINT type transposons and phage GB124, along with numerous as yet uncharacterized gut specific or gut enriched MGE, could conceivably constitute the mobile metagenome equivalent of a conserved “phylogenetic core”. It also seems most probable that MGE enriched in the human gut mobile metagenome will be associated with numerically dominant members of this community. Given the potential for a bacterial host to support a range of MGE, those elements that are most frequently associated with abundant, and presumably successful and well adapted species, in the gut microbiota are likely to confer selective advantages. While enrichment of these elements or association with numerically dominant members of the community does not necessarily indicate a role in community function or survival in the gut environment, characterization of such elements is most likely to provide insights into the role of MGE in the development and functioning of the gut microbiota, particularly given that plasmids (and most likely other MGE comprising the mobile metagenome) also appear to be susceptible to long-term habitat associated convergence of gene content in this environment.49
Plasmid Encoded Functions Enriched in the Human Gut Microbiome
In light of the prevalence of certain MGE within the human gut mobile metagenome, and the prominent role of HGT in the dissemination and ultimate enrichment of genes involved in core community level outputs, the overall abundance of genes and functions encoded by gut specific MGE is of considerable interest. It would be expected that the genes and functions encoded by MGE enriched in this community exhibit an increased prevalence in the gut microbiome as a whole. This is indeed the case for genes encoded by the CTnRINT elements described by Kurowkawa et al. (2006),20 as well as certain genes encoded by the pTRACA10 and pTRACA22 plasmids analyzed in our study.22 For CTnRINT, genes encoded by this gut associated family of MGE were found to account for around 0.8% of the 5,325 predicted ORFs identified in the combined metagenomic sequence data from 15 Japanese gut microbiomes.20 These genes were also found to be enriched in gut metagenomes of American individuals, highlighting CTnRINT as a long standing member of the gut mobile metagenome.20
Utilizing these same metagenomic data sets, we also observed the enrichment of several ORFs encoded by plasmids pTRACA10 and pTRACA22 in the human gut metagenome.22 These enriched ORFs cover a wide range of functions and include genes for phosphoesterases or phophohydrolases, replication proteins, a TA addiction module, as well as genes of unknown function.22 Of particular interest was the increased prevalence of a putative RelBE TA addiction module encoded by pTRACA22, which was unexpected in this community.22 While TA modules are generally highly abundant in free living bacteria and archaea (i.e., those not forming obligate intracellular relationships with eukaryotic host cells),95 further analysis revealed that this enrichment in the gut was confined to homologues of the pTRACA22 type RelBE module, and was not observed for MazEF, ParDE or HigBA modules.22 This naturally leads to the following questions: why is this particular family of TA modules enriched in the human gut microbiota? What role, if any, do these genes play in the overall operation of the gut microbial community?
In addressing the first question, it is important to consider whether the observed enrichment results from selection for these modules due to benefits conferred on the bacterial or human host, or is the result of the addictive nature of these modules alone.96 Perhaps the simplest explanation for the enrichment of the pTRACA22 type RelBE modules identified in our study, stems from the latter possibility, and relates to the potential for these gene systems to exist purely as selfish entities.96 It is conceivable that these pTRACA22 type TA modules present no appreciable impact on host fitness, and in terms of the gut microbiota and the human holobiont as a whole, are evolutionarily neutral.
This explanation does not appear to account for the differential enrichment of the pTRACA22 RelBE family of TA modules over other TA systems, and perhaps even over other RelBE variants.22 However, the observed trend in relative abundance of the TA systems we examined fits comfortably with this “selfish DNA” hypothesis, since there is no evidence at present which excludes the possibility of selection against the other TA systems, rather than explicit selection for the pTRACA22 sub-family. Therefore, the prevalence of the pTRACA22 type RelBE modules we identified may simply result from their addictive nature, coupled with a neutral or insignificant impact on fitness of the bacterial and ultimately the human host.
If this is so, then TA modules expanded in microbial communities such as the human gut may be under pressure to minimize any adverse effects on bacterial or human hosts. In this context it is notable that among the pTRACA22 RelE homologues we retrieved from the human gut metagenomes analyzed, certain amino acid residues were found to be completely or predominantly conserved specifically among gut associated RelE.22 This observation is more significant in light of the broad phylogenetic distribution identified for the pTRACA22 type RelE toxin sequences, and while the majority of these were affiliated with members of the Firmicutes division, representatives also grouped with members of the Bacteroidetes, Actinobacteria and Proteobacteria.22 Together, a broad phylogenetic distribution, and a conserved gut associated sequence motif, indicates the development and expansion of a gut specific RelE sub type. However, the “selfish DNA” hypothesis for the observed enrichment of pTRACA22 type RelBE modules in the human gut microbiome is neither supported, nor refuted by our findings, and further investigation of the role of these systems in the gut microbiota is required to test this hypothesis.
Alternatively, the addictive properties of TA modules could account for the observed enrichment through more nefarious mechanisms which lead to perturbations in metagenomic data sets. The contribution of TA modules to generational stability of plasmids is well established, and plasmid encoded TA modules ensure that daughter cells retain a copy of the plasmid through a post segregation killing mechanism (PSK).95,97,98 Loss of the plasmid effectively results in loss of the unstable antitoxin, leading to cell death or growth arrest through the continued activity of the stable toxin component which persists in plasmid free cells.95,97,98 PSK may account for the enrichment of certain RelBE TA modules by stabilizing metagenomic clones in the surrogate E. coli hosts used to construct metagenomic libraries. This could lead to the artificial enrichment of TA module harboring clones in the final metagenomic data set. In addition, the issue of biases generated by use of different extraction procedures and sequencing strategies used to generate metagenomic data sets, may also be an underlying factor in the observed trends. Despite this, there is much evidence indicating that the prevalence of pTRACA22 type TA modules in the human gut microbiome is not the result of bias in these data sets, and reinforces the utility of available metagenomic data.
The existence of orphan toxin genes identified in metagenomic sequences argues against bias induced by TA mediated stabilization of metagenomic clones, and no significant differences in terms of relative gene abundance were identified between the Japanese and American data sets utilized in our previous study.22 This is in spite of the reported paucity of sequences from Bacteroides sp. in the latter data set.19 Furthermore, the differential enrichment of pTRACA22 family RelBE modules compared to other TA systems in itself points to enrichment though specific selective effects, rather than a general cloning bias. This is reinforced by the well established presence and functionality of the TA systems we examined in E. coli (RelBE, MazEF, ParDE, HigBA).95,99
However, to investigate this issue more definitively, the METAHIT data set was again utilized,10 and the relative abundance of the pTRACA22 RelBE family of TA modules in the combined gut metagenomes of the 124 individuals represented was assessed (Fig. 2). This illuminated the large inter-individual variation in the relative abundance of pTRACA22 like RelBE modules between the individuals in this dataset (Fig. 2A), and demonstrated a pervading trend of a higher abundance of RelB anti-toxin in the majority of individuals (Fig. 2B). The incidence of these pTRACA22 like RelBE TA modules was high, and we were able to detect homologous TA modules in 93% of individuals represented in the METAHIT data set (Fig. 2B).
