Abstract
Autoshaping refers to a procedure during which a cue repeatedly paired with a reward elicits a conditioned response directed at either the reward delivery location (“goal-tracking”) or the cue itself (“sign-tracking”). Individual differences in expression of sign-tracking behavior may be predictive of voluntary ethanol intake. The present study was designed to explore the development of differences in sign-tracking behavior in adolescent and adult male and female rats in an 8-day autoshaping procedure. Consistency of sign-tracking and goal-tracking across age was examined by retesting adolescents again in adulthood and comparing their adult data with animals tested only as adults to explore pre-exposure effects on adult responding. In order to assess the relationship between sign-tracking and ethanol intake, voluntary ethanol consumption was measured in an 8-day, 2-hr limited access drinking paradigm following the 8-day autoshaping procedure in adulthood. Animals tested as adolescents showed notably less sign-tracking behavior than animals tested as adults, and sign-tracking behavior was not correlated across age. Animals exposed to the autoshaping procedure as adolescents demonstrated greater sign-tracking behavior as adults when compared to control animals tested only in adulthood. When examining the relationship in adulthood between sign-tracking and ethanol intake, an increase in ethanol intake among sign-trackers was found only in animals pre-exposed to autoshaping as adolescents. Whether or not these results reflect an adolescent-specific experience effect is unclear without further work to determine whether comparable pre-exposure effects are seen if the initial autoshaping sessions are delayed into adulthood.
Keywords: Sign-tracking, Autoshaping, Adolescence, Ethanol intake
1. Introduction
Adolescence is not unique to humans: the behavioral, neural and hormonal changes that characterize this developmental period are highly conserved across species (see Spear, 2000, for review). Increases in social activity, impulsivity, novelty seeking and risk-taking behavior are commonly observed during this time both in humans and in animal models of adolescence (Adriani and Laviola, 2003; Kelley et al., 2004; Laviola et al., 2003). In accordance with these behavioral changes, it is during adolescence that both drug experimentation and use are often initiated (SAMHSA, 2008). Of drugs used by adolescents, ethanol is most prevalent (Johnston et al., 2009).
In rodent subjects, adolescence is conservatively defined as postnatal days (P)28–P42. Studies measuring ethanol intake in rats often report that adolescents consume 2–3 times more ethanol than their adult counterparts (Brunell and Spear, 2005; Doremus et al., 2005; Vetter et al., 2007). Given the ethical restraints that limit human research, animal models provide an opportunity to explore factors that may contribute to the age-related susceptibility to drug-seeking behavior experienced by adolescents.
Age-related increases in drug-seeking behavior among adolescents may be due to increases or decreases in the hedonic value, or relative pleasurefulness, attributed to rewarding stimuli. One hypothesis proposes that adolescents are less sensitive than adults to the hedonic value of rewarding stimuli and are more likely to seek out natural or drug rewards to compensate for their attenuated sensitivity (Spear, 2000). Alternatively, adolescents have been suggested to avidly seek out rewarding stimuli because they attribute more positive value to the stimuli. Exploration of these hypotheses in our laboratory has produced mixed results. For example, adolescents exhibit more positive taste responses to sucrose solutions (Wilmouth and Spear, 2009) and consume more sucrose than their adult counterparts (Anderson et al., 2010), suggesting an increase in their sensitivity to hedonic rewards. Conversely, in a study using emission of 50 kHz ultrasonic vocalizations (USVs) as an index of positive affect, adolescent rats produced fewer positive USVs than their adult counterparts during social interactions with a peer (Willey et al., 2009), suggesting a decrease in sensitivity to hedonic rewards. So far, there is no clear pattern of age-related differences in hedonic sensitivity to rewarding stimuli.
An additional explanation for age-related increases in drug experimentation involves the motivational component of reward (Berridge and Robinson, 2003). According to Robinson and Berridge (1993), incentive motivation underlies behavior directed toward rewarding stimuli, with attribution of incentive salience to cues repeatedly paired with rewards also capable of eliciting similar behavior. Thus, it seems possible that age-related increases in vulnerability for drug taking among adolescents relative to adults may reflect an enhanced incentive motivation process among adolescents, resulting in increased attribution of incentive salience to cues paired with natural and drug rewards.
Cues repeatedly paired with rewarding stimuli gain incentive salience and eventually elicit conditioned responses that differ in nature among individuals (see Flagel et al., 2007). Although technically a misnomer in that no formal “shaping” occurs, autoshaping refers to a Pavlovian conditioning procedure in which a particular cue (conditioned stimulus; CS) repeatedly paired with a reward (unconditioned stimulus, US) elicits a conditioned response (CR) directed at either the reward delivery location or the cue itself. Individuals who emit a CR involving approach to the location of reward delivery are described as goal-trackers while those who approach and engage the cue itself are referred to as sign-trackers. Sign-tracking is a well-established phenomenon that has been extensively described in past research across a wide range of species (see Boakes, 1977; Brown and Jenkins, 1968; Flagel et al., 2008; and Tomie, 1996), and appears to be particularly robust when the cue also serves as a manipulandum (Tomie, 1995), with the CRs often mimicking consummatory behavior. Flagel and colleagues have examined these CRs in an autoshaping procedure and suggest that individual differences in ST and GT behavior may result from the degree to which the subjects attribute incentive salience to reward-paired cues (Flagel et al., 2007, 2008). Expression of ST, in particular, has been suggested to share a number of similarities in behavioral and neurobiological features with drug-taking behavior (see Tomie et al., 2008, for review). Indeed, many features of ST behavior, including long-term maintenance, spontaneous recovery and rapid reacquisition following extinction, resemble drug-related relapse.
Several indirect lines of evidence link propensity for ST behavior with elevated ethanol consumption as well. For instance, rats identified as impulsive in a delay discounting task have been reported not only to more readily administer drugs of abuse such as cocaine and ethanol (Poulos et al., 1995) but also to exhibit more ST behavior as well (Tomie et al., 1998). Higher corticosterone levels have been reported to be associated with elevated self-administration of ethanol (Prasad and Prasad, 1995), as well as the more rapid acquisition of ST and greater levels of ST (Tomie et al., 2000), further supporting a relationship between ST behavior and ethanol intake. Indeed, unpublished findings (reported in Tomie et al., 2000) suggest that subjects exhibiting higher levels of ST behavior tend to consume more ethanol in a home cage drinking paradigm.
If adolescents do attribute greater incentive value to rewards compared to adults, one would expect animals at this age to exhibit more ST behavior than their mature counterparts. A previous study conducted with female rats in our laboratory, however, found adolescent animals to exhibit surprisingly little ST behavior in an autoshaping procedure when compared with adults (Doremus-Fitz-water and Spear, in preparation). In order to replicate these findings and extend them to males, the present study first examined ST and GT behavior during an autoshaping procedure in adolescent and adult male and female rats. The adolescent subjects were retested in adulthood to explore expression of these behaviors across age. Control animals were tested for the first time in adulthood, thus allowing for the exploration of both age and pre-exposure effects. Finally, in order to determine the relationship between ST behavior and ethanol intake, animals were categorized as either sign-trackers or goal-trackers and then tested for voluntary ethanol consumption measured in an 8-day, 2-hr limited-access drinking paradigm following the autoshaping in adulthood.
