Abstract
Simple motor behaviors such as locomotion and respiration involve rhythmic and coordinated muscle movements that are generated by central pattern generator (CPG) networks in the spinal cord and hindbrain. These CPG networks produce measurable behavioral outputs, and thus represent ideal model systems for studying the operational principles that the nervous system uses to produce specific behaviors. Recent advances in our understanding of the transcriptional code that controls neuronal development have provided an entry point into identifying and targeting distinct neuronal populations that make up locomotor CPG networks in the spinal cord. This has spurred the development of new genetic approaches to dissect and manipulate neuronal networks both in the spinal cord and hindbrain. Here we discuss how the advent of molecular genetics together with anatomical and physiological methods has begun to revolutionize studies of the neuronal networks controlling rhythmic motor behaviors in mice.
Keywords: locomotion, respiration, spinal cord, CPG, interneuron, mouse genetics
Rhythmic motor outputs such as locomotion, respiration and mastication are, in their simplest forms, highly stereotyped motor behaviors. In fish and tadpoles locomotion primarily consists of repetitive lateral bending movements of the axis, which are produced by waves of contraction and relaxation that propagate rostrocaudally along the body axis. In contrast, terrestrial vertebrates propel themselves by flexing and extending their limbs. This more complex mode of locomotion requires extensive coordination between the forelimbs and hindlimbs on each side of the animal and between individual limb joints. The additional demands of land-based locomotion appear to have driven the evolutionary elaboration of the spinal motor circuitry in terrestrial vertebrates, both in terms of numbers and types of neurons, as well as sensory and supraspinal connectivity. In higher vertebrates, the spinal locomotor system has thus evolved into a highly dynamic network that produces a varied and flexible array of motor outputs in response to sensory feedback pathways and descending influences from rubrospinal, reticulospinal, vestibulospinal and corticospinal pathways.
Rhythmic motor circuits in the hindbrain and spinal cord
The core neuronal networks that control rhythmic respiratory and locomotor motor behaviors reside in the hindbrain and spinal cord, respectively. These CPG networks generate simple organized motor rhythms in an autonomous manner. Initial efforts to decipher the general organization of these simple motor CPGs in vertebrates relied heavily on electrophysiological and pharmacological approaches. Such efforts were greatly aided by the development of in vitro hindbrain-spinal cord preparations in neonatal rodents (Kudo and Yamada, 1987; Smith and Feldman, 1987). In addition to enabling investigators to localize two rhythmic CPGs in the medulla that drive respiratory movements, the pre-Bötzinger complex and parafacial respiratory group (Onimaru and Homma, 1987; Smith et al., 1991, this book), this in vitro preparation has been used to map the hindlimb locomotor CPG. For instance, serially sectioning the lumbar spinal cord, both rostrocaudally and dorsoventrally, has defined the minimal area needed to generate a locomotor rhythm. The area that is circumscribed encompasses laminae VII, VIII and X of the lumbar cord, with lumbar segments L1–L2 being particularly important for rhythm generation (Cazalets et al., 1995; Bracci et al., 1996; Kjaerulff and Kiehn, 1996). Forelimb movements are likewise controlled by networks distributed in the cervical spinal cord (Ballion et al., 2001). Further insights into the organization of the spinal locomotor CPG network have come from in vitro pharmacological studies. From these studies it appears that locomotor rhythmogenesis is primarily reliant on glutamatergic ipsilateral neurons (Bracci et al., 1996; Kjaerulff and Kiehn, 1997), whereas the alternating patterns of flexor-extensor and left-right activities are secured by GABAergic and glycinergic pathways (Bracci et al., 1996; Kjaerulff and Kiehn, 1996, 1997).
Over the past few years, efforts to delineate the genetic programs that pattern the caudal neuroaxis have provided new avenues for probing the composition of these CPG networks. An important thematic that has emerged has been the role that genetically-driven developmental programs play in directing neuronal specification and differentiation in the hindbrain and spinal cord. Significant progress has been made in understanding how such developmental programs determine neuronal identity and how neurons are wired together into functional motor circuits. These studies suggest commonalities in the cellular composition of CPG networks in the hindbrain and spinal cord, in so far that these circuits are built from interneuron cell types that share a similar embryonic heritage. In this review we will focus on genetic approaches used in mice to identify cells that are components of motor networks and assess their contribution to rhythmic motor behaviors, with an emphasis on locomotion.
The Developmental Program of the Caudal Neuroaxis
The developmental events that pattern the caudal neural tube play a central role in assembling sensorimotor circuits in the hindbrain and spinal cord (Jessell, 2000; Goulding, 2009). The position that a progenitor cell occupies along the dorsoventral (DV) axis confers a specific genetic code to these cells and thus serves as a major determinant of cell identity (Figure 1A). This DV patterning program segregates newborn neurons into generic populations that are arrayed as longitudinal columns along the antero-posterior axis of the hindbrain and spinal cord. As development proceeds, neurons within these columns migrate extensively to form the different laminae seen in the adult cord. Many premotor commissural interneuron cell types, for example, migrate from the intermediate zone to lamina VIII, while ventral interneurons neurons that project ipsilaterally migrate laterally to lamina VII (Figure 1C). Interestingly, the early DV organization of postmitotic neurons appears to be conserved across widely divergent vertebrate phyla from fish to mammals and it represents the developmental ground plan from which different CPG modules in the spinal cord and hindbrain emerge (Goulding, 2009; Grillner and Jessell, 2009).
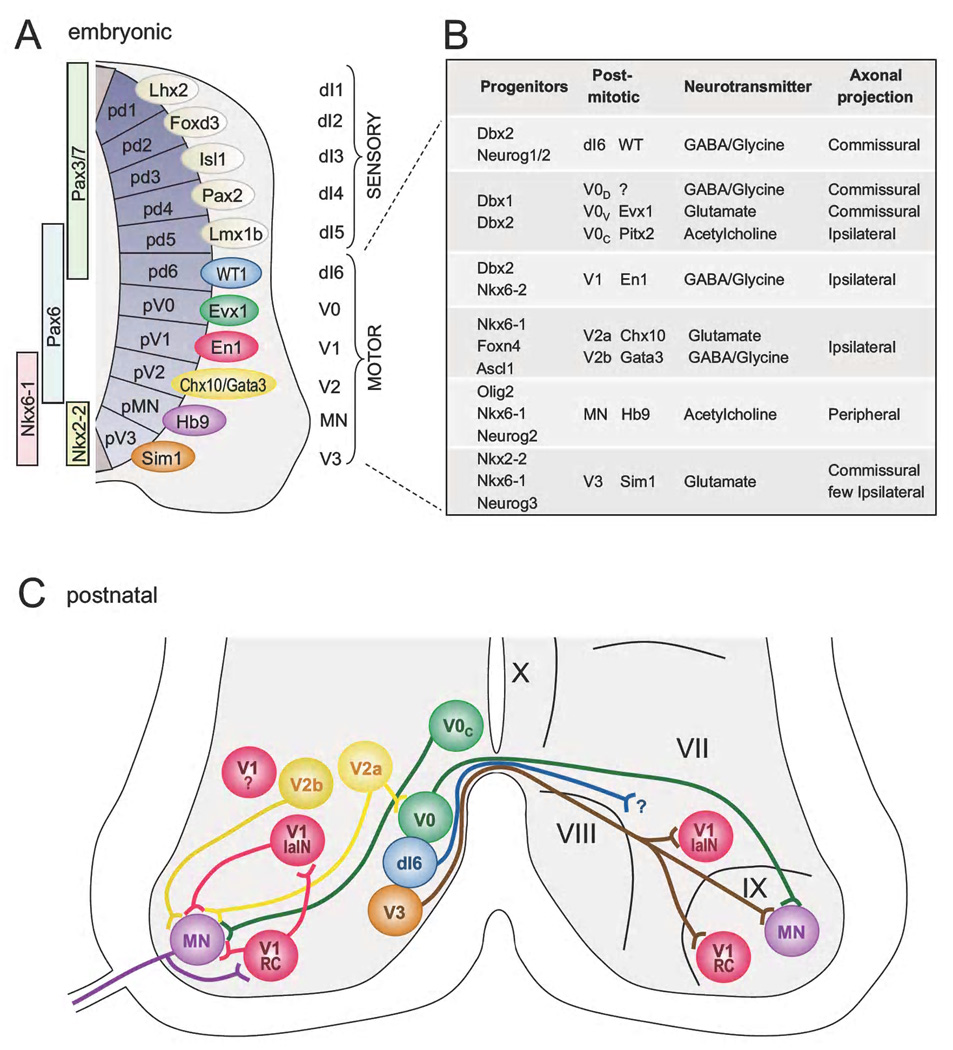
Figure 1. Genetic classification, properties and organization of spinal neurons.
