Abstract
Rac1 and Rac2, members of the small Rho GTPase family, play essential roles in coordinating directional migration and superoxide production during neutrophil responses to chemoattractants. While earlier studies in Rac1 and Rac2 knockout mice have demonstrated unique roles for each Rac isoform in chemotaxis and NADPH oxidase activation, it is still unclear how human neutrophils utilize Rac1 and Rac2 to achieve their immunological responses to foreign agent stimulation. In the current study, we used TAT-dominant negative Rac1-T17N and Rac2-T17N fusion proteins to acutely alter the activity of Rac1 and Rac2 individually in human neutrophils. We demonstrate distinct activation kinetics and different roles for Rac1 and Rac2 in response to low versus high concentrations of fMLP. These observations were verified using neutrophils from mice in which Rac1 or Rac2 was genetically absent. Based on these results, we propose a model to explain how human neutrophils kill invading microbes while limiting oxidative damage to the adjacent surrounding healthy tissue through the differential activation of Rac1 and Rac2 in response to different concentrations of chemoattractant.
Introduction
In response to inflammatory conditions, neutrophils are known to be first-line defenders in the human innate immune response system [1–3]. To fulfill this role, human neutrophils carry out two essential biological processes, chemotaxis and the production of reactive oxygen species (ROS). In chemotaxis, neutrophils acquire a polarized morphology, cross the blood vessel wall, and migrate through the adjacent epithelial tissues up a gradient of chemoattractants, such as N-formyl peptide products of bacteria, propagated from infectious sites [4]. For the production of ROS to kill microbes after their engulfment by phagocytic means, human neurophils initially generate superoxide anion via a membrane-localized NADPH oxidase [5]. Interestingly, both chemotaxis and the production of superoxide are often triggered by the same extracellular chemotactic stimuli. However, many earlier studies have also shown these processes to be initiated at different concentrations of N-formyl peptide ligand, with actin polymerization occurring at an ED50 of 0.01 nM and superoxide production occurring with an EC50 of 0.3 nM or greater [6]. This has been related to levels of receptor occupancy at various chemoattractant concentrations [6, 7]. Since during the neutrophil’s response to invading microbes, the leukocytes often are required to travel a relatively long distance across layers of healthy tissues before they eventually reach the infectious sites, controlled regulation of ROS formation during this transit is necessary to avoid causing damage to the healthy tissue by the chemotaxing leukocytes.
Over the past decade, different members of the Rho GTPase superfamily have been recognized as playing critical roles in the multiple biological responses of phagocytic leukocytes [8–10]. The Rac GTPases (Rac1 and Rac2) have garnered a great deal of attention due to their roles in reorganizing the actin-myosin cytoskeleton during chemotaxis and in controlling the activity of the NADPH oxidase during the neutrophil response to invading microbes. In human neutrophils, Rac2 is the predominant Rac isoform, making up more than 80%–95% of total Rac protein [11, 12]. Rac1 and Rac2 have more than 90% homology at the amino acid sequence level and share many similar biochemical properties [13]. Several Rac1 and/or Rac2 knockout mouse models have been studied to address the roles of Rac1 and Rac2 in neutrophil functions stimulated by different chemoattractants, including N-formylated peptides such as formyl-methionyl-leucyl-phenylalanine (fMLP) [14–21]. These studies have established that both Rac1 and Rac2 are required for normal neutrophil chemotaxis and motility in response to formyl peptides, while only Rac2 is absolutely required for fMLP-stimulated NADPH oxidase activity. Analysis of the defects in directed migration of neutrophils in Rac1−/− mice has shown that they exhibit the inability to properly orient in a chemoattractant gradient, accompanied by the formation of multiple randomly-oriented lamellipodia. They exhibit a modest defect in both F-actin assembly and the retraction of the uropod tail during migration [14, 22, 23]. The latter has been linked to specific effects of Rac1 to regulate RhoA activation in the uropod to coordinate and promote stable cell polarity during chemotaxis [22]. In contrast, Rac2−/− mice exhibit major defects in filamentous actin assembly and cell migration speed, but are still able to orient to a chemotactic gradient [14]. A subsequent study showed Rac1 to control the initial uncapping of existing actin barbed ends, whereas Rac2 regulates the extension of actin filaments via cofilin- and Arp2/3-dependent mechanisms [18].
Although these studies have shed light on the roles of Rac1 and Rac2 during neutrophil immune responses, the exact contributions of Rac1 and Rac2 GTPases to chemotaxis and motility remain unclear, and have not been examined in the context of the relative concentrations of chemoattractant. The studies presented here showed that in the low range of stimulatory fMLP concentrations, human neutrophils mainly activate Rac1 to promote initial cell spreading, leading to subsequent directional migration with regular, small lamellipodia at the leading edge and limited production of superoxide. In contrast, at high stimulatory fMLP concentrations, Rac1 is first activated to initiate the formation of lamellipodia, but then the activation of Rac2 is further required for continuous expansion of the large leading edge lamellipodia that drives effective migration, as well as superoxide formation. Our results connect the unique and overlapping roles of Rac1 and Rac2 in coordinating the directional migration of neutrophils with the production of superoxide for bacterial killing in response to changes in the chemoattractant gradients at the infectious site.
Materials and Methods
General Materials
Phorbol myristate acetate (PMA), f-Met-Leu-Phe (fMLP), wortmannin, PP2, PP3, cytochrome C, DAP1, human fibronectin, flourescein and D-glucose were purchased from Sigma-Aldrich Corporation (St. Louis, Mo). Endotoxin-free Hank’s Balanced Salt Solution (HBSS, pH 7.4) containing calcium and magnesium and phosphate-buffered saline (10 × PBS) were acquired from Invitrogein Inc. (Carlsbad, CA). Ficoll-Paque Plus gradient was purchased from GE Healthcare. Falcon 50-ml and 15-ml sterile tubes were from Fisher Scientific Inc., USA. Micropipette puller P-87 was from Sutter Instrument Company. Software package of Dynamic Image Analysis System (DIAS) was from Soll Technologies Inc. (Iowa City, IA). Monoclonal Rac1 antibody (23A8) was from Upstate-Millipore. Polyclonal Rac2 antibody R786 was generated in-lab [12, 24]. Phalloidin Alexa 568 was obtained from Sigma. HRP-conjugated secondary antibodies were from Thermo Fisher Scientific, Rockford, IL, while Alexa-488- and Alexa-563-conjugated secondary antibodies were from Molecular Probes.
TAT protein constructs
TAT protein constructs were made by fusing the TAT domain from HIV virus with the target genes at the C-terminus, and the resultant TAT fusion constructs were expressed in BL21DE3 cells and purified as described previously [25].
Isolation and maintenance of human neutrophils
Fresh human blood was collected through the normal blood donor program at the Scripps Research Institute in accordance with an institutional review board-approved protocol. The blood was drawn by venipucture, and was sedimented by 6% Dextran 500 (Pharmacosmos A/S, Denmark) at room temperature for 45 minutes. After the top layer of suspended leukocytes and a small amount of remnant red blood cells (RBCs) were separated from the majority of sedimented RBCs, this top layer of leukocytes and RBCs was subsequently loaded onto prewarmed Ficoll-Paque Plus gradient according to the Ficoll-Paque Plus manual. After centrifuging at 500 g for 30 min without braking, human neutrophils were collected, discarding the upper layers of plasma and monocytes. The isolated neutrophils were then briefly exposed to endotoxin-free water to hypotonically lyse remaining RBCs. The isolated neutrophils (up to 98% purity) were then resuspended in HBSS++ buffer (HBSS supplemented with 1 mM D-glucose) for experiments.
Determination of human neutrophil superoxide production
For human neutrophils in suspension, the cells were first prewarmed for 10 min at 37 °C and then stimulated with the final concentration of 5×10−6 M fMLP. Superoxide production of stimulated neutrophils was measured continuously for 8 min by cytochrome C assay [26].
