Abstract
Objective
Fluctuations in pain among persons with knee osteoarthritis are both common and risk factors for pain fluctuation are poorly understood. To best identify structural causes of fluctuations, multiple assessments of pain status and structural lesions are needed. Therapeutic evidence may be best gleaned if pain resolution is accompanied by diminution of lesions.
Methods
Subjects in the Multicenter Osteoarthritis Study were queried about their knee pain by interview and had knee magnetic resonance imaging at baseline, 15-month, and 30-month clinic visits. Among the knees experiencing pain fluctuation over three clinic visits we examined the relation of bone marrow lesions (BMLs), synovitis, and effusion to frequent knee pain and pain severity using conditional logistic regression.
Results
Included in the analysis were 570 subjects (651 knees). When the BML score changed from 0 to 1, 2, 3, 4, 5-6 and 7-18, the odds ratios (OR) for frequent knee pain were 1.2, 1.2, 1.5, 2.2, 2.4, and 2.5, respectively (P for trend=0.006). The corresponding ORs were 1.5, 1.5, and 2.4 when synovitis score changed from 0 to1, 2, and 3-6 (P for trend=0.045). No significant association was found between effusion and frequent knee pain. Diminishing size of BMLs was associated with resolution of knee pain (P=0.007). Similar associations were also observed between these structural lesions and knee pain severity.
Conclusion
Changes of BMLs and synovitis are associated with the fluctuation of knee pain. Pain resolution occurs more frequently when BMLs become smaller.
Keywords: Bone marrow lesions, synovitis, effusion, pain, osteoarthritis
The number of adults in the US with clinical osteoarthritis grew from 21 million to nearly 27 million from 1995 to 20051. Of those, about 10 million people had symptomatic knee OA. Pain from knee OA is a key reason for seeking medical care and is an important antecedent to disability2. While pain from knee OA has long been considered a chronic condition, most people with symptomatic knee OA experience fluctuation in pain presence and severity3. The etiology for pain fluctuation from knee OA is not well understood.
Over the last two decades, magnetic resonance imaging (MRI) has become an important tool in the evaluation and diagnosis of knee OA. Since MRI is able to visualize the subchondral bone marrow, synovial tissue, joint effusion and other abnormalities that cannot be seen with x-rays, several studies have examined the relation of the structural lesions assessed by MRI in or around the knee to knee pain4-13. Some studies found that bone marrow lesions (BMLs)5, 8, 10, 12, 13, effusions4, 9, 10 and synovitis4, 10, 11, were associated with knee pain, others, however, have failed to confirm the relation of BMLs to knee pain6, 7, 9. Some studies also demonstrated that an increase in BMLs was associated with pain onset12, 13 and that aggravating severity of synovitis was related to increasing pain severity11, nevertheless, such findings may be confounded by co-occurrence of worsening of other structural lesions. A few studies have suggested that cartilage defects may be associated with pain8, 10.
There has been a paucity of information on changes in structural lesions in relation to fluctuation of knee pain over time, especially on whether reduction or complete resolution of these structural lesions is accompanied contemporaneously by a decrease in the presence of knee pain or pain severity. The study of improvement in lesions with pain change circumvents the problem of confounding by disease worsening. Further, the relation of reduction in these lesions to reduction in pain has treatment implications. The absence of longitudinal studies with repeat MRIs may reflect logistical and methodological challenges. First, obtaining and reading knee MRIs at multiple time points in participants in a longitudinal study is expensive. Second, pain is a subjective experience and biological, psychological, and social factors play important roles in a subject's response to pain stimuli14. However, in many studies of risk factors for knee pain, the majority of these risk factors were neither collected nor adjusted for.
Since pain is a nociceptive phenomenon that requires sensory input, we hypothesize that nociceptive input must necessarily be related to some underlying structural lesions (i.e., biological origin). Such structural lesions are likely to be reversible in order to cause the fluctuation of knee pain. Therefore, we focused on three reversible structural lesions, i.e., BML's, synovitis and effusion and tested whether changes in these reversible structural lesions are associated with fluctuation of knee pain. To minimize the effect of time-invariant confounders for pain and to reduce the cost of reading knee MRIs, we conducted a self-matched case-control study15 focusing on persons with pain fluctuation to examine the relation of changes in BMLs, effusion, and synovitis to the fluctuation of knee pain among participants in the Multicenter Osteoarthritis Study (MOST).
Subjects and Methods
The MOST Study
The MOST study is a longitudinal study of risk factors for incident and progressive knee OA. Individuals were recruited from two communities in the US, Birmingham, Alabama and Iowa City, Iowa. The study protocol was approved by the institutional review boards at the University of Iowa, University of Alabama, Birmingham, University of California, San Francisco, and Boston University Medical Center. The details of the study have been published elsewhere12.
