Abstract
Efficient drug delivery to tumors is of ever increasing importance. Single-visit diagnosis and treatment sessions are the goal of future theranostics. In this paper, a non-covalent PDT cancer drug-gold nanoparticle (Au NP) conjugate system performed a rapid drug release and deep penetration of the drug into tumors within hours. The drug delivery mechanism of the PDT drug through Au NPs into tumors by passive accumulation was investigated via fluorescence imaging, elemental analysis, and histological staining. The pharmacokinetics of the conjugates over a 7 day test period showed fast drug excretion, as monitored via the fluorescence of the drug in urine. Moreover, the biodistribution of Au NPs in this study period indicated clearance of the NPs from the mice. This study suggests that the non-covalent delivery via Au NPs provides an attractive approach for cancer drugs to penetrate deep into the center of tumors.
Keywords: deep drug penetration, gold nanoparticles, drug delivery, non-covalent, PDT, pharmacokinetics
INTRODUCTION
Inefficient delivery and poor uptake of therapeutic drugs to the solid tumors hamper the efficacy of cancer treatments.1–3 Therefore, “Enhanced permeability and retention” (EPR) effect of solid tumors has been explored extensively as a target in design of drug delivery systems.4–7 Different from normal tissues, solid tumors have several abnormalities such as leaky blood vessels and poor lymph system.7–11 It is an important feature that nanosized particles can extravasate from the vasculature and passively accumulate in the tumors.5
Inorganic nanoparticles, especially gold nanoparticles (Au NPs) with good biocompatibility, versatile surface, tunable sizes and unique optical properties have received significant attention as drug delivery systems to improve targeting effect and efficacy for cancer treatment.12–21 Covalent and non-covalent attachment are the two major approaches to deliver therapeutic drugs via Au NPs.12,22–26 The covalent attachment approach not only requires structure modification of the therapeutic drugs, but triggers such as enzymes25 and light26 are also required to control the drug release. Even though the modified pro-drugs can be stabilized by a chemical bond on the Au NP surface, the efficient release and efficacy of the drugs can be reduced. Although a significant number of available effective drugs have been modified to covalently bind to the Au NPs, non-covalent attachment maintaining the active drug structure without modification provides an attractive way to bind, deliver and release the actual drug without needing such triggers. It allows the drug-loaded NPs to passively accumulate in the tumor and the non-covalently attached drug payload can be concentrated in the tumor mass.12 Depending on the respective therapeutics and targets, the drug delivery approach has to be chosen accordingly.
Photodynamic therapy (PDT) is a non-invasive treatment modality for cancers and other diseases.27–30 Tumors and cancer cells can be eradicated by the combination of light, photosensitizer and oxygen.30–32 Previous studies have proven that hydrophobic PDT drugs can induce DNA and cell membrane damage to cancer cells via peroxidation of lipids, and further damage cellular organelles such as mitochondria through the formation of reactive oxygen species (ROS).32–34 The majority of efficient photosensitizers are hydrophobic, which allow them to preferentially accumulate in the lipid bilayers of organelle membranes in cancer cells.35 The hydrophobic nature of most photosensitizers makes them insoluble under physiological conditions and hinders their systemic administration.35–37 Therefore, a relatively long time interval (~1–3 days) between intravenous drug administration and therapeutic light irradiation is required to achieve the desirable drug concentration in a tumor for effective PDT treatment.31 Moreover, PDT drugs can cause some side effects, such as prolonged skin photosensitivity, if allowed to stay in the body over long periods.31 During the treatment intervals, patients must avoid daylight exposure. Therefore, a rapid and efficient delivery of the PDT drug to the target tumor and fast clearance would be ideal for PDT treatment.
A non-covalent delivery design that can solubilize and circulate the active drug payload systemically while providing an efficient and targeted release for subsequent treatment appears to be a preferable scenario for PDT.12,38 Au NPs coated with polyethylene glycol (PEG) are promising drug delivery systems for PDT of cancer.12 The Au NP surface provides an amphiphilic environment for lipophilic PDT drugs and can physically deactivate the drug until it release from the NP.12 The PEG on the Au NP surface provides the solubility in water and minimizes any protein adsorption.19 In addition, it has been widely reported that PEGylated NPs have “stealth” character, which can postpone or prevent the rapid clearance by the reticular-endothelium system (RES).39–41 Therefore, drugs on these Au NP carriers can have a prolonged circulation time in the blood.41 Although Au NPs are not biodegradable, they have shown excellent biocompatibility and low toxicity.
The authors have previously evaluated the covalent and non-covalent PEGylated Au NP-based PDT drug delivery systems into HeLa cells.42 In contrast to the slow intracellular release and reduced drug efficacy via the covalent attachment on Au NPs, non-covalent adsorption of the PDT drug to Au NPs showed efficient drug released into HeLa cancer cells by membrane-mediated diffusion without the need to use external stimuli.42 It has been further demonstrated that the hydrophobic PDT drug silicon phthalocyanine 4 (Pc 4) non-covalently attached on PEGylated Au NPs is efficiently delivered to the tumor within minutes after intravenous injection. This is compared to the ~ 48 hours accumulation period needed for the free drug.12,43–46 Successful PDT treatment can be achieved already after 2 hours past injection. The Au NP-delivered Pc 4 can cause tissue necrosis and shrinkage in tumor size following exposure to therapeutic light.12
Since the non-covalent delivery approach for hydrophobic therapeutic drugs, including PDT drugs,12,22 via PEGylated Au NPs has attracted considerable attention, it is important to fully understand the in vivo drug delivery and release mechanism to the tumors for cancer therapy.