A) Schematic cross-section through the early embryonic mouse spinal cord. Eleven progenitor domains (pd1-pV3) have been identified in the ventricular zone that give rise to distinct postmitotic neuronal populations (dI1-V3). Selected transcription factors expressed within the progenitor domains are shown on the left and those specific to postmitotic populations of neurons born around embryonic day E11 are depicted on the right. Postmitotic neurons that contribute to the locomotor CPG machinery are shown in color, whereas neurons implicated in sensory pathways are shown in grey. B) Summary of molecular factors as well as cellular and physiological properties that characterize each locomotor neuronal class. C) Schematic of the motor circuitry in the postnatal ventral spinal cord. The color code used is the same as in (A). The locations of neuron classes and their known subsets, as well as some known connections between these classes are illustrated. p, progenitor domain.
The molecular programs that control the acquisition of DV positional identity have been explored in some detail. In short, the graded activity of several signaling molecules, sonic hedgehog (Shh) and TGF-like bone morphogenic proteins (BMPs), retinoic acid (RA) and Wnts, initiate and orchestrate the dorsoventral patterning of dividing progenitors (Chiang et al., 1996; Liem et al., 1997; Timmer et al., 2002 and reviewed in Jessell, 2000) in part by establishing dorsoventrally restricted domains of patterning factors, including the Pax3/7, Pax6, Nkx6-1 and Nkx2-2 transcription factors, within the ventricular zone of the neural tube. These patterning factors function instructively in a combinatorial manner to subdivide the neural tube into eleven progenitor zones (Goulding, 2009). As noted previously, this developmental program operates in all vertebrate embryos to activate the expression of unique sets of homeodomain (HD) and basic helix-loop-helix (bHLH) transcription factors in the postmitotic neurons that arise from these eleven progenitor domains (Goulding, 2009 and Figure1A).
Six classes of early born interneurons, the dI1–dI6 interneurons, are generated in the dorsal alar plate. Two additional late born populations of dorsal neurons have been identified that are prevalent in birds and mammals, the dILA and dILB, (Gross et al., 2002; Muller et al., 2002; Mizuguchi et al., 2006; Wildner et al., 2006). These cells are similar in molecular composition to dI4 and dI5 interneurons and it is suspected that they represent an evolutionary expansion of the early born dI4 and dI5 populations in terrestrial vertebrates (Gross et al., 2002; Muller et al., 2002; Mizuguchi et al., 2006; Wildner et al., 2006). Although the majority of dorsal cell types differentiate as sensory interneurons and sensory-relay neurons, some appear to make cellular contributions to the locomotor CPG machinery. However, most of the pre-motoneuron cell types within the locomotor CPG are derived from six generic neuronal classes that develop from basal plate progenitors: motoneurons and V0, V1, V2a, V2b and V3 interneurons (Jessell, 2000; Goulding, 2009). Derivatives of these ventral classes of interneurons settle in regions of the spinal cord that are known to contain locomotor CPG networks in quadrupedal mammals (Kiehn, 2006; Goulding, 2009). The postmitotic populations of V0, V1, V2a, V2b, and V3 interneurons are marked by the expression of the transcription factors Evx1/2, En1, Chx10, Gata2/3 and Sim1, respectively (Saueressig et al., 1999; Moran-Rivard et al., 2001; Karunaratne et al., 2002; Kimura et al., 2006; Al-Mosawie et al., 2007; Lundfald et al., 2007; Peng et al., 2007; Crone et al., 2008; Zhang et al., 2008; reviewed in Jessell, 2000; Goulding, 2009 and Figure 1A, B). dI6 neurons that arise more dorsally express Lbx1, Pax2 and Lhx1 and settle in lamina VIII (Gross et al., 2002; Muller et al., 2002), where they are thought to contribute to the premotor circuitry (Figure 1C). The hindbrain displays a similar dorsoventral organization of early neuronal cell populations. Of the fourteen progenitor domains described in the hindbrain, ten are equivalent to the spinal progenitors domains and they generate cell types that share many features with their spinal cord counterparts (Gray, 2008). Antero-posterior differentiation events that are orchestrated by the homeobox class of HD transcription factors are of primary importance for the development of hindbrain nuclei that arise from the same embryonic population. Embedded in these nuclei are circuits that control vital autonomic functions such as respiration, as well as mastication, audition and the supraspinal control of locomotion (Pagliardini et al., 2008; Dubreuil et al., 2009; Fujiyama et al., 2009; Storm et al., 2009). In the next section we will summarize what is currently known about the early development of the spinal motor circuitry.
1. Generating ventral interneurons: instructive role of progenitor domain specific transcription factors
As noted previously each of the early DV progenitor domain expresses a unique combination of transcription factors that play an instructive role in generating V0, V1, V2a, V2b and V3 interneurons (Figure 1). Changes in the expression of these factors within each progenitor domain leads to major specification defects in their neurons born. For instance, the HD transcription factor Dbx1 is an essential factor for the specification of V0 interneurons. In Dbx1 mutant mice, neural progenitors that normally give rise to V0 interneurons are respecified to produce V1 and dI6 interneurons (Pierani et al., 2001; Lanuza et al., 2004). Ablation of Nkx6-2, a homeobox transcription factor specific to the V1 progenitor domain, also leads to increased numbers of V0 interneurons at the expense of V1s (Vallstedt et al., 2001). Further ventrally, Nkx2-2 regulates V3 progenitor identity. In Nkx2-2 mutants, these cells undergo a ventral-to-dorsal transformation in fate, thereby generating motoneurons at the expense of V3 interneurons (Briscoe et al., 1999). These findings support the idea that transcription factors present in adjacent progenitor domains suppress the differentiation programs that operate in the progenitor domains they abut. They do so by cross-repressive interactions that position the boundaries between adjacent domains. Consequently, loss of one factor often results in the expansion of an adjacent progenitor domain and the cell types that arise from that domain. Other transcription factors are expressed in more than one progenitor domain, where they are required for the generation of several neuron classes. Nkx6-1 has been shown to be important for the generation of both V2 interneurons and motoneurons (Sander et al., 2000). Inactivation of Pax6, in addition to causing a loss of V1 interneurons, also alters the generation of branchiomotoneurons in the hindbrain (Burrill et al., 1997; Ericson et al., 1997; Sapir et al., 2004).
The presence of several transcription factors within each progenitor domain allows further subspecification of domains characterized by the expression of Nkx6-1 or Pax6. The V2 progenitor domain, for example, is defined by coexpression of the forkhead transcription factor Foxn4 and the bHLH transcription factor Ascl1. Interestingly, the V2 progenitor domain, unlike others in the spinal cord, gives rise to two intermingled but molecularly distinct classes, termed V2a and V2b interneurons. Foxn4 and Ascl1 are critical for establishing the V2a and V2b cell fate (Li et al., 2005; Del Barrio et al., 2007; Peng et al., 2007; Kimura et al., 2008). In V2 progenitors Foxn4 induces expression of Ascl1 and the Notch ligand Dll4, which in turn activates Notch signaling in adjacent progenitors. Active Notch signaling in progenitors undergoing a final cell division leads to the generation of V2b interneurons, whereas an absence of Notch signaling in progenitors results in differentiation into V2a interneurons (Li et al., 2005; Del Barrio et al., 2007; Peng et al., 2007; Kimura et al., 2008). A similar mechanism involving Notch signaling has been implicated in the generation of dILA and dILB interneurons in the dorsal spinal cord (Mizuguchi et al., 2006; Wildner et al., 2006).
2. Generating diversity: postmitotic transcription factors in interneuron differentiation
Neurons upon exiting the cell cycle migrate into the mantle zone. During this transition their transcription factor profile changes and they begin to express factors exclusive to postmitotic neurons. These changes in gene expression mark the acquisition of cell type specific properties such as cell body position in the cord, neurotransmitter phenotype, axonal projection pattern and synaptic target selection. V0 interneurons are commissural neurons of mixed neurotransmitter phenotypes that extend axons rostrally in the embryonic cord (Moran-Rivard et al., 2001; Pierani et al., 2001). The postmitotic transcription factor Evx1 is specific to V0V neurons that are predominantly glutamatergic, but is not expressed in dorsally derived GABA/glycinergic V0D interneurons (G. Lanuza and M. Goulding, unpublished). In Evx1 mutants, many of the V0V interneurons acquire the fate of V1 interneurons and extend axons into the ventrolateral funiculus instead of across the ventral midline. Consequently, Evx1 functions as a postmitotic determinant of V0V interneuron identity (Moran-Rivard et al., 2001; Lanuza et al., 2004). Other postmitotic neuron populations are also known to diverge into smaller subpopulations of neurons. This diversification probably represents the essential developmental and evolutionary changes needed to meet the increased requirements higher vertebrates have with respect to complex locomotor movements and respiration.