Assessment of knee pain
Two measures were used to characterize knee pain: frequent knee pain and severity of knee pain. Specifically, at the baseline and the 30-month follow-up visits, all subjects were asked a knee-specific pain question at a telephone screen before clinic as follows: “During the past 30 days, have you had pain, aching, or stiffness in your knee on most days?”, hereafter referred to as the “frequent knee pain”12, 16. Approximately one month later, subjects were invited to the clinic where the same frequent knee pain question was asked again. At the 15-month follow-up visit, only a subset of subjects were invited to attend the clinic if they : (1) had no frequent knee pain during either telephone screen or clinic visit at the baseline but developed frequent knee pain during a telephone screen at the 15-month follow-up visit, or (2) were included in a random sample of subjects who had no frequent knee pain at either telephone screen or clinic visit at the baseline and had no frequent knee pain at the 15-month telephone screen.
At each clinic visit, subjects also completed a knee-specific Western Ontario and McMaster University Osteoarthritis Index (WOMAC) pain questionnaire to assess severity of knee pain related to each of five activities (i.e., walking on a flat surface; going up or down stairs; at night while in bed; sitting or lying, and standing upright), with score ranging from 0 (no pain) to 4 (extreme pain)17.
We defined a clinic visit as a case-visit if a knee had ‘frequent knee pain’ at that visit and as a control-visit if a knee did not have pain at clinic visit(s). In addition, we divided case-visits into two groups according to the maximum item score of WOMAC pain: mild or moderate frequent knee pain (i.e., the maximum item score of WOMAC being 1 or 2), or severe or extreme frequent knee pain (i.e., the maximum item score of WOMAC being 3 or 4).
Knee MR images
At the clinic visit subjects underwent bilateral MRIs with a 1.0T extremity magnetic resonance system (OrthOne™, ONI Medical Systems., Wilmington, MA) with a circumferential extremity coil. The MR sequence protocol included fat-suppressed fast spin echo proton density (PD)-weighted sequences in the sagittal (TR=4800 ms, TE=35 ms, 3 mm slice thickness, 0 mm interslice gap, 32 slices, 288 × 192 matrix, 2 number of excitations (NEX), 140 mm2 FOV, echo train length=8) and axial planes (TR=4680 ms, TE=13 ms, 3 mm slice thickness, 0 mm interslice gap, 20 slices, 288 × 192 matrix, 2 NEX, 140 mm2 FOV, ETL=8) and a STIR sequence in the coronal plane (TR=6650 ms, TE=15 ms, TI=100 ms, 3 mm slice thickness, 0 mm interslice gap, 28 slices, 256 × 192 matrix, 2 NEX, 140 mm2 FOV, ETL=8).
All MRIs of each knee were scored at the same time in known chronological order and blinded to pain status by two musculoskeletal radiologists (AG and FR) using the Whole-Organ MRI Score (WORMS)18. Specifically, BMLs, characterized as ill-defined hyperintense signal alterations on PD or STIR MRI adjacent to the subchondral plate, were scored 0 to 3 in each of 10 sub-regions of the medial and lateral tibiofemoral compartments as well as in each of four sub-regions of the patellofemoral compartment19; synovitis, using a surrogate of hyperintense signal changes in Hoffa's fat pad, was scored 0 to 3 in the infrapatellar and intercondylar regions of Hoffa's fat pat; and effusion volume, amount of capsular distension, was scored from 0 to 3 for each knee. The changes in Hoffa's fat pad have been shown to be related to synovitis as seen on arthroscopy20. Any score of 1 or greater signifies fluid beyond physiologic. The weighted Kappa for inter-reader reliability was 0.62, 0.65, and 0.65 for BMLs, effusions, and synovitis, respectively. As part of MRI reading, cartilage morphology was scored for each MRIs using the WORMS.
Assessment of other covariates
Height was measured at the baseline clinic visit and weight was measured at each clinic visit. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Knee injury occurring within two years before baseline visit and between the consecutive visits was assessed at each clinic visit. Psychological state was evaluated using SF-12 questionnaire at each clinic visit with which we calculated a mental health component score to indicate a subject's psychological distress and psychological wellbeing21.
All participants had bilateral weight bearing, fixed flexion posteoanterior and lateral radiographic evaluation of the knee. Radiographs were scored by a musculoskeletal radiologist and a rheumatologist blinded to pain status. Each knee joint was scored for Kellgren and Lawrence grade, maximal osteophyte grade, and maximal joint space narrowing grade. Maximal osteophyte and joint space narrowing grades were determined on the posteroanterior and lateral views, thereby including both the tibiofemoral and the patellofemoral joints. A knee was considered as having radiographic OA if OA involved in either patellofemoral or tibiofemoral or both joints22.