The aim of this study is to investigate the drug release mechanism in the tumor and to provide a correlation between the drug carrier (Au NP) and the drug itself in vivo. It has been reported that nanoparticles with a hydrodynamic diameter of less than 5.5 nm can be efficiently excreted via the renal clearance path.47 We designed the Au NP core size with 5 nm diameter to remain small enough to be renally excreted while the hydrodynamic diameter of the PEGylated conjugate is 38 nm to improve stability, circulation time, and to achieve passive accumulation via the EPR effect. In addition, on a separate set of stability experiments of different PDT drug to Au NP ratios,12 the drug loading was optimized to ~30 PDT drug molecules per Au NP. This ratio was therefore also chosen for the animal studies presented here. The delivery, pharmacokinetics and excretion dynamics of Au NPs and Pc 4 have been investigated with animal in vivo fluorescence imaging, elemental gold analysis, and histological imaging over a range of time points. We examine Au NP and Pc 4 localization in the tumor, providing support for the presented drug release mechanism and EPR effect in cancerous tissues (Figure 1) and for deep penetration of the drug into the interior of the tumor. This is a completely novel approach to deliver hydrophobic drugs fast and deep into tumors based on weak chemical interactions.
Figure 1.
A) Schematic of Au NP-Pc 4 conjugates circulation into the tumor. After circulating within the blood-vascular system, Au NP-Pc 4 extravasate through the endothelial cell layer of the blood vessels into cancerous tissues via the EPR effect. B) Chemical structure of the PDT drug Pc 4. C) The hydrodynamic diameter of the Au NP-Pc 4 conjugates measured by dynamic light scattering (DLS).
MATERIALS AND METHODS
Materials
The Au NPs were synthesized by the reduction of HAuCl4 based on the modified Brust-Schiffrin method.48,49 And the synthesized NPs were coated with mPEG-SH (MW 5000, Laysan Bio). The PDT drug silicon phthalocyanine 4 (Pc 4) was then mixed with the PEGylated Au NP for 48 hours and purified based on the procedure described previously.12 The drug loading efficiency was optimized to 30 Pc 4 molecules per Au NP to obtain a stable conjugates in the aqueous phase. The final ratio of Pc 4 per Au NP in the PEGylated Au NP-Pc 4 conjugate was characterized by UV-vis spectroscopy. The average core size of Au NP was 5 nm in diameter characterized by transmission electron microscopy (JEOL JEM-1200 EX electron microscope). And the hydrodynamic diameter of the conjugates was 38.3±0.4 nm measured by dynamic light scattering (90Plus Particle Size Analyzer, Brookhaven Instruments Corporation). For intravenous injection, the administered conjugates in mice were based on a Pc 4 concentration of 1 mg kg−1. SPI-Mark™ silver enhancement kit was obtained from SPI Supplies Division of Structure Probe, Inc. (West Chester, PA) 1000 μg mL−1 gold standard solution in dilute hydrochloric acid was purchased from Inorganic Ventures. All other reagents were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA) and used as obtained.
Animal Experiments
Animal experiments were performed according to IACUC policies and guidelines of the Animal Care and Use Committee at Case Western Reserve University. Female athymic mice were obtained from the Athymic Animal Core Facility of the Cancer Research Center at Case Western Reserve University. Rat glioma (9L) cancer cell lines overexpressing both EGFR and TfR were subcutaneously implanted in the flank of athymic mice (~3 × 105 cells/implant). Tumors developed for 21 days prior to systemic injection with Au NP-Pc 4 conjugates. Animals were fed exclusively on a special rodent diet (Tekland 2018S; Harlan Laboratories, Inc.) to reduce auto-fluorescence performed under IACUC guidelines.
In Vivo Fluorescence Imaging
Fluorescence in vivo imaging experiments were carried out at Case Western Reserve University in the Case Center for Imaging Research in Cleveland, Ohio. Mice with tumors (~3–4 weeks after tumor implantation) were anaesthetized with isoflurane and injected intravenously via the tail with Au NP-Pc 4 at a dosage of Pc 4 at 1 mg kg−1 of total mouse body weight. Fluorescent multispectral images were obtained using the Maestro In Vivo Imaging System (Cambridge Research and Instrumentation, Inc., Woburn, MA). A Cy5 excitation filter (575–605 nm band-pass) and emission filter (645 nm long-pass) combination was used. Multispectral in vivo images were acquired under a constant exposure of 100 ms with a yellow filter acquisition setting of 630–850 nm in 2 nm increments. Multispectral images were unmixed into their component spectra (Pc 4, autofluorescence, and background) and these component images were used to gain quantitative information in terms of average fluorescence intensity by creating regions of interest (ROIs) around the tumors in the Pc 4 component images. Post-analysis spectral libraries were created by imaging a mouse pre- and post-injection to obtain a Pc 4 signal by subtraction of autofluorescence. Mice were imaged before injection (pre-injection), immediately following injection (10 minutes), every hour for 6 hours (unless sacrificed at 4 hours), and every 24 hours until 7 days (unless sacrificed at 24 hours). During the treatment period, mice were kept under normal ambient light cycles to mimic real-world drug applications.