2. Method
2.1. Subjects
A total of 128 male and female Sprague–Dawley rats bred in our colony at Binghamton University were used in the present study. On postnatal day (P) 1, litters were culled to 8 to 10 pups, with a ratio of six males to four females maintained whenever possible. Subjects were weaned on P21 and housed in pairs with a same-sex littermate. Animals were maintained in a temperature-controlled vivarium on a 14:10-hr light:dark cycle (lights on at 7 AM) and were at all times treated in accordance with guidelines for animal care established by the National Institutes of Health (Institute of Laboratory Animal Resources, Commission on Life Science, 1996) under protocols approved by the Binghamton University Institutional Animal Care and Use Committee. Subjects had ad libitum access to food (Purina lab chow, Lowell, MA) and water throughout the experiment.
Thirty-two subjects were assigned to each of the 2 age (adolescent: P28; adults: P69) × 2 sex conditions before further group assignments were made based on their performance during the autoshaping procedure, as described later. No more than one animal per litter was assigned to a given group in order to avoid litter effects (Holson and Pearce, 1992). All testing occurred between 1200 and 1700 hrs.
2.2. Apparatus
Twelve operant chambers measuring 30.5 × 24.1 × 21 cm housed within sound-attenuating boxes measuring 55.9 × 38 × 35.6 cm (Med Associates, St. Albans, VT) were used. The side walls of the chambers consisted of three panels with removable inserts. For the autoshaping procedure, the left wall of each chamber was outfitted with a red house light in the top right corner that was illuminated during all operant testing. The right wall of each chamber contained a trough-style food receptacle in the center panel and a retractable illuminated lever on either the left or right side of the food receptacle. Both the food trough and the lever were mounted 3 cm above the chamber floor. For adult animals, the lever measured 4.8 cm wide, whereas a mouse-sized lever measuring 1.6 cm was used for adolescent animals. Levers were illuminated only when extended out into the chamber and not when retracted into the chamber wall. Photosensors located in the food trough counted head or nose entries into the trough during the 8-s lever presentation prior to the delivery of each pellet (45 mg dustless precision banana-flavored pellets, Bio-Serv, Frenchtown, NJ) via a dispenser mounted on the exterior wall of the operant chamber. For the conditioned reinforcement test conducted on the day following the last autoshaping session (see Robinson and Flagel, 2009), the food trough was removed and the right wall of the operant chamber was reconfigured such that the retractable lever was located in the center panel, with both the left and right side panels outfitted with nosepoke hole inserts, one of which was designated as “active” and the other “inactive” (order counterbalanced across subjects).
2.3. Procedure
2.3.1. Banana pellet pre-exposure (P25–26 or P67–68)
On either P25 (for animals tested as adolescents) or P67 (for animals tested as adults), animals were rehoused in a colony room near the testing chambers with a same-sex non-littermate in standard acrylic breeder tubs with pine shavings. In order to reduce potential neophobia to the banana-flavored food pellets to be used during training, approximately 6.5 g of banana pellets were placed in the home cage of each pair of animals each day for 2 days prior to pre-training.
2.3.2. Pre-training (P27–28 or P69–70)
On each of the 2 days prior to initiation of the autoshaping procedure, animals were placed in the operant chambers with the levers remaining in the retracted position. Independently of the subjects' behavior, 25 banana pellets were delivered on a variable interval (VI) 90 s schedule during each of these pre-training sessions, with each session lasting approximately 35 to 40 min.
2.3.3. Autoshaping procedure (P29–36 or P71–78)
For the next 8 days, subjects were tested in daily autoshaping sessions. Each session consisted of twenty-five 8-s presentations of the illuminated lever on a VI 90 s schedule. Each lever presentation (CS) was followed by delivery of a banana pellet (US) as the lever retracted back into the chamber wall. The CS–US pairings occurred independently of the subjects' behavior, with each autoshaping session lasting approximately 45 min. During each 8-s lever presentation, number of head entries into the food trough and number of lever presses were measured as indices of goal-tracking and sign-tracking behavior, respectively. Latencies to emit each conditioned response were recorded during all trials.
2.3.4. Conditioned reinforcement test (P37 or P79)
The day after the final autoshaping session, all animals were given a conditioned reinforcement test (CRT) using chambers reconfigured as described earlier. During the session, each nosepoke into the active hole resulted in a 2-s presentation of the illuminated lever. Number of active hole nosepokes, number of lever presentations and number of lever presses were all measured during the CRT. A distinction was made between active nosepokes and lever presentations in that if an animal made nosepoke responses into the active hole while the lever was already out in the chamber, these responses were counted as active pokes but not lever presentations. Responses in the inactive hole, which had no consequence, were also counted as an index of general activity, and were subtracted from active nosepokes in order to calculate a nosepoke coefficient for analysis.
At the conclusion of the CRT, adolescent subjects (now P37) remained pair-housed and received no further manipulations until retesting in adulthood, beginning at the age of P69. At this time, they were exposed to pre-training, autoshaping and CRT procedures identical to those received by the animals tested only as adults.
2.3.5. Ethanol intake test (P83–90)
Two days after the CRT in adulthood, all animals were water-deprived for 22 hrs prior to the start of 8 days of 2-hr, limited access ethanol intake testing. The water deprivation schedule was maintained by providing each pair of animals with supplemental water each day after ethanol intake testing, with the amount of water provided daily titrated to permit body weight gains of 1–3 g each day. Approximately 15 minutes before each intake session, subjects were weighed and each housing pair was separated by a mesh divider to allow for assessment of individual consumption. Two bottles were prepared for each subject—one containing water and the other an ethanol solution sweetened with 0.1% saccharin. An ethanol solution of 6% was used for the first 4 days, with the concentration increased to 10% ethanol for the final 4 days. After the last intake session, blood samples were collected from the tail for analysis of blood ethanol concentrations (BECs).
2.4. Data analysis
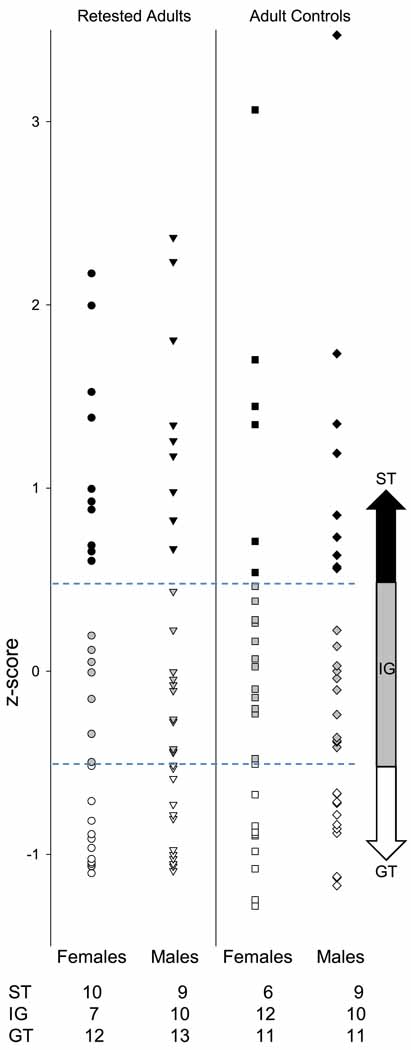
Autoshaping data for eight animals (3 retested adult females, 3 adult control females, and 2 adult control males) were lost due to malfunctioning equipment; these animals were eliminated from the study. During autoshaping, every 8-s lever presentation during which a lever press occurred was considered a ST trial. Each lever presentation during which the subject made a nosepoke response into the food trough was considered a GT trial. Prior to analysis, the number of ST trials and GT trials (each out of a possible 25) was determined for each subject each day. For classification of animals as ST and GT, the number of trials of each type were averaged over these 8 sessions for each animal and used to calculate a relative preference for ST behavior, with the following formula: [(average number of ST trials − average number of GT trials)/(average number of ST trials + average number of GT trials)]. In order to normalize the resulting scores, the means and standard deviations of each age and sex group were used to transform the data into z-scores. Within each group, subjects with a positive z-score of .5 or higher were identified as ST while subjects with a negative z-score of −0.5 or lower were identified as GT. The intermediate group of animals with z-scores in the middle of the distribution (7–12 animals per age/sex condition) was eliminated from all analyses (as in Flagel et al., 2007). Because the mean number of ST trials was so low among adolescent animals, these subjects were sorted and identified as ST or GT based on their behavior during testing in adulthood (that is, for animals tested both in adolescence and adulthood, each animal's group assignment was the same at both test ages and was based on the behavior expressed in adulthood). Sorting each group in this way resulted in group sizes of 11–13 for goal-trackers and 6–10 for sign-trackers in each age/sex condition (see Fig. 1).