Statistical analysis
We included knees that had frequent knee pain during at least one, but not all, of the three clinic visits using the self-matched case-control study design. Case-visit(s) and control-visit(s) within a knee formed a matched set; thus, all time-invariant confounders within a knee were implicitly eliminated using conditional logistic regression. Because each knee serves as its own control the effect estimate generated from the analyses can be interpreted as the change of a specific MRI lesion over time to the risk of frequent knee pain or severity of frequent knee pain categories.
We summed the scores for BMLs and synovitis across all the sub-regions for each knee at each visit. Because the number of knees with severe lesions, indicated by high score of these lesions, is relative small, to minimize the random variability and obtain robust effect estimates for these lesions, we divided the summary scores into seven categories for BMLs: 0, 1, 2, 3, 4, 5-6, and 7-18; and four categories for synovitis: 0, 1, 2, and 3-6. The scores for effusions were grouped into three categories: 0, 1 and 2-3. We examined the relation of changes in these categories of BMLs, synovitis, and effusion scores to changes in frequent knee pain status using conditional logistic regression. To examine how changes in MRI lesions were associated with development of frequent knee pain from those with resolution of frequent pain, we performed separate analyses of knees with frequent pain status switching from “absent” at an earlier clinic visit(s) to “present” at a later visit(s) and of knees switching from “present” at an earlier clinic visit(s) to “absent” at a later visit(s). In the multivariable regression model we adjusted for BMI, knee injury, mental health component score, maximum score of cartilage morphology, and other two structural lesions. Since approximately 15% of participants had two knees included in the analysis, we used a robust sandwich estimate for standard errors to accommodate the correlation between two knees within a person. Finally, we evaluated the relation of changes in each structural lesion to changes in severity of frequent knee pain using a stratified proportional odds model by amalgamating conditional logistic regression23.
Results
Included were 651 knees (570 subjects) that had had frequent knee pain present in at least one, but not all, clinic visits. Of them, 556 (86%) knees had MRIs assessed at two clinic visits (e.g., 70 were assessed at baseline and 15-month clinic visits, 486 were assess at baseline and 30-month clinic visits) and 95 at three clinic visits. The average age of subjects was 62 years (SD=7.9) and 68% were women. The mean BMI was 29.7 kg/m2 (SD=4.9). Average mental health component score at the baseline was 54.2 (range 13-69), with high score indicating better status. Slightly less than half (41.4%) of the knees had radiographic knee OA at the baseline. Over 91.5% of knees' cartilage morphology score was greater than 1.
Among the knees whose frequent pain status changed from absent to present over two consecutive clinic visits, the percentage with worsening in each structural lesions (BMLs: 36.8%; synovitis: 24.2%; and effusion: 24.4%) was higher than that of improvement in the lesions (BMLs: 27.8%; synovitis: 11.0%; and effusion: 13.2%). Among the knees whose frequent pain status changed from present to absent, percentage of improvement of BMLs (38.0%) was higher than that of worsening (25.5%), but no such difference was observed in either synovitis (11.3% vs. 12.8%) or effusion (13.7% vs. 15.5%).
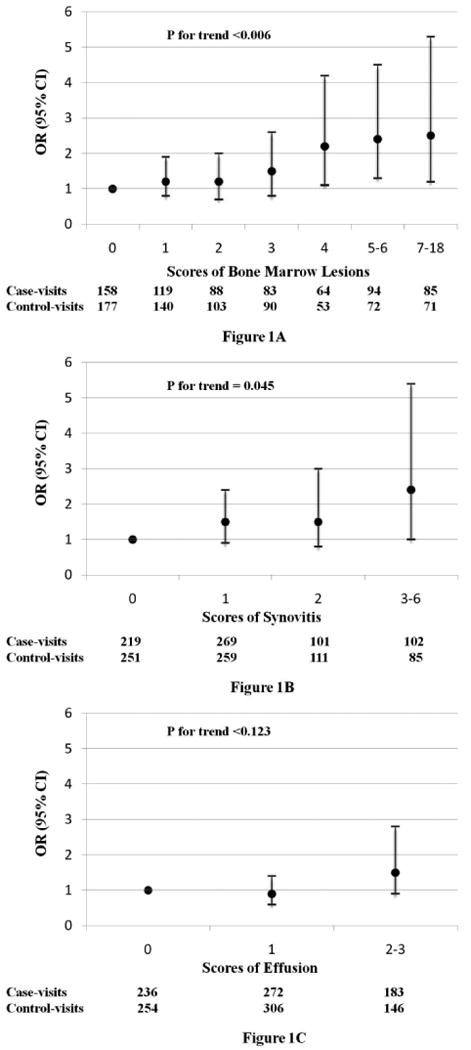