Biodistribution Experiments
Blood from the Au NP conjugate-injected mice was collected in heparinized glass tubes and stored in the refrigerator at 4°C until further processing. Tissues (tumor, heart, lung, spleen, liver, kidneys, urinary tract, large intestine, and stomach) from the mice were collected post mortem. The tissue samples were washed with normal saline, dried briefly with a paper towel and imaged with the same acquisition settings of in vivo fluorescence imaging 4 hours, 24 hours, or 7 days post-injection of the Au NP-Pc 4 conjugates. Multispectral images were unmixed into their component spectra (Pc 4, autofluorescence, and background) and Pc 4 component images were used to measure the Pc 4 intensity by creating ROIs associated with each organ. After imaging the collected tissues they were weighed and stored in a −20°C freezer until they could be analyzed for gold content via graphite furnace atomic absorption spectroscopy (GFAAS). Tissues, blood, urine and feces collected from each mouse were then digested in Parr acid digestion vessels (Parr Instrument Company, Moline, IL) with 70% nitric acid at 120°C for 2 hours. Digested samples were diluted and analyzed by GFAAS in a GTA-110 with a programmable auto-sampler (Varian, Inc., Palo Alto, CA). The wavelength of the hollow cathode gold lamp was 242.8 nm and values for the concentration of gold in the samples were calibrated by a series of gold standard solutions.
Silver-enhanced Staining of Excised Au NP-Pc 4 Injected Mice Organs
Tissue samples were fixed in 4% paraformaldehyde at room temperature for 24 hrs and embedded in a paraffin block. The tissue blocks were cut into 20 μm micrometer sections and placed on glass slides. The tissue slices were hydrated through a concentration series of ethanol (100%, 95%, 75% and 50%) and placed in distilled water for 30 minutes. The silver enhancement reagents (developer and enhancer) were mixed immediately at a 1:1 ratio before the staining. The slides were gently tapped and the silver enhancement reagent mixture was placed on the slices for 15 minutes at room temperature. The staining was stopped by rinsing the slides with distilled water for 30 minutes. The tissue slides were then co-stained with hematoxylin and eosin (H&E). After washing, the slides were dehydrated in ethanol and cleared with xylene. The slides were finally mounted with Permount and cover glass. Stained sections were observed and images were obtained using a DM4000 B inverted microscope (Leica), and QCapture Pro imaging software (version 5.1.1.14; QImaging).
RESULTS AND DISCUSSION
Delivery of Pc 4 to the Tumor
As shown in Figure 1, the PDT drug Pc 4 can be stabilized via hydrophobic interaction to the Au NP surface. And the terminal amino group on the Pc 4 axial ligand can attach to the Au NP surface through N-Au interactions. Moreover, the PEG on the NPs provides a steric repulsion to stabilize the conjugates in the aqueous phase. Pc 4 on the Au NPs is protected from light by the Au NPs via excited state energy transfer to the NPs. The fluorescence of Pc 4 has been significantly quenched on Au NPs as shown in Figure S1. After the Au NP-Pc 4 conjugates are retained in the tumor, the drug release can be triggered by the hydrophobic attraction of the drug to the lipid membrane of the cancer cells.42 The Pc 4 release from the conjugates will result in the increase of fluorescence intensity. In order to characterize the accumulation of Pc 4 delivered by the Au NPs in vivo, we accessed Pc 4 accumulation in tissues by monitoring the drug’s fluorescence (λmax,fl = 680 nm) (Figure 2 and Figure S2), whereby the observed Pc 4 fluorescence reflects the concentration of the drug, which allowed analysis and optimization of the PDT treatment. Significant Pc 4 fluorescence was observed at the tumor area just minutes after intravenous tail vein injection of the Au NP-Pc 4 conjugates (Figure 2A). Drug accumulation in the tumor was quantified by identifying the tumor as a region of interest to measure its Pc 4 fluorescence intensity (Figure 2B). The average fluorescence intensity from Pc 4 acquired in the tumor areas quickly raised within 10 minutes post-injection and reached a plateau from 1 hour to 6 hours post-injection (n=5). A decrease in tumor fluorescence was observed 24 hours post-injection. The rapid accumulation of drug within the tumor identifies a suitable treatment-time window for PDT. Au NP delivery of Pc 4 is a significant improvement in the time required by conventional means to achieve therapeutic doses in tumor tissue, reducing it from ~48 hours (conventional) to ≤ 6 hours (Au NPs).
Figure 2.
In vivo fluorescence imaging of Au NP-Pc 4 conjugates. Tumor bearing mice were injected intravenously with Au NP-Pc 4 conjugates at a Pc 4 dosage of 1 mg kg−1 mouse. (A) In vivo fluorescence imaging of the Au NP-Pc 4 conjugates injected mouse at various time points within 24 hours. Arrows indicate tumor location. (B) The average fluorescence intensities from the tumor areas of 24-hour post-injection mice (n=5). (C) Picture of the tumor at 4 hours post-injection (left) and the corresponding 3D surface plot (right) of pixel intensities (Pc 4 fluorescence) obtained from ImageJ. (D) Comparison of the fluorescence images in whole versus transected tumors at 4 hours, 24 hours and 7 days post-injection.
Tumors derived from animals treated with the Au NP-Pc 4 were transected and imaged ex vivo. Pc 4 fluorescence was distributed throughout the whole tumor section with an intense fluorescence in the tumor’s center after 4 hours of Au NP-Pc 4 injection (Figure 2C and 2D). The two-dimensional fluorescence image of the harvested tumor at 4 hours post-injection suggested Pc 4 distribution within the tumor and the corresponding surface plot of the fluorescence pixel intensities from the tumor region showed homogenous Pc 4 distribution on the tumor surface (Figure 2C).