The further specialization that occurs within these generic populations of interneurons is perhaps best exemplified by the V1 interneuron class. V1 interneurons are marked by their transient expression of the HD protein En1, although En1 has no discernable impact on V1 generic identity or diversification (Saueressig et al., 1999; Sapir et al., 2004). Recently it has been shown that En1 expressing V1 cells differentiate into Renshaw cells and Ia inhibitory interneurons (Sapir et al., 2004; Alvarez et al., 2005). Renshaw cells receive strong excitatory innervation from motoneuron axon collaterals and in turn inhibit motoneurons, as well as Ia inhibitory interneurons, via synapses that use both glycine and GABA. Remarkably, Renshaw cells and Ia inhibitory interneurons represent less than 25% of all V1-derived cells, demonstrating that there are additional uncharacterized V1-derived cell types. Current efforts to molecularly map these V1 subpopulations have relied on a combination of expression screens and candidate gene approaches. We have used genetic marking of En1 expressing V1 interneurons to purify these cells from E12.5 spinal cords. Microarray analysis of mRNA isolated from these cells has led to the identification of a number of genes that are enriched in this population (T. Hendricks and M. Goulding, unpublished). Many of them appear to be expressed in subsets of V1 interneurons and may therefore subdivide the V1 population into functional units (Table 1).
Table 1.
Genes enriched in V1 and V3 interneuron populations at E12.5
| Transcription factors | other | |
|---|---|---|
| V1 enriched genes | En1, Foxd3, Mafa Otx-like, Hoxa3, Cux2 | Nrxn3, Chmp1b, Nrip3 Kcnk13, Scn2a1 |
| V3 enriched genes | Neurog3, Olig3, Sim1 Nkx2-2, Uncx | Gtpbp6, Plekhh1, Adam11 Sema5a, Slc5a6 |
Several transcription factors exhibit more selective patterns of expression. Pitx2, for example, is differentially expressed by a small subpopulation of ipsilaterally-projecting V0 interneurons (V0C interneurons) that form cholinergic synaptic connections on motoneurons (Zagoraiou et al., 2009). In V1 inteneurons, MafA is selectively expressed in Renshaw cells, but is absent from other V1 cells (T. Hendricks, F. Stam, M. Goulding, unpublished). Glutamatergic V2a and GABA/glycinergic V2b interneuron populations are marked by their differential expression of Chx10 and Gata2/3, respectively (Al-Mosawie et al., 2007; Lundfald et al., 2007). Chx10 and Gata2/3 appear not to govern the neurotransmitter identity or axonal morphology of the V2a and V2b interneurons, however, inactivation of Gata2 in mice suggests this transcription factor has a role in maintaining postmitotic V2 cell identity (Zhou et al., 2000). V3 interneurons that express the PAS-bHLH factor Sim1 can be subdivided into dorsal and ventral populations (Zhang et al., 2008). However, none of the V3-enriched genes that have identified to date delineate these two populations (Table 1), even though both populations do exhibit differences in their axonal morphology and electrophysiological properties (E. Geiman, Y. Zhang and M. Goulding, unpublished). Although efforts to map the genetic pathways that establish interneuron diversity in the developing spinal cord are still in their infancy, they are likely to provide a clearer picture of the interneuronal composition of the spinal motor circuitry. This will in turn facilitate further anatomical, physiological and functional studies of specialized interneuron cell types so as to determine their contributions to motor control.
Genetically-defined interneuronal populations that shape the locomotor rhythm
The identification of genetic markers for different spinal interneuronal populations has laid the foundation for functional studies aimed at assessing the contribution that genetically-defined cell types make to the CPG networks controlling locomotor or respiratory rhythmogenesis. These efforts have been centered on broad interneuron classes including the V0, V1, V2a and V3 interneurons (Stepien and Arber, 2008), however, attention is now turning to subtypes within these larger populations, such as the V0C interneurons (Zagoraiou et al., 2009). In characterizing and determining the function of genetically-defined interneuron populations in the spinal cord, the following themes have emerged.
1. Modular nature of the mammalian locomotor CPG
Interneurons that make up each of the early generic populations in the embryonic cord typically share a number of common characteristics, e.g. morphology of the primary axonal process or neurotransmitter phenotype. Moreover, this subdivision of embryonic interneurons into discrete anatomical groupings has led to the suggestion that the spinal motor circuitry comprises of functional modules that are made up of genetically-defined populations of interneurons. This proposition has been confirmed by genetic studies in mice showing that particular interneuron cell types control discrete aspects of the locomotor output. Loss-of-function manipulations that target a particular interneuron population modify specific aspects of the locomotor rhythm and pattern, and leave others unchanged. Some interneuron cell types regulate left-right alternation, while others determine the speed of stepping movements or control the balance between motor outputs on the left and right sides of the cord.
In Dbx1 null mutants, where the V0 population is markedly depleted (Pierani et al., 2001; Lanuza et al., 2004), intermittent episodes of left-right synchronous activity are seen in the locomoting cord (Figure 2C). This deficit is selective, with ipsilateral flexor-extensor alternating activity being maintained and no major changes occurring in the step cycle period. In addition to identifying a neuronal population that is required for proper left-right coordination during locomotion, this study provided evidence that the locomotor CPG has a modular composition with respect to functional control by genetically-defined populations of interneurons. More recently, deletion experiments targeting V2a excitatory neurons have revealed an interesting twist to the story, by identifying V2a interneurons as an ipsilateral component of the commissural pathways that secure left-right alternation (Crone et al., 2008 and Figure 1C, 2C). Once again the effects of deleting the V2a interneurons are primarily restricted to left-right stepping behaviors. Moreover, left-right coordination is only altered when mice lacking V2a interneurons step quickly or trot, which suggests that the V2a interneurons are primarily active at higher stepping speeds (Crone et al., 2009). The observation that V2a ipsilateral neurons project onto V0 commissural interneurons confirms that the V2a cells are part of the CPG module that controls left-right coordination, and that they most likely provide rhythmic excitatory drive to commissural inhibitory neurons that generate reciprocating left-right flexor and left-right extensor activity.
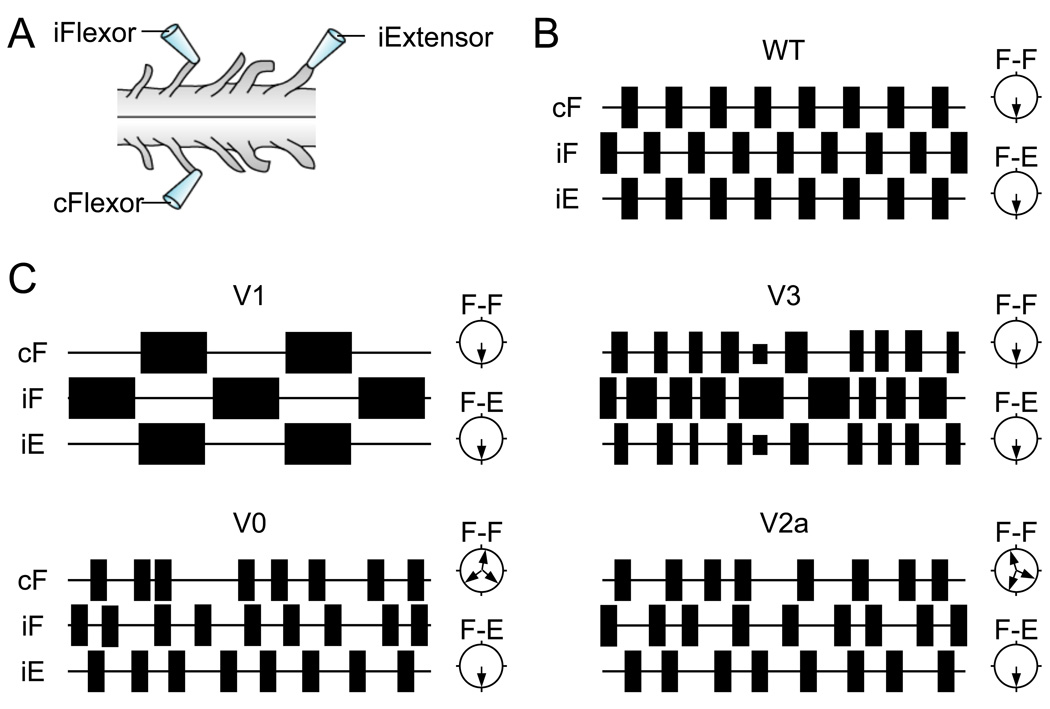
Figure 2. Fictive locomotor activities in mice defective in ventral interneurons.
