Abstract
Rationale
Anxiety disorders affect 18% of the United States adult population annually. Recent surges in the diagnosis of posttraumatic stress disorder (PTSD) from combat-exposed veterans have prompted an urgent need to understand the pathophysiology underlying this debilitating condition.
Objectives
Anxiety and fear responses are partly modulated by gamma aminobutyric acid type A (GABAA) receptor-mediated synaptic inhibition; benzodiazepines potentiate GABAergic inhibition and are effective anxiolytics. Many genetically modified mouse lines are generated and/or maintained on the C57BL/6J background, a strain where manipulation of anxiety-like behavior using benzodiazepines is difficult. Fear-potentiated startle (FPS), a test of conditioned fear, is a useful preclinical tool to study PTSD-like responses but has been difficult to establish in C57BL/6J mice.
Methods
We modified several FPS experimental parameters and developed a paradigm to assess conditioned fear in C57BL/6J mice. The 6-day protocol consisted of three startle Acclimation days, a Pre-Test day followed by Training and Testing for FPS. Subject responses to the effects of three benzodiazepines were also examined.
Results
C57BL/6J mice had low levels of unconditioned fear assessed during Pre-Test (15–18%) but showed robust FPS (80–120%) during the Test session. Conditioned fear responses extinguished over repeated test sessions. Administration of the benzodiazepines alprazolam (0.5 and 1 mg/kg, i.p.), chlordiazepoxide (5 and 10 mg/kg, i.p.), and diazepam (1, 2, and 4 mg/kg, i.p.) significantly reduced FPS to Pre-Test levels.
Conclusions
We used a modified and pharmacologically-validated paradigm to assess FPS in mice thereby providing a powerful tool to examine the neurobiology of PTSD in genetic models of anxiety generated on the C57BL/6J background.
Keywords: Anxiety, Benzodiazepine, C57BL/6J mice, Chlordiazepoxide, Diazepam, Fear-potentiated startle
Introduction
Anxiety disorders are the most prevalent types of psychiatric illness, affecting approximately 18% of the United States adult population annually (Kessler et al. 2005). Posttraumatic stress disorder (PTSD) is an often debilitating anxiety disorder that can emerge after having experienced or witnessed a traumatic event. PTSD is characterized by reexperiencing the traumatic event, avoidance, and numbing of responsiveness to stimuli associated with the event, and hyperarousal including exaggerated startle responses which may serve as an objective measure of central nervous system dysregulation (Kaplan et al. 1994; Morgan et al. 1995). Given the recent increase in combat-related PTSD (Smith et al. 2008), there is an urgent need to understand the neurobiology of fear and anxiety disorders such that more effective therapies can be developed.
Fear-potentiated startle (FPS) is a classical conditioning paradigm where a neutral conditioned stimulus (CS; e.g., tone) is paired with an aversive unconditioned stimulus (US; e.g., footshock). In both humans and rodents, presentation of a loud acoustic stimulus (e.g., a white noise burst) evokes the startle reflex which is potentiated in the presence of the CS (Brown et al. 1951; Davis 1979; Grillon et al. 1991). Consequently, the CS-elicited increase in startle amplitude serves as an operational measure of fear with excellent face validity. While FPS in rodents does not recapitulate all symptoms of PTSD, it is a useful preclinical tool to study the neurobiology underlying the acquisition and expression of fear, and its reduction by behavioral and pharmacological treatments (Davis 2006). Furthermore, because FPS has a nonzero baseline, effects of experimental manipulations on conditioned fear can be distinguished from effects on baseline startle reactivity (Falls 2002). Hence, one of the strengths of the paradigm is in its use to identify novel treatments that block fear without significant side effects (e.g., sedation) on normal behavioral output.
While the FPS paradigm has been widely used in rats for more than 50 years (Brown et al. 1951;Davis 1979), studies of FPS in mice are still limited (Di Benedetto et al. 2008; Falls 2002; Falls et al. 1997; Fendt et al. 2009; Heldt et al. 2000; Risbrough et al. 2003). Many genetically modified mouse strains designed to examine the neurobiology of anxiety disorders are generated and/or maintained on the C57BL/6J background, a strain where anxiolytic-like effects of benzodiazepines in classic tests of unconditioned anxiety (e.g., open field) has been inconsistent (Mathiasen et al. 2008).
Many variables likely affect mouse behavior and FPS has been remarkably difficult to establish in the C57BL/6J strain. For example, previous work has shown that conditioned fear responses and startle potentiation can be examined in C57BL/6J mice (Falls et al. 1997; Heldt et al. 2000; Risbrough et al. 2009; Waddell et al. 2004) but it is not uncommon to observe unconditioned effects to the tone with which the footshock was paired (>30–40%; Falls 2002). Unconditioned startle potentiation to the tone can complicate the analysis of conditioned fear responses by making it difficult to quantify the impact of anxiolytic compounds that can reduce FPS post-conditioning.
Building on pioneering work by Falls et al. (Falls 2002; Falls et al. 1997; Heldt et al. 2000; Waddell et al. 2004), we modified several parameters to develop an FPS paradigm with reduced unconditioned effects to the CS that also produces robust levels of fear to assess conditioned fear responses in C57BL/6J mice; we also examined the effects of anxiolytic medications on this response. Anxiety and fear responses are strongly modulated by GABAA receptor-mediated synaptic inhibition (Low et al. 2000; Meloni and Davis 1999). Benzodiazepines potentiate GABAergic inhibition, are generally effective anxiolytics (Garakani et al. 2006; Shader and Greenblatt 1993), and are used to characterize the predictive validity of animal models of fear and anxiety. Here we examined the effects of the benzodiazepines alprazolam, chlordiazepoxide, and diazepam on fear responses in C57BL/6J mice using this modified FPS paradigm.