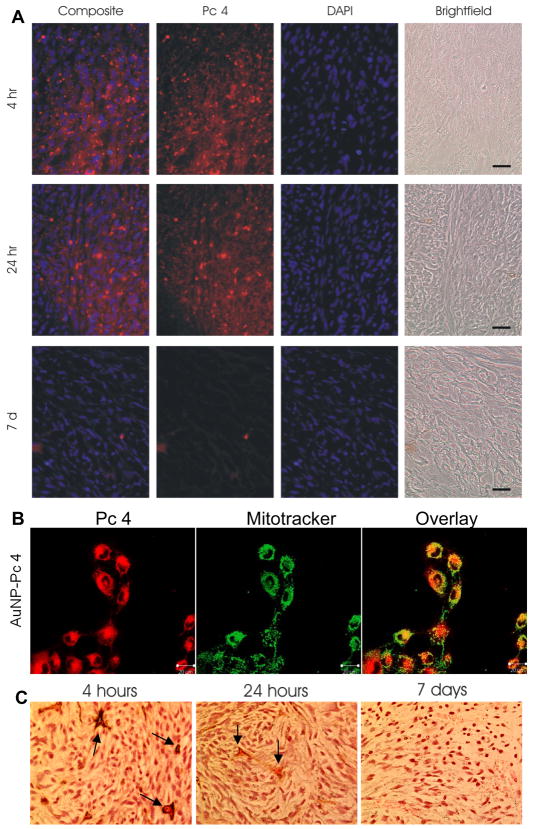
Ex vivo tumor fluorescence imaging further qualitatively verified that the best PDT treatment time must be within the first 6 hours post-injection as the 4-hour post-injection tumor showed the highest intensity compared to the 24-hour tumor (Figure 2D). After 7 days, the fluorescence from the tumor nearly disappeared. The intense Pc 4 fluorescence in the center of the tumor by 4 hours (Figure 2D) showed very efficient diffusion of the PDT drug into the tumor. The drug biodistribution in the tumor tissue was further studied by examining the Pc 4 in the tumor cryosections. Ex vivo tumor cryosections were counterstained with DAPI to visualize cell nuclei (Figure 3A). Pc 4 accumulation was observed within the cytoplasm of the cells. Similar to the ex vivo tumor images, Pc 4 fluorescence tapered off dramatically by 7 days as shown in Figure 2D and 3A. The histological study of tumor tissues indicated the accumulation of Au NPs in the perivascular space at 4 hours post-injection (Figure 3B). Au NPs in the tissue were enhanced by silver staining and visualized under microscopic magnification. The silver ions were reduced by hydroquinone to the elemental silver in presence of Au NP, which caused the growth of Au NPs.41 In addition, more Au NPs were found in the center of the tumor area 24 hours post-injection, which suggested permeation into the tumor. After 7 days, the Au NP concentration within tumor tissues dropped significantly.
Figure 3.
Au NP-Pc 4 accumulation in subcutaneous heterotopic tumors. (A) Cryosections of ex vivo tumors were counterstained with DAPI (blue) to visualize nuclei. Pc 4 fluorescence was captured using a standard Cy5 filter set (red). Images were overlaid to demonstrate Pc 4 uptake into the tumor. All images were captured at 40X magnification. Scale bar represents 100 μm. (B) Confocal images of the cancer cells incubated with Au NP-Pc 4 for 4 hours stained with mitotracker green. (C) Au NPs visualized using silver enhancement staining in ex vivo paraffin-embedded tumor sections 4 hours, 24 hours, and 7 days post-injection. Arrows indicate the blood vasculatures. The size of Au NPs was enhanced by deposition of the elemental silver on the NP surface.