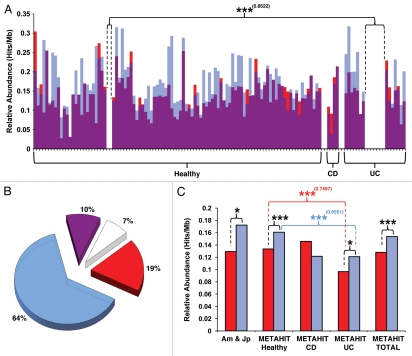
Figure 2.
Relative abundance of pTRACA22 type RelBE modules in the METAHIT dataset and variation in inflammatory bowel disease. The relative abundance of pTRACA22 type RelBE modules in the gut microbiomes of individuals represented in the METHIT10 dataset was calculated exactly as described previously in reference 22. METHIT data was searched with pTRACA22 RelB and RelE amino acid sequences using tBlastn to identify homologous sequences, and only hits generating e-values of 1 e−5 or lower with a length of 30 aa or over were considered significant, and used to calculate hits/Mb.22 For the purpose of this analysis individuals were designated as healthy unless indicated as being diagnosed with ulcerative colitis (UC) or Crohn disease (CD), regardless of age or body mass index. Statistically significant differences in relative abundance or incidence of RelBE modules within and between metagenomic datasets were explored using the χ2 distribution.22 Where significant differences in relative abundance of RelBE homologues were identified within or between groups comprised of metagenomes from the METAHIT dataset, the statistical power afforded by the available sample sizes in each group was calculated using the Piface program.111 This provides the probability that the observed differences reflect the wider population, with a probability of 0.8 generally considered to indicate adequate power. Symbols above bars indicate the level of significance in χ2 analysis, *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. Statistical power is shown in adjacent parentheses, and in all cases has been computed to p = 0.05 using variation (standard deviation) observed within the total METAHIT dataset. (A) Shows the relative abundance of pTRACA22 type RelBE homologues in the individual gut microbiomes of all 124 individuals represented in the METAHIT dataset. Each bar represents the relative abundance data (Hits/Mb) for RelB (blue) overlaid with that of RelE (red). Purple regions show the least abundant ORF in each individual metagenome, while the color of upper regions indicates the abundance of the most prevalent ORF in each individual metagenome. Individuals in which the RelB anti-toxin is most abundant are indicated by blue upper regions of bars, while those in which the RelE toxin is most abundant are indicated by red upper regions of bars. Individuals in which the level of RelB and RelE are equal are indicated by exclusively purple bars. (B) Shows the percentage of individuals represented in the entire METAHIT dataset with varying ratios of RelB:RelE abundance. Blue = % of individuals in which RelB dominates, red = % of individuals in which RelE dominates, purple = % of individuals with equal relative abundance of both RelB and RelE; white = % of individuals in which neither RelB nor RelE could be detected within the criteria employed in this study. (C) Comparison of overall relative abundance in combined human gut metagenomes of Japanese and American individuals (utilized in our original analysis), and the METAHIT dataset.10,19,20 Red bars show relative abundance of RelE homologues and Blue bars show relative abundance of RelB homologues. Am&Jp = American and Japanese gut metagenomes utilized in our original study;19,20 METAHIT Healthy = Combined gut metagenomes of individuals from the METAHIT dataset without IBD; METAHIT CD = Combined gut metagenomes of individuals from the METAHIT dataset diagnosed with Crohn disease; METAHIT UC = Combined gut metagenomes of individuals from the METAHIT dataset diagnosed with ulcerative colitis; METAHIT TOTAL = Combined gut metagenomes of all 124 individuals represented in the METAHIT dataset.
Notably the METHIT data set was generated using distinctly different methods for DNA extraction, sequencing, and library construction than the previously analyzed data sets, including the use of small and very small insert libraries unlikely to harbor intact TA modules.10 Regardless of these differences in methodology, no significant differences were observed in the relative abundance of RelBE modules between our previous analysis (based on the combined American and Japanese datasets)19,20 and that calculated from the METAHIT data (Fig. 2C).10 The variation in pTRACA22 type TA module abundance between the data sets utilized in our original study and healthy individuals in the METAHIT dataset was found to be 0.004 and 0.012 hits/Mb for RelE and RelB respectively. Thus it appears highly unlikely that the observed enrichment of the pTRACA22 family of RelBE TA modules is an artefact of the metagenomic approach.
Apart from the “selfish DNA” hypothesis, there also remains the possibility that the pTRACA22 family RelBE modules have been selected due to a beneficial function or attribute they confer upon members of the gut community or even the human host. This leads back to the question of what role these gene systems may play in the human gut microbiota or for the human holobiont as a whole, and there are a range of possible answers. TA modules have been associated with a wide variety of activities which may be relevant to microbes inhabiting the human gut. These include contribution to fitness of the bacterial host through modulation of gene expression,95,100,101 the establishment of new regulatory gene networks,102 formation of persister cells and resistance to environmental stresses such as nutrient limitation,95,103,104 as well as the potential for direct host-microbe interaction through toxic effects of RelE on eukaryotic cells.105–107 Known or proposed functions of TA modules that may be relevant to the human gut microbiome are summarized in Table 1 along with the potential utility of these attributes to gut microbes and implications for both the human host and microbiota as a whole.
Table 1.