For adherent human neutrophils, wells in the 96 well plate were pre-coated with 50 μg/ml fibronectin for 45 min, then washed three times with 1× PBS. After freshly isolated neutrophils were allowed to adhere to the fibronectin-coated surface for 30 min, the cells were stimulated with different concentrations of fMLP (as indicated), and superoxide production of the adherent neutrophils measured for 30 min by cytochrome C assay [26].
Chemotaxis assays
Sterilized coverslips were pre-coated with 50 μg/ml fibronectin for 45 min at room temperature, then washed three times with 1× PBS. Freshly isolated neutrophils were resuspended at 1×106 cells/ml in HBSS++ buffer, and allowed to adhere to the fibronectin-coated surface for 30 min before chemotactic assays (with at least 90% of cells adhered).
During a global stimulation assay, adherent human neutrophils were first incubated in HBSS++ buffer and recorded through a 100× objective on the Olympus microscope for 2 min. In the same imaging field, a 4× stock solution of fMLP was added to the experimental chamber to adjust to the final concentration of fMLP for global stimulation, and the neutrophils were recorded for an additional 12 min.
During a directional stimulation assay, adherent human neutrophils were first incubated in HBSS++ buffer and recorded through a 40× objective for 2 min. In the same imaging field, a micropipette filled with different concentrations of fMLP stock solution was lowered into the experimental chamber to create a gradient of fMLP by natural diffusion, and the neutrophils were recorded for an additional 12 min.
The movies recorded from chemotactic experiments were converted into movies with DIAS-specific format and analyzed with the DIAS software package to generate cellular behavioral parameters such as cell centroid movement, cell tracks, cell speed and cell area [27]. These data were imported into Microsoft Excel for further analysis and graphing. The cell centroid was calculated by the DIAS program from the center of geometric shape of the cell. Centroid speed is the raw measure of cell speed based on the translocation of the cell centroid.
Immunochemistry
For optimal preservation of neutrophil morphology and native cytoskeletal structure, a fixation procedure [28, 29] was adapted for human neutrophils. Briefly, after the proper treatment or stimulation, adherent neutrophils were immediately fixed in 0.7% glutaraldhyde in 1× Cramer buffer for 15 min at 0 °C, and were permeabilized with 0.1% NP40 for 10 min. Autofluorescence was immediately quenched with 1mg/ml NaBH4 for 5 min and non-specific binding was blocked with 10% goat serum in 1× PBS for 1 hour at room temperature. The actin cytoskeleton was stained with polyclonal actin antibody C4. Fixed human neutrophils were first incubated with different primary antibodies overnight at 0 °C at 1:200 dilution, then with secondary antibodies for 90 min. Finally, before the coverslips were mounted, fixed human neutrophils were counter-stained with DAPI (1:2000) and Phalloidin Alexa568 (1:400) for 30 min.
Measurement of fMLP gradients
A series of uniform fluorescein solutions from 1×10−9 M to 1×10−6 M were imaged to create a standard curve of fluorescein for estimating the concentration of fluorescein in different diffusion fields of mimicked fMLP gradients. The process of fluorescein diffusion from micropipettes loaded with either 3×10−5 or 3×10−4 M of fluorescein was recorded. Based on the standard curve of fluorescein, the concentrations of fluorescein in the diffusion fields were determined using Metamorph software package and Microsoft Excel.
Rac activation assay
Rac1 and Rac2 activation was examined as described previously [24]. In brief, stimulated adherent neutrophil extract was prepared as follows: After freshly isolated neutrophils allowed to adhere to 50 μg/ml fibronectin-coated 5-cm plates for 60 min, they were immediately stimulated with uniform fMLP at the final concentration of 1×10−7 M. The stimulation of uniform fMLP was stopped at different time points by removing the stimulation media, and placing the plates on ice and adding 450 μl of 1× cold lysis buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 30 mM MgCl2, 1% NP40 and 5% glycerol supplemented with protein inhibitors, including 1 mM PMSF, 1 mM aprotinin, 1 μM leupeptin and 1μg/ml pepstatin A. 20 μl of each cell lysate at each time point was examined on 12% SDS PAGE and blotted with the indicated Rac antibody to determine the levels of Rac1 and Rac2. To detect active Rac1 and Rac2, 400 μl of cell lysate was used with 10 μg of PBD (GST-fusion protein containing the Rac/Cdc42 binding domain of PAK1).
Knock-out mouse model and murine neutrophil chemotactic assay protocols
All procedures described were performed in accordance with the Guide for the Humane Use and Care of Laboratory Animals and were approved by the University of Toronto Animal Care Committee. Rac1-conditional null and Rac2 null mice were generated according to the protocol described by Glogauer et al. [14]. Bone marrow mouse neutrophils were isolated as described [17] and were resuspended in HBSS and 1% gelatin. The suspended mouse neutrophils (1×106 cells/ml) were allowed to adhere to 5 μg/ml fibronectin-coated glass coverslips (22×40 mm) at 37°C for 1 hour. The coverslip was inverted onto a Zigmond chamber and then 100 μL HBSS media containing fMLP (1×10−6 M) added to the right and left chambers for stimulation with uniform fMLP. Time-lapse video microscopy (Nikon Eclipse E1000) was used to examine mouse neutrophil behavior in the Zigmond chamber with a 60× objective, and images were captured at 10-second intervals. Cell-tracking software (Retrac version 2.1.01 Freeware) was used to characterize cell behaviors from the captured images. Image J software was used to quantify the spreading area and centroid movements of each cell at each time point (a total of 30 cells were analyzed for each time point).
Results
Dominant negative Rac1 and Rac2 Tat fusion proteins independently inhibit Rac1 versus Rac2 activity
Dominant negative mutants (Threonine 17 to Asparagine, T17N) of both Rac1 and Rac2 were fused to the C-terminus of the Tat domain to construct cell-permeant Rac1 and Rac2 Tat fusion proteins (Rac1-T17N and Rac2-T17N will refer to these dominant negative Tat fusion proteins). To examine whether Rac1-T17N and Rac2-T17N could specifically and independently inhibit the activation of Rac1 and Rac2, Rac1-T17N and Rac2-T17N pretreated human neutrophils in suspension were stimulated by 1×10−7 M fMLP and active Rac1-GTP and Rac2-GTP were determined by affinity-based assay [24], as shown in Supplementary Figure S1. Rac1-T17N substantially reduced Rac1 activity except at the maximum activation time point of 0.5 min (Figure S1A). Given the slight cross reactivity of the Rac1 and Rac2 antibodies, we cannot rule out that some of the activity at the 0.5 min maximum was contributed by Rac2. In contrast to Rac1, Rac2 activation was not significantly affected at any time point (Figure S1A), suggesting that Rac1-T17N treatment specifically and independently inhibits the activation of Rac1 in human neutrophils. Conversely, dominant negative Rac2-T17N dramatically suppressed Rac2 activity at all time points, while not affecting Rac1 (Figure S1B), suggesting that Rac2-T17N treatment specifically and independently inhibits the activation of Rac2 in human neutrophils. The transduction of Rac1-T17N and Rac2-T17N into human neutrophils was verified as in previous studies [25, 30] through the HA tag at the N-terminus of TAT fusion proteins (data not shown). The above data indicates that Rac1-T17N and Rac2-T17N can be transduced into human neutrophils, where they specifically and independently inhibit the activation of either Rac1 or Rac2, respectively, upon fMLP stimulation.