It is hypothesized that the Au NPs with adsorbed Pc 4 release the drug by diffusion into hydrophobic areas within tissues (e.g. cellular membranes), but the Au NPs themselves do not enter the cells and undergo a different fate. In order to support our hypothesis, we carried out quantitative chemical analysis of tissue sections for gold and Pc 4. The concentration changes of both Au NP and Pc 4 (based on fluorescence) showed a similar trend during the 7-day post-injection period (Figure 4A). This implies that Pc 4 was indeed delivered to the tumor by the Au NPs after initial tail vein injection of the conjugates. At the 4 hour post-injection time point, both Au NPs (% ID Au NP) and Pc 4 (fluorescence) showed the highest concentrations in the tumor of any time point measured. At 4 hours post-injection, Au NPs were targeted to tumors with an average accumulation of 8 ± 6 % ID presumably by the EPR effect. 30 ± 5.6 % ID Au NPs remained in the blood at four hours after injection with a circulation half-life time of ~3 hours (Figure 4B). This overlap in delivery and release dynamics supports the contention that the most suitable treatment time for PDT is within 6 hours. This passive accumulation was shown to correlate with the blood clearance of Au NPs. The relatively long circulation of the NPs in the blood allowed significant accumulation of the drug in the tumor. Pc 4 drug release from the Au NP into the cells has a half-life of 2–3 hours.42 However, the significant drug release in the tumor at 10 min to 2 hours after injections indicates that the drug release kinetics from the conjugates in vivo is different from that in vitro. The half-life of the drug release is decreased due to the more complex environment in vivo. While Au NPs mainly localized in the perivascular space, Pc 4 in the tumor tissue showed a faster diffusion into the whole tumor at 4 hours post-injection. When the Au NP-Pc 4 conjugates leak into the tumor through the endothelium of the blood vessels, the clearance of the conjugates is hampered by lack of a lymphatic recovery system.7–10 This passive accumulation of the conjugates potentially results in the diffusion of the small drug molecules to the inner layers of the tumor by simple diffusion caused by a gradient of the drug concentration within the tumor. The non-covalent delivery approach and the inherent hydrophobic nature of Pc 4 are presumably the two major factors for the efficient release into the tumor cells. Our previous in vitro studies have shown that Pc 4 can be efficiently delivered by Au NPs into cancer cells within 4 hours by membrane-mediated diffusion, while still remaining active for PDT.42 In addition, these studies have also demonstrated that only a few Au NPs are taken up into the cells. While this non-covalent delivery approach has achieved efficient drug release in vitro, it promises a similarly efficient release into tumors in the animal model. Based on a comparison with the dynamics of Au NPs, the faster diffusion of Pc 4 into the tumor (Figure 3 and 4) indicates that the drug is released from the NPs and transported via hydrophobic interaction with the plasma membranes and delivered into the cytoplasm of the tumor cells as described in Figure 1. We have further shown that the Au NP-delivered drug is mainly localized in mitochondria in the cells, similar to free Pc 4 (Figure 3B).50 There is a driving force for Pc 4 into hydrophobic environments (Figure S3). Low-density lipoproteins and other hydrophobic molecules in the blood stream or tumors are in principle possible transport mechanisms.51–54 However, the hydrodynamic diameter of the conjugates in serum did not change substantially as measured by DLS (Figure S4). This shows that the PEGylation of the Au NPs reduces effectively the protein interactions with the drug and provides longer drug circulation time in the blood stream.3,5,55,56 The observed diffusional drug uptake into the cells is therefore due to the thermodynamic driving force towards their lipid membranes. After release from the Au NP, Pc 4 selectively enters the tumor cells by diffusion following the concentration gradient from areas of higher concentration to areas of lower concentration. A significant decrease of both the Au NPs and Pc 4 within 7 days in the tumors was also observed (Figure 3).
Figure 4.
Au NP-Pc 4 accumulation in subcutaneous heterotopic tumors. (A) Graph of average Pc 4 fluorescence intensity; inset graph of Au concentration in tumors from the injected mice over a 7 day period (n=3). (B) Gold concentration in blood based on total injected dose at different time points in Au NP-Pc 4 injected mice (n=5).
Biodistribution and Clearance of Pc 4
Six hours after injection of the conjugates, Pc 4 fluorescence intensity in the whole body leveled off and little fluorescence was observed 7 days post-injection (Figure S5A). The Pc 4 fluorescence from the tumor returned almost to baseline after 7 days (Figure S5B). It was also observed that the bladder area showed significant fluorescence after injection, suggesting that Pc 4 can be excreted in the form of urine by renal clearance in a very short time. Pc 4 fluorescence could be decreased over time by chemical quenching and structural change with enzymes produced in the organs (e. g. liver). Thus, the fluorescence intensity may not be a 100% exact tracking quantification method for in vivo studies, which is generally true for in vivo fluorescence workers.
Based on the in vivo fluorescence images, most of the Pc 4 drug molecules appear to be either excreted from the body or redistributed into other organs after a week of circulation within the body. Indeed, all of the organs as well as the tumor showed an overall decrease in Pc 4 fluorescence intensity within 7 days (Figure 5). The tumor at 4 hours post-injection showed very high fluorescence intensities suggesting that the passive accumulation of Pc 4 by the NPs within the tumor was likely occurring. The liver, as part of the RES, also showed an accumulation of drug fluorescence. However, the spleen did not show much Pc 4 accumulation. Other organs such as the heart and lung showed moderate increases in Pc 4 fluorescence as well. At 4 hours post-injection, the organs of excretion and digestion, including kidneys, urinary tract, large intestine and stomach, all showed relatively intense Pc 4 fluorescence, while at 24 hours and 7 days post-injection, the excretory organs showed less intense fluorescence. Meanwhile, the fluorescence from other organs decreased significantly within 7 days. Overall, the results suggest that Pc 4 was removed from both the cardio-pulmonary (heart, lung) and the immune systems and channeled into the excretory organs where Pc 4 could be finally removed from the body by renal clearance in the form of urine and by excretion in the form of feces.
Figure 5.

Biodistribution and elimination of Au NP-Pc 4 based on Pc 4 fluorescence. The Pc 4 fluorescence images of organs from the injected mice after dissection.
Biodistribution and Clearance of Au NPs
A significant number of studies have been carried out to understand the in vivo biodistribution of the Au NPs.41,57–60 It is known that the biodistribution of Au NPs is very complex, which correlates with the surface ligands, charges, NP shapes and sizes. PEG-modified Au NPs have a long circulation time in the blood.40,41 Fifty four percent (54%) of injected Au NPs have been found in the blood at 0.5 hour and the liver only showed the most NPs after 72 hours.40 Other studies found that the biodistribution of citrate-gold nanospheres are size dependent and are mainly trapped by the liver 24 hours after administration.57,58 1.4 nm Au clusters (Au55(Ph2PC6H4SO3Na)12Cl6) and 18 nm Au NPs have been studied in rats.60 The 1.4 nm Au clusters showed some excretion by the kidneys and the hepatobiliary system 24 hours after intravenous injection. And 47.5±2.3 % of the injected dose (ID) of 1.4 nm Au clusters was found in liver, while 18 nm Au NPs showed more than 90 % of the ID in the liver with extremely low renal clearance.60 However, little information about the fate of PEGylated Au NPs as a non-covalent drug carrier has been obtained in an animal model, since most studies have focused on short term biodistribution (within 3 days). In this study, we investigated the Au NPs biodistribution up to 7 days after intravenous injection.