A) Schematic of an isolated spinal cord from a neonate mouse. When bathed with a cocktail of serotonin and N-methyl-D-aspartate, a fictive locomotor rhythm with left-right and flexor-extensor coordinations can be recorded from flexor L2 and extensor L5 lumbar ventral roots. B) Schematic of locomotor-like activity observed in wild type spinal cords. Bursts of activity are illustrated by rectangles, polar plots represent coordination between two antagonistic roots. Note the strict left-right and flexor-extensor alternations. C) Schematic of locomotor activity recorded from spinal cords of different mutant mice. Defects in V1 or V3 interneurons disturb the locomotor step cycle resulting in a slower step cycle period or an unbalanced locomotor rhythm without affecting bilateral and flexor-extensor coordination. The absence, or ablation, of V0 and V2a interneurons results in a partial impairment of bilateral alternation, which is manifested by episodes of left-right co-contraction. c, controlateral; i, ipsilateral; F, flexor; E, extensor; WT, wild type.
When a second population of excitatory commissural interneurons, the Sim1+ V3 interneurons were targeted, near normal patterns of left-right and flexor-extensor alternation were retained, even though there was a marked reduction in the coherency of the locomotor rhythm. Cords in which Sim1+ V3 interneurons were silenced did, however, exhibit a pronounced imbalance in the duration of motor bursts on each side of the cord. This typically involved bursts from one L2 ventral root being prolonged, while those in the other root were truncated (Zhang et al., 2008 and Figure 2C). V3 excitatory pathways are therefore predisposed to balancing the locomotor output between both sides of the cord, as opposed to establishing alternating patterns of left-right or flexor-extensor motor activity. Further evidence for the modular organization of the locomotor CPG network has come from experiments analyzing the role of the V1 class of interneurons. When V1 interneurons are depleted in the embryonic spinal cord, marked changes occur in the speed of locomotor rhythm (Gosgnach et al., 2006) and Figure 2. In these mice, left-right stepping and flexor-extensor reciprocation is normal, suggesting the primary role of these cells in the walking CPG is to regulate burst termination and initiation during each step cycle.
2. Functional redundancy and complexity in the locomotor CPG
Experiments demonstrating that the deletion of V0 interneurons produces only partial deficits in left-right coordination reveal a degree of redundancy in the commissural pathways that secure left-right alternation. While it seems likely that additional interneuron cell types help coordinating left-right alternation, their identity is not known. The recent demonstration that Netrin-1 mutant cords display a switch from alternating left-right to synchronous left-right locomotor activity supports the idea that multiple commissural interneurons control left-right stepping (Rabe et al., 2009). Moreover, Rabe et al. (2009) also noted that the axons of glutamatergic V3 commissural neurons still cross the ventral midline when netrin-1 is absent, demonstrating that this excitatory commissural pathway cannot secure left-right alternation. We also know that when V3 interneurons are inactivated, left-right alternation is not compromised (Zhang et al., 2008), arguing the contribution this pathway makes to left-right stepping is at best minor. The observation that mis-specification of a second class of putative excitatory commissural neurons, the Evx1+ V0V cells, also leaves left-right stepping behaviors intact (Moran-Rivard et al., 2001), leads us to believe that excitatory commissural pathways in the cord do not have essential roles in controlling left-right alternation. In support of this idea, when V3 transmission is abrogated in the Dbx1−/− mutant cord, we saw no further degradation of left-right alternation over and above that previously described for the Dbx1−/−spinal cord (Lanuza et al., 2004 and Y. Zhang and M. Goulding, unpublished).
A second example of potential redundancy in the interneuron pathways that control the locomotor output emerged from efforts to understand how the locomotor CPG produces an alternating pattern of flexor-extensor activity. Multiple manipulations that abrogate ipsilateral inhibitory V1 interneurons function leave flexor-extensor coordination intact in the isolated CPG or in adult mice (Gosgnach et al., 2006). This was somewhat surprising in so far as an earlier anatomical study had shown that V1 interneurons give rise to cells with the features of Ia disynaptic inhibitory interneurons, a key component of the reciprocal inhibition in the stretch reflex involving flexor-extensor alternation (Alvarez et al., 2005). Experiments undertaken in the lab of Eric Frank examined the integrity of disynaptic inhibitory pathways in the spinal cord of mice lacking V1 interneurons. These analyses revealed that the disynaptic inhibitory pathway between quadriceps sensory afferents and posterior biceps and semitendinosus motor neurons is still operational in Pax6 mutant animals (Wang et al., 2008) and in mice that lack V1 neurotransmission (Z. Wang, J.M. Zhang, M. Goulding, and E. Frank unpublished). It now seems that an additional population of inhibitory interneurons act in concert with the V1 population to control this aspect of the motor output (J.M. Zhang, G. Lanuza and M. Goulding, unpublished).
With the discovery of additional interneuron markers such as those described in Table 1, it is now becoming apparent that the interneuron composition of the spinal cord is quite complex. As noted previously three-quarters of the V1 interneurons remain unidentified (Sapir et al., 2004; Alvarez et al., 2005), and even less is known about the identity and function of cell types that arise from other embryonic interneuron classes. Using functional criteria to classify spinal interneurons may be problematic given the likelihood that several interneuron cell types may together regulate a particular motor behavior, and the loss of one cell population may not lead to pronounced behavioral deficits, especially when one is assaying rudimentary motor patterns such as those produced in the isolated spinal cord or in hindbrain slices. This is born out by experiments that demonstrate the slowing of the locomotor rhythm caused by V1 depletion, which is not reproduced when Renshaw cells alone are silenced (Myers et al., 2005). The complex organization of spinal motor circuitry in higher vertebrates may also be an impediment to future functional studies due to redundancy and compensation by other spinal interneuron populations that have similar or overlapping functions.
3. Comparison with respiratory CPGs
Many of the embryonic interneuron populations that are present in the spinal cord form longitudinal columns that extend into the hindbrain where they contribute to other motor networks including those controlling respiration. Furthermore, many interneuron cell types that are known to control locomotion appear to have essential roles in respiration, as inactivating them often results in perinatal lethality. For example, inhibitory cell types that are derived from the V1 and V2b interneuron populations also have essential roles in establishing a normal respiratory rhythm (J.M. Zhang, J. Ramirez, M. Goulding, J. Feldman, unpublished). In Lbx1 mutant mice, the altered organization of some hindbrain excitatory nuclei results in an immature respiratory rhythm at birth (Pagliardini et al., 2008). Among these Lbx1-expressing cells are Tlx3+ glutamatergic neurons that are important for respiration (Cheng et al., 2004). Together, these studies give heft to the argument that the respiratory and locomotor CPGs are formed from equivalent neuronal substrates in the hindbrain and spinal cord, respectively. It also highlights the potential insights that can be gained by comparative functional studies between the locomotor and respiratory CPGs with respect to the cellular organization of motor networks in the CNS. Other groups have taken the approach of probing these circuits using genes that are either expressed in the hindbrain rhombomeres from which these CPGs develop or in populations of putative respiratory interneurons (Gray et al., 2001; McKay and Feldman, 2008; Tan et al., 2008; Abbott et al., 2009; Dubreuil et al., 2009; Thoby-Brisson et al., 2009). For example, studies analyzing Phox2b mutant mice have revealed a key role for parafacial/retrotrapezoid nucleus in chemosensitivity (Dubreuil et al., 2009). Future experiments to genetically dissect these respiratory circuits are likely to use combinatorial methodologies to selectively target particular interneuron populations within a single rhombomere or region of the medulla, some of which are described in the next section.