Neutrophils exhibit chemotactic responses differentially dependent on Rac1 and Rac2 upon exposure to different levels of fMLP
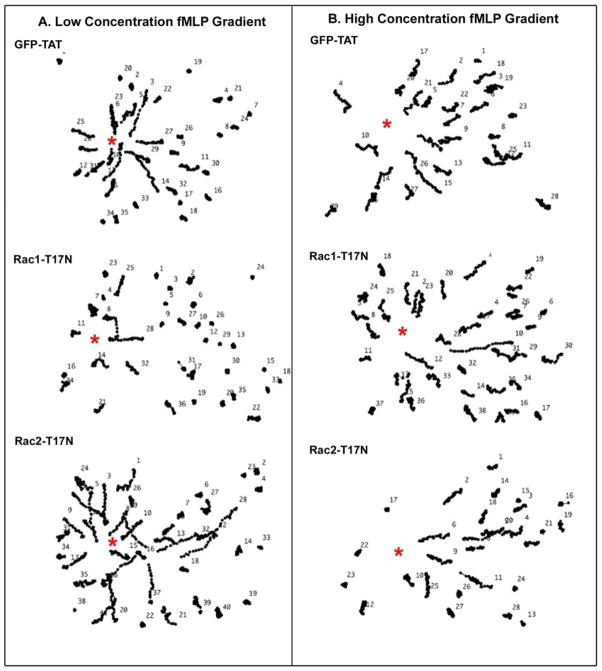
Human neutrophil chemotactic responses were examined in response to “low” versus “high” concentration gradients of fMLP (as determined in Supplemental Figure S2). We observed that both the low concentration gradient (10−8–10−9 M) of fMLP (Figure 1A) and the high concentration gradient (10−7–10−8 M) of fMLP (Figure 1B) were able to effectively induce the directional migration of control GFP-Tat-pretreated neutrophils toward the stimulant source.
Figure 1. The inhibition of Rac1 or Rac2 by Tat fusion proteins, Rac1-T17N or Rac2-T17N, resulted in different chemotactic responses in human neutrophils in high and low concentrations of fMLP gradients.
(A) In a low fMLP concentration gradient, both GFP and Rac2-T17N pretreated human neutrophils showed normal migration tracks toward the source of fMLP (labeled with red asterisk), suggesting that the inhibition of Rac1 didn’t block neutrophil chemotaxis. However, Rac1-T17N pretreated human neutrophils showed impaired migration tracks toward the source of fMLP (labeled with red asterisk), indicating that the inhibition of Rac1 was able to block neutrophil chemotaxis in a low concentration gradient of fMLP. (B)In a high fMLP concentration gradient, both GFP-Tat and Rac1-T17N pretreated human neutrophils show normal cell tracks toward the source of fMLP (red asterisk), suggesting the inhibition of Rac1 didn’t block neutrophil chemotaxis. However, Rac2-T17N pretreated human neutrophils exhibited shorter migration tracks toward the source of fMLP (red asterisk), indicating the inhibition of Rac2 was able to block neutrophil chemotaxis in a high fMLP concentration gradient. (Results are representive from 3 independent experiments)
We investigated potential differences in the Rac GTPase regulation of chemotactic responses in low vs high fMLP gradients by using dominant negative Rac1-T17N or Rac2-T17N Tat fusion proteins, respectively (Supplemental Movies 1 and 2). In the low fMLP concentration gradient, the adherent neutrophils pretreated with Rac1-T17N exhibited markedly reduced directional migration towards the chemoattractant source, as demonstrated by the much shorter cell tracks in Figure 1B and a reduced chemotactic index (Table I). In marked contrast, the Rac2-T17N pretreated neutrophils maintained their ability to migrate up the low fMLP concentration gradient, exhibiting normal directional migration to the micropipette (Figure 1B and Table I).
Conversely, in a high concentration gradient of fMLP, the Rac2-T17N-pretreated neutrophils were now not able to effectively migrate directionally to the point source, as demonstrated by their generally shorter cell tracks in response to the chemotactic stimulation (Figure 1A) and reduced chemotactic index (Table I). However, in a high concentration gradient of fMLP, the neutrophils pretreated with Rac1-T17N were able to chemotax normally (Figure 1A and Table ), unlike the loss of chemotaxis observed at low fMLP concentrations.
The above results indicate that: a) the inhibition of Rac1 activation (with Rac1-T17N) or Rac2 activation (with Rac2-T17N) had independent effects on chemotactic responses to fMLP in neutrophils; and b) that inhibiting the activation of Rac1 vs Rac2 resulted in distinct inhibitory effects on neutrophil chemotaxis that depended on the relative concentration gradient of fMLP to which the cells were exposed. Neutrophil chemotaxis in a low concentration gradient of fMLP required Rac1 activation, but not activation of Rac2, while neutrophil chemotaxis in a high concentration gradient of fMLP was mainly dependent on the activation of Rac2.
Human neutrophils show similar chemotactic behavioral responses to low vs high uniform fMLP vs fMLP gradients
To further elucidate the Rac GTPase isoform signaling dependence during chemotaxis, neutrophil chemotactic behaviors in fields of different concentration gradients of fMLP versus fields of different uniform concentrations of fMLP were compared (Supplemental Movie 3). As shown in Figure 2A and 3A, resting adherent neutrophils had a smooth spherical shape before stimulation with fMLP. As the neutrophils were stimulated with either a high concentration gradient of fMLP (Figure 2B) or by a high uniform concentration of fMLP (Figure 3B), they showed a very similar behavioral pattern. This started with spreading and expansion of the cell periphery, resulting in an up to 3-fold increase in cell area, and was followed by the formation of a large lamellipodium at the leading front of the neutrophil, associated with rapid chemotactic movement (Figure 2B vs 3B and Supplemental Movie 3).
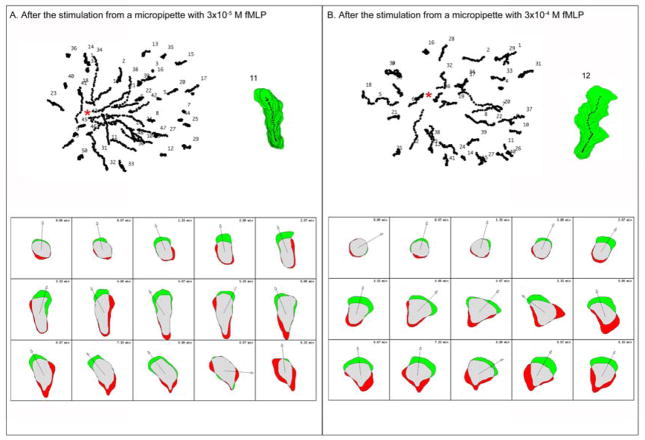
Figure 2. Human neutrophils show different morphological changes in response to different fMLP concentration gradients.
(A) Human neutrophils were stimulated in a low concentration fMLP gradient. All the cell tracks are shown at the top left of panel A and the gradient source was indicated by the red asterisk. The cell stack of sample cell 11 is shown at the top right of panel A. At the bottom of panel A, the morphological changes of sample cell 11 at various times are shown in the form of a schematic cell, in which the green regions represent the expansion area of the cell and the red regions represent the retraction area of the cell. (B) Human neutrophils were stimulated in a high concentration fMLP gradient. All the cell tracks are shown at the top left of panel B and the gradient source was indicated by the red asterisk. The cell stack of sample cell 12 is shown at the top right of panel B. At the bottom of panel B, the morphological changes of sample cell 12 are shown in the form of schematic cells, as described above.
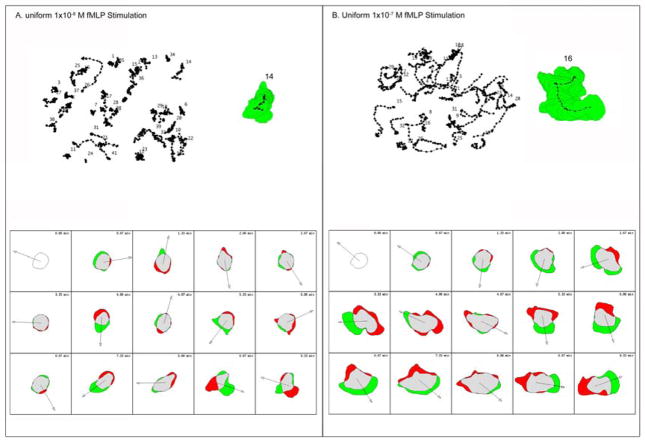
Figure 3. Human neutrophils show different morphological changes in response to different concentrations of uniform fMLP.