The Au NP-Pc 4 conjugates in this study have an average Au NP core size of 5 nm in diameter and can easily be quantified with GFAAS to determine biodistribution (Figure 6A and Figure S6). Blood, urine, feces, organs and tumors were collected at different time points after injection and analyzed for Au content. Most tissues including the tumor reached maximum gold accumulation by 4 hours followed by a gradual decrease over time. A significant exception to this was observed in both the liver and spleen where accumulation of the Au continued over time to reach a maximum at the latest time point measured, 7 days. Au NPs showed the highest concentration per gram of tissue in the spleen after 7 days post-injection (Figure 6A), while the liver showed the highest Au concentration in terms of % ID by accumulation of over 30 % of the Au NPs (Figure S6) after 7 days.
Figure 6.
Assessment of Au NP-Pc 4 biodistribution on Au content. (A) Au (μg) per gram of sample in organs. Mice were injected with Au NP-Pc 4 (n≥3 per each time point). The total gold content in tissue samples was evaluated by GFAAS. (B) Histology studies of the organ tissues. Organ samples were removed from mice injected with Au NP-Pc 4 after 4 hours, 24 hours, and 7 days post-injection. The paraffin-embedded tissue slices were stained with hematoxylin, eosin, and silver enhancement reagents. The black and brownish spots indicate Au NPs in the tissues. Images were captured at 40X magnification. Scale bar represents 100 μm.
Au NPs were found in the kidneys and urinary tract over the next 7 day study. Urine samples from 4 hours, 24 hours, and 7 days showed the existence of the NPs, indicating that the Au NP-Pc 4 could be excreted by renal clearance. In addition, the Au NPs were found in feces samples from both 24 hours and 7 days samples. In the tumor, heart, lung, kidney and urinary track, the Au NP concentration decreased significantly after 7 days. Interestingly, the large intestine and stomach did not seem to exhibit a time dependent increase or decrease in Au accumulation.
We found that PEGylated Au NPs circulating in the blood can be slowly opsonized and removed by the RES from the bloodstream, which is consistent with previous studies on nanomaterials such as polymeric NPs,61 single-walled carbon nanotubes,62 and quantum dots63 (Figure 6B). Silver stained tissue samples showed significant accumulation of Au NPs in the liver and spleen (part of the RES) up to 7 days post-injection, which correlated well with the GFAAS analysis of the Au NPs in the organs (Figure 6). At 4 hours post-injection, the NPs in the liver were mainly found in the blood vessels and liver sinusoids. After 24 hours, the NPs were cleared from the blood vessels but remained in the liver sinusoids. A small number of Au NPs were observed in the hepatocytes in the liver. Histological examination suggested that macrophages in the spleen tissue were heavily loaded with the NPs (Figure 6B). Moreover, Au NPs were located in the glomerulus of the kidney, which supported the observation that the NPs could presumably be filtered by renal clearance in the form of urine. Over time, the number of Au NPs in the kidney decreased. A few Au NPs were found in the stroma of the urinary tract, further suggesting excretion of Au NPs through renal clearance.
Overall, PEGylated Au NPs, as the drug carriers circulating in the blood, can be slowly opsonized and removed from the bloodstream, followed by accumulation in the liver and spleen. When the NPs circulate in the body, the excretion process is fast. Once the NPs are entrapped in the liver and spleen, this process slows down and depends on the digestion rate and metabolic activity of such cells as the Kupffer cells and macrophages. A significant number of Au NPs was found in the urine and feces sampled within 7 days. Therefore, the kidneys and urinary tract are important excretion organs for Au NPs. In addition, part of the Au NPs can be excreted by the hepatobiliary system (such as stomach and large intestine).
Correlation Between Distributions of Au NPs and Pc 4 in vivo
This study also demonstrates the consequences of the non-covalent drug delivery, which result in the efficient drug delivery into the tumor cells and simultaneously in a minimal uptake of the NPs into the cells. Based on the mechanisms and driving forces described above, the drug separates from the Au NP carrier while it is retained in the tumor tissue and taken up into the tumor cells by membrane mediated diffusion. The PEG coated Au NPs do not necessarily need to enter into the cancer cells to deliver drugs. After the drug is deposited into the cells, the NPs keep circulating in the body and have their own biodistribution. Eventually, both the NP and the Pc 4 have different distribution profiles upon injection of the Au NP-Pc 4 conjugates into the mice. In the tumor site, the EPR effect was the driving force to accumulate the conjugates into the tumor area where they could interact with tumor tissue and deliver drugs, as indicated in the accumulation of both entities in the tumor at 4 hours post injection (Figure 5 and Figure 6A). While the drug localizes predominately in the tumor, the NPs have been filtered by the liver and spleen. Consequently, there is not a strict correlation between Au NP and Pc 4 concentrations in the tissues such as liver and spleen. We already have successfully treated tumors in mice via PDT. However, due to the biomedical nature of these experiments, and will publish the outcome in a more biomedical oriented journal in the near future.