Methods and experimental design
Subjects
In Experiment 1, we used 12-week-old male C57BL/6J mice (n=27) from Jackson Laboratory (Bar Harbor, ME, USA). In Experiment 2 (n=110), we used 12-week-old male C57BL/6J mice (original stock from Jackson Laboratory) bred in our colony at McLean Hospital (Belmont, MA, USA). All subjects were group housed (2–4 mice/cage) in Super Mouse 750™ cages containing a LifeSpan™ Rodent Enrichment insert (Lab Products, Seaford, DE, USA) and covered by micro-isolator non-wire bar lids; these cages could be maintained either on or off individual ventilation (IV).
Subjects were maintained on a 12:12-h light–dark cycle (lights on at 0600 hours) with food (Purina Lab Diet 5P76, PMI Nutrition International, Brentwood, MO, USA) and water available ad libitum. Subjects were acclimated to the behavioral suite for 2 weeks prior to testing; during this and the subsequent experiment time, subjects were maintained off IV. Experiments were conducted between 0900 and 1700 hours to avoid ceiling effects that would arise from the normal nocturnal elevation of startle responses (Chabot and Taylor 1992). All animal procedures were approved by the Institutional Animal Care and Use Committee of McLean Hospital and in accordance with the NIH Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Apparatus
FPS was measured using the MedAssociates Inc. (St. Albans, VT, USA) Startle Reflex System and Advanced Startle software program. Plexiglas and wire grid animal holders (ENV-264C) were attached to a load cell platform (PHM-250) contained within sound-attenuating cubicles. Cage movement resulted in displacement of the load cell stabilimeter where the resultant voltage was amplified and digitized into arbitrary units by an analog-to-digital converter (ANL-925C Amplifier) interfaced to a personal computer. Startle amplitude was proportional to the amount of cage movement and defined as the maximum peak-to-peak voltage occurring within the first 100 ms after onset of the startle stimulus. High-frequency speakers (5–40 kHz) were located 4 cm behind the chambers and delivered the acoustic stimuli. Footshocks were delivered by Stand Alone Stimulators/Scramblers (ENV-414) connected to the wire grid floors of the animal holders. All stimuli were calibrated using the MedAssociates software packages.
Experimental paradigm
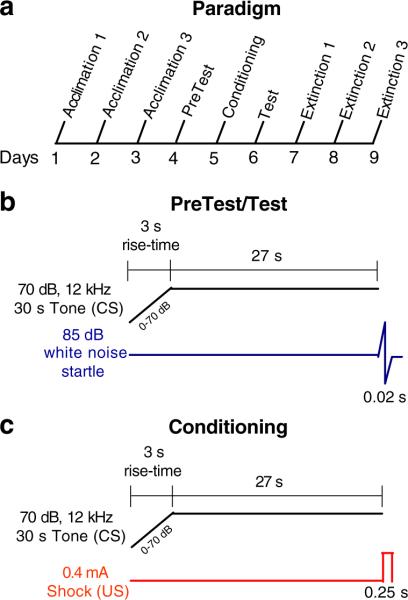
FPS was assessed using a 6-day paradigm (Fig. 1a) adapted from the one originally developed by Falls for C57BL/6J mice (Falls 2002). One modification was the introduction of a 3-s rise time during the onset of the tone CS (Fig. 1b and c), which eliminated transient startle responses we observed when the tone had an immediate onset (see Fig. S1). Before each daily session, mice were hand carried in their home cages from the housing room to a separate room within the behavioral suite that contained the experimental equipment.
Fig. 1.
a Schematic of the fear-potentiated startle (FPS) paradigm and 3 consecutive days of Extinction. b Presentation of the auditory stimuli on Tone + Startle trials during PreTest and Test; the startle response is depicted as a sine wave. c On the day of Conditioning, subjects are presented with the same tone CS as in b that is immediately followed by the unconditioned stimulus (US)
On days 1–3 (Acclimation), subjects were given a 5-min period during which no acoustic stimuli were presented, followed by a semi-random presentation of fifty 20-ms white noise startle stimuli (ten each of 70, 80, 85, 90, and 100 dB) with a 30-s inter-trial interval (ITI). The Acclimation days have the dual advantage of familiarizing subjects to handling and the apparatus as well as for assessing startle intensity function. Baseline activity was calculated by examining load cell displacement (also measured as startle amplitude in arbitrary units) in the 500 ms before startle onset for all trials and averaged across the experimental session to determine whether responses to the lowest white noise stimulus (70 dB) were distinguishable from general movement on the load cell.
On day 4 (PreTest), subjects were given a 5-min Acclimation period followed by presentation of 10 Leader startle stimuli (85 dB; 1 min ITI) to habituate startle responses to a baseline level. Subsequently, mice received a semi-random presentation of 20 Startle Only trials (85 dB, 1 min ITI) and 20 Tone + Startle Trials where the white noise startle stimulus was preceded by presentation of a 30-s, 12-kHz, 70-dB tone with a 3-s rise time (Fig. 1b). This tone is identical to the one used as the CS on the following Conditioning Day. On day 5 (Conditioning), after a 5-min baseline period, subjects received ten Tone + Shock fear conditioning trials where the CS was immediately followed by a 0.25-s, 0.4-mA foot shock US with a random ITI (120, 180, or 240 s) (Fig. 1c). Shock reactivity (total cage displacement in response to the shock) was sampled for the entire duration of the foot shock (i.e., 0.25 s). On day 6 (Test), FPS was assessed by presenting the same experimental session given during PreTest (Fig. 1b). To assess Extinction of FPS, subjects were given the same experimental session as on days 4 and 6 for three consecutive days following Test (days 7–9; Extinction Days 1–3). There was no background noise in the chambers during any part of the experimental paradigm.