Changes in severity of BMLs, synovitis, and effusion (i.e., either worsening or improving) in relation to the risk of frequent knee pain (i.e., either present or absent) is depicted in Figures 1-A, 1-B, and 1-C, respectively. The multivariable adjusted odds ratios of frequent knee pain status were 1.1, 1.2, 1.5, 2.2, 2.4, and 2.5 for changes in scores of BML severity from 0 to 1, 2, 3, 4, 5-6, and 7-18 (P for trend=0.006, OR (95% CI) per one unit increase in BML = 1.1 (1.0, 1.2)), respectively. The odds ratios for frequent knee pain were 1.5, 1.5, and 2.4 for a change in scores of synovitis severity from 0 to 1, 2, and 3-6, respectively (P for trend =0.045, OR (95% CI) per unit increase in synovitis = 1.3 (1.0, 1.7)) (Figure 1-B). However, no significant dose-response relationship was observed for effusion (Figure 1-C). The values of maximum rescaled R2 were 5.88%, 7.86%, 7.13%, and 7.34%, respectively, for the models that included all other covariates only (i.e., BMI, knee injury, mental health component score, and maximum score of cartilage morphology), all covariates plus BMLs, all covariates plus synovitis, and all covariates plus effusion. These findings suggested that BMLs had better predictive ability for fluctuation of knee pain than either synovitis or effusion. When all variables were included in the mode, the value of maximum rescaled R2 was 9.62%. The results did not change materially when we limited the analyses to the subjects who had only one knee that experienced pain fluctuation.
Figure1A-1C. Relation of BMLs, Effusion, and Synovitis to Fluctuation of Frequent Knee Pain.
Results of the genetic association analysis of (a) CWP and (b) total number of pain sites for SNPs genotyped in the discovery and validation cohorts are shown. Estimates and 95%CI are shown in red for the discovery cohort and in green for the validation cohort. The pair-wise LD between the associated SNPs is shown in (c) (coloured by D′ (low D′=white, high D′=red and numbered by r2 (no number where r2=1)).**recessive model, *dominant model. Rs9526245 was genotyped as a proxy (r2=1) for rs9316235 in the validation cohort.
Results of separate analyses of the knees with frequent pain status switching from absent at an early clinic visit(s) to present at later visit(s) and vice versa are shown in Table 1. Worsening of synovitis and effusions were associated with an increased risk of frequent knee pain, whereas reduction or complete resolution of BMLs or of effusions was associated with a decreased risk of frequent knee pain.
Table 1. Changes in severity of BMLs, synovitis, and effusion and the direction of changes in frequent knee pain.
| Scores of Structural lesions | Change in frequent knee pain status from an earlier to a later clinic visit | |||||
|---|---|---|---|---|---|---|
| From absent to present | From present to absent | |||||
| Control-visits | Case-visits | OR (95% CI)* | Control- visits | Case-visits | OR (95% CI)* | |
| BMLs | ||||||
| 0 | 84 | 81 | 1.0 (reference) | 93 | 85 | 0.3 (0.1-0.9) |
| 1 | 70 | 66 | 1.1 (0.6, 2.3) | 70 | 59 | 0.3 (0.1-1.0) |
| 2 | 55 | 47 | 0.7 (0.3, 1.6) | 48 | 47 | 0.3 (0.1-1.1) |
| 3 | 49 | 43 | 0.7 (0.3, 1.6) | 41 | 43 | 0.5 (0.2-1.6) |
| 4 | 34 | 39 | 0.9 (0.3, 2.3) | 19 | 30 | 1.0 (0.3-3.4) |
| 5-6 | 39 | 60 | 1.3 (0.5, 3.4) | 33 | 37 | 0.7 (0.3-1.8) |
| 7-18 | 46 | 60 | 1.3 (0.5, 3.6) | 25 | 28 | 1.0 (reference) |
| P for trend | 0.450 | 0.007 | ||||
| Synovitis | ||||||
| 0 | 126 | 107 | 1.0 (reference) | 125 | 118 | 0.4 (0.1-1.4) |
| 1 | 151 | 160 | 1.2 (0.7, 2.3) | 108 | 123 | 1.0 (0.3-2.8) |
| 2 | 56 | 64 | 2.1 (0.9, 5.1) | 55 | 42 | 0.6 (0.2-1.8) |
| 3-6 | 44 | 65 | 4.4 (1.4, 14.0) | 41 | 46 | 1.0 (reference) |
| P for trend | 0.011 | 0.342 | ||||
| Effusions | ||||||
| 0 | 162 | 148 | 1.0 (reference) | 92 | 106 | 3.0 (1.1-8.2) |
| 1 | 139 | 138 | 1.4 (0.8, 2.6) | 167 | 144 | 0.8 (0.4-1.5) |
| 2-3 | 76 | 110 | 2.3 (1.0, 5.3) | 70 | 79 | 1.0 (reference) |
| P for trend | 0.031 | 0.039 | ||||
Odds ratio (OR) for a specific structural lesion was mutually adjusted for other two structural lesions in addition to BMI, knee injury, mental health status, and maximal cartilage morphology score.
