Abstract
Cocaine self-administration in rodents has been used widely as a preclinical model of cocaine use in humans. In laboratory animals, estradiol enhances behavioral sensitization to cocaine and the acquisition of cocaine self-administration in female rats. The rewarding effect of cocaine has been shown to be enhanced following behavioral sensitization in male rats. This experiment examined whether behavioral sensitization to cocaine would promote cocaine-taking behavior in female rats, and whether estradiol could further modulate cocaine-taking behavior in cocaine-sensitized rats. Ovariectomized female rats were pretreated with either cocaine or saline for 4 days per week for 3 weeks. Self-administration sessions started 2 weeks after the last dose of drug. Female Sprague–Dawley rats received either estradiol or oil 30 min prior to the start of each session and self-administration was carried out 5 days per week for 4 weeks. The dose of cocaine self-administered each week was as follows (in mg/kg/infusion): week 1, 0.1; week 2, 0.1; week 3, 0.15; and week 4, 0.4. The rats that received cocaine pretreatment took fewer days to acquire cocaine self-administration and took more cocaine than rats that received saline pretreatment. Estradiol enhanced cocaine intake during the last six self-administration sessions after acquisition but did not affect acquisition of self-administration at the lowest doses of cocaine used. In conclusion, cocaine sensitization promotes the acquisition of cocaine self-administration in female rats. Furthermore, prior cocaine experience is more powerful than estradiol at enhancing acquisition, while estradiol enhances intake of cocaine after acquisition of self-administration.
Keywords: Behavioral sensitization, Cocaine, Self-administration, Sex differences, Estradiol, Estrogen
Introduction
Many factors influence whether an individual will become addicted to drugs of abuse: gender/sex, hormonal status, prior stress history, prior drug history, and individual personality characteristics (Becker and Hu, 2008; Belin et al., 2008; Dalley et al., 2007; Davis et al., 2008; Miczek et al., 2008; Piazza et al., 1989; Thomas et al., in press). Cocaine self-administration in rodents has been used widely as a preclinical model of cocaine use in humans. In laboratory animals, prior experience with psychomotor stimulants has been shown to enhance acquisition of self-administration. In male rats, behavioral sensitization induced either by stress or repeated amphetamine (AMPH) treatment predisposes rats to self-administer a low dose of AMPH (Miczek et al., 2008; Piazza et al., 1990; Sofuoglu et al., 2002). Animals that have been previously sensitized to AMPH also acquire cocaine self-administration faster (Horger et al., 1992; Valadez and Schenk, 1994) and accelerate escalation of cocaine intake when they are given extended access to cocaine self-administration (Ferrario and Robinson, 2007). Similarly, rats that are pre-exposed to cocaine acquire cocaine self-administration faster and develop a preference for a dose of cocaine that non-exposed rats do not prefer more than saline (Childs et al., 2006; Horger et al., 1990).
The relationship between sensitization and acquisition of cocaine self-administration has been studied in male rats, as discussed above, but not in females. Clinical and preclinical studies indicate that there are sex/gender differences in response to cocaine (Becker and Hu, 2008). In women, cocaine cues induce more cocaine craving in female than male addicts (Robbins et al., 1999). Women also report less pleasure and dysphoria and greater anxiety in response to cocaine than do men (Kosten et al., 1993, 1996; Lukas et al., 1996; Singha et al., 2000). Female substance users have greater cocaine use and are more likely to be dependent on cocaine than males (Lejuez et al., 2007). Furthermore, women cocaine addicts report greater subjective effect of smoked or intravenous cocaine administration in the follicular phase than in the luteal phase, and progesterone treatment modulates cocaine intake, which suggest a role of ovarian hormones on cocaine-induced responses (Evans et al., 2002; Sofuoglu et al., 1999, 2002).