(A) Human neutrophils were stimulated with a low uniform concentration of fMLP. All the cell tracks are shown at the top left of panel A. The cell stack of sample cell 14 is shown at the top right of panel A. At the bottom of panel A, the morphological changes of sample cell 14 are shown in the form of a schematic cell, in which the green regions represent the expansion area of the cell and the red regions represent the retraction area of the cell. (B) Human neutrophils were stimulated in a high uniform concentration of fMLP. All the cell tracks are shown at the top left of panel B. The cell stack of sample cell 16 is shown at the top right of panel B. At the bottom of panel B, the morphological changes of sample cell 16 are shown schematically, as described above.
In contrast to this behavior, when human neutrophils were stimulated with either a low concentration gradient of fMLP (Figure 2A) or by a low uniform concentration of fMLP (Figure 3A), they responded initially with the spreading at the cell periphery, but to a lesser extent than seen in the high concentration of fMLP (Figure 2A vs 3A and Supplemental Movie 3). This was followed with relatively slow migration of the cells in uniform low fMLP that was supported by small lamellipodia that formed randomly around the cell periphery, as shown in the cell tracks of Figure 2A and 3A. Similarly, the cells in the low fMLP gradient, although they assumed a more elongated appearance as they responded to the chemoatractant gradient, also only extended small lamellipodia at the leading edge (Supplementary Movie 3).
Because of the apparent similarity in neutrophil chemotactic behavioral patterns between the fields of uniform fMLP and the fields of fMLP gradients, and the ability to obtain higher magnification images of cells upon uniform fMLP stimulation, this condition was subsequently used for more accurate morphological analysis and evaluation of the relative roles of Rac1 and Rac2 in chemotactic responses.
Formation of the large leading edge lamellipodia requires Rac1 for its initiation and Rac2 for continuous expansion at the leading front
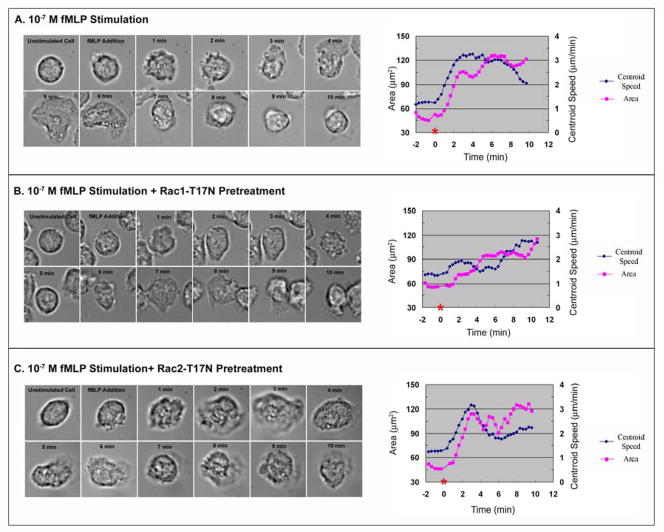
In order to dissect individual roles of Rac1 and Rac2 for morphological changes at the leading edge of chemotaxing neutrophils, they were pretreated with dominant negative Rac1 vs Rac2 TAT fusion proteins, stimulated with a high concentration of uniform fMLP, and then analyzed with DIAS software package for detailed dissection of cellular behavioral changes (Supplemental Movie 4). Compared to untreated neutrophils, pretreatment with Rac1-T17N resulted in significant changes of neutrophil chemotactic behaviors but didn’t inhibit overall neutrophil motility, consistent with previous knockout studies [14, 17, 22]. At the initial stage, untreated neutrophils experienced rapid cell spreading (Stage 1: 0–4 minutes in Supplemental Movie 4 and sequential DIC images of Figure 4A), characterized by an increase of cell area and centroid movement, as shown in Figure 4A. This initial phase of rapid cell spreading was characteristically missing in Rac1-T17N-treated neutrophils (Stage 1 in Supplemental Movie 4 and sequential DIC images of Figure 4B). This was evident also from the quantitative parameters of cell area and centroid movement shown in Figure 4B.
Figure 4. Differential Rac GTPase regulation of neutrophil responses to a high concentration of uniform fMLP.
(A) Left- Sequential DIC images of an untreated human neutrophil are shown during stimulation with uniform 10−7 M fMLP. Right- the measurements of cell area and centroid speed averaged from 30 cells were plotted against the time, where the time of fMLP addition (zero) is labeled with a red asterisk on the X-axis. (B) Sequential DIC images of a Rac1-T17N pretreated human neutrophil are shown during stimulation with uniform 10−7 M fMLP (left side of panel B). At the right side of panel B, the measurements of cell area and centroid speed averaged from 30 cells were plotted against the time, where the time of fMLP addition (zero) is labeled with a red asterisk on the X-axis. (C) Sequential DIC images of a Rac2-T17N pretreated human neutrophil are shown during a uniform 10−7 M fMLP stimulation (left side of panel C). At the right side of panel C, the measurements of cell area and centroid speed averaged from 30 cells are plotted against the time, where the time of fMLP addition (zero) is labeled with a red asterisk on the X-axis. (Results are collected from three independent experiments)
Following this initial cell spreading, untreated chemotaxing neutrophils started to expand a large lamellipodium at the leading edge to support continuous rapid migration (Stage 2: 4–6 minutes in Supplemental Movie 4 and sequential DIC images of Figure 4A), quantitatively characterized by the maintenance of a large cell area and by a further increase of centroid movement (Figure 4A). Conversely, Rac1-T17N treated neutrophils during Stage 2 showed a 25%–40% decrease in centroid movement compared to control neutrophils (Figure 4A vs 4B) and had a markedly reduced cell area compared to control neutrophils (Figure 4A vs 4B), consistent with the absence of the formation of the large lamellipodium at the leading edge during this time period (Supplementary Movie 4 and Figure 4B vs 4A).
After 8 minutes of fMLP stimulation, untreated neutrophils retracted the single large lamellipodium and started to form much smaller lamellipodia at the leading edge to support and maintain cell migration (Stage 3: after 7 minutes in Supplemental Movie 4). This was associated with a decrease in cell area after 8 minutes (Figure 4A). However, during Stage 3, Rac1-T17N treated neutrophils started to form a relatively large lamellipodium at the leading edge (Stage 3 in Supplemental Movie 4 and sequential DIC images in Figure 4B), demonstrated by the increase of cell area and centroid movement (Figure 4B), suggesting that there was an abnormal delay in the formation of the large lamellipodium in Rac1-T17N pretreated neutrophils. In contrast, pretreatment with Rac2-T17N inhibited neutrophil chemotaxis overall (Supplemental Movie 4), consistent with prior knockout studies [15, 16, 18, 20]. Compared to control neutrophils, Rac2-T17N treated cells were able to undergo the initial cell spreading response to fMLP stimulation (Stage 1:0–4 minutes in Supplemental Movie 4 and sequential DIC images of Figure 4C), demonstrated by the increase of cell area and centroid movement. However, during Stage 2 and 3, Rac2-T17N treated neutrophils had a 30% decrease in centroid movement compared to control cells (Figure 4A vs Figure 4C). They also did not form the large lamellipodium at the leading edge over the time course of imaging (Stage 2 and 3 in Supplemental Movie 4 and sequential DIC images of Figure 4C).
Overall, the absence of initial cell spreading in Rac1-T17N treated neutrophils and the ability of Rac2-T17N treated neutrophils to spread during Stage 1 suggest that the activation of Rac1, but not Rac2, is crucial for early neutrophil spreading. Furthermore, the delayed formation of the large lamellipodium in Rac1-T17N treated neutrophils and the absence of the large lamellipodium in Rac2-T17N treated neutrophils imply that the formation of the large lamellipodium in chemotaxing neutrophils during Stage 2 may be initially triggered by Rac1 activation. However, the subsequent expansion of the large lamellipodium appears to require the further activation of Rac2.