As shown in Table 2, the multivariable adjusted cumulative ORs for the severity of frequent knee pain increased as severity of BMLs and synovitis increased or vice versa, suggesting that changes in severity of these two lesions were associated with fluctuation of severity of frequent knee pain. When we performed the analyses on changes in each structural lesion among the knees that experienced improvement of severity of frequent knee pain and knees that experienced worsening of severity of frequent knee pain (Table 3), the results were similar to those presented in Table 1.
Table 2. Changes in severity of BMLs, synovitis, and effusion in relation to severity of frequent knee pain.
| Structural Lesions | Severity of Frequent Knee Pain at a Clinic Visit | Crude OR (95% CI) | Adjusted OR (95% CI)* | ||
|---|---|---|---|---|---|
| No frequent knee pain | Mild/moderate | Severe/extreme | |||
| BMLs | |||||
| 0 | 177 | 132 | 26 | 1.0 (ref) | 1.0 (ref) |
| 1 | 140 | 99 | 20 | 1.2 (0.8, 1.8) | 1.3 (0.8, 2.1) |
| 2 | 103 | 74 | 14 | 1.3 (0.8, 2.2) | 1.2 (0.7, 2.0) |
| 3 | 90 | 65 | 17 | 1.4 (0.8, 2.4) | 1.4 (0.8, 2.4) |
| 4 | 53 | 53 | 11 | 2.1 (1.1, 4.0) | 1.9 (1.0, 3.8) |
| 5-6 | 72 | 74 | 20 | 2.7 (1.4, 5.2) | 2.2 (1.1, 4.4) |
| 7-18 | 71 | 64 | 21 | 3.0 (1.5, 6.0) | 2.2 (1.0, 4.7) |
| P for trend | 0.047 | ||||
| Synovitis | |||||
| 0 | 251 | 180 | 39 | 1.0 (ref) | 1.0 (ref) |
| 1 | 259 | 211 | 57 | 1.7 (1.0, 2.7) | 1.3 (0.8, 2.3) |
| 2 | 111 | 83 | 18 | 1.8 (0.9, 3.4) | 1.5 (0.7, 2.8) |
| 3-6 | 85 | 87 | 15 | 2.5 (1.1, 5.6) | 2.4 (1.0, 5.6) |
| P for trend | 0.052 | ||||
| Effusions | |||||
| 0 | 254 | 191 | 45 | 1.0 (ref) | 1.0 (ref) |
| 1 | 306 | 220 | 51 | 1.2 (0.8, 1.9) | 0.9 (0.6, 1.5) |
| 2-3 | 146 | 150 | 33 | 2.7 (1.5, 4.8) | 1.6 (0.9, 3.0) |
| P for trend | 0.059 | ||||
Odds ratio (OR) for a specific structural lesion was mutually adjusted for other two structural lesions in addition to BMI, knee injury, mental health status, and maximal cartilage morphology score.
Table 3. Changes in severity of BMLs, synovitis, and effusion in relation to the direction of changes in severity of frequent knee pain.