New genetic approaches for studying motor circuits in the spinal cord
The elaboration of a genetic classification schemes for the interneuron cell types involved in spinal motor control has opened up new routes for manipulating the spinal motor system and determining how specific motor behaviors are generated. The approaches used so far involve deleting or inactivating broad interneuron classes and assessing how this affects network activity (Table 2). This is usually achieved by generating transgenic animals in which a recombination event such as Cre-mediated excision is used to express an effector molecule in a particular group of neurons (Figure 3). Class specific promoters in combination with site-specific recombinases allow ablating, silencing or activating a population. Most of what we know about the function of genetically-defined spinal interneuron classes comes from in vitro analysis using the isolated spinal cord preparation, in which fictive locomotor activity is determined by extracellular ventral root recordings (Lanuza et al., 2004; Gosgnach et al., 2006; Crone et al., 2008; Zhang et al., 2008). Such studies have provided a broad functional overview of the cellular makeup of the spinal locomotor CPG, however, finer aspects of motor control have been largely ignored due to the limitations of these approaches. Nonetheless an arsenal of second-generation tools is being assembled that builds on the lessons learnt from these cruder studies.
Table 2.
Genetic tools in locomotion and respiration
| Method | Target of Action |
Effect | Inducer | Caveats | Advantages |
|---|---|---|---|---|---|
| Knock out (a–f) |
gene inactivation | loss of protein function |
- |
|
|
| DTA (b,g) |
inhibition of RNA translation |
cell death | - |
|
|
| TeNT (h) |
cleavage of VAMP-2 synaptic protein |
silencing (synaptic transmission) |
- |
|
|
| AlstR (b, h) |
activation of GIRK channel |
silencing (hyperpolarisation) |
Alst (peptide) |
|
|
| ChR2 (i) |
expression of light sensitive cation channel |
activation (depolarisation) |
Blue Light |
|
|
DTA, Diphteria Toxin A; DTR, Diphteria Toxin Receptor; TeNT, Tetanus toxin light chain; AlstR, Allatostatin Receptor; Alst, Allatostatin; ChR2, Channelrhodopsin 2; BBB, blood brain barrier; GIRK, G-protein-coupled inwardly-rectifying potassium channel; VAMP-2, vesicle-associated membrane protein 2 (synaptobrevin2). a, (Lanuza et al., 2004); b, (Gosgnach et al., 2006); c, (Pagliardini et al., 2008); d, (Rose et al., 2009); e, (Dubreuil et al., 2009); f, (Wallen-Mackenzie et al., 2006); g, (Crone et al., 2008); h, (Zhang et al., 2008); i, (Hagglund et al., 2010).
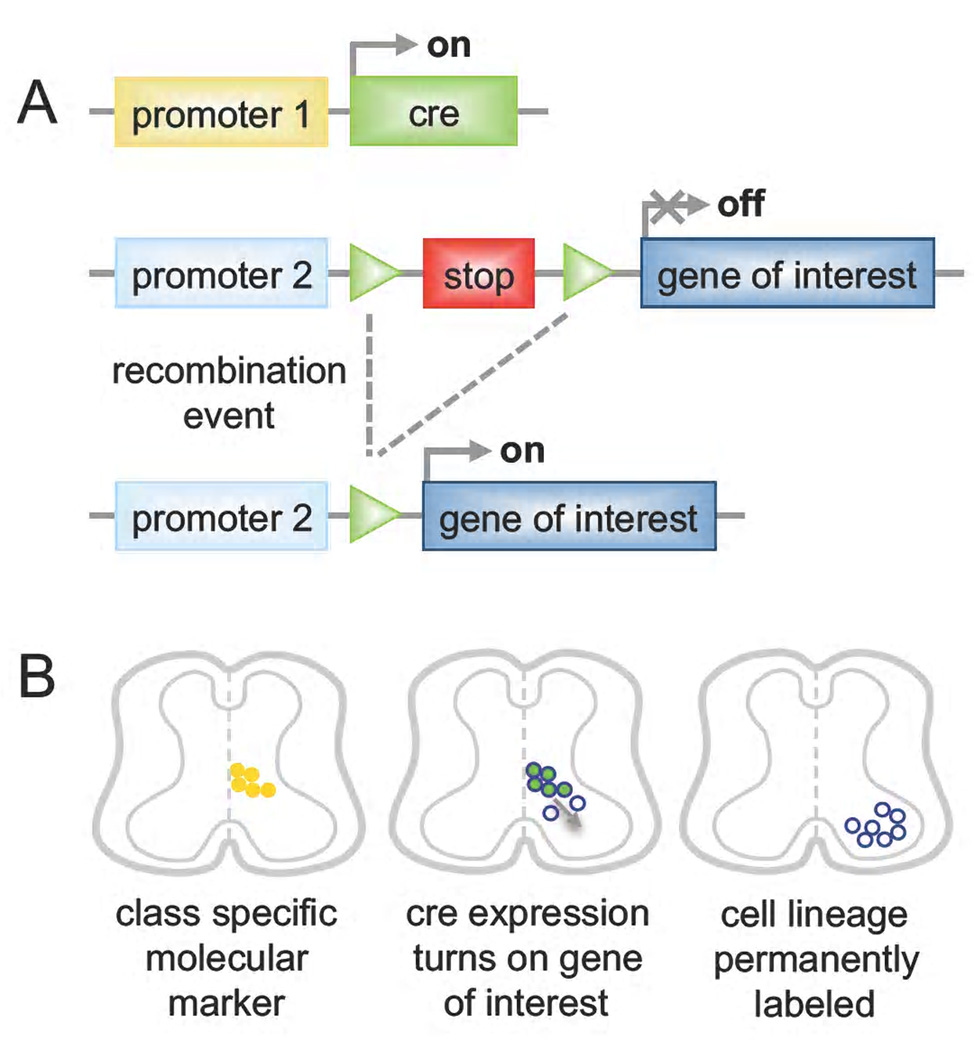
Figure 3. Cre-dependent manipulation of defined neuronal populations.
A) Two different alleles are used in combination to genetically manipulate defined populations of cells. First, the site-specific Cre recombinase is expressed from a promoter (1) specific to one neuronal class. A second allele is used to express a gene of interest from a promoter (2) that is, in general, constitutive and broadly active but can also be neuronal or cell type specific. Transcriptional STOP sequences flanked by loxP sites (green triangles) are interposed between the promoter and the coding sequence such that Cre-mediated recombination of loxP sites and excision of the STOP cassette is required to express the gene of interest. This strategy can be used to express a reporter gene such as GFP for morphological and axonal characterization, and fate mapping of the genetic lineage or to express an effector gene like TeNT or DTA, resulting in cell silencing or ablation for functional studies. B) Schematic picture showing genetic lineage labeling in the spinal cord. The promoter (1) of a molecular marker defining a neuronal class (yellow dots) is used to drive Cre expression in the same cells (green dots). Recombination induces expression of the gene of interest (blue circles), which is dependent on promoter (2). At later stages of development recombined cells have migrated to settle in their final location. Although these cells no longer express the molecular marker nor Cre, they are permanently labeled (blue circles).
1. Defective cell generation or cell ablation
One of the routes that has been successfully used to analyze motor circuits in the hindbrain and spinal cord makes use of mouse mutants in which transcription factors or signaling molecules that specify neuronal identity and connectivity during development have been inactivated (Figure 1). Although these factors are required for the generation of distinct populations of spinal interneurons, they are often expressed elsewhere in the developing mouse, which leads to perinatal lethality when these genes are inactivated. As locomotor like activity can usually be recorded in neonate mice using the isolated spinal cord preparation, such mutant animals have been used to look at motor activity in the hindbrain and spinal cord (Lanuza et al., 2004; Myers et al., 2005; Gosgnach et al., 2006). An additional issue is the broad expression of many factors within the spinal cord or hindbrain, in so far as removing these factors typically affects large neuronal populations, or results in complex cell fate changes that make interpretation of any observed motor deficit difficult. While approaches that selectively ablate a population may avoid some of the complex cell fate changes associated with inactivating developmental control genes, in many instances the effects may still be quite widespread. Expression of the diphtheria toxin A protein (DTA) in molecularly defined cells has been used to selectively eliminate entire classes of interneurons. Examples come from V1 and V2a interneurons, which were successfully ablated using the Rosa-stop-DTA ablator allele and a Chx10-stop-DTA allele, respectively (Gosgnach et al., 2006; Crone et al., 2008). However, use of this strategy is limited as molecular markers of spinal interneurons are often also expressed in non-neuronal tissues, which causes additional phenotypes in mice genetically modified to kill one population. For this reason, cell ablation approaches are often not possible due to collateral tissue damage that results in embryonic lethality. Examples of this are the V1 and V3 interneuron-specific factors En1 and Sim1 that are expressed in muscle cells or V2b marker Gata3, which is expressed in the placenta and in endothelial cells (Ng et al., 1994; Chen and Johnson, 2002; Coumailleau and Duprez, 2009). Using a neuronal driver such as Eno2-Stop-DTA allele restricts ablation to neurons, thus avoiding the effects in non-neural tissue (Kobayakawa et al., 2007).
