Abstract
To provide effective host defense, a healthy immune system must recognize microbial threats and coordinate a protective inflammatory response. Yet the maintenance of overall homeostasis also dictates an absolute requirement for the efficient recognition and clearance of the host’s own dead and dying cells, and these functions must somehow also be balanced to avoid autoimmune disease. In recent studies we have characterized a class of naturally arising regulatory antibodies (NAbs) to oxidation-associated phospholipid antigens on apoptotic-cell (AC) membranes that discriminate apoptotic from healthy cells. When augmented by appropriate constant region effector functions, these antibodies enhance the phagocytic clearance of ACs, blunt inflammatory responses transduced by membrane and endosomal Toll-like receptors (TLRs), and block the development of inflammatory arthritis. These NAbs have also been implicated in lupus as well as atherosclerosis. We describe a model of immune homeostasis, termed the inhibitory dual receptor hypothesis, in which NAbs to ACs oppose the development of autoimmune and inflammatory disease.
Introduction
One of the most fundamental challenges to the immune system is the efficient recognition and clearance of the body’s own cells, which because of senescence or injury enter programmed death pathways. Apoptotic cell (AC) clearance is therefore important for resolving the cellular consequences of normal development and tissue remodeling that begins during embryogenesis and continues throughout life. These death pathways can also result from tissue injury that can follow exposure to environmental factors such as smoking or ultraviolet light. Hence throughout the lifespan of multicellular organisms there is an absolute need for the clearance of cell corpses, which are not uncommon since more than 1011 cells in our bodies die each day by apoptosis.
The clearance of ACs by the human immune system constitutes an immense and fundamental challenge. Multiple pathways therefore exist to clear these ACs in order to prevent the development of inflammatory immune responses that may be triggered by progression from cellular apoptosis to secondary necrosis, with release of self-antigens and activation of Pattern Recognition Receptors, such as Toll-like receptors (TLRs).
Walport and colleagues developed the “waste disposal” hypothesis to rationalize how defects in the removal of dying cells and cell debris, as occurs in C1q deficiency or other clearance pathways, can lead to systemic autoimmunity and SLE (1). The innate immune system has therefore developed a specialized multi-step process, which has been termed efferocytosis (taken from the Latin effero, meaning to take to the grave or to bury). Efferocytosis involves numerous surface ligands, bridging molecules, phagocyte receptors, and signaling transducers (reviewed in (2)). These pathways for the clearance of apoptotic cells likely represent one of the most primitive and highly conserved functions of the innate immune system, and due to the overarching importance of AC clearance, redundant systems have been developed to ensure the efficiency of these processes. We postulate that regulatory natural antibodies to AC, as part of the repertoire of the earliest immune cells of the adaptive immune system, arose to provide additional levels of such protection for the host (3).
IgM natural autoantibodies are present from birth
At birth, we already have substantial levels of circulating IgM antibodies, which reflect a functional neonatal B-cell compartment poised and ready to contribute to neonatal host defense. These IgM antibodies arise in the womb from neonatal B lymphocytes that express clonally distributed B-cell antigen receptors (BCRs). The conservation of certain antibody specificities across species may suggest selection to prime immune defenses from infectious agents. Yet it has also been postulated that part of this early B-cell repertoire may result from an evolutionary pressure to provide important housekeeping functions (reviewed in (4)). This latter topic has received little attention in recent years.
Regulatory autoantibodies recognize AC-specific membrane phospholipid determinants
B-1 cells represent a distinct tier of mature B cells, which arise during the perinatal period and are the predominant source of circulating IgM in mice. The prototypic murine T15 B-1 cell clonotype is a well-characterized example of a natural antibody (NAb)-producing set of B cells that is defined by specific H-L paired canonical antibody gene rearrangements without hypermutation. This clone spontaneously arises and becomes highly represented within the first week of life, even in mice raised under germ-free conditions (reviewed in (5). Thus, microbial ligands are not primary mediators of T15 B-cell clonal selection.