Verification of the distinct roles of Rac1 versus Rac2 using knockout mice
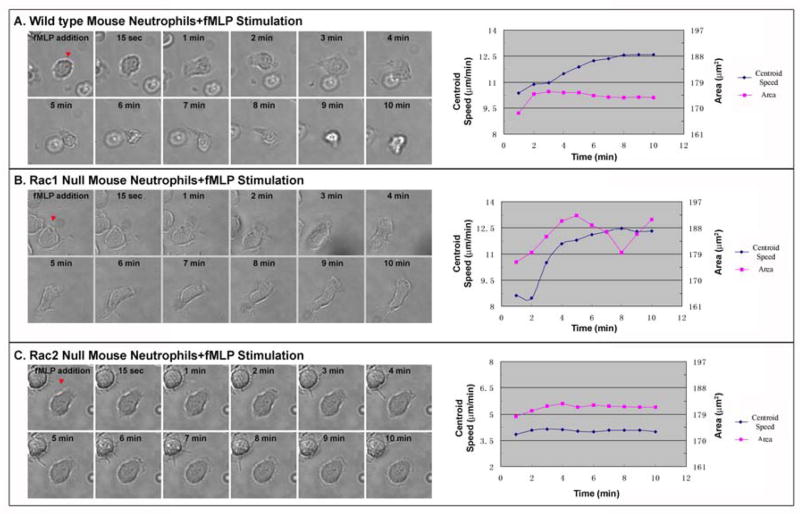
To verify Rac1 and Rac2 functions during neutrophil chemotactic responses to high concentration of uniform fMLP, neutrophils from conditional Rac1 knockout and Rac2 knockout mice were examined. In the Rac1 conditional knockout mice, we observed that neutrophils were defective in the initial increase of cell area due to cell spreading from 1–3 min post-stimulation, but subsequently exhibited a delayed spreading response over 2–5 min that was coupled with a delayed increase in centroid movement, as compared to wild type mouse neutrophils (Figure 5A vs 5B). These results are similar to our observations in human neutrophils treated with Rac1-T17N (Figure 4B), although the time course is slightly different. In contrast, in the Rac2 knockout mice, the neutrophils showed an increase of cell area (albeit weaker) due to cell spreading within the first 2–4 min, but exhibited almost no increase in centroid movement during the experimental period, as compared to wild type neutrophils (Figure 5A vs 5C). The Rac2-deficient cells also never formed a large leading edge lamellipodium and were largely non-responsive to the chemoattractant. These results confirm our observations with human neutrophils treated with Rac2-T17N (Figure 4C). Taken together, our observations suggest that Rac1 activation is an important determinant for initiating cell spreading and the initial formation of the lamellipodium, while Rac2 activation is required for the continuous expansion and maintenance of the leading edge lamellipodium upon fMLP stimulation.
Figure 5. Differential Rac GTPase regulation of neutrophil responses to a high concentration of uniform fMLP verified using mouse neutrophils genetically deficient in Rac1 or Rac2.
(A) Sequential DIC images of a wild type mouse neutrophil are shown during fMLP stimulation at the left of panel A. At the right of panel A, the measurements of cell area and centroid speed averaged from 30 cells were plotted against time. (B) Sequential DIC images of Rac1−/− mouse neutrophils are shown during fMLP sitmulation at the left of panel B. At the right of panel B, the measurements of cell area and centroid speed averaged from 30 cells were plotted against time. (C) Sequential DIC images of Rac2−/− mouse neutrophils are shown during fMLP stimulation at the left of panel C. At the right of panel C, the measurements of cell area and centroid speed averaged from 30 cells were plotted against time. (Results are collected from three independent experiments)
The activation and redistribution of Rac1 and Rac2 correlate with leading edge morphological changes in chemotaxing neutrophils
To determine if Rac1 and Rac2 activity and/or subcellular distribution was consistent with the distinct roles of each GTPase in human neutrophil chemotactic behavior, neutrophils were stained with Rac1- and Rac2-selective antibodies (Figure S3) after stimulation with uniform 1×10−7 M fMLP for various times (Figure 6). Rac1 was initially observed throughout the cytoplasm, but became colocalized with F-actin in the cell periphery at ~ 0.5 min after stimulation with 1×10−7 M fMLP (Figure 6A). At this time the stimulated neutrophils began to spread, as shown in the corresponding DIC-DAPI overlaid image of Figure 6A (see also Figure 4). Following this initial morphological change over the course of the first 2–3 minutes, the colocalization of Rac1 and F-actin was maintained, but as the chemotaxing neutrophil started to form the large lamellipodium at the leading edge, Rac1 accumulation disappeared from the expanding lamellipodium, which was marked by the presence of strong F-actin staining (between 3 and 5–6 minutes in Figure 6A). Colocalization between Rac1 and F-actin did not reappear at the leading edge until the small lamellipodia become the dominant force for cell migration again (at 7 minutes in Figure 6A). Consistent with these observations, the activation pattern of Rac1 detected by PBD pull-down assay showed that the activation of Rac1 peaked by 0.5 minute, remained elevated throughout first 4-minute period of observation, but was inactivated from 4 minutes to 7 minutes, the period during which the expansion of the large lamellipodium occurred (Figure 7A and 7C). Rac1 activity was observed to be increased again after 7 minutes (Figure 7A and 7C), consistent with the formation of smaller lamellipodium at this time and the localization of Rac1 to these structures (Figure 6A).
Figure 6. Adherent human neutrophils stimulated by1×10−7 M fMLP show a differential subcellular distribution of Rac1 and Rac2 with time of stimulation.
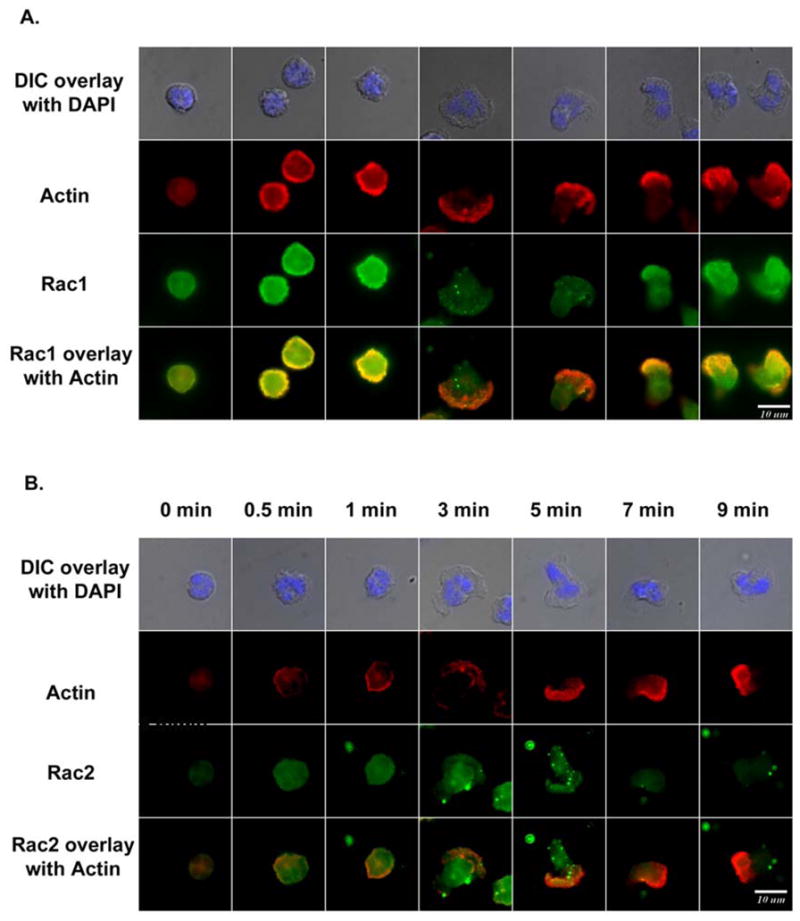
(A) Freshly prepared human neutrophils were allowed to adhere to fibronectin-coated surface for 1 hour, stimulated with the final concentration of 1×10−7 M fMLP, and immunostained for Rac1 (green) and F-actin (red) at 0 min, 0.5 min, 1 min, 3 min, 5 min, 7 min and 9 min with Rac1-specific antibody (Upstate, 23A8) and phalloidin. (B) Freshly prepared human neutrophils were allowed adhere to fibronectin-coated surface for 1 hour, stimulated with the final concentration of 1×10−7 M fMLP, and immunostained for Rac2 (green) and F-actin (red) at 0 min, 0.5 min, 1 min, 3 min, 5 min, 7 min and 9 min with Rac2-specific antibody (R786) and phalloidin. In both A and B, the morphological changes were monitored through the corresponding DIC-DAPI images (top panels). (The result is representive of two independent experiment)
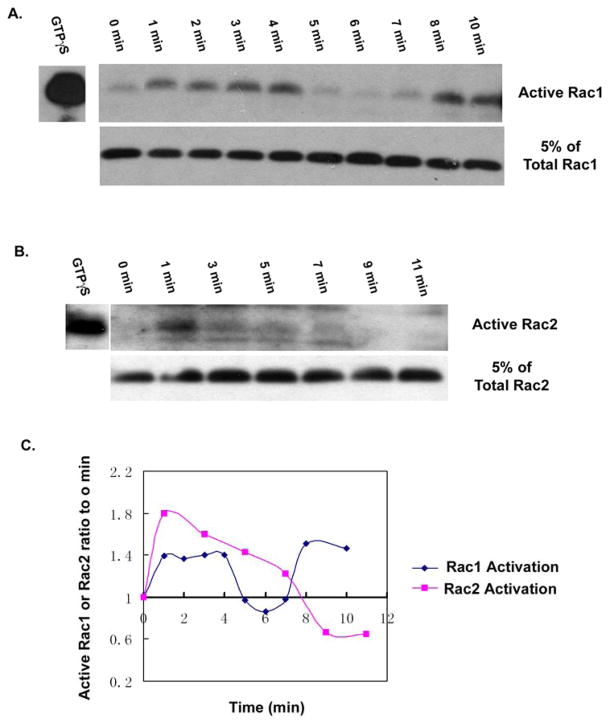
Figure 7. Rac1 and Rac2 are differently activated by uniform fMLP stimulation in human neutrophils.