Consistent with the results of clinical studies, there are also sex differences in response to cocaine in rats. Female rats acquire cocaine self-administration more rapidly and self-administer more cocaine than do male rats (Hu et al., 2004), and they are more sensitive to the priming effect of cocaine during reinstatement (Becker and Hu, 2008; Lynch and Carroll, 2000). Moreover, estradiol enhances the acquisition of cocaine self-administration, cocaine intake, and the motivation to take cocaine in female rats, but not males (Becker and Hu, 2008; Hu and Becker, 2008; Hu et al., 2004; Jackson et al., 2006; Lynch, 2006). In this report, we explore the relations between prior drug history and the gonadal hormone estradiol on cocaine self-administration in female rats.
Materials and methods
Animals
Female Sprague–Dawley rats (Harlan, Indianapolis, IN), weighing 225–250 g at the start of the experiment, were housed three to four per cage when they arrived. The animals were housed in a room maintained at 20–21 °C, with a 14:10 (light:dark) cycle, lights on at 5:30 am, and free access to phytoestrogen-free rodent chow (2014 Teklad Global, 14% protein rodent maintenance diet, Harlan rat chow; Harlan Teklad, Madison, WI) and water available ad libitum. All procedures were performed according to a protocol approved by the University of Michigan Committee for Use and Care of Animals.
All rats were bilaterally ovariectomized (OVX) approximately 1 week after they arrived as described previously (Hu and Becker, 2003). Animals were assigned to one of the four treatment groups: (1) control rats that received saline treatment (0.1 ml/kg, i.p.) during sensitization and 0.1 ml peanut oil (oil, s.c.) treatment during self-administration (SalOil, n=12); (2) control rats that received saline treatment during sensitization and 5 μg estradiol benzoate (EB) dissolved in 0.1 ml peanut oil (s.c.) treatment during self-administration (SalEB, n= 12); (3) rats that received cocaine treatment (described below) during sensitization and oil treatment during self-administration (CocOil, n= 13); and (4) rats that received cocaine treatment during sensitization and EB treatment during self-administration (CocEB, n= 12).
Cocaine sensitization
The testing chamber consisted of a round chamber (bottom diameter: 41 cm; top diameter: 50 cm; height: 42 cm) with a cone (bottom diameter: 16 cm; height: 42 cm) in the middle, which allowed the rat to move freely around the cone. Two weeks after OVX, animals received an injection of oil subcutaneously and were placed in the testing chamber for a 30-min habituation period. Then each rat received an injection of either saline or cocaine according to their treatment group and was put back into the testing chamber for 1 h. Animals were treated 4 days per week, followed by 3 days off, for three consecutive weeks, for a total of 12 testing days (Hu and Becker, 2003). No cocaine or saline were administered on the 3 days off. Intermittent treatment was used here because it was better at producing behavioral sensitization than continuous treatment (Robinson and Becker, 1986).
Activity was recorded on day 1, day 5 (first day of the second week), day 9 (first day of the third week), and day 12 (last day). On the above recording days, digital cameras were mounted on the top of each chamber to automatically track rats’ activity. Behavior was analyzed by the Any-Maze system (Stoelting, Inc., Wood Dale, IL). On days when behavior was recorded, animals were allowed to habituate to the apparatus for 30 min; they were then given 5 mg/kg cocaine (i.p.) or saline injections (i.p.) and put back in the testing chamber for 1 h. Following that, they received 15 mg/kg cocaine or saline injections and were placed in the testing chamber for an additional hour. The two doses of cocaine were used during recording days so that sensitization of locomotor behavior could be determined at the lower dose. At 15 mg/kg animals will exhibit stereotypy after sensitization, and locomotor behavior is no longer directly related to the magnitude of the behavioral response (Robinson and Becker, 1986). On days when behavior was not recorded, animals received 15 mg/kg cocaine (i.p.) or saline after the habituation period as described above and then were placed in the testing chamber for 1 h.