2. Manipulating cell activity
A different approach to assessing neuronal function involves silencing or blocking neurotransmission in selected neurons and analyzing the effects on particular behaviors. Synaptic transmission can be abolished in a particular spinal cell class by expression of the light chain of the tetanus toxin (TeNT) (Yamamoto et al., 2003; Zhang et al., 2008). TeNT prevents neurotransmitter release at the synapse level shutting down the activation or inhibition of post synaptic targets of the cells expressing TeNT. This approach has the advantage that the genetically targeted cells are still present, receive contacts and synaptics inputs. However, expression of TeNT is difficult to assess functionally. While VAMP2 expression can be used as a read out of TeNT activity, electrophysiological studies provide the most compelling validation of TeNT activity. For example, experiments recently undertaken in the cerebellum revealed TeNT expression in cerebellar granule cells disrupts synaptic transmission to Purkinje cells (Kim et al., 2009). A different strategy for acute silencing involves expressing the insect allatostatin G-coupled protein receptor, AlstR (Gosgnach et al., 2006; Zhang et al., 2008). Upon allatostatin ligand binding, this receptor activates inwardly rectifying K+ channels (GIRK channels) leading to transient hyperpolarization and decreased excitability of the neurons in which it is expressed (Lechner et al., 2002; Tan et al., 2008). Neuronal signaling can also be altered by inactivating critical components of the neurotransmission machinery. This approach was successfully used by Zagoraiou et al. (2009) since they specifically knock-out choline acetyl transferase (ChAT) in Pitx2+ V0C cells thus preventing synaptic transmission from this particular neuronal population (Zagoraiou et al., 2009). Finally, genetic systems that can be used to stimulate or increase the excitability of defined neurons are being developed in mice, and in zebrafish (Armbruster et al., 2007; Wyart et al., 2009). Neurons can be remotely activated by the expression of channelrhodopsin 2 protein (ChR2), a microbial light sensitive monovalent cation channel that allows entrance of Na+ ions in the cell following exposition to blue light (Nagel et al., 2003; Luo et al., 2008). Thus, illumination of ChR2 expressing neurons leads to their depolarization and activation (Boyden et al., 2005). ChR2 expression was recently targeted to VGlut2 neurons in the hindbrain and the spinal cord and illumination of each region was seen to induce and maintain locomotor-like activity in the spinal preparation. This finding confirms that glutamatergic neurons have a key role in elaborating the locomotor rhythm (Hagglund et al., 2010). Approaches using “designer” muscarinic GPCR receptors that can be exclusively activated by synthetic ligands provide another avenue for selectively stimulating particular interneuron cell types (Armbruster et al., 2007).
3. Case history: genetic manipulations involving V1 interneurons
Many of the aforementioned techniques for genetically manipulating neurons have now been trialed, each of which has advantages and disadvantages. However, by judiciously tailoring the approach used, one can undertake elegant manipulations of these spinal motor networks. Loss of cells is readily quantifiable, whereas silencing activity requires electrophysiological techniques to confirm reduced activity in the targeted neurons. In the Goulding lab, several genetic approaches have been used to remove the V1 class from the spinal circuits. These include developmental deficits in V1 specification (Pax6−/−), V1 specific cell ablation (En1-DTA) or blocking transmission/silencing (En1-TeNT and En1-AlstR, respectively). All four methods cause a strong slowing of the locomotor-like rhythm while preserving other aspects of the motor output, i.e. rhythmogenesis, left-right and flexor-extensor alternation (Figure 2C). The phenotypes of the Pax6 mutant and En1-DTA mice are very similar, whereas En1-TeNT mice produce a comparatively slower motor rhythm. While cell “silencing” with AlstR produces a slow rhythm when compared to wild-type animals, the rhythm is significantly faster than that observed in En1-TeNT cords. This, in all likelihood, represents partial silencing/inactivation of the V1 population. The rhythm in the En1-AlstR cords also returns to its normal frequency approximately 15 min after exposure to allatostatin, which probably reflects desensitization of the GIRK channels following prolonged exposure to allatostatin.
4. Studies in adult behaving mice
Functional analysis in vitro using the isolated spinal cord preparation usually only allows identification of gross defects in motor activity. Analyzing complex motor behaviors at adult stages in genetically-manipulated mice is often not possible because of neonatal lethality and deficits in more anterior regions of the CNS or in non-neuronal structures. The transcription factor Lbx1 for instance, which is important for specifying dorsal neural fate including the dI6 interneurons, is expressed in the diaphragm (Gross et al., 2000; Storm et al., 2009) and hindbrain, where it is involved in respiratory rhythmogenesis (Pagliardini et al., 2008). Nevertheless, genetically altering the circuits has allowed locomotor studies in adult behaving mice in few instances so far. Silencing the V3 cells in adult mice by using the AlstR system results in defects in walking (Zhang et al., 2008). Similarly to the findings from in vitro experiments, the conditional silencing of these commissural interneurons in adult mice produced less stable and robust locomotor cycles during walking, thus validating the fictive locomotion analysis performed in the neonatal spinal cord.
Studying locomotor behaviors in adult mice can also be used to refine observations that have been made using the isolated spinal cord preparation. In vitro studies of V2a interneurons in the neonatal cord (Crone et al., 2008) were extended by adult kinematic analyses showing that V2a interneurons are needed for left-right stepping at faster gait speeds (Crone et al., 2009). Acute silencing of lumbar level V3 interneurons was used to confirm the altered rhythm that occurs in vitro following TeNT blockade of V3 transmission (Zhang et al., 2008). Finer aspects of motor control have also been tested in behaving mice. For example, analysis of the V0C population in adult mice unraveled the specific function of these neurons during particular locomotor behavior. EMG recordings from gastrocnemius and tibialis anterior muscles, respectively ankle extensor and flexor, during walking and swimming tasks revealed that in the absence of V0C neuron activity, the task-dependent modulation of the gastrocnemius muscle was impaired (Zagoraiou et al., 2009). In vitro approaches would have not revealed these kinds of modulatory functions, in particular with respect to smaller neuronal populations.
5. Looking forward
A next and crucial step for understanding the functional contribution these spinal interneuron classes make to motor control involves acutely eliminating each of them locally from spinal networks in the adult. It is yet not clear whether there are developmental compensatory changes that occur in spinal neuronal networks when a particular neuronal component is removed from the circuit as it is being wired. Loss of cells, especially as circuits are forming, may result in network rearrangement that could eventually compensate for the absence of these cells. In particular, little is known about the consequences on growing axons seeking out for synaptic partners when their target cells are absent. Inducing cell ablation after the circuits have wired and fully matured may therefore be critical. An elegant approach is to render mouse cells susceptible to the diphtheria toxin by specific expression of a simian or human diphtheria toxin receptor (DTR). Selective cell death can then be achieved by injecting the diptheria toxin intraperitoneally at desired time points. Using such strategy, functional compensation has been reported in the circuits controlling feeding and nociception (Luquet et al., 2005; Cavanaugh et al., 2009).
Most molecular markers of interneurons being also expressed in supraspinal neurons or other tissue types, new strategies need to be implemented where genetic targeting is restricted to neuronal classes only in the spinal cord. This would allow revealing the exclusive role of these subpopulations in shaping motor output during walking, without affecting the functionality of other tissues/organs, or regions of the nervous system. For this purpose, approaches using intersecting combinations of expressed genes are promising. This has successfully been done using Cre driver lines in combination with Flp driver lines (Awatramani et al., 2003). Several dual recombinase-response alleles have now been generated that allow cell labeling or silencing (Farago et al., 2006; Jensen et al., 2008; Kim et al., 2009; Yamamoto et al., 2009). This strategy could allow dissecting the roles of genetically defined cells present in multiple regions in the rostro-caudal axis. For instance, silencing of all neurons of the Atoh1 lineage, including granule cells in the cerebellum and the dI1 class of spinal cells, results in a robust motor coordination defect in behaving mutant mice (Kim et al., 2009). Generating Flp lines specific to the cerebellum or the spinal cord only would allow testing the contribution of each region. The intersectional strategy also represents an attractive method for subdividing the current classes into smaller subsets. Within the V1 interneuron class defined by expression of En1, only the Renshaw cells also co-express the Ca2+ binding protein calbindin (Carr et al., 1998; Sapir et al., 2004). Using these two genes in a combinatorial manner to target this subset of V1 interneurons would thus allow to functionally testing the role of this subpopulation. Double recombinase approaches could also be helpful to unravel the functional contribution of specific cell types related to respiration. Patterning programs in the hindbrain specify interneurons that integrate different functional circuitry according to their antero-posterior localization. The main respiratory nuclei derive from rhombomeres 1, 4–6 and 7–8. Manipulating one interneuronal population in specific rhombomeres would allow one to unravel the roles different classes of hindbrain interneurons play in respiratory rhythmogenesis. Together with inducible systems for activation and silencing of neurons the dual recombinase approach represents an elegant way to study specific neuronal populations in adult mice. Nevertheless, in addition to generating novel genetic tools for studying locomotion, more effort needs to be invested in developing robust and precise behavioral tests that are able to detect the subtle locomotor phenotypes when smaller groups of cells are inactivated in adult mice.