Animal holders were wiped with distilled water in between subjects and then wiped clean with 70% ethanol at the end of each daily session. Home-cage bedding changing only occurred after completion of Acclimation Day 1 and Extinction Day 1 to minimize the impact of cage-changing stress on behavior.
Drugs
Alprazolam (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sterile water and administered at either 0.5 or 1 mg/kg. Chlordiazepoxide hydrochloride (Sigma-Aldrich) was dissolved in 0.9% sterile saline and administered at either 5 or 10 mg/kg. Diazepam (BIOMOL International, Plymouth Meeting, PA, USA) was dissolved in a 10% (2-Hydroxypropyl)-β-cyclodextrin solution (Sigma-Aldrich) and administered at doses of 1, 2, or 4 mg/kg (Straub et al. 2010). Drugs were injected intraperitoneally in a volume of 10 ml/kg 30 min before Test start; subjects did not receive drug on any other daily session, including extinction days.
Experiment 1: paired (1A) and paired versus unpaired (1B) CS-US acquisition of FPS in C57BL/6J mice
Experiment 1A
FPS was first assessed in vendor-obtained C57BL/6J mice (n=12) and then in mice bred in our colony (n=15; original stock obtained from Jackson Laboratory) using our 6-day paradigm to determine (1) whether these mice exhibited similar or different levels of fear conditioning compared to previously published studies (Falls 2002; Waddell et al. 2004) and (2) whether differences in rearing and housing conditions could impact startle responding (Kallnik et al. 2007). One animal was euthanized after Extinction 1 due to health issues; data from this animal were included in the analysis.
Experiment 1B
A second group of vendor-obtained mice (n=15) was tested independently by another investigator (K.M.M.). Subjects were housed in the McLean Animal Care Facility under similar conditions as animals in Experiment 1A and tested in a physically different laboratory at McLean Hospital that also had the same MedAssociates startle equipment described above. Half of the animals (paired group, n=8) were trained and tested identically to animals in Experiment 1A; in this group the tone and shock were explicitly paired as previously described. The other half (unpaired group, n=7) were exposed to ten pseudorandom tone and foot shock presentations with randomized intervals between the offset of the tone and the onset of the shock ranging from 50–150 s so that the CS and US were never explicitly paired.
Experiment 2: effect of benzodiazepines on FPS in C57BL/6J mice
Previous work showed that benzodiazepines significantly reduce FPS in the DBA/1J strain (Risbrough et al. 2003). Here, we evaluated whether administration of moderate, non-baseline-startle-reducing (Guscott et al. 2000; Risbrough et al. 2003) doses of the benzodiazepines alprazolam (n=8/dose), chlordiazepoxide (n=7–8/dose) or diazepam (n=16/dose) would reduce FPS in C57BL/6J mice.
Data analysis
Mean startle amplitudes were calculated by averaging across each of the startle-eliciting intensities during Acclimation, and across Leader, Startle Only, and Tone + Startle trials during the PreTest, Test, and Extinction sessions. Fear potentiation was defined as increased startle responding during trials when the CS was presented (Tone + Startle trials) after Tone + Shock conditioning compared to preconditioning (PreTest). Percent FPS was calculated from data on Test day using mean startle amplitude values with the following formula: [((Tone + Startle) − Startle Only)/Startle Only]×100. In order to establish treatment groups with equivalent levels of baseline startle and unconditioned effects of the tone on startle, subjects were matched into the different drug groups using startle amplitude and percent change in startle amplitude on Tone + Startle trials versus Startle Only trials during the Pretest (same formula used to calculate percent FPS); this value represents the unconditioned effect of the tone on startle.
Data were expressed as mean ± SEM and analyzed using the SAS statistical software version 9.1 (SAS Institute, Inc., Cary, NC, USA). Data were analyzed with ANOVAs and t-tests, as appropriate; significant main effects and interactions were followed up with additional ANOVAs and Tukey's post-hoc tests. The significance level for all tests was set at p<0.05.
Results
Experiment 1: paired (1A) and paired versus unpaired (1B) CS-US acquisition of FPS in C57BL/6J mice
Experiment 1A
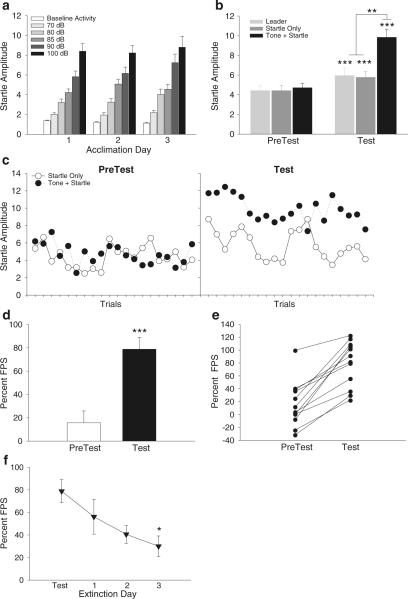
Subjects showed an intensity-dependent increase in startle responding during each Acclimation day that did not change across days (F(10,132)=1.46, p=0.16; Fig. 2a). A mid-level startle response was achieved when white noise startle bursts were presented at 85 dB. Results of a 2×3 factor ANOVA (test session × trial type) showed that PreTest startle amplitudes did not differ across the Leader, Startle Only, and Tone + Startle trials (F(2,33)=0.12, p=0.891; Fig. 2b). In contrast, while the mean startle amplitude did not differ between Leader and Startle Only trials during Test, startle responding on the Tone + Startle trials was significantly greater than either of the other two trial types (F(2,33)=9.55, p=0.005; Fig. 2b). Startle amplitudes for all three trial types during Test were also significantly elevated compared to the respective trial conditions in the PreTest (significant main effect of test session, F(1,33)=18.03, p<0.001; posthoc tests p<0.05). There was also a significant test session × trial type interaction (F(2,33)=4.50, p=0.018; Fig. 2b) reflecting the potentiation of startle on Tone + Startle trials during Test.