(A) Freshly prepared human neutrophils were allowed to adhere to fibronectin coated surface for 1 hour, were stimulated with the final concentration of 1×10−7 M fMLP for the time period indicated, and active Rac1 was detected by affinity-based PBD pulldown assay as described in Materials and Methods.. (B) Adherent human neutrophils were stimulated by 1×10−7 M fMLP for the times indicated. Active Rac2 was detected by affinity-based PBD pulldown assay. (C) The ratio of active Rac at each time point as compared to time zero was described as [Active Rac (time X)/Total Rac (time X)]/[Active Rac (0 min)/Total Rac (0 min)]. Therefore, if the ratio of active Rac to the zero time is 1, it means no activation of Rac. If the ratio is more than 1, it means activation of Rac, and if the ratio is less than 1, it means inhibition of Rac activation. The activation of Rac1 was increased throughout the first 4 minutes of stimulation, then dropped sharply between 4–6 minutes, but increased again after 7 minutes. Conversely, the activation of Rac2 was increased throughout the first 7 minutes, then decreased sharply after this time. (The result is representive of two independent experiments)
The Rac2 distribution pattern during 1×10−7 M fMLP stimulation was substantially different from that of Rac1. After 1×10−7 M fMLP stimulation, Rac2 changed from entirely cytosolic to association with the region that ultimately initiated expansion of the large lamellipodia at around 3 minutes (Figure 6B). After 3 minutes, Rac2 continued to accumulate at the front of the large expanding lamellipodium, where it co-localized with F-actin. Such co-localization was maintained until ~7 minutes, when the neutrophils began to form smaller lamellipodia (Figure 6B). Consistent with these observations, the PBD activity assay showed that the activation of Rac2 was initially detected and peaked at 0.5 min, and was maintained at an elevated level for 6–7 minutes (Figure 7B and 7C). Thus, Rac2 activity ended as the expansion of the large lamellipodium was completed. The initial rapid burst of Rac2 activation observed at 0.5 min. was likely required for supporting superoxide production, which we observed to peak at 0.5–2 minutes post stimulation (data not shown). Rac2 is known to be required for fMLP-induced NADPH oxidase activation [5, 15, 31].
These activation and distribution patterns of Rac1 and Rac2 are thus consistent with a role for Rac1 activation in the initiation of cell spreading, perhaps the initiation of the large lamellipodium, and the appearance of smaller lamellipodia. In contrast, Rac2 seems to be important for extending the Rac1-initiated lamellipodium to form a large lamellipodia at the leading edge which supports rapid migration to the chemotactic source.
Discussion
We report here that Rac1 and Rac2 play distinct roles in regulating human neutrophil morphological responses to stimulation with different concentrations of chemoattractant. This was determined using Tat-dominant negative Rac1-T17N or Rac2-T17N fusion proteins to acutely inhibit fMLP-stimulated changes in Rac1 or Rac2 activity, and was verified using mouse neutrophils in which either Rac1 or Rac2 was genetically deleted. The differential effects of the dominant negative Rac1 versus Rac2 proteins, which are believed to act by sequestering GEFs involved in GTPase activation, suggests that these two Rac isoforms respond to distinct GEFs during chermotaxis. Indeed, several Rac-specific GEFs have been implicated in fMLP-stimulated neutrophil activation, including Vav1 [32, 33], P-Rex1 [34–36], and Dock2 [37]. Selectivity for Rac2 has been reported for Vav1 [38] and P-Rex1 [34].
The differential changes in neutrophil responses observed in the presence of the Tat-Rac1-T17N or the Tat-Rac2-T17N were confirmed in neutrophils derived from the Rac1 and Rac2 knockout mice. Chemotactic defects have been reported previously in both the Rac2 [16, 20, 39] and Rac1 [14, 17, 18, 39] knockout mice. Rac1 deficiency was characterized by normal cell motility, but inability to sense a chemotactic gradient [14]. In the absence of Rac2, orientation to the gradient was normal, but the cells failed to migrate efficiently [16, 18, 20, 39]. In the current study, we observed that Tat-Rac1-T17N-mediated inhibition resulted in a loss of the initial spreading response to chemoattractant, as well as a delay in formation of the large lamellipodium at the leading edge (Figure 4B and Supplemental Movie 4). In contrast, the initial spreading response was intact in Rac2-inhibited cells, but the leading lamellipodium did not form at all when Rac2 was inhibited (Figure 4C and Supplemental Movie 4). These results appear consistent with a prior study showing that Rac1 induces the uncapping of actin filaments to drive rapid formation of free actin barbed ends, while Rac2 produces a slower and more sustained activation of cofilin- and ARP2/3-dependent de novo actin polymerization [18].
Consistent with both early and late roles of Rac1 in the chemotactic responses of human neutrophils, we also observed that there was a bi-phasic activation of Rac1 (Figure 6A and 7C). The early response from 1 to 4 minutes was associated with the initial spreading response to chemoattractant. This was followed by a second increase in Rac1 activity beginning at 8 minutes that occurred after the loss of the large lanmellipodium, and concomitant with the formation and maintenance of multiple smaller lamellipodia (Figure 6A and 7C). Rac2 activity exhibited a major peak at 0.5–1 min that has been shown to be associated with initiation of NADPH oxidase activation [40], then remained elevated over the baseline until 7–8 min, when it dropped to unstimulated levels. This change of Rac2 activity correlated well with the formation and maintenance of a single large lamellipodium at the leading front and efficient chemotactic motility (Figure 6B and 7C). The timing of Rac1 and Rac2 activation we observed matched very well with changes in intracellular distribution of Rac1 versus Rac2 in the stimulated neutrophils (Figure 6).