Proposed or confirmed functions of TA addiction modules and relevance to the human gut microbiota
| Confirmed or proposed function of TA modules | Significance/relevance to human gut microbiome1 |
|
The stabilization of plasmids though post-segregational killing (PSK) by TA modules should allow the retention of plasmids within the gut microbiome in the absence of direct selective pressure for functions they encode.24 Chromosomally encoded TA modules may stabilize neighbouring gene regions, and ensure vertical transmission of associated genes is maintained even in the absence of direct selective pressure for functions they encode.95 Together, these stabilization effects may serve to retain important functions within the gut microbiome that are not under continual selection. These may be functions required only intermittently, or during key stages of holobiont development, such as transmission of the microbiota to new hosts, or initial formation of the community. |
|
Chromosomally encoded TA modules may function as anti-addiction modules, preventing PSK by plasmid encoded TA systems and destabilising the plasmid within the host bacterial population.112 Both plasmid and chromosomally encoded TA systems may also function as phage exclusion mechanisms, restricting the capacity of bacteriophage to infect a bacterial population.113 These attributes could constitute a mechanism which controls the rate of HGT in the gut microbiome by restricting the range of MGE that may participate in this process. TA modules may also limit the impact of bacteriophage on the gut microbiota, stabilising the community against bacteriophage “attacks”. This attribute fits well with the observed enrichment of pTRACA22 type RelBE modules in the gut microbiome,22 and is of particular interest in light of the potential role of bacteriophage in dysbiosis of the gut microbiota in CD.109 |
|
Specific mechanisms through which plasmids and other MGE alter global gene expression of the bacterial host have recently been described.45,114 Modulation of gene expression and the formation of new regulatory gene networks in bacterial cells have also been linked with TA modules.95,100–102 These potential functions of TA systems could serve to facilitate rapid adaptation of gut associated bacterial species to the GIT, or changing conditions within this environment. This may be of significance during colonization of pristine gut environments, as well as survival of bacterial cells in the external environment during transmission to new hosts. This feature of TA systems may also allow members of an established gut microbiota to deal with shifts in environmental conditions, such as changes in host diet. |
|
TA modules have been consistently associated with the formation of persister cells, and survival of environmental stresses.96,104,105 These include nutrient limitation and exposure to antimicrobial agents, both of which are likely to be encountered during colonization of the human gut. In addition, the ability to survive under nutrient limiting conditions may also be of benefit to members of an established community. For example the ability to survive under nutrient limiting conditions may be important during changes in host diet which reduce the levels of usable nutrients to particular members of the gut community, or during periods of host starvation. In particular, wide variation in diet and periods of starvation were undoubtedly more common in the ancestors of modern humans, and access to a consistent food supply is still a problem for a significant fraction of the human population alive today. Alternatively, the association of persister cells with survival of exposure to antimicrobial agents, may indicate a potential role for TA systems in the ability of the gut microbiota to recover following exposure to antibiotics. |
|
The recent finding that TA modules may play a role in regulating aspects of biofilm formation is also of relevance to the human gut microbiome.115 The formation of biofilm communities associated with the gut mucosa, or the surface of indigestible food particles, is generally considered to be an important and consistent behaviour in the gut microbial community, which may be involved in the persistence of microbes in the gut, and also in the pathogenesis of intestinal diseases.116,117 |
|
Perhaps the most contentious of the proposed functions for RelBETA modules is the potential for a direct role of these gene systems in host-microbe interaction.22 This theory stems from the observation that RelE toxins are active in eukaryotic cells via the same mechanism employed to inhibit growth of or kill bacterial cells.105–107 Importantly, in cultured human cells expression of bacterial RelE leads to cell death by induction of apoptosis. As such these systems may contribute to homeostasis of the intestinal epithelium, as has been documented for other activities of the gut microbiota. However, this activity has to date only been observed using artificial mammalian gene expression systems,105–107 and it is not yet known if RelE expressed by microbial cells in the gut microbiota, or in even in model co-culture systems, will recreate these effects. The observed enrichment of the pTRACA22 type RelBE in the human gut microbiome argues for further study of this potential activity of RelBETA modules.22 |
Previously presented functions or activities of TA modules which may be relevant to the human gut microbiota are described, and the significance of these functions to the gut microbiota is considered. All functions of TA that are discussed are based on previous studies which have provided evidence for particular roles of TA modules, and relevant literature cited. However, further research is required to ascertain the relevance of these functions, and the enriched pTRACA22 type RelBE modules, if any, to the human gut microbiome.
Importantly, in the case of mechanisms underlying bacterial colonization and persistence in the gut, these must not only permit bacteria to overcome particular environmental stresses, but must do so without any negative effect on fitness of the human host, and TA modules would appear to fulfil this remit. It is also conceivable that discrete functions mediated by TA modules are not only differentially selected for in various host bacteria, (with different host species benefiting from distinct functions of the same TA module), but are also of most benefit at particular stages in the development of the gut microbiota or under specific environmental parameters which are not a constant feature of the gut environment. If so, the observed prevalence of these TA modules may result from the wide range of functions potentially mediated by TA (Table 1), which make them of benefit to a broad range of community members under a wide range of conditions. The addictive properties of these gene systems would only serve to reinforce any positive selection for these modules and stabilize them within the community.
When considered from the perspective of the human host and within the context of the hologenome theory of human evolution, there also exists the possibility that the primary reason for the observed enrichment may be selection for these pTRACA22 type RelBE TA modules at the level of the human host. In this case, carriage of these modules may impose a fitness cost on the bacterial host which is ultimately offset by increased fitness of the holobiont as a whole. However, further study is required to determine if these modules simply persist as selfish entities in the human hologenome, with no tangible impact on the human holobiont or contribute to functions undertaken by the gut microbiota and its interaction with the human host.
MGE, Community Output and Intestinal Disease: A Preliminary Investigation
Our original comparative metagenomic analysis also hinted at the inter-individual variation of the gut mobile metagenome,22 and this has been further supported by the analysis of the METAHIT dataset presented in this article (Fig. 1). Variation in this sphere of the human hologenome, as with other sections, has the potential to contribute to differences in the metabolic output of gut communities between individuals.22 In this respect the gut mobile metagenome may be an auxiliary, flexible gene pool that introduces additional variation at a level distinct from that of the principal gut metagenome (which is based on the core genome content of constituent species). Changes in the structure and metabolic output of the human gut microbiota are being increasingly linked to human disease, and there is potential for the mobile metagenome to contribute to such changes.
Bacteriophage in particular may be involved in the pathogenesis of disorders such as inflammatory bowel diseases (IBD). As well as mediating HGT and harboring genes involved in important activities of the gut microbiota, bacteriophage may also be a major force behind changes in the species composition of the gut community within an individual. Selective attack on dominant species in the gut microbiota opens new niches for less abundant members, and phage driven population shifts are well documented in other bacterial ecosystems.108 This aspect of phage-bacteria interaction is of particular significance in light of recent observations by Lepage et al. (2008),109 who described distinct differences in abundance of bacteriophage between healthy individuals and those suffering from Crohn disease (CD). This raises the possibility that bacteriophage drive the dysbiosis of the gut microbiota observed in CD patients, and that phage driven shifts in community structure may be involved in disease initiation or progression.109 Alternatively, the observed potential for bacteriophage to translocate from the gut environment and themselves act as antigens raises the possibility that phage play a direct role in the pathogenesis of CD,94 and also fits with their increased prevalence in CD patients.109 In both hypotheses, the relapsing and remitting nature of CD may correspond to predator-prey population dynamics of relevant phage and their bacterial hosts.