CONCLUSION
In this paper, the drug delivery mechanism and pharmacokinetics of non-covalent Au NP-Pc 4 conjugates was studied over a period of 7 days. No adverse effects of the conjugates were observed in the mice in this study. A PDT treatment time window from 1 hour to 6 hours after intravenous administration has been determined. A correlation of the drug and Au NPs in the tumor was examined. The non-covalent delivery approach provided surprisingly efficient release and penetration of the drug into the tumor. This is a novel approach to deliver not only PDT drugs, but also other hydrophobic drugs fast and deep into tumors. Over the 7 day study period, fast drug excretion has been observed by in vivo and ex vivo fluorescence imaging. Although Au NPs have an overall longer retention time, especially in liver and spleen, their excretion pathways have been identified. Both the drug and the Au NPs were found to be excreted from the body by renal clearance and the hepatobiliary system. This study demonstrates efficient non-covalent drug delivery by PEGylated gold NPs on the example of Pc 4, but may have ramifications for a much wider range of hydrophobic drugs.
Supplementary Material
Acknowledgments
We thank Prof. Hisashi Fujioka for helpful discussion and Dr. James Faulk for assistance in gold GFAAS analysis. This work was financially supported by the BRTT Center for Targeted Nanoparticles for Imaging and Therapeutics at Case Western Reserve University (to CB). This work was also supported by an ongoing center grant from the National Foundation for Cancer Research (to JPB) and a fellowship award (to AMB). In addition, the project described was supported by Grant Number K01EB006910 (to AMB) from the National Institute of Biomedical Imaging and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.
References
- 1.Heldin CH, Rubin K, Pietras K, Ostman A. Nat Rev Cancer. 2004;4:806. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 2.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, Ruoslahti E. Cancer Cell. 2009;16:510. doi: 10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langer R. Nature. 1998;392:5. [PubMed] [Google Scholar]
- 4.Torchilin VP. Adv Drug Deliv Rev. 2006;58:1532. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nat Nanotechnol. 2007;2:751. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 6.Cho KJ, Wang X, Nie SM, Chen Z, Shin DM. Clin Cancer Res. 2008;14:1310. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 7.Maeda H. Adv Enzyme Regul. 2001;41:189. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura Y, Maeda H. Cancer Res. 1986;46:6387. [PubMed] [Google Scholar]
- 9.Baban DF, Seymour LW. Adv Drug Deliv Rev. 1998;34:109. doi: 10.1016/s0169-409x(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 10.Iyer AK, Khaled G, Fang J, Maeda H. Drug Discov Today. 2006;11:812. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Allen TM, Cullis PR. Science. 2004;303:1818. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Y, Samia AC, Meyers JD, Panagopoulos I, Fei BW, Burda C. J Am Chem Soc. 2008;130:10643. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elbakry A, Zaky A, Liebl R, Rachel R, Goepferich A, Breunig M. Nano Lett. 2009;9:2059. doi: 10.1021/nl9003865. [DOI] [PubMed] [Google Scholar]
- 14.Jain PK, Huang X, El-Sayed IH, El-Sayed M. Acc Chem Res. 2008;41:1578. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 15.Gao J, Gu H, Xu B. Acc Chem Res. 2009;42:1097. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh P, Han G, De M, Kim CK, Rotello VM. Adv Drug Deliv Rev. 2008;60:1307. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee DK, Fong LS, Zhang Y. Adv Drug Deliv Rev. 2008;60:1627. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Drug Deliv. 2004;11:169. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 19.Paciotti GF, Kingston DGI, Tamarkin L. Drug Dev Res. 2006;67:47. [Google Scholar]
- 20.Boisselier E, Astruc D. Chem Soc Rev. 2009;38:1759. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 21.Hone DC, Walker PI, Evans-Gowing R, FitzGerald S, Beeby A, Chambrier I, Cook MJ, Russell DA. Langmuir. 2002;18:2985. [Google Scholar]
- 22.Kim CK, Ghosh P, Pagliuca C, Zhu Z, Menichetti S, Rotello VM. J Am Chem Soc. 2009;131:1360. doi: 10.1021/ja808137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong R, Han G, Fernandez JM, Kim BJ, Forbes NS, Rotello VM. J Am Chem Soc. 2006;128:1078. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- 24.Gibson JD, Khanal BP, Zubarev ER. J Am Chem Soc. 2007;129:11653. doi: 10.1021/ja075181k. [DOI] [PubMed] [Google Scholar]
- 25.Hwu JR, Lin YS, Josephrajan T, Hsu M, Cheng F, Yeh C, Su W, Shieh D. J Am Chem Soc. 2009;131:66. doi: 10.1021/ja804947u. [DOI] [PubMed] [Google Scholar]
- 26.Agasti SS, Chompoosor A, You C, Ghosh P, Kim CK, Rotello VM. J Am Chem Soc. 2009;131:5728. doi: 10.1021/ja900591t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dougherty TJ, Grinday GB, Fiel R, Weishaupt KR, Boyle DG. J Natl Cancer Inst. 1975;55:115. doi: 10.1093/jnci/55.1.115. [DOI] [PubMed] [Google Scholar]