Fig. 2.
Subject startle responses during Experiment 1A. a Averaged startle amplitude during each Acclimation Day. b Averaged startle amplitude for each trial type on PreTest and Test. c Time course of startle responding on Startle Only and Tone + Startle trials across PreTest and Test. d Mean percent FPS on PreTest versus Test and e the corresponding scatterplot of individual responses. f Extinction of startle responses occurs over 3 consecutive days following Test. Data are presented as mean ± standard error of the mean (SEM) except for 1c and 1e where individual data points are presented as the mean only. *p<0.05, **p<0.01, ***p<0.0001
As illustrated in Fig. 2c, startle amplitude across individual trials was similar for both trial types during PreTest, indicating a general lack of an unconditioned effect of the tone on startle. However, after fear conditioning, there was a significant potentiation of startle on Tone + Startle trials compared to Startle Only trials across the entire Test session even though startle amplitude habituated across trials for both trial types.
Figure 2d shows the percent change in startle amplitude on Tone + Startle trials compared with Startle Only trials. During PreTest, the 16% change in startle amplitude, reflected a relatively small unconditioned effect of the tone on startle. In contrast, after fear conditioning, animals showed robust FPS (paired t-test, t(11)=7.38, p<0.0001) compared to PreTest; a scatterplot of these data shows the dynamic variability of responses between subjects (Fig. 2e). The data show variability in the unconditioned effect of the tone on startle (PreTest data) and FPS (Test data) between animals but demonstrate that all subjects exhibited startle potentiation after conditioning.
As shown in Fig. 2e, one animal had an extremely high (99%) unconditioned effect of the tone on startle during PreTest. Hence, if this animal had been statistically excluded from the analysis (i.e., >3 standard deviations above the mean), the overall percent change in mean startle amplitude on PreTest would have been 8% with overall levels of FPS during Test not being dramatically affected (75% with this animal excluded versus 79% overall). Repeated presentation of the test session over 3 consecutive days following Test showed that potentiation of the startle response gradually decreased and nearly reached baseline PreTest levels by Extinction Day 3. Results of a one-way repeated measures ANOVA showed that FPS on Extinction Day 3 was significantly less than that on Test (F(3,42)=3.59, p=0.02; Fig. 2f).
Colony-bred mice exhibited nearly identical patterns of responding to vendor-obtained mice throughout each day of the test paradigm, demonstrating that differences in rearing and housing conditions did not impact FPS in this mouse strain (see Fig. S2).
Experiment 1B
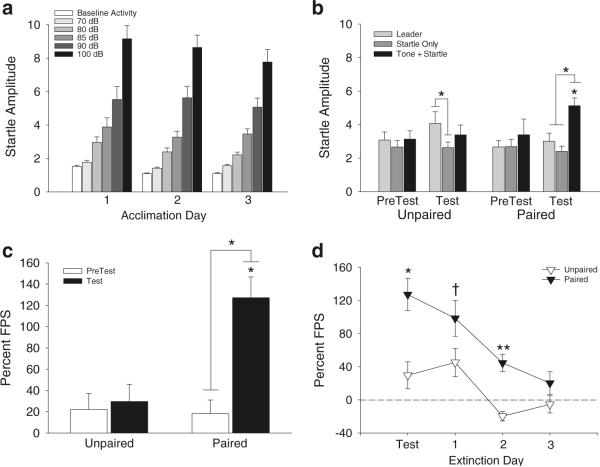
During all 3 days of Acclimation, subjects responded identically to those in Experiment 1A (Fig. 3a). Figure 3b shows the averaged Pretest and Test startle data from animals in the Unpaired and Paired conditioning groups. A three-way ANOVA with conditioning group (Unpaired and Paired groups) as a between-subjects factor, and trial type (Leaders, Startle Only, Tone + Startle) and test session (Pretest and Test) as within-subjects factors revealed a significant main effect of trial type (F(2,26)=14.0, p<0.0001) and a significant conditioning group × trial type interaction (F(2,26)=7.92, p=0.005). Post hoc comparisons indicated that startle was significantly potentiated on Tone + Startle trials compared to Leader and Startle Only trials for animals in the Paired conditioning group, but not the Unpaired group, on the Test day. Potentiation of startle in the presence of the tone in the Paired group was also significantly different from Pretest Tone + Startle trials for this group (posthoc test p<0.05), as well as in comparison to Tone + Startle trials for the Unpaired group on the Test day (posthoc test p<0.05; Fig. 3b). The only other significant pairwise comparison was an elevation in startle on Leader trials versus Startle Only trials for the Unpaired group on the Test day (posthoc test p<0.05), which may reflect context conditioning in animals that did not receive explicit pairing of the shocks with the tone (Fig. 3b). However, this effect gradually extinguished over the presentation of the ten Leader trials (data not shown), which likely accounts for the observation that startle on Startle Only trials was at PreTest levels.
Fig. 3.