Conclusion
The delineation of the transcriptional code for neuronal cell populations in the ventral spinal cord together with the emergence of several genetic approaches to manipulate cells of interest have paved the way for genetically and functionally dissecting the neural circuits controlling locomotion. The embryonic building blocks that make up these motor circuits are shared between the networks controlling respiration and locomotion. Thus, understanding how neuronal cell populations that comprise the hindlimb locomotor CPG are organized should provide important insights into how other CPGs function. It is also hoped that efforts to understand motor behaviors such as locomotion and respiration will provide some of the keys for unlocking more complex neuronal networks.
The studies described in this review highlight some of the fundamental findings that have shaped our understanding of how interneurons in the ventral spinal cord form neuronal circuits that elaborate coordinated rhythmic motor outputs. However, there is a high degree of complexity in the composition of these circuits, with recent studies revealing the existence of specialized subpopulations within the generic populations of interneurons that emerge from the ventral spinal cord. Although, significant progress has been made in understanding the coarse function of large cell groups in controlling particular aspects of locomotor rhythm and pattern generation, many more investigations are waiting to be undertaken which elucidate the role of smaller subpopulations in fine tuning and shaping locomotor output. Such efforts will depend heavily on the identification of specialized neuronal cell types and the development of new methodologies to genetically target and manipulate them.
Acknowledgements
KSG is funded by a Feodor Lynen Fellowship from Alexander von Humboldt foundation. Research in the Goulding lab is supported by grants from the National Institutes of Health (NS031249, NS031978 and NS037075) and the Christopher and Dana Reeve Foundation. We would particularly like to thank Tim Hendricks, Floor Stam and other members of the Goulding Lab for allowing us to cite their unpublished findings.
Abbreviations
- Alst
Allatostatin
- AlstR
Allatostatin receptor
- bHLH
basic helix-loop-helix
- BMPs
bone morphogenetic proteins
- ChAT
cholineacetyl transferase
- ChR2
channelrhodopsin 2
- CPGs
Central Pattern Generators
- DTA
Diphteria toxin A subunit
- DTR
Diphteria toxin receptor
- DV
dorsoventral
- EMG
electromyogram
- GIRK
G-protein-coupled inwardly-rectifying potassium channel
- GPCR
G-protein-coupled receptor
- HD
homeodomain
- RA
retinoic acid
- Shh
sonic hedgehog
- TeNT
light chain of the tetanus toxin
- TGF
Transforming growth factor
References
- Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29(no. 18):5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mosawie A, Wilson JM, Brownstone RM. Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur J Neurosci. 2007;26(no. 11):3003–3015. doi: 10.1111/j.1460-9568.2007.05907.x. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Jonas PC, Sapir T, Hartley R, Berrocal MC, Geiman EJ, Todd AJ, Goulding M. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J Comp Neurol. 2005;493(no. 2):177–192. doi: 10.1002/cne.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104(no. 12):5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani R, Soriano P, Rodriguez C, Mai JJ, Dymecki SM. Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nat Genet. 2003;35(no. 1):70–75. doi: 10.1038/ng1228. [DOI] [PubMed] [Google Scholar]
- Ballion B, Morin D, Viala D. Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat. Eur J Neurosci. 2001;14(no. 10):1727–1738. doi: 10.1046/j.0953-816x.2001.01794.x. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(no. 9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Bracci E, Ballerini L, Nistri A. Localization of rhythmogenic networks responsible for spontaneous bursts induced by strychnine and bicuculline in the rat isolated spinal cord. J Neurosci. 1996;16(no. 21):7063–7076. doi: 10.1523/JNEUROSCI.16-21-07063.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O'Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398(no. 6728):622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Moran L, Goulding MD, Saueressig H. PAX2 is expressed in multiple spinal cord interneurons, including a population of EN1+ interneurons that require PAX6 for their development. Development. 1997;124(no. 22):4493–4503. doi: 10.1242/dev.124.22.4493. [DOI] [PubMed] [Google Scholar]
- Carr PA, Alvarez FJ, Leman EA, Fyffe RE. Calbindin D28k expression in immunohistochemically identified Renshaw cells. Neuroreport. 1998;9(no. 11):2657–2661. doi: 10.1097/00001756-199808030-00043. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106(no. 22):9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci. 1995;15(no. 7 Pt 1):4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Johnson RL. Interactions between dorsal-ventral patterning genes lmx1b, engrailed-1 and wnt-7a in the vertebrate limb. Int J Dev Biol. 2002;46(no. 7):937–941. [PubMed] [Google Scholar]
- Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, Gray PA, Arata S, Shirasawa S, Bouchard M, Luo P, Chen CL, Busslinger M, Goulding M, Onimaru H, Ma Q. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci. 2004;7(no. 5):510–517. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383(no. 6599):407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Coumailleau P, Duprez D. Sim1 and Sim2 expression during chick and mouse limb development. Int J Dev Biol. 2009;53(no. 1):149–157. doi: 10.1387/ijdb.082659pc. [DOI] [PubMed] [Google Scholar]
- Crone SA, Quinlan KA, Zagoraiou L, Droho S, Restrepo CE, Lundfald L, Endo T, Setlak J, Jessell TM, Kiehn O, Sharma K. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60(no. 1):70–83. doi: 10.1016/j.neuron.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci. 2009;29(no. 21):7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Barrio MG, Taveira-Marques R, Muroyama Y, Yuk DI, Li S, Wines-Samuelson M, Shen J, Smith HK, Xiang M, Rowitch D, Richardson WD. A regulatory network involving Foxn4, Mash1 and delta-like 4/Notch1 generates V2a and V2b spinal interneurons from a common progenitor pool. Development. 2007;134(no. 19):3427–3436. doi: 10.1242/dev.005868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009;29(no. 47):14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90(no. 1):169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Farago AF, Awatramani RB, Dymecki SM. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 2006;50(no. 2):205–218. doi: 10.1016/j.neuron.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Fujiyama T, Yamada M, Terao M, Terashima T, Hioki H, Inoue YU, Inoue T, Masuyama N, Obata K, Yanagawa Y, Kawaguchi Y, Nabeshima Y, Hoshino M. Inhibitory and excitatory subtypes of cochlear nucleus neurons are defined by distinct bHLH transcription factors, Ptf1a and Atoh1. Development. 2009;136(no. 12):2049–2058. doi: 10.1242/dev.033480. [DOI] [PubMed] [Google Scholar]
- Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440(no. 7081):215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10(no. 7):507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA. Transcription factors and the genetic organization of brain stem respiratory neurons. J Appl Physiol. 2008;104(no. 5):1513–1521. doi: 10.1152/japplphysiol.01383.2007. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4(no. 9):927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19(no. 6):572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross MK, Dottori M, Goulding M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron. 2002;34(no. 4):535–549. doi: 10.1016/s0896-6273(02)00690-6. [DOI] [PubMed] [Google Scholar]
- Gross MK, Moran-Rivard L, Velasquez T, Nakatsu MN, Jagla K, Goulding M. Lbx1 is required for muscle precursor migration along a lateral pathway into the limb. Development. 2000;127(no. 2):413–424. doi: 10.1242/dev.127.2.413. [DOI] [PubMed] [Google Scholar]
- Hagglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat Neurosci. 2010;13(no. 2):246–252. doi: 10.1038/nn.2482. [DOI] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11(no. 4):417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1(no. 1):20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Karunaratne A, Hargrave M, Poh A, Yamada T. GATA proteins identify a novel ventral interneuron subclass in the developing chick spinal cord. Dev Biol. 2002;249(no. 1):30–43. doi: 10.1006/dbio.2002.0754. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kim JC, Cook MN, Carey MR, Shen C, Regehr WG, Dymecki SM. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron. 2009;63(no. 3):305–315. doi: 10.1016/j.neuron.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26(no. 21):5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Satou C, Higashijima S. V2a and V2b neurons are generated by the final divisions of pair-producing progenitors in the zebrafish spinal cord. Development. 2008;135(no. 18):3001–3005. doi: 10.1242/dev.024802. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci. 1996;16(no. 18):5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Crossed rhythmic synaptic input to motoneurons during selective activation of the contralateral spinal locomotor network. J Neurosci. 1997;17(no. 24):9433–9447. doi: 10.1523/JNEUROSCI.17-24-09433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450(no. 7169):503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Kudo N, Yamada T. Morphological and physiological studies of development of the monosynaptic reflex pathway in the rat lumbar spinal cord. J Physiol. 1987;389:441–459. doi: 10.1113/jphysiol.1987.sp016665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42(no. 3):375–386. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- Lechner HA, Lein ES, Callaway EM. A genetic method for selective and quickly reversible silencing of Mammalian neurons. J Neurosci. 2002;22(no. 13):5287–5290. doi: 10.1523/JNEUROSCI.22-13-05287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Misra K, Matise MP, Xiang M. Foxn4 acts synergistically with Mash1 to specify subtype identity of V2 interneurons in the spinal cord. Proc Natl Acad Sci U S A. 2005;102(no. 30):10688–10693. doi: 10.1073/pnas.0504799102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91(no. 1):127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Lundfald L, Restrepo CE, Butt SJ, Peng CY, Droho S, Endo T, Zeilhofer HU, Sharma K, Kiehn O. Phenotype of V2-derived interneurons and their relationship to the axon guidance molecule EphA4 in the developing mouse spinal cord. Eur J Neurosci. 2007;26(no. 11):2989–3002. doi: 10.1111/j.1460-9568.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57(no. 5):634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(no. 5748):683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- McKay LC, Feldman JL. Unilateral ablation of pre-Botzinger complex disrupts breathing during sleep but not wakefulness. Am J Respir Crit Care Med. 2008;178(no. 1):89–95. doi: 10.1164/rccm.200712-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi R, Kriks S, Cordes R, Gossler A, Ma Q, Goulding M. Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat Neurosci. 2006;9(no. 6):770–778. doi: 10.1038/nn1706. [DOI] [PubMed] [Google Scholar]
- Moran-Rivard L, Kagawa T, Saueressig H, Gross MK, Burrill J, Goulding M. Evx1 is a postmitotic determinant of v0 interneuron identity in the spinal cord. Neuron. 2001;29(no. 2):385–399. doi: 10.1016/s0896-6273(01)00213-6. [DOI] [PubMed] [Google Scholar]
- Muller T, Brohmann H, Pierani A, Heppenstall PA, Lewin GR, Jessell TM, Birchmeier C. The homeodomain factor lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron. 2002;34(no. 4):551–562. doi: 10.1016/s0896-6273(02)00689-x. [DOI] [PubMed] [Google Scholar]
- Myers CP, Lewcock JW, Hanson MG, Gosgnach S, Aimone JB, Gage FH, Lee KF, Landmesser LT, Pfaff SL. Cholinergic input is required during embryonic development to mediate proper assembly of spinal locomotor circuits. Neuron. 2005;46(no. 1):37–49. doi: 10.1016/j.neuron.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100(no. 24):13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YK, George KM, Engel JD, Linzer DI. GATA factor activity is required for the trophoblast-specific transcriptional regulation of the mouse placental lactogen I gene. Development. 1994;120(no. 11):3257–3266. doi: 10.1242/dev.120.11.3257. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1987;403(no. 2):380–384. doi: 10.1016/0006-8993(87)90080-1. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Ren J, Gray PA, Vandunk C, Gross M, Goulding M, Greer JJ. Central respiratory rhythmogenesis is abnormal in lbx1- deficient mice. J Neurosci. 2008;28(no. 43):11030–11041. doi: 10.1523/JNEUROSCI.1648-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CY, Yajima H, Burns CE, Zon LI, Sisodia SS, Pfaff SL, Sharma K. Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord. Neuron. 2007;53(no. 6):813–827. doi: 10.1016/j.neuron.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron. 2001;29(no. 2):367–384. doi: 10.1016/s0896-6273(01)00212-4. [DOI] [PubMed] [Google Scholar]
- Rabe N, Gezelius H, Vallstedt A, Memic F, Kullander K. Netrin-1-dependent spinal interneuron subtypes are required for the formation of left-right alternating locomotor circuitry. J Neurosci. 2009;29(no. 50):15642–15649. doi: 10.1523/JNEUROSCI.5096-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MF, Ren J, Ahmad KA, Chao HT, Klisch TJ, Flora A, Greer JJ, Zoghbi HY. Math1 is essential for the development of hindbrain neurons critical for perinatal breathing. Neuron. 2009;64(no. 3):341–354. doi: 10.1016/j.neuron.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Paydar S, Ericson J, Briscoe J, Berber E, German M, Jessell TM, Rubenstein JL. Ventral neural patterning by Nkx homeobox genes: Nkx6.1 controls somatic motor neuron and ventral interneuron fates. Genes Dev. 2000;14(no. 17):2134–2139. doi: 10.1101/gad.820400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir T, Geiman EJ, Wang Z, Velasquez T, Mitsui S, Yoshihara Y, Frank E, Alvarez FJ, Goulding M. Pax6 and engrailed 1 regulate two distinct aspects of renshaw cell development. J Neurosci. 2004;24(no. 5):1255–1264. doi: 10.1523/JNEUROSCI.3187-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saueressig H, Burrill J, Goulding M. Engrailed-1 and netrin-1 regulate axon pathfinding by association interneurons that project to motor neurons. Development. 1999;126(no. 19):4201–4212. doi: 10.1242/dev.126.19.4201. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254(no. 5032):726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987;21(no. 2–4):321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Stepien AE, Arber S. Probing the locomotor conundrum: descending the 'V' interneuron ladder. Neuron. 2008;60(no. 1):1–4. doi: 10.1016/j.neuron.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Storm R, Cholewa-Waclaw J, Reuter K, Brohl D, Sieber M, Treier M, Muller T, Birchmeier C. The bHLH transcription factor Olig3 marks the dorsal neuroepithelium of the hindbrain and is essential for the development of brainstem nuclei. Development. 2009;136(no. 2):295–305. doi: 10.1242/dev.027193. [DOI] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBotzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11(no. 5):538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci. 2009;12(no. 8):1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Timmer JR, Wang C, Niswander L. BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development. 2002;129(no. 10):2459–2472. doi: 10.1242/dev.129.10.2459. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Muhr J, Pattyn A, Pierani A, Mendelsohn M, Sander M, Jessell TM, Ericson J. Different levels of repressor activity assign redundant and specific roles to Nkx6 genes in motor neuron and interneuron specification. Neuron. 2001;31(no. 5):743–755. doi: 10.1016/s0896-6273(01)00412-3. [DOI] [PubMed] [Google Scholar]
- Wallen-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygard A, Enjin A, Fujiyama F, Fortin G, Kullander K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci. 2006;26(no. 47):12294–12307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li L, Goulding M, Frank E. Early postnatal development of reciprocal Ia inhibition in the murine spinal cord. J Neurophysiol. 2008;100(no. 1):185–196. doi: 10.1152/jn.90354.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildner H, Muller T, Cho SH, Brohl D, Cepko CL, Guillemot F, Birchmeier C. dILA neurons in the dorsal spinal cord are the product of terminal and non-terminal asymmetric progenitor cell divisions, and require Mash1 for their development. Development. 2006;133(no. 11):2105–2113. doi: 10.1242/dev.02345. [DOI] [PubMed] [Google Scholar]
- Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, Baier H, Isacoff EY. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature. 2009;461(no. 7262):407–410. doi: 10.1038/nature08323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Shook NA, Kanisicak O, Yamamoto S, Wosczyna MN, Camp JR, Goldhamer DJ. A multifunctional reporter mouse line for Cre- and FLP-dependent lineage analysis. Genesis. 2009;47:107–114. doi: 10.1002/dvg.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Wada N, Kitabatake Y, Watanabe D, Anzai M, Yokoyama M, Teranishi Y, Nakanishi S. Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J Neurosci. 2003;23(no. 17):6759–6767. doi: 10.1523/JNEUROSCI.23-17-06759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64(no. 5):645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, Fan CM, Goulding M. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron. 2008;60(no. 1):84–96. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yamamoto M, Engel JD. GATA2 is required for the generation of V2 interneurons. Development. 2000;127(no. 17):3829–3838. doi: 10.1242/dev.127.17.3829. [DOI] [PubMed] [Google Scholar]