T15-NAbs bind to phosphorylcholine (PC) determinants, which is a small phospholipid head group that is an immunodominant determinant on pneumococci and several other common microbial pathogens, and T15-NAbs contribute to host defense to PC-containing pneumococci and other microbes and provide optimal protection from systemic infection (reviewed in (5)). More recently, PC determinants were also identified on oxidatively modified low-density lipoprotein (OxLDL) generated during atherogenesis (6). Significantly, we found that pneumococcal immunization, which induced active B-cell responses with raised T15 antibody levels, greatly ameliorated atherosclerosis in this murine model of hyperlipidemia and chronic inflammation (7). Surprisingly, although it was first assumed that NAb binding interactions with components of atherosclerotic plaques were responsible, infusions of these anti-PC antibodies failed to enhance in vivo clearance of labeled OxLDL (8), which indicated that the actual basis for this vaccine-mediated atheroprotection needed an alternative explanation.
Instead, our investigations have suggested that it is the cross-reactivity of these same NAbs with PC epitopes on ACs that is most likely the source of the protective effects seen in this atherosclerosis model. By immune recognition of the PC head group, T15-NAbs can discriminate dead/dying cells from healthy cells (reviewed in (5)). In explanation, the PC head group is also a component of neutral phospholipids (e.g. phosphatidyl choline) in the outer leaflet of host cell membranes. In healthy cells, PC is sequestered and not available for immune recognition. Yet, during apoptotic death the PC head group becomes exposed due to oxidative damage to the polyunsaturated fatty acid side chains of phospholipids, to generate reactive compounds such as 1-palmitoyl-2-(5-oxovaleroyl)-snglycero-3-phosphorylcholine_(POVPC), which is an oxidative breakdown product of phosphatidylcholine (reviewed in (5)). A second major set of IgM NAbs recognizes malondialdehyde, MDA, which is a major degradation product of unsaturated lipids reacting with reactive oxidation species. Thereby, damaged cells undergoing apoptosis are flagged by the emergence of PC and MDA neo-epitopes. These oxidation-associated neo-epitopes are then recognized by commonly expressed NAbs, thereby enabling discrimination between apoptotic and healthy cells (6, 7, 9-12).
The levels of these NAbs appear to be driven by exposure to ACs. After intravenous administration of ACs, without adjuvant or other stimulants, the induced humoral response has been evaluated with an 800-feature antigen microarray, and these surveys showed that anti-AC responses are dominated by antibodies to antigens involving the simple compounds, PC and MDA (10). Indeed at a cellular level, PC and MDA-albumin antigens were together recognized by more than 55% of all induced splenic IgM-secreting cells after AC immunization (10). Hence, induced anti-AC responses are heavily dominated by these same NAbs, which are also present in naïve mice. As discussed below, these NAbs were shown to form immune complexes with ACs, which we postulate enhance complex synaptic interactions with immune cells that can regulate key innate immune functions.
Regulatory properties of anti-ACM antibodies involve recruitment of MBL and C1q to AC-complexes
The phagocytic clearance of ACs can involve innate immune recognition molecules, such as C1q and mannose binding lectin (MBL), that can be directly deposited from plasma onto late apoptotic and early necrotic cells, where they serve as “eat me” signal that aids phagocytic clearance by macrophages (Mφ and dendritic cells (DCs) (2, 13, 14). MBL is a multimeric lectin protein member of collectin family structurally akin to C1q (reviewed in (15). In vitro studies have shown that C1q and MBL also have redundant capacities to directly down-modulate innate immune cell responses. However, this process of direct opsonin mediated phagocytosis is relatively inefficient, and recent studies have shown that that C1q- and MBL-mediated AC phagocytic clearance can be enhanced several-fold by the addition of polyclonal natural IgM (9, 10, 16, 17), or by a monoclonal IgM with unmutated Ab variable regions identical to the classical T15 B-1 cell clonotype that binds PC-containing antigens (9, 10, 16). Significantly, anti-PC IgM antibodies shifted the recruitment of C1q and MBL onto ACs at much earlier stages of apoptosis that have the greatest immunomodulatory properties, which likely explains the associated immune modulating activity (9, 10).