A model for differential regulation of neutrophil response to infection by Rac1 vs Rac2
During the immunological responses of human neutrophils after their initial emigration out of the blood vessel, they encounter an increasing gradient of chemotactic signals diffused from the infectious sites [2]. In vivo and vitro, isolated human neutrophils have been shown to be responsive to stimulation with fMLP in a range of 1×10−9 M to 1×10−7 M [41–43]. In the current study, we establish that human neutrophils respond to increasing concentrations of fMLP through the differential activation of Rac1 and Rac2 (Figure 1). In response to a low concentration gradient of fMLP (ranging from 10 −9 M to 10 −8 M), which mimics the situation at the time of neutrophil’s emigration from the blood vessel, we found that inhibition of Rac1, but not Rac2, by dominant negative Rac1 TAT protein was able to impede neutrophil chemotaxis (Figure 1B). The activation of Rac1 correlates well with the low concentrations of N-formyl peptides that initiate chemotaxis [6]. A morphological characteristic in neutrophils responding to such a low concentration fMLP gradient is the relatively small and short lamellipodia at the leading edge. These are likely to be supported by the uncapping of existing actin free barbed ends through Rac1 activation, as reported [18]. This low range of fMLP concentration elicits only very limited production of superoxide, less than one tenth of Rac2-dependent superoxide production observed at 10−7 M fMLP (data not shown). At this stage, such a limited amount of superoxide formation will cause minmal damage to the surrounding healthy tissue (see Model - Figure 8)
Figure 8. Model proposed to explain the regulation of Rac1 and Rac2 in a chemoattractant gradient from blood vessels to infectious sites.
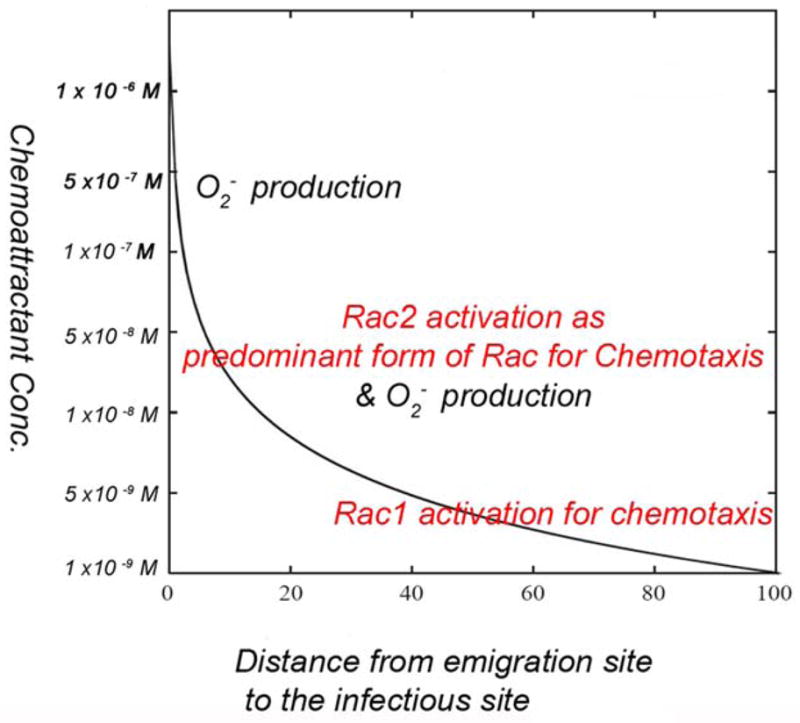
In this model, the X axis represents the distance from the emigration sites, where neutrophils migrate out of the blood vessels, to the infectious sites, where neutrophils exhibit inflammatory responses, such as superoxide production. The Y axis represents the predicted and deduced concentration range of fMLP (in log scale) over the distance from the emigration sites, where the fMLP concentration is lowest (1×10-9 M) to the infectious sites, where the fMLP concentration is highest (1×10-6 M). At the emigration site where the concentration of fMLP is low, neutrophils will activate Rac1 as the predominant form of Rac for initiating chemotaxis and directional migration. As neutrophils move towards the infectious sites, they experience a higher concentration of fMLP and activate both Rac1 and Rac2 to support both chemotaxis and inflammatory activity, such as superoxide production. Once neutrophils eventually reach the infectious site, under the high concentration of fMLP, only Rac2 is activated and the cells stop moving, allowing the neutrophils to stay at the infectious site for performing their inflammatory functions.
In response to the high concentration fMLP gradient, which mimics the situation at the vicinity of infectious sites, we suggest that human neutrophils initially elicit Rac1 activation as part of sensing the chemoattractant gradient (Figure 1A) and to initiate chemotactic responses such as cell spreading and the early increase in cell motility. This is evidenced by the lack of these responses in Rac1T17N-pretreated neutrophils and in Rac1 conditional knock-out mouse neutrophils. Following these initial responses, we suggest that human neutrophils then rely on the activation of Rac2 to support efficient rapid migration towards the gradient source. The latter is supported by Rac2-regulated Arp2/3- and cofilin-mediated actin polymerization for continuous expansion of the leading lamellipodium. This stage would also enable the cells to respond with a burst of superoxide formation (See model- Figure 8). The differential responses of neutrophils to low versus high concentrations of fMLP may relate to the levels of receptor occupancy, as previously described [6, 7]. These studies showed that whereas chemotactic responses occurred at low levels of occupied fMLP receptor, the activation of NADPH oxidase required much higher receptor occupancy by ligand.
In summary, our studies of human and mouse neutrophils in response to different concentrations of fMLP suggest that during chemotaxis from blood vessel to infectious sites, neutrophils initiate different chemotactic responses through the differential activation of Rac1 and Rac2 (Figure 9). Over the low concentration range, neutrophils activate Rac1 to sustain directional migration to the source, but without the triggering of the superoxide burst that is detrimental to the surrounding healthy tissue. As human neutrophils approach the infectious sites, where they experience high concentrations of chemoattractant, the activation of Rac2 at the leading front is required for maintaining a continuous fast expansion the large leading edge lamellipodium, as well as supporting NADPH oxidase activation in a timely manner. Distinct Rho GTPase GEFs may be essential factors in controlling this differential activation of Rac1 and Rac2 by chemoattractants.
Supplementary Material
Acknowledgments
H. Zhang designed and carried out experiments, analyzed data, and wrote the paper, C.S. carried out experiments with murine neutrophils, M.G. analyzed data and directed the murine studies, and G.M.B. analyzed data, directed the study overall, and wrote the paper. We thank Ben Bohl and Bruce Fowler for technical assistance, and Ms. Emilie Broderick for editorial assistance. This is manuscript #20003 from the Department of Immunology and Microbial Science, TSRI.
Footnotes
Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produces version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
This work was supported by Grant from the National Institutes of Health (GM039434) to G.M.B. and a Canadian Institutes of Health Research (CIHR) operating grant to M.G..