In the case of the human gut specific phage infecting Bacteroides GB124, the observation that the host species for these phage may be a strain of Bacteroides ovatus also has implications for their involvement in CD. The titre of serum antibodies against Bacteroides ovatus is significantly elevated in patients with CD, in contrast to that of other common gut associated Bacteriodes sp. (B. fragilis and B. vulgatus).110 While it is presently unclear if this is indicative of a role for B. ovatus in the pathogenesis of CD, the immunogenicity of this organism, and the increased levels of both IgG and IgA anti-ovatus antibodies in CD patients, suggests a possible protective role for bacteriophage capable of killing this organism.
The METAHIT data set also allowed us to explore more fully the incidence and relative abundance of plasmids captured in our original study (pTRACA10, pTRACA17, pTRACA18, pTRACA20, pTRACA22 and pTRACA30) among individuals of different nationalities, genders or disease status (Fig. 1). For all plasmids a large inter-individual variation in incidence and relative abundance was observed (Fig. 1B inset), but when group data was analyzed, no significant difference between healthy individuals by nationality or gender was identified, with the exception of relative abundance of pTRACA10 in healthy Spanish compared to healthy Danish individuals, although power calculations indicated this is almost certainly not a true representation of the wider population (Fig. 1B). The incidence of all plasmids was found to vary between different groups, particularly healthy individuals and those diagnosed with CD, but no significant differences were observed (Fig. 1A). However, significant differences in relative abundance of pTRACA10, pTRACA22 and pTRACA30 were identified in individuals with CD or ulcerative colitis (UC), compared to healthy individuals, but in most cases available sample sizes do not provide adequate statistical power to infer an association in the wider population (Fig. 1B).
Interestingly, the relative abundance of pTRACA22 type TA modules was also altered in both CD and UC disease states (Fig. 2C). In the CD cohort the pervading trend for higher abundance of RelB antitoxin over the RelE toxin in healthy individuals was reversed, with RelE toxin elevated and RelB antitoxin reduced in comparison to healthy individuals and those with UC, although this was not statistically significant (Fig. 2C), despite the distinct separation of the gut microbiomes of healthy individuals and those with CD and UC reported by Qin et al. (2010).10 In the case of UC, a statistically significant reduction in both RelB and RelE homologues was observed, and analysis of individual gut metagenomes showed that in 33.33% of this cohort no pTRACA22 type RelBE modules could be detected, compared to only 2.02% of individuals in the healthy group (Fig. 2A).
These observations potentially associate pTRACA22 type TA modules with aspects of these diseases (Fig. 2). While several suggested functions of TA modules, particularly their potential toxicity towards eukaryotic cells (Table 1),105–107 appear to fit with an association with IBD, the actual role of these gene systems in the human gut remains unknown, and toxicity of RelE has not yet been demonstrated outside artificial delivery to, and expression in mammalian cells via specialized vectors.105–107 Overall, the most probable explanation remains that the observed changes are the result of the well established population shifts characteristic of CD, rather than a direct involvement in the disease, and it is perhaps not surprising that the mobile metagenome also appears disrupted in individuals diagnosed with these diseases.
While these initial findings are intriguing and indicate that this aspect of the gut metagenome should be subjected to further study, it should also be noted that only a small number of individuals with CD or UC (n = 4, and n = 21 respectively) are present in the METAHIT data set. Although significant differences were observed between the metagenomes analyzed here, power calculations clearly demonstrate that in most cases the small numbers of gut metagenomes from individuals with CD or UC do not provide adequate power (generally accepted to be 0.8 at p = 0.05). Where statistical power is low, the probability that the observed differences are truly reflective of the wider population is correspondingly low, and as such any apparently significant differences should be treated with caution.
However, adequate statistical power appears to be available to demonstrate the greater number of individuals in which pTRACA22 type RelBE modules were undetected among the UC group (Fig. 2A); and possibly the greater relative abundance of pTRACA10 in individuals with UC compared to healthy individuals (power = 0.796; Fig. 1C). Although, for functions analyzed here, even when sufficient samples are available to provide the required statistical power, it is not yet clear if a statistically significant difference between gut metagenomes from distinct groups equates to any meaningful biological effect and vice versa.
Nonetheless, although far from proven a role for the mobile metagenome in intestinal disease cannot yet be precluded, particularly as MGE may also be enriched or reduced by the microbial dysbiosis characteristic of IBD (Fig. 1B). These initial findings are in agreement with numerous other studies, including the original METHIT study,10 showing differences in structure and function of the gut microbiome in IBD patients and potentially extends the dysbiosis of the gut microbiota observed in IBD to the mobile metagenome and specifically the plasmid population (Fig. 1B). Such disturbances could lead to alterations in the abundance of MGE and the genes they encode, including pTRACA22 type RelBE modules, in the gut microbiomes of individuals with IBD and therefore perturb or amplify any effects they may have on the human host. Further research will be required to substantiate or refute these preliminary findings, establish their relevance to initiation, progression or diagnosis of IBD, and answer the question: Do attributes of, or functions encoded by, the human gut mobile metagenome play any role in the pathogenesis of IBD or other intestinal disorders?
Summary
If we accept the paradigm of humans as chimeras of both eukaryotic and prokaryotic cells, with the gut microbiota constituting a “virtual organ”, and forming an integral part of the human (holo)genome, then we must also accept that our knowledge of how our bodies function, and the mechanisms of many prevalent diseases will remain obscure until we have also gained an in-depth understanding of our prokaryotic components. The gut microbiota especially is far more versatile than any of our own tissues (both metabolically and genetically), and undoubtedly contributes greatly to differences in health and disease risk, not only between individuals but also within an individual over time.
The gut mobile metagenome is a particularly enigmatic and little explored aspect of the human holobiont. MGE encoding genes required for bacteria to establish symbiotic relationships with metazoan host organisms, as well as MGE conferring a direct benefit to the metazoan host, have already been described among the mobile metagenomes of microbial communities colonizing plants, invertebrates and also humans.24,67–69,71,76–79 Therefore genes important for life in the gastrointestinal tract, as well as activities that impact on host-microbe or microbe-microbe interactions, are likely to be encoded by the gut mobile metagenome.
Perhaps of more significance is the overall capacity of the mobile metagenome to move information both into and out of the gut community. This has profound implications for the human holobiont, in that the prokaryotic mobile metagenome effectively links the human hologenome with the wider prokaryotic world. In this view, the hologenome of an individual human may be likened to an island in an ocean of prokaryotic genetic information. The primary eukaryotic core of the human hologenome constitutes the rocky heart of the island which changes only slowly. Our principal prokaryotic metagenome sits at the interface between the island and the ocean forming a beach of relatively stable but ever shifting sands. The tertiary mobile metagenome forms the shallow waters of the surrounding reef which connects to the wider ocean beyond, and through which new genetic material enters the lagoon, washes onto the prokaryotic beach and is distributed along the shore.