Startle responses of subjects following paired and unpaired CS-US presentations in Experiment 1B. a Average baseline activity and startle amplitude during each Acclimation Day and on PreTest and Test b for subjects in the Paired and Unpaired conditioning groups. c Mean percent FPS for both groups of subjects on PreTest and Test. d Extinction of startle responses for subjects in each conditioning group across 3 consecutive days following Test. The dashed line in (3D) reflects the zero baseline. All data are presented as mean ± SEM. *p<0.05, **p<0.01, †p=0.07 nearly significant difference
Figure 3c illustrates the percent change in startle amplitude on Tone + Startle trials compared with Startle Only trials for the Unpaired and Paired groups. A two-way ANOVA revealed significant main effects of conditioning group (F(1,13)=6.95, p=0.02) and test session (F(1,13)=15.20, p=0.001) as well as a significant conditioning group × test session interaction (F(1,13)=11.49, p=0.004). Subjects in the Unpaired group had low levels of potentiated startle during the PreTest that did not change after conditioning. Subjects from the Paired group also had low levels of potentiated startle during PreTest but had a significant increase in FPS during Test (posthoc p<0.05) similar to subjects in Experiment 1A; FPS during Test for the Paired group was also significantly greater than Test FPS in the Unpaired group (posthoc p<0.05).
FPS in the Paired group extinguished with repeated testing over 3 consecutive days following Test (Fig. 3d; data points are the same as in Fig. 3c). A two-way ANOVA showed significant main effects of conditioning group (F(1,13)=12.51, p=0.0036) and test session (F(3,39)=22.56, p<0.0001) and a significant conditioning group×test session interaction (F(3,39)=3.48, p=0.024). Pairwise comparisons indicated that the difference in FPS levels between the Paired and Unpaired groups approached significance during the first extinction day (F(1,13)=3.58, p=0.08), was significantly different on Extinction Day 2 (F(1,13)=27.05, p=0.0002), and was not significantly different between groups on Extinction Day 3.
Experiment 2: effect of benzodiazepines on FPS in colony-bred C57BL/6J mice
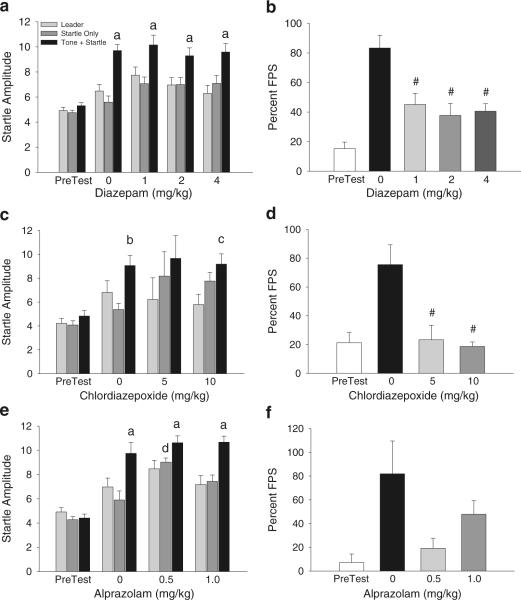
Figure 4 shows the effects of pretreatment with diazepam, chlordiazepoxide, and alprazolam on the expression of FPS. A two-way ANOVA with trial type as a within-subjects comparison and dose of diazepam as a between-subjects comparison indicated a significant main effect of trial type (F(2,180)=30.61, p<0.0001). Individual post hoc comparisons showed that all groups had significantly elevated startle on Tone + Startle trials compared to both Startle Only and Leader trials (p<0.05) (Fig. 4A). The slight increase in baseline startle on Startle Only trials in animals treated with diazepam was not significantly different from vehicle-treated mice (Fig. 4A). A one-way ANOVA revealed a significant main effect of drug treatment on percent FPS (F(3,60)=8.19, p<0.0001) (Fig. 4B); individual pairwise comparisons demonstrated that all doses of diazepam significantly reduced percent FPS during Test compared to vehicle-treated mice (p<0.05 for all values).
Fig. 4.
Impact of benzodiazepines on FPS in Experiment 2. a Mean startle responses by trial type and b percent FPS were examined during PreTest and Test in vehicle and diazepam-treated subjects. c Mean startle amplitude and d percent FPS wasalsoexaminedinsubjects that received vehicle or chlordiazepoxide on Test. e Startle amplitude by trial type and f percent FPS values for alprazolam-treated mice during Test. Data are presented as mean ± SEM; p<0.05 for all values. a startle amplitude on Tone + Startle trials significantly different than both Leader and Startle Only trials; b startle amplitude on Tone + Startle trials significantly different than Startle Only trials; c startle amplitude on Tone + Startle trials significantly different than Leader trials. d startle amplitude on Startle Only trials for the mice treated with the low dose of alprazolam significantly higher than the same trial type in vehicle-treated mice; #, percent FPS for drug-treated mice significantly different from vehicle
Similarly, a two-way ANOVA with trial type as a within-subjects comparison and dose of chlordiazepoxide as a between-subjects comparison revealed a significant main effect of trial type (F(2,57)=8.16, p<0.001; Fig. 4C). Individual post hoc comparisons indicated that startle amplitude on Tone + Startle trials was significantly elevated compared to Startle Only trials in vehicle-treated mice (p< 0.05) (Fig. 4C). In mice treated with the highest dose of chlordiazepoxide, startle responding on Tone + Startle trials was significantly higher than Leader trials only (p<0.05) (Fig. 4C). While there was an overall slight increase in baseline startle on Startle Only trials in chlordiazepoxide-treated mice (both doses) compared to those that received vehicle, this increase was not statistically significant. As shown in Fig. 4D, a one-way ANOVA revealed a significant main effect of drug treatment on percent FPS during Test (F(2,19)=9.84, p<0.01); both doses of chlordiazepoxide significantly reduced percent FPS compared to vehicle-treated mice (p<0.05).