Fig. 1.
Group assignment data. Z-scores based on number of ST and GT trials for individual animals are plotted for each age/sex condition. Black symbols indicate the animals classified as sign-trackers (z-scores above 0.5) whereas white symbols indicate animals identified as goal-trackers (z-scores below −0.5). Gray symbols are the z-scores of an intermediate group of animals that were eliminated from all analyses. Group sizes are listed below each column of symbols.
Data were subjected to Levene's test for homogeneity of variance and analyzed using analyses of variance (ANOVAs). All ST data violated the assumption of homogeneity of variance and were subjected to a square root transformation to improve homogeneity prior to analysis. Some data collected from the CRT also violated this assumption and were likewise transformed before analysis as noted below. All significant effects and interactions were further explored using Fisher's LSD post-hoc tests. The first set of analyses focused on comparisons between data collected from adolescent and adult animals to examine age differences in ST and GT behavior. A second set of analyses then examined the effects of prior autoshaping experience during adolescence on adult ST and GT behavior by comparing autoshaping behavior and subsequent ethanol intake in animals previously tested as adolescents and retested during as adults vs. control animals tested for the first time as adults. For ease in comparison across test conditions, Figs. 2–5 display data from both age and pre-exposure analyses, thereby allowing all groups to be visually compared at once. In these figures, the left and right columns (solid lines) depict data from the same animals tested during adolescence (left column) and adulthood (right column), with data from adult control animals shown in the center column (dashed lines). Age effects can be seen by comparing the left and center columns of these figures whereas pre-exposure effects are evident from comparing the center and right columns.
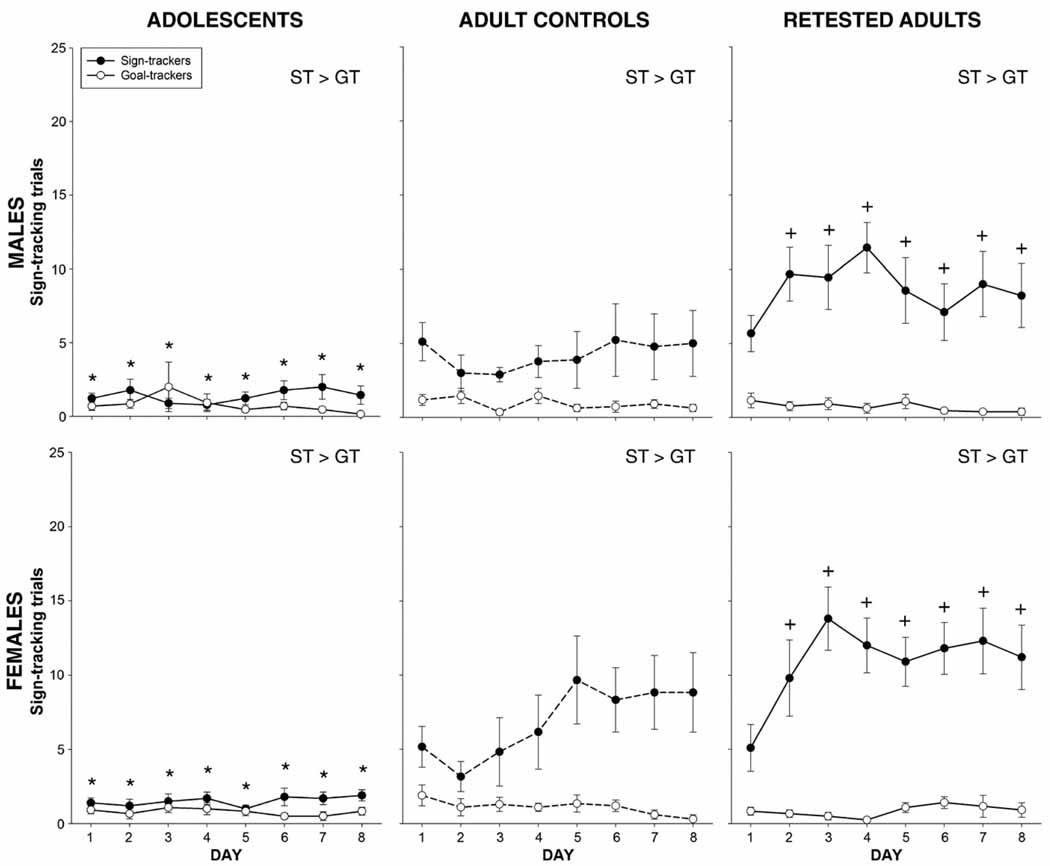
Fig. 2.
Number of trials with a sign-tracking response. Regardless of age/pre-exposure, sex and day, sign-trackers had more ST trials than goal-trackers. * indicates a decrease relative to adult control sign-trackers, collapsed across sex. + indicates an increase relative to adult control sign-trackers, collapsed across sex. Sex effects are described in the text.