References
- 1.Kobayashi SD, Voyich JM, Burlak C, DeLeo FR. Neutrophils in the innate immune response. Arch Immunol Ther Exp (Warsz) 2005;53:505–517. [PubMed] [Google Scholar]
- 2.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 4.Fenteany G, Glogauer M. Cytoskeletal remodeling in leukocyte function. Curr Opin Hematol. 2004;11:15–24. doi: 10.1097/00062752-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bokoch GM, Zhao T. Regulation of the phagocyte NADPH oxidase by Rac GTPase. Antioxid Redox Signal. 2006;8:1533–1548. doi: 10.1089/ars.2006.8.1533. [DOI] [PubMed] [Google Scholar]
- 6.Sklar LA, Hyslop PA, Oades ZG, Omann GM, Jesaitis AJ, Painter RG, Cochrane CG. Signal transduction and ligand-receptor dynamics in the human neutrophil. Transient responses and occupancy-response relations at the formyl peptide receptor. J Biol Chem. 1985;260:11461–11467. [PubMed] [Google Scholar]
- 7.Korchak HM, Wilkenfeld C, Rich AM, Radin AR, Vienne K, Rutherford LE. Stimulus response coupling in the human neutrophil. Differential requirements for receptor occupancy in neutrophil responses to a chemoattractant. J Biol Chem. 1984;259:7439–7445. [PubMed] [Google Scholar]
- 8.Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163–171. doi: 10.1016/j.tcb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Cicchetti G, Allen PG, Glogauer M. Chemotactic signaling pathways in neutrophils: from receptor to actin assembly. Crit Rev Oral Biol Med. 2002;13:220–228. doi: 10.1177/154411130201300302. [DOI] [PubMed] [Google Scholar]
- 10.Bokoch GM. Chemoattractant signaling and leukocyte activation. Blood. 1995;86:1649–1660. [PubMed] [Google Scholar]
- 11.Heyworth PG, Bohl BP, Bokoch GM, Curnutte JT. Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. Evidence for its interaction with flavocytochrome b558. J Biol Chem. 1994;269:30749–30752. [PubMed] [Google Scholar]
- 12.Quinn MT, Evans T, Loetterle LR, Jesaitis AJ, Bokoch GM. Translocation of Rac correlates with NADPH oxidase activation. Evidence for equimolar translocation of oxidase components. J Biol Chem. 1993;268:20983–20987. [PubMed] [Google Scholar]
- 13.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 14.Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, Foerster I, Marks P, Downey GP, Dinauer M, Kwiatkowski DJ. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol. 2003;170:5652–5657. doi: 10.4049/jimmunol.170.11.5652. [DOI] [PubMed] [Google Scholar]
- 15.Kim C, Dinauer MC. Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J Immunol. 2001;166:1223–1232. doi: 10.4049/jimmunol.166.2.1223. [DOI] [PubMed] [Google Scholar]
- 16.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 17.Sun CX, Downey GP, Zhu F, Koh AL, Thang H, Glogauer M. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood. 2004;104:3758–3765. doi: 10.1182/blood-2004-03-0781. [DOI] [PubMed] [Google Scholar]
- 18.Sun CX, Magalhaes MA, Glogauer M. Rac1 and Rac2 differentially regulate actin free barbed end formation downstream of the fMLP receptor. J Cell Biol. 2007;179:239–245. doi: 10.1083/jcb.200705122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamauchi A, Kim C, Li S, Marchal CC, Towe J, Atkinson SJ, Dinauer MC. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J Immunol. 2004;173:5971–5979. doi: 10.4049/jimmunol.173.10.5971. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Yamauchi A, Marchal CC, Molitoris JK, Quilliam LA, Dinauer MC. Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J Immunol. 2002;169:5043–5051. doi: 10.4049/jimmunol.169.9.5043. [DOI] [PubMed] [Google Scholar]
- 21.Yang FC, Atkinson SJ, Gu Y, Borneo JB, Roberts AW, Zheng Y, Pennington J, Williams DA. Rac and Cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc Natl Acad Sci U S A. 2001;98:5614–5618. doi: 10.1073/pnas.101546898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pestonjamasp KN, Forster C, Sun C, Gardiner EM, Bohl B, Weiner O, Bokoch GM, Glogauer M. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 2006;108:2814–2820. doi: 10.1182/blood-2006-01-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh AL, Sun CX, Zhu F, Glogauer M. The role of Rac1 and Rac2 in bacterial killing. Cell Immunol. 2005;235:92–97. doi: 10.1016/j.cellimm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 25.Vocero-Akbani A, Chellaiah MA, Hruska KA, Dowdy SF. Protein transduction: delivery of Tat-GTPase fusion proteins into mammalian cells. Methods Enzymol. 2001;332:36–49. doi: 10.1016/s0076-6879(01)32190-0. [DOI] [PubMed] [Google Scholar]
- 26.Mayo LA, Curnutte JT. Kinetic microplate assay for superoxide production by neutrophils and other phagocytic cells. Methods Enzymol. 1990;186:567–575. doi: 10.1016/0076-6879(90)86151-k. [DOI] [PubMed] [Google Scholar]
- 27.Soll DR, Wessels D, Voss E, Johnson O. Computer-assisted systems for the analysis of amoeboid cell motility. Methods Mol Biol. 2001;161:45–58. doi: 10.1385/1-59259-051-9:045. [DOI] [PubMed] [Google Scholar]
- 28.Eddy RJ, Pierini LM, Maxfield FR. Microtubule asymmetry during neutrophil polarization and migration. Mol Biol Cell. 2002;13:4470–4483. doi: 10.1091/mbc.E02-04-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson JM, Vandre DD. Antigen retrieval in cells and tissues: enhancement with sodium dodecyl sulfate. Histochem Cell Biol. 2001;116:119–130. doi: 10.1007/s004180100299. [DOI] [PubMed] [Google Scholar]
- 30.Zhao T, Bokoch GM. Critical role of proline-rich tyrosine kinase 2 in reversion of the adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J Immunol. 2005;174:8049–8055. doi: 10.4049/jimmunol.174.12.8049. [DOI] [PubMed] [Google Scholar]
- 31.Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254:1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 32.Kim C, Marchal CC, Penninger J, Dinauer MC. The hemopoietic Rho/Rac guanine nucleotide exchange factor Vav1 regulates N-formyl-methionyl-leucyl-phenylalanine-activated neutrophil functions. J Immunol. 2003;171:4425–4430. doi: 10.4049/jimmunol.171.8.4425. [DOI] [PubMed] [Google Scholar]
- 33.Roepstorff K, Rasmussen I, Sawada M, Cudre-Maroux C, Salmon P, Bokoch G, van Deurs B, Vilhardt F. Stimulus-dependent regulation of the phagocyte NADPH oxidase by a VAV1, Rac1, and PAK1 signaling axis. J Biol Chem. 2008;283:7983–7993. doi: 10.1074/jbc.M708281200. [DOI] [PubMed] [Google Scholar]
- 34.Dong X, Mo Z, Bokoch G, Guo C, Li Z, Wu D. P-Rex1 is a primary Rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr Biol. 2005;15:1874–1879. doi: 10.1016/j.cub.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Zhao T, Nalbant P, Hoshino M, Dong X, Wu D, Bokoch GM. Signaling requirements for translocation of P-Rex1, a key Rac2 exchange factor involved in chemoattractant-stimulated human neutrophil function. J Leukoc Biol. 2007;81:1127–1136. doi: 10.1189/jlb.0406251. [DOI] [PubMed] [Google Scholar]
- 36.Welch HC, Condliffe AM, Milne LJ, Ferguson GJ, Hill K, Webb LM, Okkenhaug K, Coadwell WJ, Andrews SR, Thelen M, Jones GE, Hawkins PT, Stephens LR. P-Rex1 regulates neutrophil function. Curr Biol. 2005;15:1867–1873. doi: 10.1016/j.cub.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 37.Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, Watanabe K, Sanematsu F, Sasazuki T, Sasaki T, Fukui Y. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol. 2006;174:647–652. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim C, Marchal CC, Penninger J, Dinauer MC. The hemopoietic/Rac guanine nucleotide exchange factor Vav1 regulates N-formyl-methionyl-leucyl-phenylalanine-activated neutrophil functions. J Immunol. 2003;171:4425–4430. doi: 10.4049/jimmunol.171.8.4425. [DOI] [PubMed] [Google Scholar]
- 39.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 40.Zhao T, Benard V, Bohl BP, Bokoch GM. The molecular basis for adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J Clin Invest. 2003;112:1732–1740. doi: 10.1172/JCI19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shields JM, Haston WS. Behaviour of neutrophil leucocytes in uniform concentrations of chemotactic factors: contraction waves, cell polarity and persistence. J Cell Sci. 1985;74:75–93. doi: 10.1242/jcs.74.1.75. [DOI] [PubMed] [Google Scholar]
- 42.Geiger J, Wessels D, Soll DR. Human polymorphonuclear leukocytes respond to waves of chemoattractant, like Dictyostelium. Cell Motil Cytoskeleton. 2003;56:27–44. doi: 10.1002/cm.10133. [DOI] [PubMed] [Google Scholar]
- 43.Maher J, Martell JV, Brantley BA, Cox EB, Niedel JE, Rosse WF. The response of human neutrophils to a chemotactic tripeptide (N-formyl-methionyl-leucyl-phenylalanine) studied by microcinematography. Blood. 1984;64:221–228. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.