Acknowledgements
I would like to thank Dr. Caroline Jones for valuable discussion and constructive criticism of the manuscript, and Elizabeth Cheek for expert advice regarding statistical analyses of the data presented. Research in the laboratory of Dr. B.V. Jones is currently supported by funding from the Medical Research Council, The Hospital Infection Society, The Society for Applied Microbiology, The Royal Society and the Centre for Biomedical and Health Science Research at the School of Pharmacy and Biomolecular Sciences, University of Brighton.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/14108
References
- 1.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 3.O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev. 2008;32:723–725. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg E, Sharon G, Zilber-Rosenberg I. The hologenome theory of evolution contains Lamarckian aspects within a Darwinian framework. Environ Microbiol. 2009;11:2959–2962. doi: 10.1111/j.1462-2920.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 6.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilks M. Bacteria and early human development. Early Hum Dev. 2007;83:165–170. doi: 10.1016/j.earlhumdev.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Turnbaugh PJ, Quince C, Faith JJ, McHardy AC, Yatsunenko T, Niazi F, et al. Organismal, genetic and transcriptional variation in the deeply sequenced gut microbiomes of identical twins. Proc Natl Acad Sci USA. 2010;107:7503–7508. doi: 10.1073/pnas.1002355107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin J, Li R, Raes J, Arumugaum M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet JP, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11:2574–2584. doi: 10.1111/j.1462-2920.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 12.Backhed F, Ding H, Wang T, Hooper LV, Koh Y, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 2006;127:423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 15.Hooper LV, Xu J, Falk PG, Midtvedt T, Gordon JI. A molecular sensor that allows a gut commensal to control its nutrient foundation in a competitive ecosystem. Proc Natl Acad Sci USA. 1999;96:9833–9838. doi: 10.1073/pnas.96.17.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stappenback TS, Hooper LV, Gordon JI. Development regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoetendal EG, Vaughan EE, de Vos WM. A microbial world within us. Mol Microbiol. 2006;59:1639–1650. doi: 10.1111/j.1365-2958.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- 18.Lombardo P. Access to mutualistic endosymbiotic microbes: an underappreciated benefit of group living. Behav Ecol Sociobiol. 2008;62:479–497. [Google Scholar]
- 19.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurokawa K, Itoh T, Kuwahara T, Oshima K, Toh H, Toyoda A, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14:169–181. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones BV, Begley M, Hill C, Gahan CGM, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA. 2008;105:13580–13585. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones BV, Sun F, Marchesi JR. Comparative metagenomic analysis of plasmid encoded functions in the human gut microbiome. BMC Genomics. 2010;11:46. doi: 10.1186/1471-2164-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones BV, Marchesi JR. Transposon-aided capture (TRACA) of plasmids resident in the human gut mobile metagenome. Nat Methods. 2007;4:55–61. doi: 10.1038/nmeth964. [DOI] [PubMed] [Google Scholar]
- 24.Jones BV, Marchesi JR. Accessing the mobile metagenome of the human gut microbiota. Mol Biosyst. 2007;3:749–758. doi: 10.1039/b705657e. [DOI] [PubMed] [Google Scholar]
- 25.Nikolich MP, Hong G, Shoemaker NB, Salyers AA. Evidence for natural horizontal transfer of tetQ between bacteria that normally colonize humans and bacteria that normally colonize livestock. Appl Environ Microbiol. 1994;60:3255–3260. doi: 10.1128/aem.60.9.3255-3260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salyers AA, Shoemaker NB, Stevens AM, Li LY. Conjugative transposons: an unusual and diverse set of integrated gene transfer elements. Microbiol Rev. 1995;59:579–590. doi: 10.1128/mr.59.4.579-590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazimierczal KA, Scott KP, Kelly D, Aminov RI. Tetracycline resistome of the organic pig gut. Appl Environ Microbiol. 2009;75:171–1722. doi: 10.1128/AEM.02206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoemaker NB, Vlamakis H, Hayes K, Salyers AA. Evidence for extensive resistance gene transfer among Bacteroides spp. and among Bacteroides and other genera in the human colon. Appl Environ Microbiol. 2001;67:561–568. doi: 10.1128/AEM.67.2.561-568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta A, Vlamakis H, Shoemaker N, Salyers AA. A new Bacteroides conjugative transposon that carries an ermB gene. Appl Environ Microbiol. 2003;69:6455–6463. doi: 10.1128/AEM.69.11.6455-6463.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang YP, Wang GR, Shoemaker NB, Whitehead TR, Salyers AA. Distribution of the ermG gene among bacterial isolates from porcine intestinal contents. Appl Environ Microbiol. 2005;71:4930–4934. doi: 10.1128/AEM.71.8.4930-4934.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salyers AA, Gupta A, Wang YP. Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends Microbiol. 2004;12:412–416. doi: 10.1016/j.tim.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Kazimierczak KA, Flint HJ, Scott KP. Comparative analysis of sequences flanking tet(W) resistance genes in multiple species of gut bacteria. Antimicrob Agents Chemother. 2006;50:2632–2639. doi: 10.1128/AAC.01587-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melville CM, Brunel R, Flint HJ, Scott KP. The Butyrivibrio fibrisolvens tet(W) gene is carried on the novel conjugative transposon TnB1230, which contains duplicated nitroreductase coding sequences. J Bacteriol. 2004;186:3656–3659. doi: 10.1128/JB.186.11.3656-3659.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melville CM, Scott KP, Mercer DK, Flint HJ. Novel tetracycline resistance gene, tet(32), in the Clostridium-related human colonic anaerobe K10 and its transmission in vitro to the rumen anaerobe Butyrivibrio fibrisolvens. Antimicrob Agents Chemother. 2001;45:3246–3249. doi: 10.1128/AAC.45.11.3246-3249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott KP, Melville CM, Barbosa TM, Flint HJ. Occurrence of the new tetracycline resistance gene tet(W) in bacteria from the human gut. Antimicrob Agents Chemother. 2000;44:775–777. doi: 10.1128/aac.44.3.775-777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shears P, Suliman G, Hart CA. Occurrence of multiple antibiotic resistant and R plasmids in Enterobacteriaceae isolated from children in the Sudan. Epidem Inf. 1988;100:73–81. doi: 10.1017/s0950268800065572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trobos M, Lester CH, Olsen JE, Frimondt-MØller N, Hmmerum AM. Natural transfer of sulphonamide and amplicllin resistance between Escherichia coli residing in the human intestine. J Antimicrob Chemother. 2009;63:80–86. doi: 10.1093/jac/dkn437. [DOI] [PubMed] [Google Scholar]