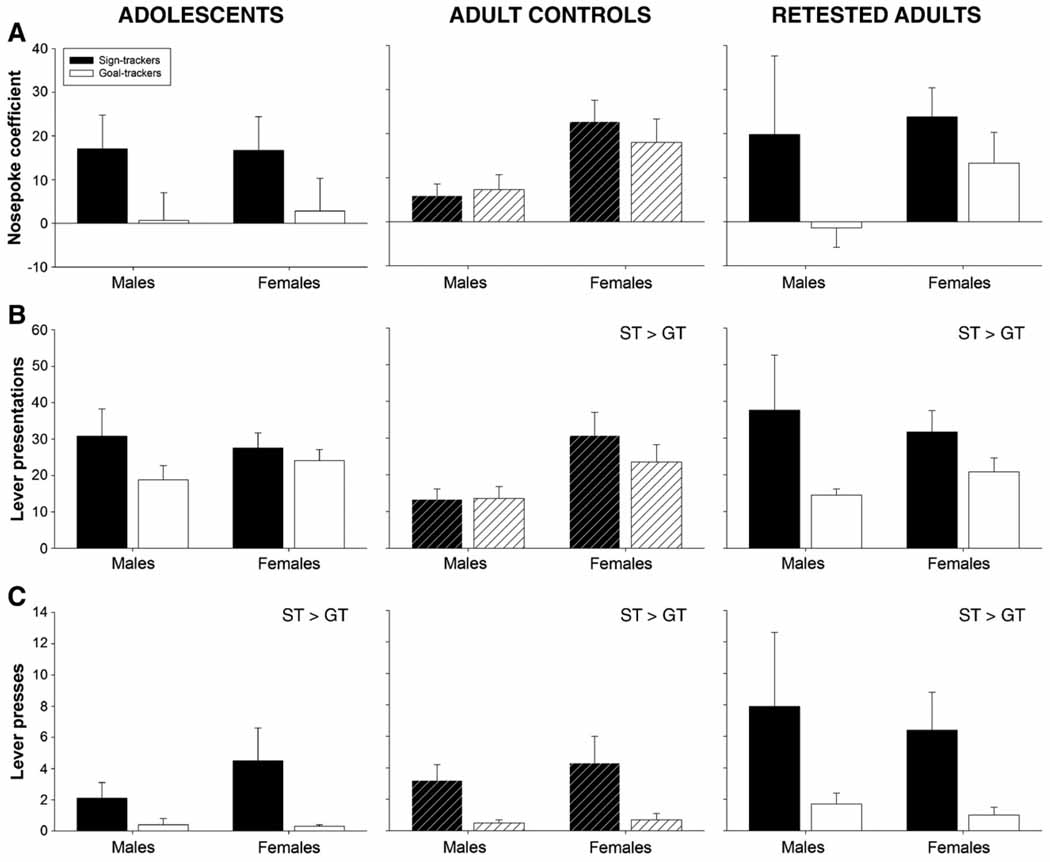
Fig. 5.
Conditioned reinforcement test data. Collapsed across sex, increases among signtrackers relative to goal-trackers are indicated in the text on the graphs. Sex differences are described in the text.
3. Results
3.1. Age effects: adolescents vs. adult controls
3.1.1. Autoshaping
3.1.1.1. ST trials
Square root transformed ST trial data for each day exhibited by animals categorized as ST or GT were subjected to a 2 age (adolescent, adult) × 2 sex (male, female) × 2 group (ST, GT) × 8 day repeated measures ANOVA, revealing main effects of group [F(1,72) = 55.8, p<0.001] and age [F(1,72) = 29.5, p<0.001] both tempered by a significant group × age interaction [F(1,72) = 16.8, p<0.001]. When collapsed across day, sign-trackers of both ages had more ST trials than goal-trackers, with adolescent sign-trackers exhibiting fewer ST trials than adult sign-trackers (see Fig. 2.) Additionally, post-hoc tests conducted on data collapsed across age to explore a group × day interaction [F(7,504) = 4.0, p<0.001] found that animals identified as sign-trackers exhibited significantly more ST trials than those classified as goal-trackers on all 8 days, an effect that tended to become more pronounced over days. Though not a significant effect, there was also a tendency for female rats to exhibit more ST trials than males (p<0.07).
3.1.1.2. GT trials
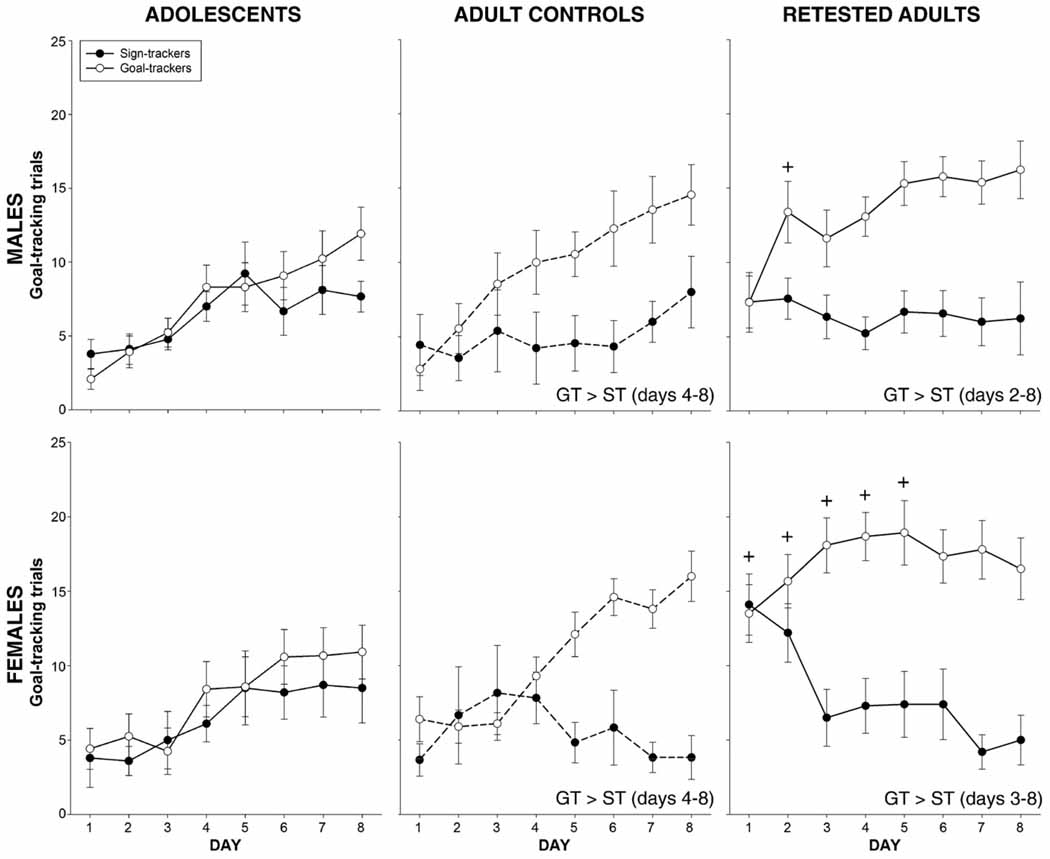
Analysis of GT trials revealed main effects of group [F(1,72) = 10.9, p<0.01] and day [F(7,504) = 21.9, p<0.001], along with significant interactions of group × day [F(7,504) = 6.9, p<0.001] and age × group × day [F(7,504) = 2.5, p<0.02]. As shown in Fig. 3, adult controls categorized as goal-trackers exhibited more GT trials than those identified as sign-trackers on days 4–8 whereas no group differences were evident among adolescents.
Fig. 3.
Number of trials with a goal-tracking response. Goal-trackers had more GT trials than sign-trackers on days indicated in the text on each graph. + indicates an increase relative to adult control goal-trackers of the same sex. Sex effects are described in the text.
3.1.1.3. Overall responding
In order to compare acquisition of conditioned responding across age and sex, the number of trials each day during which either a ST or GT response occurred was analyzed via a 2 age (adolescent, adult) × 2 sex (male, female) × 8 day repeated measures ANOVA. Only significant effects of age [F(1,76) = 4.7, p<0.04] and day [F(7,532) = 25.1, p<0.001] emerged, with adults showing more behavioral responses than adolescents and with all animals showing more responses on days 3–8 relative to day 1. Data are shown in Table 1.
Table 1.