Examination of startle amplitude in animals pretreated with alprazolam using a two-way ANOVA revealed a significant main effect of trial type (F(2,63)=18.70, p< 0.0001); like the diazepam-treated mice, individual post hoc comparisons showed that all groups had significantly elevated startle on Tone + Startle trials compared to both Startle Only and Leader trials (p<0.05) (Fig. 4E). The same two-way ANOVA also revealed a significant main effect of drug treatment (F(2,63)=5.79, p<0.01) (Fig. 4E); unlike the previous two treatment groups, a post hoc one-way ANOVA revealed that there was a significant increase in startle amplitude on Startle Only trials for animals treated with the low dose of alprazolam compared to vehicle-treated mice (F(2,21)=7.63, p<0.01; Fig. 4E). While percent FPS during Test was reduced for both doses of alprazolam compared to vehicle, a one-way ANOVA revealed a nearly significant main effect (F(2,21)=3.04, p=0.06; Fig. 4F).
Discussion
Building on pioneering work by Falls et al. (Falls 2002; Falls et al. 1997; Heldt et al. 2000; Waddell et al. 2004), we modified and pharmacologically validated an FPS paradigm to assess PTSD-like responses in C57BL/6J mice. While some aspects of his FPS paradigm remained the same, we modified several features that helped decrease unconditioned effects to the tone. Features we did not alter included presentation of white noise startle bursts at a 20-ms duration, ten pairings of a 0.4-mA, 0.25-s footshock with a 30-s, 12-kHz, 70-dB tone, use of an intermediate shock intensity to avoid the nonmonotonic inverse relationship between shock intensity and FPS magnitude (Davis 1979; Davis and Astrachan 1978), and use of the PreTest prior to conditioning to minimize unconditioned startle effects to novel stimuli seen in mice (Falls et al. 1997; Heldt et al. 2000) and rats (Davis 1974; Walker and Davis 1997).
Several parametric features were changed following preliminary empirical studies designed to replicate Falls's original paradigm (Falls et al. 1997). First, we modified the presentation of the 12-kHz, 70-dB tone, which had no onset rise time in the Falls paradigm. In preliminary studies, presentation of this abrupt auditory stimulus caused a transient startle response on most trials that was substantially reduced when the tone onset had a 3-s rise time (see Fig. S1A). Even though startle in response to the onset of the tone occurred 30 s before presentation of the startle-eliciting white noise burst, we posit that this initial response may have played a role in generating higher levels of what is referred to in the literature as the unconditioned effects of the tone on startle (Falls 2002; Falls et al. 1997; Heldt et al. 2000; Waddell et al. 2004) (see Fig. S1B). This response is frequently observed in C57BL/6J mice and may be a part of normal startle reactivity in this strain.
It is unclear why startle to the tone would subsequently lead to a larger startle response to the white noise burst on Tone + Startle trials compared to trials where the startle stimulus alone was presented (i.e., Startle Only trials), only that we observed nearly equivalent levels of startle on both trial types in the PreTest when we used a tone with a 3-s rise time at the onset. Here, gradually increasing the intensity of the tone from 0 to 70 dB over 3 s prevented startling to the tone onset and helped reduce the unconditioned effect of the tone on startle (see Fig. S1). Hence, the unconditioned effect of the tone on startle may partially arise from a sensorimotor process instead of reflecting an innate fear of the novel stimulus per se. Second, the Falls protocol averages startle amplitudes across three different startle intensities at the higher range of auditory intensity (e.g., 95, 105, 110 dB) to calculate FPS. Although our Acclimation data does indeed show an intensity-dependent increase in startle, we used an intermediate startle of 85 dB for all startle presentations during PreTest and Test.
The results of Experiment 1 show that the FPS paradigm modifications helped reduce the unconditioned effect of the tone on startle as seen in the roughly equivalent levels of startle across all of the Leader, Startle Only, and Tone + Startle Trials in the PreTest. After conditioning, the mean startle amplitudes were significantly greater on Tone + Startle trials than on Startle Only trials and remained elevated throughout the duration of the Test session; the robust potentiation of startle is consistent with levels reported by Falls (2002). Repeated presentation of the experimental paradigm given on both PreTest and Test for 3 consecutive days following Test showed that FPS gradually extinguished as the CS began to lose its emotional salience. This paradigm may also be useful in examining extinction which is commonly accepted to represent new learning and memory (Myers and Davis 2007; Quirk and Mueller 2008).
Although rearing and housing conditions (e.g., environmental enrichment), subject age, and other factors can impact a variety of murine behaviors, including anxiety-like and startle behaviors (Falls et al. 2010; Kallnik et al. 2007), in our hands, colony-bred C57BL/6J mice exhibited nearly identical patterns of startle responses to the vendor-obtained animals of the same strain (see Fig. S2). Our data demonstrate that conditioned fear responses in this strain using our modified paradigm were conserved across different rearing conditions.
As expected, subjects in Experiment 1B in the Unpaired conditioning group did not show startle potentiation during Test compared to subjects in the Paired group demonstrating that startle potentiation in the Paired group was indeed a function of the cue-specific conditioning similar to Falls (Falls 2002;Falls et al. 1997; Heldt et al. 2000; Waddell et al. 2004). Interestingly, startle amplitude for the Unpaired group during Test Leader trials, that is the initial trials to assess baseline levels of startle after subjects were placed back into the startle chambers after conditioning, were elevated when compared to startle amplitude in the Startle Only trials. Animals in this group likely formed an association between the startle chambers (i.e., context) and the aversive conditioning that represents contextual fear (Blanchard and Blanchard 1972; Bolles and Fanselow 1980) which is reflected as enhanced levels of startle in Leader trials. FPS to contextual stimuli can occur when rodents are presented with footshocks that are not specifically paired with a CS (Campeau et al. 1991;Rescorla and Wagner 1972). According to Grillon (2002), context conditioning is not dependent on the same neural structures needed for cued fear conditioning and may serve as a better preclinical model of some elements of human anxiety.