| Scores of Structural Lesions | Change in Severity of Frequent Knee Pain from an Earlier to a Later Clinic Visit | |||||||
|---|---|---|---|---|---|---|---|---|
| Pain severity worsened | Pain severity improved | |||||||
| No frequent knee pain | Mild/moderate | Severe/extreme | OR (95% CI) | No frequent knee pain | Mild/moderate | Severe/extreme | OR (95% CI) | |
| BMLs | ||||||||
| 0 | 84 | 65 | 16 | 1.0 (reference) | 93 | 73 | 13 | 0.3 (0.1-1.0) |
| 1 | 70 | 52 | 13 | 1.2 (0.6, 2.7) | 70 | 51 | 10 | 0.3 (0.1-1.1) |
| 2 | 55 | 38 | 8 | 0.7 (0.3, 1.5) | 48 | 42 | 8 | 0.3 (0.1-1.0) |
| 3 | 49 | 28 | 12 | 0.6 (0.3, 1.5) | 41 | 40 | 6 | 0.4 (0.1-1.3) |
| 4 | 34 | 30 | 9 | 0.8 (0.3, 2.2) | 19 | 28 | 2 | 0.9 (0.3-3.2) |
| 5-6 | 39 | 42 | 17 | 1.3 (0.5, 3.6) | 33 | 34 | 4 | 0.5 (0.2-1.4) |
| 7-18 | 46 | 47 | 13 | 1.1 (0.4, 3.2) | 25 | 20 | 8 | 1.0 (reference) |
| P for trend | 0.781 | 0.011 | ||||||
| Synovitis | ||||||||
| 0 | 126 | 84 | 23 | 1.0 (reference) | 125 | 101 | 17 | 0.3 (0.1-1.2) |
| 1 | 151 | 112 | 43 | 1.1 (0.6, 2.0) | 108 | 110 | 21 | 0.8 (0.2-2.8) |
| 2 | 56 | 50 | 13 | 2.1 (0.8, 5.6) | 55 | 38 | 5 | 0.4 (0.1-1.5) |
| 3-6 | 44 | 56 | 9 | 3.6 (1.1, 11.4) | 41 | 39 | 8 | 1.0 (reference) |
| P for trend | 0.027 | 0.175 | ||||||
| Effusion | ||||||||
| 0 | 162 | 113 | 33 | 1.0 (reference) | 92 | 93 | 17 | 3.4 (1.2-9.2) |
| 1 | 139 | 107 | 29 | 1.5 (0.7, 3.2) | 167 | 122 | 25 | 0.9 (0.4-1.7) |
| 2-3 | 76 | 82 | 26 | 2.7 (0.9, 8.1) | 70 | 73 | 9 | 1.0 (reference) |
| P for trend | 0.048 | 0.019 | ||||||
Odds ratio (OR) for a specific structural lesion was mutually adjusted for other two structural lesions in addition to BMI, knee injury, mental health status, and maximal cartilage morphology score.
In addition, we performed the analyses described above according to baseline knee ROA status. Associations between changes in severity of BMLs and frequent knee pain were similar among knees with and knees without baseline ROA; however, a statistically significant association between changes in severity of effusion and knee pain fluctuation was observed only among knees with knee ROA at baseline whereas changes in severity of synovitis with knee pain fluctuation was found among knees without baseline knee ROA.
Discussion
The results of our study indicate that changes in severity of BMLs and synovitis were associated with the fluctuation of frequent knee pain and pain severity, and of the two BMLs was the better predictor for fluctuation of knee pain than synovitis. Furthermore, improvement of BMLs was associated with a decreased risk of frequent knee pain and of less severe pain, whereas worsening of synovitis and effusion was associated with an increased risk of frequent knee pain and of more severe pain.
Previous studies have focused on risk factors for radiographic knee OA24, and few have attempted to study risk factors for pain from knee OA; thus, factors associated with symptomatic knee OA are largely unknown. Clinically, physicians often notice that patients with knee OA experience pain exacerbation over the course of the disease25. Such pain patterns were also observed in the Framingham OA study; roughly a third of participants with symptomatic knee OA had improvement of their symptoms over time26. Recently, two studies3, 27 reported that joint pain among persons with knee or hip OA is often intermittent and variable, and adaptation and avoidance strategies modify the experience of pain. All these findings indicate that pain from knee OA often fluctuates and suggest that factors that vary over time may play roles in pain fluctuation.
To our knowledge, only a few studies have been conducted to evaluate the association between structural lesions detected by MRIs and the presence of pain or on pain severity from knee OA4-13; results, however, are inconsistent. In a case-control study, the proportion of BML scores worsening was higher in the knees with onset of frequent pain (49.1%) than in knees without frequent pain (26.8%), and two or more units increase in BMLs score were associated with a 3-fold increased risk of frequent pain12. In a longitudinal study Hill et al. found that, compared with the baseline visit, about 15-20% of knees' synovitis scores improved and 20% deteriorated at a 30-month follow-up visit; changes in the synovitis score were modestly associated with the change in pain severity, with a one unit increase in summary score of synovitis resulting in a 3.2 mm increase in VAS pain score (0-100 scale)11. However, this study did not examine whether reduction or complete resolution of synovitis was associated with a decreased risk of knee pain or pain severity. Our study demonstrated that improvement of BMLs, but not improvement of synovitis or effusions, was associated with a decreased risk of frequent knee pain and pain severity. Given the vast number of Americans with knee OA and the dearth of effective treatment for pain among those suffering with the disease, a better understanding of risk factors for pain is sorely needed. If our findings are confirmed by future studies and these structural lesions do indeed contribute to the occurrence of frequent knee pain and to pain severity, treatments and therapies that target these specific features can be developed.