Overall number of conditioned responses during autoshaping sessions (collapsed across sex).
| Age | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 |
|---|---|---|---|---|---|---|---|---|
| Adolescents | 4.4 ± 0.7 | 5.5 ± 0.6 | 6 ± 0.8 | 8.5 ± 0.7 | 8.9 ± 1.0 | 9.5 ± 0.9 | 10.2 ± 0.9 | 10.5 ± 0.9 |
| Adults | 6.9 ± 0.9 | 6.4 ± 0.9 | 8.4 ± 1.1 | 9.6 ± 1.0 | 10.5 ± 1.0 | 12.0 ± 1.2 | 12.2 ± 1.0 | 12.9 ± 1.2 |
3.1.2. Latency data
3.1.2.1. Latency to lever-press
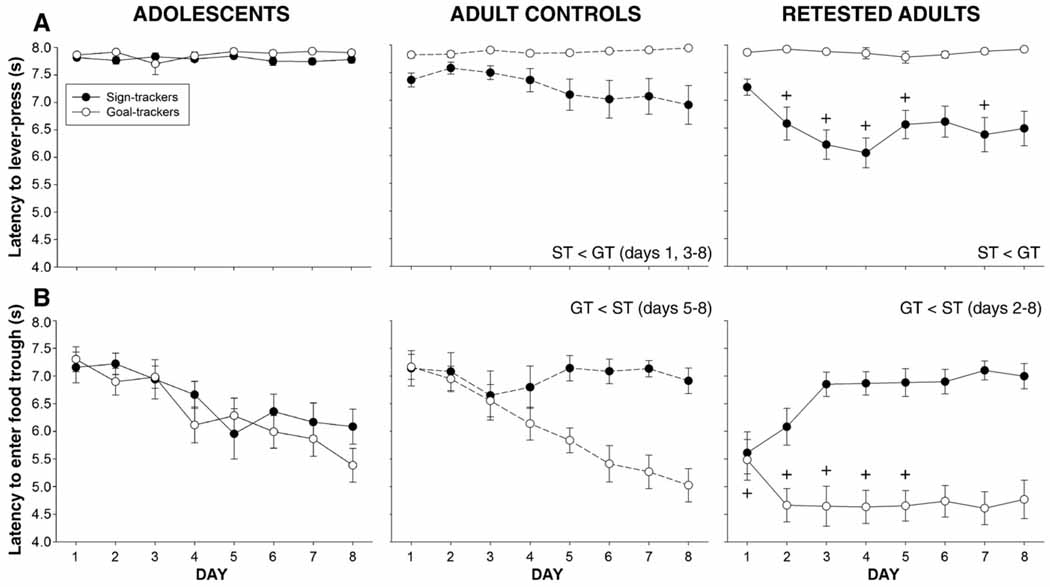
Latency data were subjected to a 2 age (adolescent, adult) × 2 sex (male, female) × 2 group (ST, GT) × 8 day repeated measures ANOVA. Analysis revealed significant effects of age [F(1,72) = 7.3, p<0.01], group [F(1,72) = 64.2, p<0.001], and interactions of these effects with day and each other, all tempered by a day × age × group interaction [F(7,483) = 2.1, p<0.05]. Data are shown in Fig. 4A. Post hoc analyses indicated that among adolescent animals, latencies did not change across day and adolescents assigned to the sign-tracker group did not differ from the goal-tracker group. Among adults, however, sign-trackers had shorter latencies than goal-trackers on days 1 and 3–8. Whereas adult sign-trackers had shorter latencies on days 6–8 relative to day 1, latencies did not change across days among adult goal-trackers.
Fig. 4.
Latency data. Differences between sign-trackers and goal-trackers are described in the text on each graph. + indicates a shorter latency relative to adult controls assigned to the same group. Data are collapsed across sex.
3.1.2.2. Latency to enter food trough
Analysis of latency data revealed significant effects of group [F(1,69) = 4.8, p<0.01] and day [F(7,483) = 13.7, p<0.001] which interacted with each other and with age [F(7,483) = 2.8, p<0.01]. Data are shown in Fig. 4B. Post hoc analysis indicated that adolescent sign-trackers and goal-trackers did not differ from each other, with latencies in both groups decreasing over days (shorter latencies than on day 1 on days 5–8 for sign-trackers and days 4–8 for goal-trackers). Adult goal-trackers had shorter latencies than sign-trackers on days 5–8, reflecting the shorter latencies of adult goal-trackers on days 4–8 than day 1. Latencies did not change across days in adult sign-trackers.
3.1.3. Conditioned reinforcement test
Nosepoke coefficient (active nosepokes–inactive nosepokes), lever presentations and lever presses served as the independent variables of interest during CRT, and were subjected to a 2 age × 2 sex × 2 group (ST, GT) factorial ANOVA. As shown in Fig. 5A and B, analysis of nosepoke coefficients and lever presentations revealed no effects of age and only a trend for sign-trackers to have both higher coefficients and lever presentations than goal-trackers (p < .07 and p < .09, respectively). A main effect of sex was seen in the analysis of lever presentations, with females generally earning significantly more presentations of the lever than males [F(1,72) = 5.2, p<0.03]. Although not a significant interaction, adolescent male sign-trackers tended to earn more lever presentations than all other groups of males, a trend not seen in females.
Lever press data were subjected to a square root transformation in order to resolve a violation of the assumption of homogeneity of variance. Analysis of these transformed data revealed a significant main effect of group assignment [F(1,72) = 23.5, p<0.001], with the ST group emitting more lever presses than the GT group. Neither age nor sex influenced lever pressing behavior (see Fig. 5C).
3.2. Pre-exposure effects: retested adults vs. adult controls
In order to compare animals pre-exposed during adolescence and retested in adulthood with animals tested for the first time in adulthood, the adult test data were analyzed as in the preceding age analyses, but with pre-exposure condition replacing age as a factor.
3.2.1. Autoshaping
3.2.1.1. ST trials
Analysis of the square root transformed ST data revealed main effects of group [F(1,72) = 193.6, p<0.001] and pre-exposure [F(1,72) = 6.8, p<0.02], both of which interacted with day [F(7,504) = 3.7, p<0.001]. In addition to sign-trackers exhibiting more ST trials than goal-trackers regardless of their pre-exposure condition, post-hoc tests indicated that retested adult sign-trackers exhibited more ST trials than sign-trackers tested for the first time as adults, an effect seen on days 2–8 (see Fig. 2). Additionally, there was a main effect of sex [F(1,72) = 4.2, p<0.05] that was tempered by an interaction involving day [F(7,504) = 2.1, p<0.05], with females exhibiting significantly more ST trials than males on days 5–6.
3.2.1.2. GT trials
Analysis of GT behavior revealed main effects of group [F(1,72) = 35.5, p<0.001] and pre-exposure [F(1,72) = 10.8, p<0.01], with goal-trackers generally exhibiting more GT trials than sign-trackers as expected, and retested adults also generally exhibiting more GT trials than animals tested for the first time as adults. These main effects were tempered by numerous significant interactions including a 4-way interaction involving all factors [F(7,504) = 36.9, p<0.02]. These data are shown in Fig. 3. Post hoc analyses revealed that female goal-trackers exhibited more GT trials than female sign-trackers on days 3–8 among retested females and on days 5–8 for control females. Similarly, among males, goal-trackers exhibited more GT trials than sign trackers on days 2–8 among retested males and on days 4–8 for control males. Retested animals exhibited significantly more GT trials than control adults on days 1–5 for females, but only on day 2 for males.