Anti-ACM antibodies enhance the phagocytic clearance of ACs by Mφ and DCs
In many settings, Mφ are primarily responsible for the clearance of ACs, which is essential to prevent the release of pro-inflammatory factors and autoantigens that can select pathogenic B- and T-cell clones. There are many subsets of DCs that vary by surface phenotype and functional capacity. Certain DCs share the phagocytic properties of activated Mφ, especially at early stages of differentiation and/or those expressing CD103 and high levels of CD11c. Phagocytosis of ACs by such DCs is part of a process of constant steady-state sampling and presentation of self-antigens, which in vivo experiments suggest actively reinforces immune tolerance (18).
Regulatory NAbs, such as the prototypic monoclonal T15 IgM (from the E06 hybridoma (12)), have been shown to greatly enhance in vivo clearance of ACs by peritoneal Mφ and also in vitro clearance by DCs (9, 10). Notably, the effects of NAb on AC engulfment by DCs was dependent on C1q- and/or MBL recruitment (9; 10), while there was neither an absolute requirement for C4, C3, iC3b or other breakdown products nor for activation of the downstream complement cascade, although this topic is controversial (19, 20). Moreover, circulating NAbs to PC and MDA in murine neonatal and adult sera had similar properties to enhance AC phagocytosis by DCs (10).
Anti-ACM antibodies block TLR-mediated responses of Mφ and DCs
Inflammation is a protective host response to foreign challenge or tissue injury that is ultimately beneficial as it leads to the restoration of tissue structure and function. The resolution of an inflammatory response is therefore essential for homeostasis. Both the exposure to ACs and their clearance have been recognized as important mechanisms for the control and resolution of inflammation in vivo (reviewed in (2)).
In studies of the effects of NAbs on inflammatory responses, the IgM anti-ACM inhibited in vivo responses to endotoxin (TLR4 agonist) and poly I:C (TLR3 agonist), with suppression of blood levels of inflammatory cytokines, such as IL-6, IL-12p70, IL-17, TNFα and the chemokines, MIP-1α, MCP-1 KC and IP-10, which have all been implicated in human autoimmune disease (9). There was also inhibition of activation marker expression on splenic Mφs and DCs, which included CD40, CD86 and MHC II, although this could also have reflected changes in splenic cellular representation and/or altered phagocyte trafficking (9). This same natural antibody was capable of dose-dependent suppression of in vitro IL-6 and TNFα responses of a monocyte-like cell line, RAW264.7, to the TLR4 agonist LPS (9).
The effects on DCs may potentially be even more important, as DCs serve as sentinel immune cells and when induced to fully mature, lose phagocytic capacity, up-regulate costimulatory molecules and chemokine receptors, migrate to draining lymph nodes, and become potent antigen-presenting cells. Moreover, when certain types of DCs are fully activated they can also be high-level producers of a range of cytokines and chemokines. We found that the IgM anti-ACM also blocked responses of DCs to agonists to TLR3, TLR4, TLR7 and TLR9, with inhibition of DC production of IL-6, IL-12p70, IL-17, TNFα and a range of chemokines (9). IgM anti-ACM also suppresses IFN related genes, including IP-10 (9) and IFN-β1 and IRF-4 (unpublished). However, we were surprised to find that the anti-ACM NAbs did not induce these bone marrow-derived DCs to produce IL-10 or TGF-β, factors implicated in the suppression of inflammatory responses in other settings. Thus, the nature of this anti-inflammatory activity of anti-ACM antibodies appears to utilize different mechanisms in vitro. However, it remains possible that IL-10 and TGFβ may be induced by anti-ACM NAbs in other cell types during in vivo responses.