The use of traditional epidemiologic study designs to assess the dynamic relation of structural changes to the risk of knee pain and pain severity poses significant methodological challenges. Pain is a subjective experience. Various risk factors, such as genetic predisposition28, prior experience29, personal perception30, expectations31, and social-cultural environment32, play important roles in a subject's response to painful stimuli. Unless all of these risk factors are measured and adjusted for, studies aiming to evaluate the effect of pathological lesions in or around the knee on the risk of knee pain or on pain severity are susceptible to confounding bias. Second, the high cost of reading knee MRIs makes it expensive to examine the relation of changes in the structural lesions detected by MRIs to the fluctuation of knee pain in a large scale of longitudinal study.
We used the self-matched case-control study design15, 33, akin to the case-crossover study34, to evaluate the association between three structural lesions and fluctuation of knee pain. Since each knee serves as its own control, confounders, either known or unknown, that do not vary over time within a knee but that may differ among persons are eliminated. The self-matched case-control study design allows us to examine knee-specific rather than population-average effect of change in reversible structural lesions over time on the fluctuation of frequent knee pain or pain severity; thus it would allow us to have more confidence in making causal inference. Our findings, if confirmed by other studies, will be an incentive to invest in treatments that target these structural abnormalities.
While cartilage is aneural, we postulate that cartilage loss could contribute to pain indirectly. For example, cartilage debris could be ingested by synovial cells, inducing synovitis35, 36. Further, since an effusion must arise from the synovium, cartilage loss may indirectly be related even to effusion. A few studies have found that cartilage defects are associated with knee pain8, 10. However, when we controlled for cartilage morphology score, the association between BMLs, synovitis and effusion with knee pain did not change materially. In other work, we will explore the relation of cartilage loss with pain but note here that synovitis would be a mediating factor in tying cartilage loss to pain. Thus, one could not validly examine the role of cartilage loss with pain while adjusting for synovitis. We did not find that psychological factors, indicated by mental health component score from the SF-12, were associated with presence of structural lesions; however, psychological factors may affect pain reporting. Nevertheless adjustment for mental health score did not alter the effect of BMLs, synovitis and effusion on knee pain. Interestingly, when adding knee injury into the multivariable regression model, the effect of synovitis and effusion was attenuated, suggesting that knee injury may cause the occurrence of synovitis or effusion of the knee.
Our study has some limitations. First, the weighted kappa statistic of the measurement of each of the three structural lesions was slightly above 0.60; thus our assessment of these lesions may be susceptible to misclassification. However, the readers were unaware of the pain status of the knee when scoring structural lesions; thus the true effect sizes associated with these structural lesions are likely to be attenuated owing to non-differential misclassification bias. Second, we used non-contrast MRIs to assess severity of synovitis. However, as of today large epidemiological studies have mostly not used contrast for assessing synovitis in view of potential toxicity involved with the intravenous administration of contrast agents and associated costs. In addition, some studies have shown that synovitis assessed on non-contrast MRIs is associated with knee pain11. It is beyond the scope of our study to determine what caused the occurrence and resolution of BMLs, synovitis and effusion. Nevertheless, future studies aimed at understanding the causes of these reversible lesions will guide investigators to search for more rational targeted therapies for knee pain. Third, other potential risk factors for knee pain, such as physical activity, muscle weakness, and weather-related factors, were not collected at each clinic visits. Finally, we used R2 value, albeit small, to rank the importance of each structural lesion on frequent knee pain severity. However, it is well known that R2 generated from the logistic regression model is of marginal use, and such measure should not be interpreted as actual percent of variance of variable explained.
In conclusion, our study showed that changes in scores of BMLs and synovitis were associated with the fluctuation of frequent knee pain and pain severity and the effect of BMLs was greater than synovitis. Improvement of BMLs over time was associated with concomitant reduction in pain presence and pain severity, whereas worsening of synovitis and effusion over time was associated with an increase in knee pain presence and severity. These findings have implications for treatment and prevention in symptoms of knee OA.