Catheter implantation
Ten days after sensitization treatment, rats received indwelling intravenous jugular catheters connected to a backport. Rats were anesthetized with a combination of ketamine (45 mg/kg, i.p.) and medetomidine (0.3 mg/kg, i.p.). Catheters were constructed by gluing Silastic tubing (0.51 mm ID, 0.94 mm OD; Dow Corning, Midland, MI) to an external guide cannula. The cannulae were then glued to polypropylene mesh with cranioplastic cement. The free end of the Silastic tubing was inserted into the right jugular vein and secured with 4.0 silk sutures around the venous tissue. The catheters exited dorsally on the animal’s back. Dummy stylets were inserted into the catheters when rats were not connected to infusion pumps. On the 3 days immediately after the surgery, catheters were flushed with a 0. 1-ml heparinized saline (30 U/ml in 0.9% sterile saline buffered at pH 7.4) and a 0. 1-ml gentamicin solution (0.8 mg/ml) to prevent occlusions and to circumvent microbial buildup in the catheter. Thereafter, catheters were flushed daily with a 0. 1-ml gentamicin solution (0.8 mg/ml) to circumvent microbial buildup in the catheter. For each self-administration session, catheters were flushed with 0.1 ml of saline before sessions began and with 0.1 ml of gentamicin after the session ended. Twenty-four hours before the first weekly test session, and immediately after the last weekly test session, 0.1 ml of Pentothal (thiopental sodium, 20 mg/ml in sterile water) was infused to check for catheter patency. Rats that did not show a loss of muscle tone within 5 s were removed from analysis.
Cocaine self-administration
The self-administration apparatus was a standard operant chamber (25 × 27 × 30 cm) that was situated in a sound-attenuating cabinet (Med Associates, Inc., Georgia, VT). There were a red house light, a tone generating apparatus, and two nose-poke holes in each chamber. Rats were connected to an infusion syringe and tethered via a steel cable to a swivel. The swivel was mounted on a counterbalanced arm so that the animal could move freely in the test cage. The infusion pumps were mounted on the outside doors of the cabinets. At the start of the experiment, the red house lights were illuminated and remained on for the whole session so that the rat’s behavior could be monitored through an observation camera on the cabinet door. Nose pokes in the active hole resulted in an intravenous injection of 50 μl of cocaine-HCl in saline delivered over 2.8 s, doses of cocaine were adjusted for body weight as described below. This process was accompanied by a compound stimulus consisting of a white stimulus light and a tone (85 dB). There was a 5-s time-out period after each infusion, during which time further nose pokes had no programmed consequences. But nose pokes were still recorded. Nose pokes in the inactive hole were also recorded but had no programmed consequences.
Seventeen days after the last treatment with cocaine or saline (approximately 5 days after recovery from catheter surgery), rats began cocaine self-administration. Rats received either EB or Oil (s.c), according to their treatment group, 30 min before the self-administration session started. Rats were allowed to nose poke to obtain an i.v. infusion of cocaine on an FR1 schedule of reinforcement. Rats did not receive any training for nose poking; they were not primed with cocaine nor were animals food-deprived at any time. The self-administration tests occurred 5 days per week with 2 days off for 4 weeks. No cocaine, saline treatment, or hormone was administered during the 2 days off. The doses of cocaine that were self-administered during each week were 0.1, 0.1, 0.15, and 0.4 mg/kg/infusion. We have seen an effect of estradiol on acquisition of cocaine self-administration at 0.3 mg/kg/infusion and we reasoned that lower doses would be needed to pull out differences due to prior sensitization, so 0.1 and 0.15 mg/kg/infusion were used for initial acquisition. The dose of 0.4 mg/kg/infusion was used to demonstrate that all animals were capable of acquiring cocaine self-administration. The test sessions lasted 120 min each day during the first 3 weeks and 60 min during the last week. The session time was reduced in the last week to prevent rats from an overdose.
Acquisition was defined as more than 20 nose pokes in the active hole during 2-h sessions, or more than 10 nose pokes for 1-h sessions, on three consecutive days. Nose poking in the active hole also had to be more than twice that in the inactive hole. This criterion was chosen based on the average number of nose pokes in the inactive hole obtained in 2-h test sessions during the first week, which was not greater than 10. This criterion has been used in other self-administration studies (Hu et al. 2004; Jackson et al. 2006). Rats that acquired cocaine self-administration according to this criterion showed a stable increase in cocaine intake over the remaining test days.