- 38.Sommer MOA, Dantas G, Church GM. Functional characterisation of the antibiotic resistance reservoir in the human microflora. Science. 2009;325:1128–1131. doi: 10.1126/science.1176950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goran MG, Carmeli Y, Schwaber MJ, Chmelnitsky I, Schechner V, Navon-Venezia S. Transfer of carbapenem-resistant plasmid from Klebsiella pneumonias ST258 to Escherichia coli in patient. Emerg Infect Dis. 2010;16:1014–1017. doi: 10.3201/eid1606.091671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Licht TN, Wilcks A. Conjugative gene transfer in the gastrointestinal environment. Adv Appl Microbiol. 2006;58:77–95. [PubMed] [Google Scholar]
- 41.Li Y, Canchaya C, Fang F, Raftis E, Ryan KA, van Pijkeren JP, et al. Distribution of megaplasmids in Lactobacillus salivarius and other Lactobacilli. Appl Environ Microbiol. 2007;189:6128–6139. doi: 10.1128/JB.00447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oshima K, Toh H, Ogura Y, Sasamoto H, Morita H, Park SH, et al. Complete genome sequence and comparative analysis of the wild-type commensal Escherichia coli strain SE11 isolated from a healthy adult. DNA Res. 2008;15:375–386. doi: 10.1093/dnares/dsn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Millette M, Dupont C, Sharek F, Ruiz MT, Archambault D, Lacroix M. Purification and identification of the pediocine produced by Pediococcus acidilactici MM33, a new human intestinal strain. J Appl Microbiol. 2007;104:269–275. doi: 10.1111/j.1365-2672.2007.03583.x. [DOI] [PubMed] [Google Scholar]
- 44.Claesson MJ, Li Y, Leahy S, Canchaya C, van Pijkeren JP, Cerdeño-Tárraga C, et al. Multireplicon genome architecture of Lactobacillus salivarius. Proc Natl Acad Sci USA. 2006;103:6718–6728. doi: 10.1073/pnas.0511060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moon K, Sonnenburg J, Salyers A. Unexpected effect of a Bacteriodes conjugative transposons, CtnDOT, on chromosomal gene expression in its bacterial host. Mol Microbiol. 2007;64:1562–1572. doi: 10.1111/j.1365-2958.2007.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chassey B, Gibson EM, Giuffrida A. Evidence for plasmid associated lactose metabolism in Lactobacillus casei ssp. casei. Curr Microbiol. 1978;1:141–144. doi: 10.1007/BF02601666. [DOI] [PubMed] [Google Scholar]
- 47.Muriana PM, Klaenhammer RT. Conjugal transfer of plasmid-encoded determinants for bacteriocin production and immunity in Lactobacillus acidophilus 88. Appl Environ Microbiol. 1987;53:553–560. doi: 10.1128/aem.53.3.553-560.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tannock GW, Munro K, Harmsen HJ, Welling GW, Smart J, Gopal PK. Analysis of the faecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl Environ Microbiol. 2000;66:2578–2588. doi: 10.1128/aem.66.6.2578-2588.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaneveld JR, Lozupone C, Gordon JI, Knight R. Ribosomal RNA diversity predicts genome diversity in gut bacteria and their relatives. Nucleic Acids Res. 2010;38:3869–3879. doi: 10.1093/nar/gkq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blum-Oehler G, Oswald S, Eitejörge K, Sonnenborn U, Schulze J, Kruis W, et al. Development of strain-specific PCR reactions for the detection of the probiotic Escherichia coli strain Nissle 1917 in faecal samples. Res Microbiol. 2003;154:59–66. doi: 10.1016/s0923-2508(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 51.Shankar N, Baghdayan AS, Gilmore MS. Modulation of virulence within an pathogenicity island in a vancomycin resistant Enterococcus faecalis. Nature. 2002;417:746–750. doi: 10.1038/nature00802. [DOI] [PubMed] [Google Scholar]
- 52.Tzipori S, Gibson R, Montanaro J. Nature and distribution of mucosal lesions associated with enteropathogenic and enterohemorrhagic Escherichia coli in piglets and the role of plasmid-mediated factors. Infect Immun. 1989;57:1142–1150. doi: 10.1128/iai.57.4.1142-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dudley EG, Abe C, Ghigo JM, Latour-Lambert P, Hormazabal JC, Nataro JP. An IncI1 plasmid contributes to the adherence of the atypical enteroaggregative Escherichia coli strain C1096 to cultured cells and abiotic surfaces. Infect Immun. 2006;74:2102–2114. doi: 10.1128/IAI.74.4.2102-2114.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rotger R, Casadesús J. The virulence plasmids of Samonella. Internatl Microbiol. 1999;2:177–184. [PubMed] [Google Scholar]
- 55.Boyd EF, Hartl D. Salmonella virulence plasmid: Modular acquisition of the spv virulence region by an F-plasmid in Salmonella enterica subspecies I and insertion into the chromosome of subspecies II, IIa, IV and Vii isolates. Genetics. 1998;149:1183–1190. doi: 10.1093/genetics/149.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venkatesan MM, Goldberg MB, Rose DJ, Grotebeck EJ, Burland V, Blattner FR. Complete DNA sequence and analysis of the large virulence plasmid of Shigella flexneri. Infect Immun. 2001;96:3271–3285. doi: 10.1128/IAI.69.5.3271-3285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiong Z, Tang X, Yang F, Zhang X, Yang J, Chen L, et al. Comparison of the virulence plasmid genomes of two strains of Shigella which lost the ability to bind Congo red. Sci China C Life Sci. 2006;49:141–148. doi: 10.1007/s11427-006-0141-3. [DOI] [PubMed] [Google Scholar]