Acknowledgments
Supported by the National Institute on Aging (NIA): Felson – 1 U01 AG18820; Torner – 1 U01 AG18832; Lewis – 1 U01 AG18947; Nevitt – 1 U01 AG19069. Also supported by NIH AR47785
References
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadler NM. Knee pain is the malady--not osteoarthritis. Ann Intern Med. 1992;116:598–9. doi: 10.7326/0003-4819-116-7-598. [DOI] [PubMed] [Google Scholar]
- 3.Gooberman-Hill R, Woolhead G, Mackichan F, Ayis S, Williams S, Dieppe P. Assessing chronic joint pain: lessons from a focus group study. Arthritis Rheum. 2007;57:666–71. doi: 10.1002/art.22681. [DOI] [PubMed] [Google Scholar]
- 4.Hill CL, Gale DG, Chaisson CE, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28:1330–7. [PubMed] [Google Scholar]
- 5.Felson DT, Chaisson CE, Hill CL, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–9. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 6.Sowers MF, Hayes C, Jamadar D, et al. Magnetic resonance-detected subchondral bone marrow and cartilage defect characteristics associated with pain and X-ray-defined knee osteoarthritis. Osteoarthritis Cartilage. 2003;11:387–93. doi: 10.1016/s1063-4584(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 7.Link TM, Steinbach LS, Ghosh S, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226(2):373–81. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 8.Zhai G, Blizzard L, Srikanth V, et al. Correlates of knee pain in older adults: Tasmanian Older Adult Cohort Study. Arthritis Rheum. 2006;55:264–71. doi: 10.1002/art.21835. [DOI] [PubMed] [Google Scholar]
- 9.Kornaat PR, Bloem JL, Ceulemans RY, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology. 2006;239:811–7. doi: 10.1148/radiol.2393050253. [DOI] [PubMed] [Google Scholar]
- 10.Torres L, Dunlop DD, Peterfy C, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14:1033–40. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Hill CL, Hunter DJ, Niu J, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felson DT, Niu J, Guermazi A, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 13.Davies-Tuck ML, Wluka AE, Wang Y, English DR, Giles GG, Cicuttini F. The natural history of bone marrow lesions in community-based adults with no clinical knee osteoarthritis. Ann Rheum Dis. 2009;68:904–8. doi: 10.1136/ard.2008.092973. [DOI] [PubMed] [Google Scholar]
- 14.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365:965–73. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang B, Wise B, Niu J, Zhu Y. Statistical approaches to evaluating the effect of risk factors on the pain of knee osteoarthritis in longitudinal studies. Current opinion in rheumatology. 2009;21:513–9. doi: 10.1097/BOR.0b013e32832ed69d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neogi T, Felson D, Niu J, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 18.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Roemer FW, Frobell R, Hunter DJ, et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthritis Cartilage. 2009;17:1115–31. doi: 10.1016/j.joca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Madrid F, Karvonen RL, Teitge RA, Miller PR, An T, Negendank WG. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magnetic resonance imaging. 1995;13:177–83. doi: 10.1016/0730-725x(94)00119-n. [DOI] [PubMed] [Google Scholar]
- 21.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Felson DT, Nevitt MC, Yang M, et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol. 2008;35:2047–54. [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee B, Ahn J, Liu I, Rathouz PJ, Sanchez BN. Fitting stratified proportional odds models by amalgamating conditional likelihoods. Stat Med. 2008;27:4950–71. doi: 10.1002/sim.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 25.Hochberg M. Osteoarthritis: A. Epidemiology, Pathology, and Pathogenesis. In: Klippel J, editor. Primer on the Rheumatic Diseases. 12. Atlanta: Arthritis Foundation; 2001. pp. 285–9. [Google Scholar]
- 26.Felson DT, Zhang Y, Hannan MT, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38:1500–5. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 27.Allen KD, Coffman CJ, Golightly YM, Stechuchak KM, Keefe FJ. Daily pain variations among patients with hand, hip, and knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:1275–82. doi: 10.1016/j.joca.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7744–51. doi: 10.1073/pnas.96.14.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colloca L, Benedetti F. How prior experience shapes placebo analgesia. Pain. 2006;124:126–33. doi: 10.1016/j.pain.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Dworkin SF. Relying on objective and subjective measures of chronic pain: Guidelines for use and interpretation. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. New York: Guilford Press; 2001. pp. 619–38. [Google Scholar]
- 31.Wager TD. Expectations and anxiety as mediators of placebo effects in pain. Pain. 2005;115:225–6. doi: 10.1016/j.pain.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Giardino ND, Jensen MP, Turner JA, Ehde DM, Cardenas DD. Social environment moderates the association between catastrophizing and pain among persons with a spinal cord injury. Pain. 2003;106:19–25. doi: 10.1016/s0304-3959(03)00226-4. [DOI] [PubMed] [Google Scholar]
- 33.Wise BL, Niu J, Zhang Y, et al. Psychological factors and their relation to osteoarthritis pain. Osteoarthritis Cartilage. 2010;18:883–7. doi: 10.1016/j.joca.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–53. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 35.Myers SL, Flusser D, Brandt KD, Heck DA. Prevalence of cartilage shards in synovium and their association with synovitis in patients with early and endstage osteoarthritis. J Rheumatol. 1992;19:1247–51. [PubMed] [Google Scholar]
- 36.Lindblad S, Hedfors E. Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 1987;30:1081–8. doi: 10.1002/art.1780301001. [DOI] [PubMed] [Google Scholar]