Statistics
Data were subjected to analysis of variance (ANOVA), Student’s t-test, or log-rank test. Differences were considered significant if p≤0.05.
Results
Induction of cocaine sensitization
The difference in distance traveled over 60 min on day 1 vs. day 12 was calculated to assess sensitization. As expected, cocaine treatment produced behavioral sensitization (t(24)=2.046, p=0.05), and saline treatment resulted in behavioral habituation (t(23)= −4.964, p<0.0001). The cocaine-treated group was significantly different from the saline group (p< 0.04; Fig. 1).
Fig. 1.
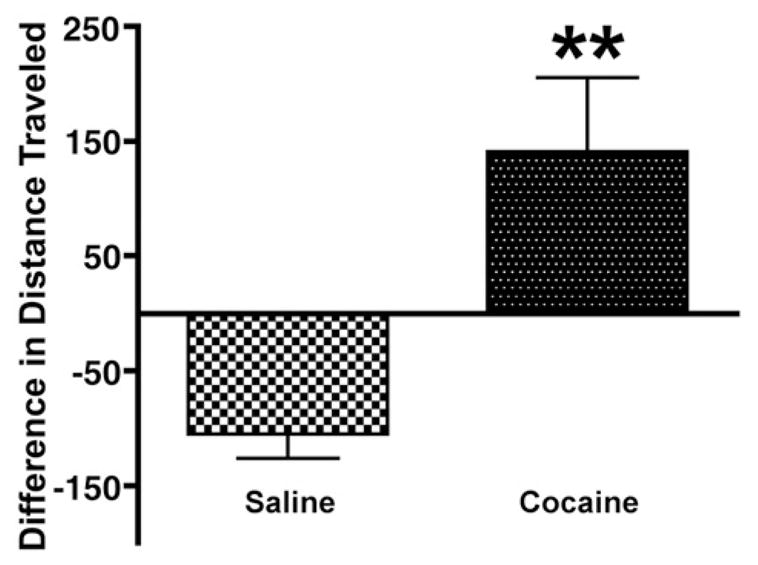
Difference in distance traveled (centimeters, mean+SEM) after cocaine (5 mg/kg) or saline injections (day 12-day 1). The cocaine-treated group exhibited significant behavioral sensitization and was significantly different from the saline-treated group (**p< 0.04).
Acquisition of cocaine self-administration
Prior sensitization enhanced acquisition of cocaine self-administration. By the end of the first 4 days of self-administration, half of the rats from the CocEB group had acquired self-administration, whereas only 30% of rats from other groups had done so (Fig. 2). After 10 days of self-administration, 80% of rats from the CocEB group and more than 60% from the CocOil group had acquired self-administration whereas only 40% of saline-pretreated animals with or without EB had acquired self-administration. The dose of cocaine was increased to 0.15 mg/kg/infusion for days 11–15 and all the rats in the SalEB group acquired self-administration at this dose; most of the rats in the SalOil group also acquired self-administration at this dose. One SalOil animal acquired self-administration only when the dose was increased to 0.4 mg/kg/infusion during days 15 to 20. A log-rank test of survival curves for the day of acquisition showed that cocaine-pretreated rats acquired self-administration faster than saline-pretreated rats χ2(1)=4.018, p= 0.045. Individual comparisons between each pair of acquisition curves indicated that the CocEB group acquired more rapidly than did the SalOil group, χ2 (1)=4.586, p= 0.032. The results of a two-way ANOVA indicated a trend for an overall effect of prior cocaine treatment to decrease the day of acquisition, F(1,45)=3.68, p= 0.06. Subsequent pairwise comparisons did not indicate differences between individual groups.
Fig. 2.