- 28.Dougherty TJ. Crit Rev Oncol Hemat. 1984;2:83. doi: 10.1016/s1040-8428(84)80016-5. [DOI] [PubMed] [Google Scholar]
- 29.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. J Natl Cancer Inst. 1998;90:889. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castano AP, Mroz P, Hamblin MR. Nat Rev Cancer. 2006;6:535. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolmans DEJGJ, Fukumura D, Jain RK. Nature Rev. 2003;3:380. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 32.Oleinick NL, Morris RL, Belichenko T. Photochem Photobiol Sci. 2002;1:1. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- 33.Girotti AW. J Photochem Photobiol B: Biol. 2001;63:103. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 34.Hong IS, Greenberg MM. J Am Chem Soc. 2005;127:10510. doi: 10.1021/ja053493m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konan YN, Gurny R, Allemann E. J Photochem Photobiol B: Biol. 2002;66:89. doi: 10.1016/s1011-1344(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 36.van Nostrum CF. Adv Drug Deliv Rev. 2004;56:5. doi: 10.1016/j.addr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Kessel D. Adv Drug Deliv Rev. 2004;56:7. doi: 10.1016/j.addr.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR, Morgan J, Dougherty TJ, Prasad PN. J Am Chem Soc. 2003;125:7860. doi: 10.1021/ja0343095. [DOI] [PubMed] [Google Scholar]
- 39.Gref R, Lück M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Müller RH. Colloids Surf B: Biointerfaces. 2000;18:301. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 40.Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. J Controlled Release. 2006;114:343. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Nano Lett. 2009;9:1909. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Y, Samia AC, Li J, Kenney ME, Resnick A, Burda C. Langmuir. 2010;26:2248. doi: 10.1021/la902390d. [DOI] [PubMed] [Google Scholar]
- 43.Oleinick NL, Antunez AR, Clay ME, Rihter BD, Kenney ME. Photochem Photobiol. 1993;57:242. doi: 10.1111/j.1751-1097.1993.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 44.Miller JD, Baron ED, Scull H, Hsia A, Berlin JC, McCormick T, Colussi V, Kenney ME, Cooper KD, Oleinick NL. Toxicol Appl Pharmacol. 2007;224:290. doi: 10.1016/j.taap.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai L, Guo J, Bontempo FA, III, Eiseman JL. Photochem Photobiol. 2009;85:1011. doi: 10.1111/j.1751-1097.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- 46.Colussi VC, Feyes DK, Mulvihill JW, Li YS, Kenney ME, Elmets CA, Oleinick NL, Mukhtar H. Photochem Photobiol. 1999;69:236. [PubMed] [Google Scholar]
- 47.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Nat Biotechnol. 2007;25:1165. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duan H, Nie S. J Am Chem Soc. 2007;129:2412. doi: 10.1021/ja067727t. [DOI] [PubMed] [Google Scholar]
- 49.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. J Chem Soc, Chem Comm. 1994;7:801. [Google Scholar]
- 50.Morris RL, Azizuddin K, Lam M, Berlin J, Nieminen AL, Kenney ME, Samia ACS, Burda C, Oleinick NL. Cancer Res. 2003;63:5194. [PubMed] [Google Scholar]
- 51.Kongshaug M, Moan J, Brown SB. British J Cancer. 1989;59:184. doi: 10.1038/bjc.1989.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maziere JC, Morliere P, Santus R. J Photochem Photobiol B: Biol. 1991;8:351. doi: 10.1016/1011-1344(91)80111-t. [DOI] [PubMed] [Google Scholar]
- 53.Haylett AK, Moore JV. J Photochem Photobiol B: Biol. 2002;66:171. doi: 10.1016/s1011-1344(02)00241-5. [DOI] [PubMed] [Google Scholar]
- 54.Polo L, Valduga G, Jori G, Reddi E. Int J Biochem Cell Biol. 2002;34:10. doi: 10.1016/s1357-2725(01)00092-9. [DOI] [PubMed] [Google Scholar]
- 55.Torchilin VP. J Controlled Release. 2001;73:137. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Chan JM, Gu FX, Rhee J-W, Wang AZ, Radovic-Moreno AF, Alexis F, Langer R, Farokhzad Omid C. ACS Nano. 2008;2:1696. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sonavane G, Tomod K, Makin K. Colloids Surf B: Biointerfaces. 2008;66:274. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Sadauskas E, Wallin H, Stoltenberg M, Vogel U, Doering P, Larsen A, Danscher G. Particle Fibre Toxicol. 2007;4:10. doi: 10.1186/1743-8977-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipka J, Semmler-Behnke, Manuela, Sperling RA, Wenk A, Takenaka S, Schleh C, Kissel T, Parak WJ, Kreyling WG. Biomaterials. 2010;31:6574. doi: 10.1016/j.biomaterials.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Semmler-Behnke M, Kreyling WG, Lipka J, Fertsch S, Wenk A, Takenaka S, Schmid G, Brandau W. Small. 2008;4:2108. doi: 10.1002/smll.200800922. [DOI] [PubMed] [Google Scholar]
- 61.Moghimi SM, Hunter AC, Murray JC. Pharmacol Rev. 2001;53:283. [PubMed] [Google Scholar]
- 62.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H. Nat Nanotechnol. 2006;2:47. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 63.Choi HS, Ipe BI, Misra P, Lee JH, Bawendi MG, Frangioni JV. Nano Lett. 2009;9:2354. doi: 10.1021/nl900872r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.