- 58.Zagaglia C, Casalino M, Colonna B, Conti C, Calconi A, Nicoletti M. Virulence plasmids of enteroinvasive Escherichia coli and Shigella flexneri integrate into a specific site on the host chromosome: Integration greatly reduces expression of a plasmid-carried virulence genes. Infect Immun. 1991;59:729–799. doi: 10.1128/iai.59.3.792-799.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCormick B, Siber AM, Maurelli AT. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infect Immun. 1998;66:4237–4243. doi: 10.1128/iai.66.9.4237-4243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gigho JM. Natural conjugative plasmids induce bacterial biofilm development. Nature. 2001;412:4442–4445. doi: 10.1038/35086581. [DOI] [PubMed] [Google Scholar]
- 61.Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol. 2007;9:1101–1111. doi: 10.1111/j.1462-2920.2007.01281.x. [DOI] [PubMed] [Google Scholar]
- 62.Huycke MM, Gaskins HR. Commensal bacteria, redox stress and colorectal cancer: mechanisms and models. Exp Biol Med. 2004;229:586–597. doi: 10.1177/153537020422900702. [DOI] [PubMed] [Google Scholar]
- 63.Claesson MJ, Cusak S, O'Sullivan O, Greene-Dinaz R, de Weerd H, Flannery E, et al. Composition, variability and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centinarians. Plos ONE. 2010;5:10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pang AW, MacDonald JR, Pinto D, Wei J, Rafiq MA, Conrad DF, et al. Towards a comprehensive structural variation map of an individual human genome. Genome Biol. 2010;11:52. doi: 10.1186/gb-2010-11-5-r52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.The Chimpanzee Sequencing and Analysis Consortium, author. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- 67.Denarie J, Boistard P, Cassedelbart F, Atherly AG, Berry JO, Russell P. Indigenous plasmids of Rhizobium. Int Rev Cytol. 1981;13:225–246. [Google Scholar]
- 68.Martinez E, Romero D, Palacios R. The Rhizobium genome. Crit Rev Plant Sci. 1990;9:59–93. [Google Scholar]
- 69.Top EM, Moenne-Loccoz Y, Pembroke T, Thomas CM. Phenotypic traits conferred by plasmids. In: Thomas CM, editor. The Horizontal Gene Pool, Bacterial Plasmids and Gene Spread. Amsterdam: Harwood Academic Publishers; 2000. pp. 249–285. [Google Scholar]
- 70.Oliver KM, Degnan PH, Hunter MS, Moran NA. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science. 2009;325:992–994. doi: 10.1126/science.1174463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hehemann JK, Correc G, Barbeyon T, Helbert W, Czjzek M, Michel G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464:908–912. doi: 10.1038/nature08937. [DOI] [PubMed] [Google Scholar]
- 72.Luzopone CA, Hamady M, Cantral BL, Coutinho PM, Henrissat B, Gordon JI, et al. The convergence of carbohydrate active gene repertoires in human gut microbes. Proc Natl Acadn Sci USA. 2008;105:15076–15081. doi: 10.1073/pnas.0807339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu J, Mahowals MA, Ley RE, Lozupone CA, Hamady M, Martens EC, et al. Evolution of symbiotic bacteria in the distal human intestine. PLOS Biol. 2007;5:156. doi: 10.1371/journal.pbio.0050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Louis P, McCrae SI, Charrier C, Flint HJ. Organization of butyrate synthetic genes in human colonic bacteria: phylogenetic conservation and horizontal gene transfer. FEMS Microbiol Lett. 2007;269:240–247. doi: 10.1111/j.1574-6968.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 75.Netherwood T, Bowden R, Harrison P, O'Donnell AG, Parker DS, Gilbert HJ. Gene transfer in the gastrointestinal tract. Appl Environ Microbiol. 1999;65:5139–5141. doi: 10.1128/aem.65.11.5139-5141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bardin S, Dan S, Osteras SM, Finan TM. A phosphate transport system is required for symbiotic nitrogen fixation by Rhizobium meliloti. J Bacteriol. 1996;178:4540–4547. doi: 10.1128/jb.178.15.4540-4547.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen HC, Gartner E, Rolfe BG. Involvement of genes on a megaplasmid on the acid-tolerant phenotype of Rhizobium leguminsarum. Appl Environ Microbiol. 1993;59:1058–1064. doi: 10.1128/aem.59.4.1058-1064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baldani JI, Weaver RW. Survival of clover rhizobia under heat and drought stresses in soil after curing plasmids. Soil Biol Biochem. 1992;24:737–742. [Google Scholar]
- 79.Barbour WM, Elkan GH. Relationship of the presence and copy number of plasmids to exopolysaccharide production and symbiotic effectiveness in a Rhizobium fredii ASDA 206. Appl Environ Microbiol. 1989;55:813–818. doi: 10.1128/aem.55.4.813-818.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McNiel NI. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. 1984;39:338–342. doi: 10.1093/ajcn/39.2.338. [DOI] [PubMed] [Google Scholar]
- 81.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 82.Cummings JH, Antoine JM, Azpiroz, Bourdet-Sicard R, Brandtzaeg P, Calder PC, et al. PASSCLAIM1—Gut health and immunity. Eur J Nut. 2004;43:118–173. doi: 10.1007/s00394-004-1205-4. [DOI] [PubMed] [Google Scholar]
- 83.McIntyre A, Gibson PR, Young GP. Butyrate production from dietary fiber and protection against large bowel cancer in a rat model. Gut. 1993;34:86–391. doi: 10.1136/gut.34.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sponheimer M, Passey BH, de Ruiter DJ, Guatelli-Steinberg D, Cerling TE, Lee-Thorp JA. Isotopic evidence for dietary variability in the early hominin Paranthropus robustus. Science. 2006;314:980–9822. doi: 10.1126/science.1133827. [DOI] [PubMed] [Google Scholar]
- 85.Sponheimer M, Lee-Thorp JA. Isotopic evidence for dietary variability in the early hominid Australopithecus africanus. Science. 1999;283:368–370. doi: 10.1126/science.283.5400.368. [DOI] [PubMed] [Google Scholar]
- 86.Igimi S, Ryu CH, Park SH, Sasaki Y, Sasaki T, Kumagai S. Transfer of conjugative plasmid pAMbeta1 from Lactococcus lactis to mouse intestinal bacteria. Lett Appl Microbiol. 1996;23:31–35. doi: 10.1111/j.1472-765x.1996.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 87.Tuohy K, Davies M, Rumsby P, Rumney C, Adams MR, Rowland IR. Monitoring transfer of recombinant and non-recombinant plasmids between Lactococcus lactis strains and members of the human gastrointestinal microbiota in vivo -impact of donor cell number and diet. J Appl Microbiol. 2002;93:954–964. doi: 10.1046/j.1365-2672.2002.01770.x. [DOI] [PubMed] [Google Scholar]