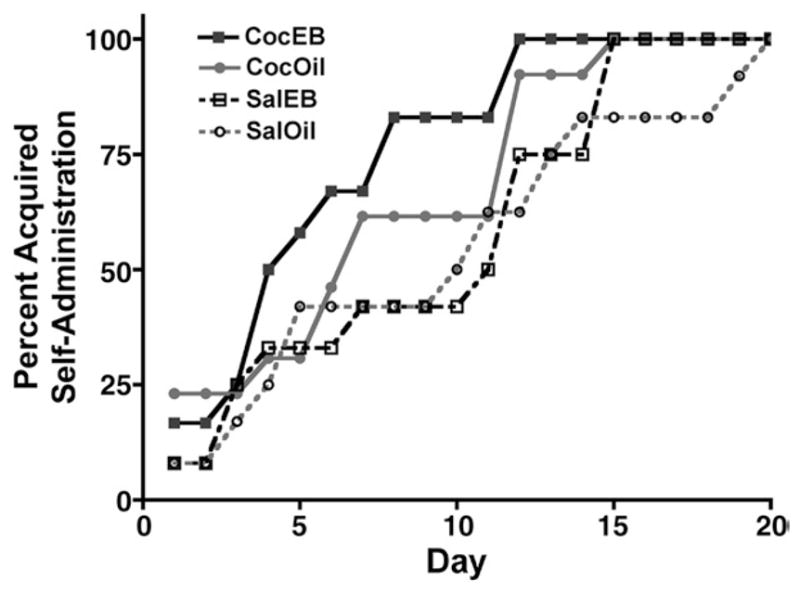
(A) The self-administration acquisition curves for each group. Cocaine-pretreated rats acquired cocaine self-administration more rapidly than saline-pretreated animals. In individual group comparisons, the CocEB group acquired self-administration faster than SalOil group (p<0.05).
Cocaine intake
As illustrated in Fig. 3, prior cocaine sensitization enhanced the total intake (mg/kg) of cocaine (Fig. 3A; main effect of treatment, F(1,45)=9.844, p= 0.003) and the total number of cocaine infusions received (Fig. 3B; main effect of treatment, F(1,45) =8.595, p= 0.005). Post hoc comparisons of individual groups during each week showed that the CocEB group took more cocaine than SalOil and SalEB groups in terms of the total intake of cocaine (p< 0.05) during weeks 2 and 3. The CocOil group took more cocaine than the SalOil group during week 3 (p< 0.05). Additionally, the CocEB group received more infusions than the SalOil group during weeks 2, 3, and 4 (p< 0.01). Thus, even though the CocEB group did not receive significantly more cocaine (mg/kg) than other groups during week 4, this group was making more reinforced responses compared with the OilSal group.
Fig. 3.
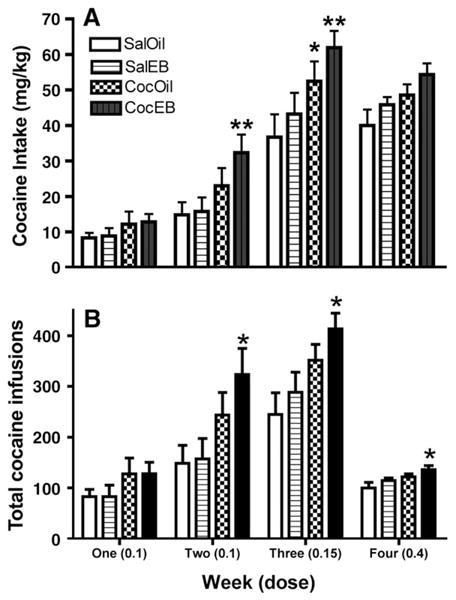
Cocaine sensitization enhanced cocaine self-administration. (A) Total cocaine intake (mg/kg, mean+SEM) per week for each group of rats. (B) Total number of cocaine infusions (mean+SEM) per week for each group of rats. *Significantly different from the SalOil group. **Significantly different from the SalOil and SalEB groups.
During the last six self-administration sessions, both prior cocaine sensitization and EB treatment enhanced cocaine intake (Fig. 4; two-way ANOVA, main effect of drug pretreatment (F(1,45)=8.698, p= 0.005) and a main effect of EB treatment (F(1,45)=3.836, p= 0.056)). One animal in the SalOil group reach criteria for acquisition during these sessions; all other animals had already reached criteria.