Regulatory NAbs may block development of inflammatory autoimmune disease
As inflammatory pathways involving Mφ, DC, and TLR have been implicated in the pathogenesis of autoimmune arthritis, we studied collagen-induced arthritis (CIA) in DBA/1 mice. Significantly, pretreatment with the IgM anti-PC NAb markedly reduced clinical disease activity, synovial leukocytic infiltrates, and bone and joint damage (9). Notably, there were no differences in IgG anti-Collagen type II (CII) levels induced by collagen immunization in the different treatment groups, suggesting that T15-NAb primarily inhibited the IgG immune complex mediated end-organ inflammatory response.
To further define the role of the adaptive immune system in this process, the effects of T15-NAb were studied on arthritis induced by the passive transfer of anti-CII IgG, in which the innate immune system dominates pathogenic pathways and lymphocytes do not a play central role. Here, T15-NAb treatment also significantly diminished joint swelling (9). Taken together, these findings indicate that the regulatory properties of T15-NAb in these models of arthritis act through the blunting of pro-inflammatory effector mechanisms mediated by the recruitment of IgG-autoantibody immune complexes.
Regulatory NAbs and the dual inhibitory receptor hypothesis
To better understand the potential mechanistic basis for the properties of regulatory NAbs, we have compared these pathways with those of pathogenic antibodies as formulated by the Activating dual receptor hypothesis of Marshak-Rothstein (21). This model explained that pathogenic IgG-autoantibodies, which can form immune complexes with RNA- and DNA-containing nuclear Ags from our own cells, can stimulate immune cells though TLR receptors and FcγR on myeloid and plasmacytoid DC, or alternatively through TLRs and BCR on B cells (22). For DC activation, an IgG-nuclear antigen complex (that includes IgG of activating subclasses) can trigger and be internalized via interactions with ITAM-associated FcγR (and especially FcγRIIIa)(23), which results in the delivery of the DNA or RNA-containing complex to otherwise sequestered endosomal lysosomal compartment. These IgG-immune complexes deliver activating ligands to endosomal TLR7 and TLR9 molecules and their co-receptors. Interactions with pathogenic autoantibody-RNA/DNA complexes set off proinflammatory cascades, with central roles for Mitogen Activated Protein (MAP) Kinase pathways, and especially the primary MAP Kinase, p38. This cascade, with activation of the intertwined NF-κB pathways, results in activation and nuclear entry of a range of pro-inflammatory transcription factors. Presumably, pathogenic autoantibodies in inflammatory arthritis also involve other currently unknown ligands for innate immune Pattern Recognition receptors.
We postulate that the presence of a regulatory NAb acts through an anti-inflammatory mechanism, the dual inhibitory receptor pathway. In this model, the anti-ACM autoantibodies are counter-regulatory factors for the pathogenic IgG autoantibodies to nucleoproteins that are striking features of SLE. In Table I, the features of pathogenic IgG lupus anti-nuclear autoantibodies are contrasted with those of counter-regulatory anti-ACM NAbs. Both types of autoantibodies recognize autoantigens that are generated during apoptosis and/or secondary necrosis. Yet, there are important differences. AC-membranes, themselves, have immune modulatory activities, which we propose are essential for the properties of anti-ACM Abs. We postulate that the homeostatic properties of natural Abs to ACM act by coordinating and enhancing specific interactions with two types of membrane-associated inhibitory receptors; receptors for early complement factors (C1q and MBL) that have known anti-inflammatory activities (15) and to receptors for ACs (Figure 1). Our future studies will test whether the anti-ACM antibodies specifically increase accessibility on immune-complexed AC of phosphatidylserine residues, which are reported to mediate binding to a range of putative AC receptors (reviewed in (2)).
Table I.