3.2.2. Latency data
3.2.2.1. Latency to lever-press
Latency data were subjected to a 2 pre-exposure × 2 sex × 2 group × 8 day repeated measures ANOVA. Main effects of pre-exposure [F(1,72) = 7.3, p<0.01], group [F(1,72) = 64.2, p<0.001] and day [F(7,504) = 3.0, p<0.01] emerged that were all tempered by a significant interaction of all three factors [F(7,504) = 3.6, p<0.001]. Data are shown in the center and right columns of Fig. 4B. Post hoc analyses revealed that among adult controls, sign-trackers had shorter latencies than goal-trackers on days 5–8 whereas retested adults sign-trackers had shorter latencies than goal-trackers on all 8 days. Further, retested adult sign-trackers had shorter latencies than adult control sign-trackers on days 2–5 and 7.
3.2.2.2. Latency to enter food trough
Analysis of latency data revealed significant effects of pre-exposure [F(1,72) = 11.1, p<0.01] and group [F(1,72) = 35.8, p<0.001], which both interacted with day and each other. These effects were all tempered by a significant pre-exposure × group × day interaction [F(7,504) = 3.3, p<0.01]. Data are shown in center and right columns of Fig. 4B. Post hoc analyses revealed that among adult controls, goal-trackers had shorter latencies than sign-trackers on days 5–8 and among retested adults, goal-trackers had shorter latencies than sign-trackers on days 2–8. Adult control goal-trackers had shorter latencies relative to day 1 on days 3–8 whereas latencies did not change in sign-trackers. In retested adults, latencies for goal-trackers were shorter on days 2–8 relative to day 1 and latencies for sign-trackers actually increased from day 1 to days 3–8. When comparing retested adults with control adults, retested goal-trackers had shorter latencies than control goal-trackers on days 1–5.
3.2.3. Conditioned reinforcement test
As shown in Fig. 5A and B, main effects of sex were seen in the analyses of nosepoke coefficients [F(1,72) = 4.4, p<0.04] and the square root of the number of lever presentations [F(1,72) = 5.5, p<0.03], with females having a higher coefficient and earning more lever presentations than males. The analysis of lever presentations also revealed a main effect of group [F(1,72) = 5.7, p<0.02], with sign-trackers earning more presentations than goal-trackers. Although there was no group × pre-exposure interaction, these effects appear to be largely driven by the retested adults.
Lever press data (see Fig. 5C) violated the assumption of homogeneity of variance; an inverse transformation [1/(x + 1)] addressed this issue most effectively. Analysis of these transformed data revealed only a main effect of group, with animals identified as sign-trackers pressing the lever significantly more often than goal-trackers [main effect of group; F(1,72) = 19.1, p<0.001]. Neither pre-exposure nor sex influenced lever pressing behavior.
3.2.4. Correlation of behavior across age
The relationship between ST and GT behavior in adolescence and adulthood was explored using Pearson's r correlation. No significant correlation was observed for ST behavior across age (r = 0.27; r2 = 0.073); however, GT was weakly but significantly correlated across age (r = 0.31; r2 = 0.096, p<0.05).
3.2.5. Sign-tracking behavior and ethanol intake
3.2.5.1. Ethanol intake
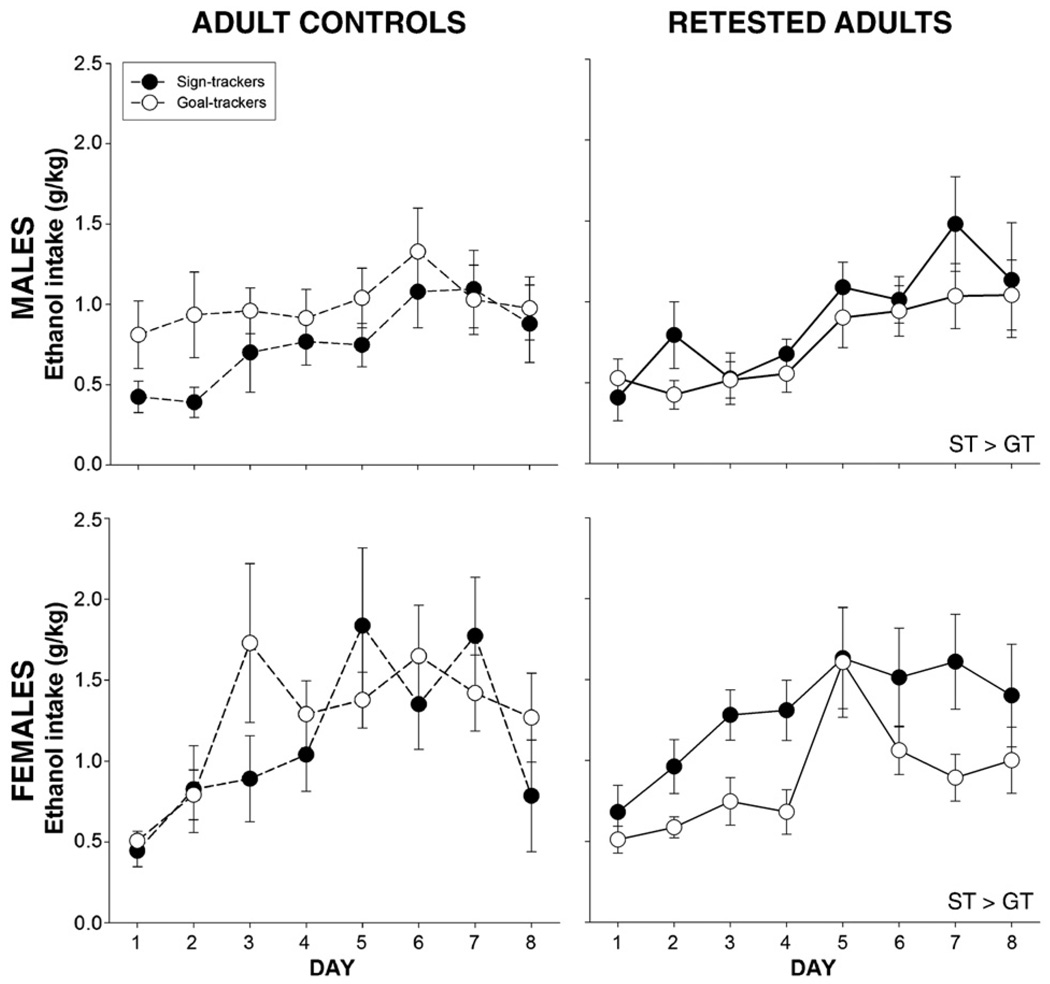
Ethanol consumption data across the 8 days of intake testing were subjected to a 2 pre-exposure × 2 sex × 2 group × 8 day repeated measures ANOVA. A significant pre-exposure × group interaction [F(1,72) = 4.9, p<0.03] revealed that among animals retested as adults, sign-trackers consumed more ethanol than goal-trackers, a pattern not observed in animals tested for the first time in adulthood. Main effects of sex [F(1,72) = 7.6, p<0.01], day [F(7,504) = 20.2, p<0.001], and their interaction [F(7,504) = 3.1, p<0.01] were evident, however, with females consuming significantly more than males on days 3–5 and with animals drinking more during later intake sessions (days 3–8 for females and days 5–8 for males) compared to day 1 (Fig. 6).
Fig. 6.
Ethanol intake in adulthood. Collapsed across day and sex, retested adult signtrackers consumed more ethanol than retested adult goal-trackers, an effect not seen in the adult controls. Sex differences are described in the text.