Fig. 4.
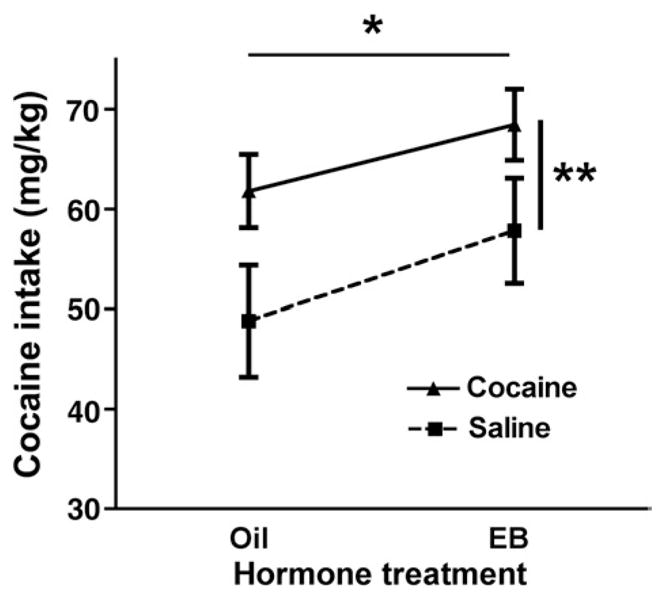
Total cocaine intake over the last six sessions for each group (Mean± SEM). *Significant effect of hormone treatment. **Significant effect of drug pretreatment.
To determine whether there group differences in general activity, nose pokes in the inactive hole were analyzed. There was no significant difference in inactive nose pokes among the four groups of rats during the first 3 weeks. Animals that received EB treatment during week 4, however, exhibited significantly more nose poking in the inactive hole than did oil-treated animals (two-way ANOVA, main effect of hormone treatment, F(1,45)=8.786, p= 0.005), when the highest dose of cocaine was self-administered.
Discussion
This is the first study to demonstrate that prior sensitization to cocaine significantly accelerates acquisition of cocaine self-administration and cocaine intake in female rats, and estradiol further enhances cocaine intake. These findings are consistent with previous reports that prior sensitization enhances self-administration of cocaine or AMPH in male rats (Ferrario and Robinson, 2007; Horger et al., 1992; Pierre and Vezina, 1997; Vezina et al., 2002). An effect of estradiol at promoting the acquisition of cocaine self-administration and cocaine intake has been previously found with doses of cocaine between 0.3 and 0.5 mg/kg (Hu and Becker, 2008; Hu et al., 2004; Jackson et al., 2006). We did not see an effect of estradiol on the acquisition of cocaine self-administration in this study using the low dose of 0.1 mg/kg/infusion. These results suggest that the effect of estradiol to enhance drug self-administration depends on the dose of cocaine being self-administered and that prior cocaine sensitization has a more powerful effect than estradiol at enhancing the acquisition of cocaine self-administration, especially at the low doses of cocaine used here.
It is known that sensitization powerfully enhances the acquisition of drug self-administration at sub-threshold doses but has no effect on acquisition of self-administration or drug intake at high doses because all animals rapidly acquire self-administration (Lorrain et al., 2000; Mendrek et al., 1998). Even though drug-pretreated and drug-naïve animals are not distinguishable when they self-administer high doses of drug with little work demand, they do differ in the motivation to take high dose of drugs when the work demands are increased in a progressive ratio schedule. Animals that have been sensitized work harder to obtain drugs than do drug-naïve rats (Lorrain et al., 2000; Mendrek et al., 1998). Estradiol is also known to enhance females’ motivation to take cocaine as exhibited by higher breaking point on a progressive ratio schedule (Becker and Hu, 2008). Further research is needed to determine whether estradiol and cocaine sensitization interact or have additive effects on a female rats’ motivation to work for high dose of cocaine on a progressive ratio schedule.