- 88.Fang F, Li Y, Bumann M, Raftis EJ, Casey PG, Cooney JC, et al. Allelic variation of bile salt hydrolase genes in Lactobacillus salivarius does not determine bile resistance levels. J Bacteriol. 2009;191:5743–5757. doi: 10.1128/JB.00506-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006;72:1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, et al. Environmental genome shotgun sequencing of the Sargasso Sea. Science. 2004;304:66–74. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- 91.Tringe SG, von Mering C, Kobayashi A, Salamov AA, Chen K, Chang HW, et al. Comparative metagenomics of microbial communities. Science. 2005;308:554–557. doi: 10.1126/science.1107851. [DOI] [PubMed] [Google Scholar]
- 92.Breitbart M, Hewson I, Felts B, Mahaffy JM, Nulton J, Salamon P, et al. Metagenomic analyses of an uncultured viral community from human feces. J Bacteriol. 2003;185:6220–6223. doi: 10.1128/JB.185.20.6220-6223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ebdon J, Muniesa M, Taylor H. The application of a recently isolated strain of Bacteroides (GB-124) to identify human sources of faecal pollution in a temperate river catchment. Water Res. 2007;41:3683–3690. doi: 10.1016/j.watres.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 94.Górski A, Wazna E, Dabrowska BW, Dabrowska K, Switala-Jelen K, Miedzybrodzki R. Bacteriophage translocation. FEMS Immunol Med Microbiol. 2006;46:313–319. doi: 10.1111/j.1574-695X.2006.00044.x. [DOI] [PubMed] [Google Scholar]
- 95.Pandey DP, Gerdes K. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 2005;33:966–976. doi: 10.1093/nar/gki201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Melderen L, De Bast MS. Bacterial toxin-antitoxinsystems: More than selfish entities? Plos Genet. 2009;5:1000437. doi: 10.1371/journal.pgen.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Overgaard M, Borch J, Jørgensen MG, Gerdes K. Messenger RNA interferase RelE controls relBE transcription by conditional cooperativity. Mol Microbiol. 2008;69:841–857. doi: 10.1111/j.1365-2958.2008.06313.x. [DOI] [PubMed] [Google Scholar]
- 98.Gerdes K, Møller-Jensen J, Bugge Jensen R. Plasmid and chromosome partitioning: surprises from phylogeny. Mol Microbiol. 2000;37:455–466. doi: 10.1046/j.1365-2958.2000.01975.x. [DOI] [PubMed] [Google Scholar]
- 99.Tsilibaris V, Maenhaut-Michel G, Mine N, Van Melderen L. What is the benefit to Escherichia coli of having multiple toxin-antitoxin systems in its genome? J Bacteriol. 2007;189:6101–6108. doi: 10.1128/JB.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Christensen SK, Pedersen K, Hansen FG, Gerdes K. Toxin-antitoxin loci as stress-response-elements: ChpAK/MazF and ChpBK cleave translated RNAs and are counteracted by tmRNA. J Mol Biol. 2003;332:809–819. doi: 10.1016/s0022-2836(03)00922-7. [DOI] [PubMed] [Google Scholar]
- 101.Christensen SK, Mikkelsen M, Pedersen K, Gerdes K. ReIE, a global inhibitor of translation, is activated during nutritional stress. Proc Natl Acad Sci USA. 2001;98:14328–14333. doi: 10.1073/pnas.251327898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anantharaman V, Aravind L. New connections in the prokaryotic toxin-antitoxin network: Relationship with the eukaryotic nonsense-mediated RNA decay System. Genome Biol. 2003;4:81. doi: 10.1186/gb-2003-4-12-r81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vázquez-Laslop N, Lee H, Neyfakh AA. Increased persistence in Escherichia coli caused by controlled expression of toxins or other unrelated proteins. J Bacteriol. 2006;188:3494–3497. doi: 10.1128/JB.188.10.3494-3497.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nieto C, Sadowy E, de la Campa AG, Hryniewicz W, Espinosa M. The relBE2Spn toxin-antitoxin system of Streptocococcus pneumonia: Role in antibiotic tolerance and functional conservation in clinical isolates. Plos One. 2010;5:11289. doi: 10.1371/journal.pone.0011289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamamoto TA, Gerdes K, Tunnacliffe A. Bacterial toxin RelE induces apoptosis in human cells. FEBS Lett. 2002;519:191–194. doi: 10.1016/s0014-5793(02)02764-3. [DOI] [PubMed] [Google Scholar]
- 106.Kristoffersen P, Jensen GB, Gerdes K, Piskur J. Bacterial toxin-antitoxin gene system as containment control in yeast cells. Appl Environ Microbiol. 2000;66:5524–5526. doi: 10.1128/aem.66.12.5524-5526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andreev D, Hauryliuk V, Terenin I, Dmitriev S, Ehrenberg M, Shatsky I. The bacterial toxin ReIE induces specific mRNA cleavage in the A site of the eukaryote ribosome. RNA. 2008;14:233–239. doi: 10.1261/rna.693208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rohwer F. Prangishvili D, Lindell D. Roles of viruses in the environment. Environ Microbiol. 2009;11:2771–2774. doi: 10.1111/j.1462-2920.2009.02101.x. [DOI] [PubMed] [Google Scholar]
- 109.Lepage P, Colombet J, Marteau P, Sime-Ngando T, Doré J, Leclerc M. Dysbiosis in inflammatory bowel disease: a role for bacteriophages? Gut. 2008;57:424–425. doi: 10.1136/gut.2007.134668. [DOI] [PubMed] [Google Scholar]
- 110.Saitoh S, Noda S, Aiba Y, Takagi A, Sakamoto M, Benno Y, et al. Bacteroides ovatus as the predominant commensal intestinal microbe causing a systemic antibody response in inflammatory bowel disease. Clin Diagnost Lab Immunol. 2002;9:54–59. doi: 10.1128/CDLI.9.1.54-59.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lenth RV. Java applets for power and sample size. [Piface.jar Version 1.70, March 2009], http://www.stat.uiowa.edu/∼rlenth/Power.
- 112.Saavedra De Bast M, Mine N, Van Melderen L. Chromosomal toxin-antitoxin systems may act as anti-addiction modules. J Bacteriol. 2008;190:4603–4609. doi: 10.1128/JB.00357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pecota CD, Wood TK. Exclusion of T4 phage by the hok/sok killer locus from plasmid R1. J Bacteriol. 1996;178:2044–2050. doi: 10.1128/jb.178.7.2044-2050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Doyle M, Fookes M, Ivens A, Mangan MW, Wain J, Dorman CJ. A H-NS-like stealth protein aids horizontal gene transmission in bacteria. Science. 2007;315:251–252. doi: 10.1126/science.1137550. [DOI] [PubMed] [Google Scholar]
- 115.Kim Y, Wang X, Ma Q, Zhang XS, Wood TK. Toxin-antitoxin systems in Escherichia coli influence biofilm formation through YigK (TabA) and fimbriae. J Bacteriol. 2009;191:1258–1267. doi: 10.1128/JB.01465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bollinger RR, Barbas AS, Bush EL, Lin SS, Parker W. Biofilms in the normal human large bowel: Fact rather than fiction. Gut. 2007;56:1481–1482. [PMC free article] [PubMed] [Google Scholar]
- 117.Kleessen B, Blaut M. Modulation of gut mucosal biofilms. Br J Nutr. 2005;93:35. doi: 10.1079/bjn20041346. [DOI] [PubMed] [Google Scholar]