Anxiety and fear responses are strongly modulated by GABAA receptor-mediated synaptic inhibition (Low et al. 2000; Meloni and Davis 1999). Although there are conflicting reports about the efficacy of benzodiazepines in reducing FPS in humans (Baas et al. 2002; Bitsios et al. 1999; Riba et al. 2001; Rodriguez-Fornells et al. 1999), rodent studies have demonstrated that diazepam and chlordiazepoxide reduce FPS in rats (Davis 1979) and DBA/1J mice (Risbrough et al. 2003). Since benzodiazepines can reduce baseline startle responses (Guscott et al. 2000; Risbrough et al. 2003), most likely due to their sedative effects, we used alprazolam, chlordiazepoxide, and diazepam at anxiolytic (Kilfoil et al. 1989; Low et al. 2000; Rudolph et al. 1999), and non-baseline-startle-reducing doses (Davis 1979; Risbrough et al. 2003) as tools to determine whether manipulation of the GABAergic system would reduce FPS in C57BL/6J mice.
One of our main findings, as shown in Experiment 2, was that pretreatment with diazepam, chlordiazepoxide, or alprazolam substantially blocked FPS in C57BL/6J mice on Test; this is consistent with another report showing that diazepam and chlordiazepoxide block FPS in DBA/1J mice (Risbrough et al. 2003). Furthermore, administration of each of these benzodiazepines did not reduce baseline startle as reflected by startle amplitudes in the Leader and Startle Only trials. In fact, startle amplitude in these trials was slightly elevated in all animals that received drug compared to vehicle-treated subjects and was significantly elevated in mice treated with the lose dose of alprazolam although it is not clear why this is the case. While the elevation of baseline startle accounted for the largest decrease in percent FPS during Test for this specific group of alprazolam-treated mice, the overall pattern observed across all drug-treated groups was a consistent reduction of FPS that was not a function of elevated baseline startle.
Pretreatment with chlordiazepoxide in this study yielded the largest reduction of FPS with percent FPS values reaching PreTest level; drug-treated mice had approximately 55% less FPS than vehicle-treated subjects. Since each drug has its own biotransformation profile yielding slightly different active metabolites (Trevor and Way 1987), it is possible that the additional two active metabolites (i.e., desmethylchlordiazepoxide and desmethyldiazepam), produced during chlordiazepoxide biotransformation may have contributed to the greater FPS-reducing effects seen following administration of this drug. Differences in the amount of FPS reduction seen between drugs could also be attributed to differences in the anxiolytic potency of each compound.
In conclusion, while these data illustrate the continuing challenge in assessing anxiety- and fear-related behaviors in the C57BL/6J strain of mice, results of this study support a modified, pharmacologically-validated paradigm to assess FPS in mice thereby providing a powerful tool to assess the neurobiology of anxiety and fear in preclinical models of anxiety generated on the C57BL/6J background.
Supplementary Material
Acknowledgements
The project described was supported by a grant from the Andrew P. Merrill Memorial Fellowship Foundation and a fellowship from the National Institute of Neurological Disorders and Stroke Neuroscience Scholars Program to KSS, a Collaborative Initiative Award from the Howard Hughes Medical Institute to WAC, and Award Number R01 MH080006 from the National Institute of Mental Health to UR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-010-2026-1) contains supplementary material, which is available to authorized users.
References
- Baas JM, Grillon C, Bocker KB, Brack AA, Morgan CA, 3rd, Kenemans JL, Verbaten MN. Benzodiazepines have no effect on fear-potentiated startle in humans. Psychopharmacology. 2002;161:233–247. doi: 10.1007/s00213-002-1011-8. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Philpott A, Langley RW, Bradshaw CM, Szabadi E. Comparison of the effects of diazepam on the fear-potentiated startle reflex and the fear-inhibited light reflex in man. J Psychopharmacol. 1999;13:226–234. doi: 10.1177/026988119901300303. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Fanselow MS. A perceptual-defense-recuperative model of fear and pain. Behav Brain Sci. 1980;3:291–323. [Google Scholar]
- Brown JS, Kalish HI, Farber IE. Conditioned fear as revealed by the magnitude of startle response to an auditory stimulus. J Exp Psychol. 1951;41:317–327. doi: 10.1037/h0060166. [DOI] [PubMed] [Google Scholar]
- Campeau S, Hayward MD, Hope BT, Rosen JB, Nestler EJ, Davis M. Induction of the c-fos-proto-oncogene in rat amygdala during unconditioned fear and conditioned fear. Brain Res. 1991;565:349–352. doi: 10.1016/0006-8993(91)91669-r. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Taylor DH. Daily rhythmicity of the rat acoustic startle response. Physiol Behav. 1992;51:885–889. doi: 10.1016/0031-9384(92)90131-k. [DOI] [PubMed] [Google Scholar]
- Davis M. Sensitization of the rat startle response by noise. J Comp Physiol Psychol. 1974;87:571–581. doi: 10.1037/h0036985. [DOI] [PubMed] [Google Scholar]
- Davis M. Diazepam and flurazepam: effects on conditioned fear as measured with the potentiated startle paradigm. Psychopharmacology. 1979;62:1–7. doi: 10.1007/BF00426027. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Astrachan DI. Conditioned fear and startle magnitude: effects of different footshock or backshock intensities used during training. J Exp Psychol Anim Behav Process. 1978;4:95–103. doi: 10.1037//0097-7403.4.2.95. [DOI] [PubMed] [Google Scholar]
- Di Benedetto B, Kallnik M, Weisenhorn DM, Falls WA, Wurst W, Holter SM. Activation of ERK/MAPK in the lateral amygdala of the mouse is required for acquisition of a fear-potentiated response. Neuropsychopharmacology. 2008;34:356–366. doi: 10.1038/npp.2008.57. [DOI] [PubMed] [Google Scholar]