Consistent with previous findings, estradiol enhanced cocaine intake during the last six test sessions with cocaine doses of 0.15 and 0.4 mg/kg. One thing that should be kept in mind is that prior to this time, many animals were still acquiring cocaine self-administration. It is possible that a greater effect of estradiol may have emerged if self-administration had been extended for a longer time. There was also a difference between oil and estradiol-treated animals in nose pokes in the inactive hole during the last week of self-administration with 0.4 mg/kg cocaine. This difference most likely reflects greater habituation by the oil-treated rats since the estradiol-treated rats did not exhibit an increase in inactive nose pokes compared to the previous weeks. Nevertheless, it is possible that the estradiol-treated animals are developing greater sensitization of motor activity than the oil-treated animals.
In conclusion, this study demonstrates that prior cocaine sensitization of female rats enhances the acquisition of cocaine self-administration and cocaine intake, as has been seen in male rats. Furthermore, prior cocaine experience is more powerful than estradiol at enhancing the acquisition of cocaine self-administration at the low dose of cocaine tested in this study. This study adds important evidence to the current literature supporting the relationship between cocaine sensitization and cocaine self-administration in females rats as is seen in males. In females, estradiol has an additional additive effect on cocaine intake, above the effect of sensitization to cocaine. Understanding the mechanism(s) underlying these behavioral changes will be important for our understanding of cocaine abuse in women and for developing appropriate treatments.
Acknowledgments
This research was supported by a grant from the NIH DA012677.
References
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320 (5881):1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Shoaib M, Stolerman IP. Cocaine self-administration in rats with histories of cocaine exposure and discrimination. Psychopharmacology. 2006;186:168–176. doi: 10.1007/s00213-006-0364-9. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement [see comment] Science. 2007;315 (5816):1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BA, Clinton SR, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively-bred high-responder and low-responder rats. Pharmacol Biochem Behav. 2008;90:331–338. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159 (4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Robinson TE. Amphetamine pretreatment accelerates the subsequent escalation of cocaine self-administration behavior. Eur Neuropsychopharmacol. 2007;17 (5):352–357. doi: 10.1016/j.euroneuro.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Horger BA, Shelton K, Schenk S. Preexposure sensitizes rats to the rewarding effects of cocaine. Pharm Biochem Behav. 1990;37:707–711. doi: 10.1016/0091-3057(90)90552-s. [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology. 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23 (2):693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB. Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug Alcohol Depend. 2008;94:56–62. doi: 10.1016/j.drugalcdep.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29 (1):81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10 (1):63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH. Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry. 1996;39:147–148. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Bornovalova MA, Curtin JJ, Reynolds EK, Daughters SB. Risk factors in the relationship between gender and crack/cocaine. Exp Clin Psychopharmacol. 2007;15:165–175. doi: 10.1037/1064-1297.15.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Arnold GM, Vezina P. Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav Brain Res. 2000;107 (1–2):9–19. doi: 10.1016/s0166-4328(99)00109-6. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology. 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14 (1):34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology. 2000;148 (2):196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Blaha C, Phillips A. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology. 1998;135:416–422. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245 (4925):1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;514 (1):22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology. 1997;129 (3):277–284. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53 (3):223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396 (2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Singha AK, McCance-Katz EF, Petrakis I, Kosten TR, Oliveto A. Sex differences in self-reported and physiological response to oral cocaine and placebo in humans. Am J Drug Alcohol Abuse. 2000;26 (4):643–657. doi: 10.1081/ada-100101900. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharm Biochem Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Thomas MB, Hu M, Lee TM, Bhatnagar S, Becker JB. Sex-specific susceptibility to cocaine in rats pre-exposed to prenatal stress. Physiology and Behavior. 2009;97 (2):270–277. doi: 10.1016/j.physbeh.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Valadez A, Schenk S. Persistence of the ability of amphetamine preexposure to facilitate acquisition of cocaine self-administration. Pharmacol Biochem Behav. 1994;47:203–205. doi: 10.1016/0091-3057(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22 (11):4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