3.2.5.2. Blood ethanol concentrations
Analysis of BEC data collected immediately after the final intake session revealed no significant effects of retesting, sex, or group assignment (data not shown). BEC values at the end of the 2-hr session averaged 18 ± 3 mg/dl.
4. Discussion
Adolescent animals expressed significantly less ST behavior than their adult counterparts. Age disparities were not as pronounced for GT behavior, with adolescents and adults exhibiting similar numbers of GT trials on most days. Although ST behavior was not correlated across age, animals pre-exposed to the autoshaping procedure during adolescence exhibited more ST behavior when retested as adults than control animals tested for the first time in adulthood. Assessment of voluntary ethanol intake in adulthood revealed group differences only in the animals previously exposed to autoshaping as adolescents, with sign-trackers consuming more ethanol than goal-trackers.
Regardless of sex, adolescents exhibited less ST behavior than adult animals. These results replicate and extend previous work conducted in female rats from our laboratory (Doremus-Fitzwater and Spear, in preparation), providing further support for the suggestion that adolescents do not express greater incentive motivation to reward-paired cues than adults. The minimal expression of ST behavior in adolescents does not appear to be related simply to age differences in learning. Previous drug self-administration studies have demonstrated that adolescent rats are capable of learning a response contingency involving lever-pressing (see Harvey et al., 2009 and Shramet al., 2008). Indeed, the analysis of overall conditioned responding revealed that although adults emitted more behavioral responses than adolescents, overall responding did not differ across days as a function of age. These results suggest that despite possible age differences in motivation to exhibit both behaviors, adolescents and adults demonstrated similar rates of acquisition of the behavioral responses during autoshaping. Latency data further support that the results are not attributable merely to age differences in learning the CS–US association. Both adolescents and adults exhibited shorter latencies to enter the food trough over days, suggesting that animals of both ages learned the predictive value of the lever cue. Yet, unlike adult animals, adolescents exhibited no change in their latencies to lever-press across days. These findings support that adolescents are truly not expressing sign-tracking behavior.
Age differences were not apparent during the CRT. That is, despite the minimal expression of ST behavior among adolescents compared to adults during autoshaping, behaviors leading to lever presentation, number of lever presentations, and lever presses did not differ between adolescents and adults during CRT. According to Robinson and Flagel, incentive stimuli exhibit three properties: they are capable of eliciting approach, they can strengthen instrumental responding, and they can serve as conditional reinforcers during learning (2009). Sign-tracking during autoshaping involves cue-induced approach, whereas the CRT examines the ability of the reward-paired cue to serve as a reinforcer in an operant situation. Thus, although assessment of ST behavior during autoshaping and examination of lever presses during CRT have both been used as indices of incentive motivation, the two measures address somewhat different properties of incentive stimuli. This possibility might explain why the age effects observed during autoshaping were not evident during the CRT in the present study. One potentially important difference between responding during autoshaping and during the CRT is whether the subject has control over the presentation of the stimulus; during the autoshaping trial, lever presentation is independent of any response whereas lever presentation during the CRT is a consequence of a specific behavioral response. To our knowledge, the degree to which a required behavioral response increases in the investment of a subject in a stimulus has not been systematically examined. The dramatically different age-related effects observed here when measuring behavior toward a passively presented versus elicited lever hint that exploration of this topic could yield interesting results. Moreover, including a control lever in future work that is explicitly unpaired with reward delivery during autoshaping and also tested during the CRT could help determine whether the subjects attribute incentive salience only to the reward-paired lever.
A recent study has demonstrated differences between adolescents and adults during an operant learning task (Sturman et al., 2010). Food-restricted animals of both ages were trained to nosepoke in the presence of a cue light for a food pellet. Though adolescents and adults demonstrated similar learning for the first training sessions, adolescent animals made more “task-irrelevant” nosepokes while adults made more premature nosepokes. The authors interpreted the irrelevant pokes as exploratory behavior and the premature pokes as a result of focused attention on the operant task. This study draws attention to potential age differences in strategy during an operant training task that may potentially apply to Pavlovian conditioning situations as well. If these same strategies apply, adults may exhibit more ST trials than adolescents because they are more focused on the CS–USpairing.During extinction trials in the Sturman et al. (2010) study, adolescents exhibited more perseverative responding than adults, particularly under conditions of food deprivation and presentation of a cue previously paired with the reward, suggesting that motivational factors may affect adolescents and adults differently.
Adolescents have demonstrated conditioned place preference, another type of Pavlovian conditioned approach behavior, to a variety of stimuli including social partners (Douglas et al., 2004), novel objects (Douglas et al., 2003), and drugs of abuse such as nicotine (Shram and Lê, 2010; Vastola et al., 2002) and cocaine (Badanich et al., 2006). Conversely, a study examining conditioned place preference to location of food delivery among food-deprived animals found that adolescents did not spend more time in the food-paired environment, although adult animals did (Rubinow et al., 2009). Although place preference conditioning and autoshaping involve the measurement of very different behaviors, these findings may suggest that although food rewards are sufficiently salient to elicit approach in adult animals, they may not be for adolescents. Given that the nature of the reward may strongly influence conditioning behavior directed at the reward-paired cue, the banana pellets used in the present study perhaps might not be as salient a reward in non-food-deprived animals as social interaction or cocaine for adolescents, and hence may have contributed to the limited expression of ST behavior among adolescents in the present study. Further investigation of motivational effects (including food deprivation and isolate housing) on expression of ST behavior in adolescent animals is currently underway.
Neither expression of ST nor GT behavior in adolescence was strongly related to adult expression of these behaviors. When adolescent-tested animals were retested in adulthood, only a modest correlation between their GT behavior in adolescence and in adulthood emerged, whereas ST behavior was not significantly related at the two ages. Thus, sign-tracking and goal-tracking do not appear to be stable, intrinsic traits, but may emerge over time, with their development and expression depending on earlier life experiences. Indeed, evidence for effects of prior experience was revealed in the current study, with subjects previously tested in adolescence demonstrating elevated ST behavior and shorter latencies to lever-press when tested again in adulthood relative to the subjects tested only as adults. One possible explanation for this increase in adult ST behavior following adolescent pre-exposure to the autoshaping procedure is that adolescent pre-exposure increases attribution of incentive salience to reward-paired cues later in adulthood. Yet, such speculations must remain tentative given that the present study does not address the issue of whether the unexpected increase in adult ST behavior following pre-exposure in adolescence merely represents a pre-exposure effect or whether the effect is specific to adolescence.
In the analyses of the adult data, animals identified as sign-trackers during the autoshaping phase earned significantly more lever presentations and made more lever presses during the CRT than goal-trackers. These data support the idea that sign-trackers attribute greater incentive salience to reward-paired cues as Robinson and Flagel (2009) suggested; accordingly, they make more responses that result in presentation of the cue previously paired with the reward and then press the lever more when it is available. Although no pre-exposure effects were significant in the analysis of adult CRT data, some indirect comparisons support perhaps a slight potentiating effect of prior autoshaping experience in adolescence on number of earned lever presentations. That is, in the age analysis for adolescent and adult controls, there was only a trend for sign-trackers to earn more lever presentations than goal-trackers, whereas this difference was significant effect in the analysis of retested and control adults. Albeit indirect, these findings provide further support for the notion that the animals retested in adulthood became more sensitive to the CS–US association and may have found the lever more rewarding.