Proposed properties of opposing pathogenic and protective autoantibodies
| Pathogenic lupus autoantibodies |
Protective anti-ACM autoantibodies |
|||
|---|---|---|---|---|
|
Isotype/subclass
(in mouse) |
Commonly murine IgG2a, IgG2b or IgG2c that strongly interacts with activating FcγR. In humans IgG1 and IgG3 |
Ref. (22, 23) |
IgM and perhaps IgG that do not interact with activating FcγR |
Ref. (6, 7, 9- 11) |
|
When are these
autoantibodies expressed? |
During active immune disease |
(21) | From perinatal period and throughout life. |
(unpubl ished) (26) |
|
What happens
during active disease? |
Often increase during active disease |
(21) | May decrease during active disease |
(24,25 ) |
|
Cellular location
of targeted autoantigens |
Normally sequestered intracellular (i.e., nuclear) |
(21) | PL-related neoantigens form naturally during apoptosis. Rapidly expressed & accessible on AC membranes beginning from early apoptotic death. |
(6, 9, 10) |
|
Postulated that
location of receptors of autoantibody- complexes involved in mechanism of action |
Coordinated interactions with activating membrane FcγR and BCR and otherwise inaccessible endosomal receptors (TLR7 & TLR9) |
(21) | Cell membrane receptors constitutively expressed on MΦ and DC, and may be inducible on lymphocytes after certain forms of activation |
(9,10) |
Figure 1. Hypothesis: Anti-ACM NAbs enhance triggering via inhibitory dual receptors.
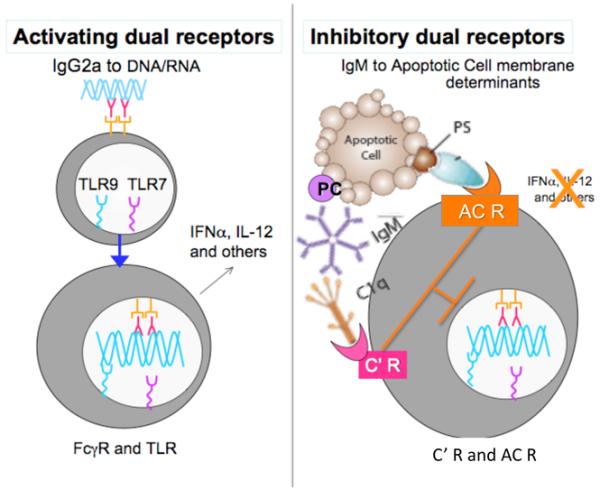
At left, the pathogenic autoantibody pathway can involve autoantigens, which in health are primarily sequestered in the nucleus. In murine lupus models, these IgG2a autoantibodies are made under Th1-biased autoimmune responses. These immune complexes can trigger activating FcγR, which causes internalization followed by interactions with the stimulatory TLR7 and TLR9 otherwise sequestered within intracellular compartments. At right, the opposing pro-homeostatic anti-ACM antibodies, which are present in the circulation from birth, inhibit the pro-inflammatory influences of TLR ligands, as well as the activating activity of pathogenic lupus autoantibodies to DNA and RNA Abs. A depiction of C1q is shown, while MBL may play similar or overlapping roles. C’ R, complement receptor(s); AC R, apoptotic cell receptor(s).
As described above, prominent amongst the properties of anti-ACM Abs is the capacity to enhance the efficiency of AC clearance, which may also effectively reduce the availability of sequestered autoantigens that can drive the production of pathogenic autoantibodies. IgM anti-ACM can also directly protect from the development of inflammatory arthritis, and a protective role of such NAbs have also been implicated in atherogenesis (7). We are currently exploring how anti-ACM antibodies may block central inflammatory signal transduction pathways common to a range of TLR responses. Conversely, we wonder whether some IgG anti-ACM may be capable of forming complexes that trigger pro-inflammatory activating FcγR that may in fact worsen disease, and we speculate that such opposing activities may contribute to the accelerated atherosclerosis associated with rheumatoid arthritis and SLE. In fact recent studies support the notion that both IgG and IgM anti-PC antibodies are commonly elevated in patients with SLE compared to healthy controls, while patients with SLE without a history of cardiovascular events had significantly higher anti-PC IgM levels (24, 25)(manuscript submitted).