- Falls WA. Fear-potentiated startle in mice. Curr Prot Neurosci. 2002:8.11B.1–811B.16. doi: 10.1002/0471142301.ns0811bs19. [DOI] [PubMed] [Google Scholar]
- Falls WA, Carlson S, Turner G, Willott JF. Fear-potentiated startle in two strains of inbred mice. Behav Neurosci. 1997;111:855–861. [PubMed] [Google Scholar]
- Falls WA, Fox JH, MacAulay CM. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behav Brain Res. 2010;207:321–331. doi: 10.1016/j.bbr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Fendt M, Burki H, Imobersteg S, Lingenhohl K, McAllister K, Orain D, Uzunov D, Chaperon F. Fear-reducing effects of intra-amygdala neuropeptide Y infusion in animal models of conditioned fear: an NPY Y1 receptor independent effect. Psychopharmacology. 2009;206:291–301. doi: 10.1007/s00213-009-1610-8. [DOI] [PubMed] [Google Scholar]
- Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med. 2006;73:941–949. [PubMed] [Google Scholar]
- Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biol Psychiatry. 2002;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;25:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Guscott MR, Cook GP, Bristow LJ. Contextual fear conditioning and baseline startle responses in the rat fear-potentiated startle test: a comparison of benzodiazepine/gamma-aminobutyric acid-A receptor agonists. Behav Pharmacol. 2000;11:495–504. doi: 10.1097/00008877-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Heldt S, Sundin V, Willott JF, Falls WA. Posttraining lesions of the amygdala interfere with fear-potentiated startle to both visual and auditory conditioned stimuli in C57BL/6J mice. Behav Neurosci. 2000;114:749–759. [PubMed] [Google Scholar]
- Kallnik M, Elvert R, Ehrhardt N, Kissling D, Mahabir E, Welzl G, Faus-Kessler T, Hrabe de Angelis M, Wurst W, Schmidt J, Holter SM. Impact of IVC housing on emotionality and fear learning in male C3HeB/FeJ and C57BL/6J mice. Mamm Genome. 2007;18:173–186. doi: 10.1007/s00335-007-9002-z. [DOI] [PubMed] [Google Scholar]
- Kaplan HI, Sadock BJ, Grebb JA. Synopsis of psychiatry. 7th edn. Williams and Wilkins; Baltimore: 1994. [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilfoil T, Michel A, Montgomery D, Whiting RL. Effects of anxiolytic and anxiogenic drugs on exploratory activity in a simple model of anxiety in mice. Neuropharmacology. 1989;28:901–905. doi: 10.1016/0028-3908(89)90188-3. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy J-M, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Mathiasen LS, Mirza NR, Rodgers RJ. Strain- and model-dependent effects of chlordiazepoxide, L-838, 417 and zolpidem on anxiety-like behaviours in laboratory mice. Pharmacol Biochem Behav. 2008;90:19–36. doi: 10.1016/j.pbb.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Davis M. Muscimol in the deep layers of the superior colliculus/mesencephalic reticular formation blocks expression but not acquisition of fear-potentiated startle in rats. Behav Neurosci. 1999;113:1152–1160. doi: 10.1037//0735-7044.113.6.1152. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Grillon C, Southwick SM, Davis M, Charney DS. Fear-potentiated startle in posttraumatic stress disorder. Biol Psychiatry. 1995;38:378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academy; Washington: 1996. [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: current theory and research. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Riba J, Rodriguez-Fornells A, Urbano G, Morte A, Antonijoan R, Barbanoj MJ. Differential effects of alprazolam on the baseline and fear-potentiated startle reflex in humans: a dose-response study. Psychopharmacology. 2001;157:358–367. doi: 10.1007/s002130100816. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Brodkin JD, Geyer MA. GABA-A and 5HT1A receptor agonists block expression of fear-potentiated startle in mice. Neuropsychopharmacology. 2003;25:654–663. doi: 10.1038/sj.npp.1300079. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Geyer MA, Hauger RL, Coste S, Stenzel-Poore M, Wurst W, Holsboer F. CRF1 and CRF2 receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2009;34:1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Riba J, Gironell A, Kulisevsky J, Barbanoj MJ. Effects of alprazolam on the acoustic startle response in humans. Psychopharmacology. 1999;143:280–285. doi: 10.1007/s002130050948. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy J-M, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Shader RI, Greenblatt DJ. Use of benzodiazepines in anxiety disorders. N Engl J Med. 1993;328:1398–1405. doi: 10.1056/NEJM199305133281907. [DOI] [PubMed] [Google Scholar]
- Smith TC, Ryan MAK, Wingard DL, Slymen DJ, Sallis JF, Kritz-Silverstein D. New onset and persistent symptoms of post-traumatic stress disorder self reported after deployment and combat exposures: prospective population based US military cohort study. Br Med J. 2008;336:366–371. doi: 10.1136/bmj.39430.638241.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub CJ, Carlezon WA, Jr, Rudolph U. Diazepam and cocaine potentiate brain stimulation reward in C57BL/6J mice. Behav Brain Res. 2010;206:17–20. doi: 10.1016/j.bbr.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Trevor AJ, Way WL. Sedative-Hypnotics. In: Katzung BG, editor. Basic and clinical pharmacology. 3rd edn. Appleton and Lange; Norwalk: 1987. pp. 241–253. [Google Scholar]
- Waddell J, Dunnett C, Falls WA. C57BL/6J and DBA/2J mice differ in extinction and renewal of extinguished conditioned fear. Behav Brain Res. 2004;154:567–576. doi: 10.1016/j.bbr.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle paradigm. Biol Psychiatry. 1997;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.