Adolescent pre-exposure to autoshaping affected the later relationship between ST behavior and ethanol consumption: among animals previously tested during adolescence, sign-trackers consumed more ethanol than goal-trackers, an effect that appears to be more pronounced in retested females. These group differences were not evident among animals tested only as adults. The data seem to suggest that any experiences that enhance ST behavior may also increase ethanol consumption, an interpretation in agreement with the theory that ST behavior may be indicative of addiction vulnerability (Tomie et al., 2008; Flagel et al., 2009). Adolescent experience has previously been reported to influence behavior in adulthood. In a study by Walker and Ehlers, a group of adolescent Wistar rats traversed a runway to gain access to a sweetened 10% ethanol solution while their yoked-control counterparts were allowed the same access to ethanol in an environment similar to the home cage. When tested for ethanol consumption in adulthood, the subjects required to cross the runway to access the ethanol as adolescents consumed more than the control animals that were simply provided the same amount of ethanol (Walker and Ehlers, 2009). The results of this study provide an interesting example of differences between ethanol as a reward when it is actively earned by the subject rather than passively presented, and emphasize the importance of the motivational component of the adolescent experience.
To our knowledge, this is the first study to directly examine ST behavior in both males and females. A modest pattern of sex differences was revealed. In the analyses focusing on comparing adolescents and adults, females earned more lever presentations than males during the CRT, data that initially suggest that females may have attributed greater incentive value to the lever. However, no sex differences emerged during analysis of ST and GT trials in these across-age analyses. CRT data in the comparison focusing on retested adults versus animals tested only in adulthood followed a similar pattern, with females having a higher nosepoke coefficient and earning more lever presentations than males. In the test/retest analyses of autoshaping data, however, retested adult females were found to exhibit more GT trials than males during earlier testing sessions, with no sex differences seen in number of ST trials. Given that incentive salience is a process that sensitizes (Robinson and Berridge, 2001) and evidence that female rats show greater stimulant sensitization than males (Cailhol and Mormede, 1999; Robinson and Becker, 1982;Wooters et al., 2006),we had expected to uncover greater incentive motivation (indexed by a greater number of ST trials) among female than male rats. It is possible that examination of total number of lever presses, rather than trials during which lever presses occurred, might have proved to be a more sensitive measure of subtle sex differences. In terms of ethanol intake testing in adulthood, regardless of ST/GT assignment and test/retest condition, adult females consumed more ethanol than males, replicating typical sex differences in ethanol intake in adult rats commonly reported in our lab (Doremus et al., 2005; Vetter-O'Hagen et al., 2009).
Adolescence has been previously described as a period of addiction vulnerability that may be due to alternations in motivational neurocircuitry during development (Chambers et al., 2003). The results of the present study suggest that although expression of ST behavior is limited under autoshaping conditions among adolescents, pre-exposure to an autoshaping procedure at that time enhances later expression of ST in adults, and is related to increased ethanol consumption. Whether or not these findings reflect an adolescent-specific experience effect is unclear without further work to determine whether comparable pre-exposure effects are seen if the initial autoshaping sessions are delayed into adulthood.
Acknowledgments
The research presented in this manuscript was supported by grants DA 019071 and AA 018026 to LPS.
Abbreviations
- ST
sign-tracking
- GT
goal-tracking
- CRT
conditioned reinforcement test
- BEC
blood ethanol concentration
References
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003 Aug;117(4):695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male Sprague-Dawley rats: Impact of age and stress. Alcohol Clin Exp Res. 2010;34(12):2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006 Nov 21;550(1–3):95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003 Sep;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurvitz HMB, editors. Operant Pavlovian Interactions. Hillsdale, NJ: Erlbaum Associates; 1977. pp. 67–97. [Google Scholar]
- Brown PL, Jenkins HM. Auto-shaping of the pigeon's key-peck. J Exp Anal Behav. 1968;(11):1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29(9):1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain Res. 1999 Sep 18;842(1):200–205. doi: 10.1016/s0006-8993(99)01742-4. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29(10):1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol Behav. 2003;80(2–3):317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004 Nov;45(3):153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Flagel S, Watson S, Robinson T, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology. 2007;191(3):599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186(1):48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology. 2009;56 Suppl 1:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RC, Dembro KA, Rajagopalan K, Mutebi MM, Kantak KM. Effects of self-administered cocaine in adolescent and adult rats on orbitofrontal cortex-related neurocognitive functioning. Psychopharmacology. 2009;206(1):61–71. doi: 10.1007/s00213-009-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992 May–Jun;14(3):221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Guide for the care and use of laboratory animals. Washington DC: National Academy Press; 1996. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2008 (NIH Publication No. 09-7401) Bethesda, MD: National Institute on Drug Abuse; 2009. [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann NY Acad Sci. 2004 Jun;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003 Jan–Mar;27(1–2):19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995 Dec;6(8):810–814. [PubMed] [Google Scholar]
- Prasad C, Prasad A. A relationship between increased voluntary alcohol preference and basal hypercorticosteronemia associated with an attenuated rise in corticosterone output during stress. Alcohol. 1995 Jan–Feb;12(1):59–63. doi: 10.1016/0741-8329(94)00070-t. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Behavioral sensitization is accompanied by an enhancement in amphetamine-stimulated dopamine release from striatal tissue in vitro. Eur J Pharmacol. 1982 Nov 19;85(2):253–254. doi: 10.1016/0014-2999(82)90478-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993 Sep–Dec;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001 Jan;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009 May 15;65(10):869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow MJ, Hagerbaumer DA, Juraska JM. The food-conditioned place preference task in adolescent, adult and aged rats of both sexes. Behav Brain Res. 2009 Mar 2;198(1):263–266. doi: 10.1016/j.bbr.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Lê AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behav Brain Res. 2010 Jan 20;206(2):240–244. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33(4):739–748. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sturman DA, Mandell DR, Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behav Neurosci. 2010 Feb;124(1):16–25. doi: 10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Results from the 2007 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-34, DHHS Publication No. SMA 08-4343) Rockville, MD: 2008. Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Tomie A. Cam: An animal learning model of excessive and compulsive implement assisted drug-taking in humans. Clin Psychol Rev. 1995;15(3):145–167. [Google Scholar]
- Tomie A. Locating reward cue at response manipulandum (CAM) induces symptoms of drug abuse. Neurosci Biobehav Rev. 1996 Autumn;20(3):505–535. doi: 10.1016/0149-7634(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Ethanol induces impulsive-like responding in a delay-of-reward operant choice procedure: impulsivity predicts autoshaping. Psychopharmacology. 1998;139(4):376–382. doi: 10.1007/s002130050728. [DOI] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Individual differences in Pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacol Biochem Behav. 2000;65(3):509–517. doi: 10.1016/s0091-3057(99)00241-5. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58(1):121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002 Sep;77(1):107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31(7):1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O'Hagen CS, Varlinskaya EI, Spear LP. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. 2009 doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behav Neurosci. 2009 Aug;123(4):926–935. doi: 10.1037/a0016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey AR, Varlinskaya EI, Spear LP. Social interactions and 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behav Brain Res. 2009 Aug 24;202(1):122–129. doi: 10.1016/j.bbr.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: taste reactivity and voluntary sucrose consumption. Pharmacol Biochem Behav. 2009 Jun;92(4):566–573. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacol Berl. 2006 Sep;188(1):18–27. doi: 10.1007/s00213-006-0445-9. [DOI] [PubMed] [Google Scholar]