In conclusion, investigations into regulatory natural autoantibodies are beginning to characterize the pathways responsible for the potent capacity for controlling inflammatory responses driven by either innate or adaptive immune pathways. These studies are already shedding light on previous unsuspected connections at the interface of the adaptive and innate immune systems that may contribute to the accelerated atherosclerosis in patients with SLE, RA and related diseases. These observations may also provide the blueprints for the development of a novel therapeutic approach, which enhances physiologic anti-inflammatory pathways to control pathways responsible for the tissue damage occurring in chronic autoimmune disease.
Acknowledgments
Financial support: Grants from the NIH; R01 AI068063 and ARRA supplement, R01AI090118, and from the ACR REF Within Our Reach campaign, the Alliance for Lupus Research, the Arthritis Foundation, and the P. Robert Majumder Charitable Trust Foundation.
Footnotes
Conflicts: GJS has served as a consultant for Neostasis Inc.
References
- 1.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol. 2004;22:431–56. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 2.Erwig LP, Henson PM. Immunological consequences of apoptotic cell phagocytosis. Am J Pathol. 2007;171(1):2–8. doi: 10.2353/ajpath.2007.070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverman GJ, Gronwall C, Vas J, Chen Y. Natural autoantibodies to apoptotic cell membranes regulate fundamental innate immune functions and suppress inflammation. Discov Med. 2009;8(42):151–6. [PubMed] [Google Scholar]
- 4.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7(6):812–8. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 5.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 6.Shaw PX, Goodyear CS, Chang M-K, Witztum J, Silverman GJ. The Autoreactivity of Anti-Phosphorylcholine Antibodies for Atherosclerosis-Associated Neo-antigens and Apoptotic Cells. J Immunol. 2003;170:6151–7. doi: 10.4049/jimmunol.170.12.6151. [DOI] [PubMed] [Google Scholar]
- 7.Binder C, Hörkkö S, Dewan A, Chang M-K, Kieu E, Goodyear C, Shaw P, Palinski W, Witztum J, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: Molecular mimicry between oxidized LDL and Streptococcus pneumoniae. Nature Medicine. 2003;9:736–43. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 8.Reardon CA, Miller ER, Blachowicz L, Lukens J, Binder CJ, Witztum JL, Getz GS. Autoantibodies to OxLDL fail to alter clearance of injected OxLDL in apolipoprotein E deficient mice. J Lipid Res. 2004;45(7):1374–54. doi: 10.1194/jlr.M400075-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Chen YF, Khanna S, Goodyear CS, Park Y-B, Raz E, Thiel S, Boyle DB, Corr M, Kono DH, Silverman GJ. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that enhances phagocytic clearance, suppresses TLR responses and inhibits inflammatory arthritis. J Immunol. 2009;183:1346–59. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YF, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–43. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw PX, Hörkkö S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105(12):1731–40. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang MK, Bergmark C, Laurila A, Hörkkö S, Han KH, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA. 1999;96(11):6353–8. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194(6):781–95. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nauta AJ, Castellano G, Xu W, Woltman AM, Borrias MC, Daha MR, van Kooten C, Roos A. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol. 2004;173(5):3044–50. doi: 10.4049/jimmunol.173.5.3044. [DOI] [PubMed] [Google Scholar]
- 15.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44(1-3):33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur J Immunol. 2005;35(1):252–60. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- 17.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IgM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38(4):259–64. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 19.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188(7):1359–68. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188(12):2313–20. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6(11):823–35. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199(12):1631–40. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171(6):3296–302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 24.Su J, Hua X, Concha H, Svenungsson E, Cederholm A, Frostegard J. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology (Oxford) 2008;47(8):1144–50. doi: 10.1093/rheumatology/ken120. [DOI] [PubMed] [Google Scholar]
- 25.Silverman GJ, Germar K, Goodyear CS, Andrews KA, Ginzler EM, Tsao BP. Genetic imprinting of autoantibody repertoires in SLE patients. Clin Exp Immunol. 2008;153:102–116. doi: 10.1111/j.1365-2249.2008.03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Hörkkö S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119(5):1335–49. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]


