Abstract
Aim
To determine if the association between hyperuricaemia and poor outcomes in heart failure (HF) varies by chronic kidney disease (CKD).
Methods and results
Of the 2645 systolic HF patients in the Beta-Blocker Evaluation of Survival Trial with data on baseline serum uric acid, 1422 had hyperuricaemia (uric acid ≥6 mg/dL for women and ≥8 mg/dL for men). Propensity scores for hyperuricaemia, estimated for each patient, were used to assemble a matched cohort of 630 pairs of patients with and without hyperuricaemia who were balanced on 75 baseline characteristics. Associations of hyperuricaemia with outcomes during 25 months of median follow-up were examined in all patients and in those with and without CKD (estimated glomerular filtration rate of <60 mL/min/1.73 m2). Hyperuricaemia-associated hazard ratios (HRs) and 95% confidence intervals (CI) for all-cause mortality and HF hospitalization were 1.44 (1.12–1.85, P = 0.005) and 1.27 (1.02–1.58, P = 0.031), respectively. Hazard ratios (95% CIs) for all-cause mortality among those with and without CKD were 0.96 (0.70–1.31, P = 0.792) and 1.40 (1.08–1.82, P = 0.011), respectively (P for interaction, 0.071), and those for HF hospitalization among those with and without CKD were 0.99 (0.74–1.33, P = 0.942) and 1.49 (1.19–1.86, P = 0.001), respectively (P for interaction, 0.033).
Conclusion
Hyperuricaemia has a significant association with poor outcomes in HF patients without CKD but not in those with CKD, suggesting that hyperuricaemia may predict poor outcomes when it is primarily a marker of increased xanthine oxidase activity, but not when it is primarily due to impaired renal excretion of uric acid.
Keywords: Heart failure, Hyperuricaemia, Chronic kidney disease, Outcomes
Introduction
Studies of hyperuricaemia and outcomes in heart failure (HF) are limited by small sample size, short follow-up, lack of definitive endpoints, failure to adjust for prognostically important covariates, and use of traditional regression-based risk adjustment.1–5 Further, little is known about whether the association between hyperuricaemia and poor outcomes in HF is a direct effect of uric acid or is mediated by xanthine oxidase. Because uric acid is primarily eliminated via the kidneys, hyperuricaemia in HF patients without chronic kidney disease (CKD) may be considered primarily due to increased production of uric acid and thus a marker of increased xanthine oxidase activity.6 On the other hand, hyperuricaemia in patients with CKD may in large part be considered due to impaired renal excretion of uric acid and thus not associated with increased xanthine oxidase activity. Therefore, we hypothesized that if high serum uric acid is associated with poor outcomes in those without CKD but not in those with CKD, it would suggest lack of an intrinsic effect of high serum uric acid levels, but rather an effect of increased xanthine oxidase activity.
In the Beta-Blocker Evaluation of Survival Trial (BEST), extensive data on baseline characteristics including serum uric acid and various outcomes were collected on 2708 HF patients.7 The aims of the current investigation were to (i) investigate the effect of hyperuricaemia in a propensity-matched cohort of chronic advanced systolic HF patients who would be well-balanced on all measured baseline characteristics, and (ii) to gain further insight into the mechanism of action of uric acid in HF by examining the effect of uric acid on subgroups of patients with and without CKD.
Methods
Setting and patients
We used a public-use copy of the BEST data obtained from the National Heart, Lung and Blood Institute (NHLBI), which had data on 2707 patients (one patient did not consent to be included in the public-use copy of the data). The design and results of the BEST trial have been previously described.7 Briefly, 2708 chronic advanced (mean duration of HF, 49 months) systolic (mean left and right ventricular ejection fraction, 23 and 35%, respectively) HF patients from the USA and Canada were randomized to receive bucindolol or placebo between 1995 and 1998. All patients had New York Heart Association class III–IV symptoms and over 90% were receiving an angiotensin-converting enzyme (ACE) inhibitor, loop diuretic, and digitalis.
Baseline hyperuricaemia
Data on baseline serum uric acid were available from 2645 (98%) patients and 1422 (54%) had hyperuricaemia defined as serum uric acid of >6 mg/dL for women and >8 mg/dL for men. There is no standard definition for hyperuricaemia in advanced systolic HF and values >7 mg/dL have been used to define hyperuricaemia in men with mild to moderate HF.8–10 Therefore, we used a cut-off of >8 mg/dL to define high serum uric acid for men, and used a 25% lower cut-off of >6 mg/dL for women.11
Outcomes
Primary outcomes were all-cause mortality and HF hospitalization during 25 months of median follow-up (range, 0.03–50 months). Secondary outcomes were cardiovascular mortality, HF mortality, sudden cardiac death, and all-cause hospitalization, and combined endpoint of HF hospitalization or all-cause mortality. All outcomes in BEST were centrally adjudicated.
Assembly of study cohort
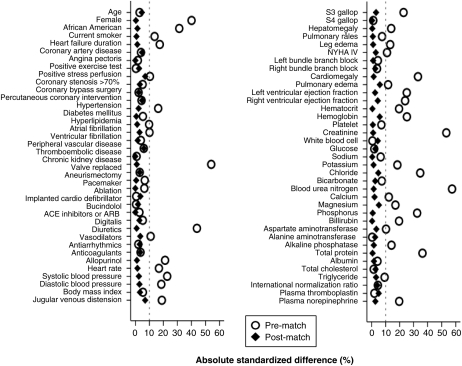
To reduce significant imbalances in baseline characteristics between patients with and without hyperuricaemia (Table 1), we used propensity score matching to assemble a balanced cohort. The propensity score for hyperuricaemia for a patient would be that patient's probability of having hyperuricaemia based on his/her measured baseline characteristics. We estimated propensity scores for hyperuricaemia for each of the 2645 patients, using a non-parsimonious multivariable logistic regression model.12,13 In the model, hyperuricaemia was the dependent variable and 75 baseline characteristics displayed in Figure 1 were used as covariates. We then used a greedy matching protocol to match 630 pairs of patients with and without hyperuricaemia who had similar propensity scores.14,15 Because propensity score models are sample-specific adjusters and are not intended to be used for out-of-sample prediction or estimation of coefficients, measures of predictive fitness and discrimination are not important for assessment of the model's effectiveness. Instead, the model's effectiveness is best assessed by its ability to balance covariates between the groups. As such we estimated absolute standardized differences, expressed as a percentage of the pooled standard deviations, to assess post-match balance and presented them as a Love plot (Figure 1). An absolute standardized difference of 0% indicates no residual bias and values <10% are generally considered inconsequential.
Table 1.
Baseline patient characteristics by high uric acid before and after propensity matching
| n (%) or median (interquartile range) | Before propensity matching |
After propensity matching |
||||
|---|---|---|---|---|---|---|
| Normal uric acida (n = 1223) | High uric acidb (n = 1422) | P-value | Normal uric acida (n = 630) | High uric acidb (n= 630) | P-value | |
| Age, years | 61 (18) | 61 (18) | 0.775 | 61 (19) | 62 (17) | 0.411 |
| Female | 162 (13) | 417 (29) | <0.001 | 121 (19) | 122 (19) | 1.000 |
| African American | 199 (16) | 416 (29) | <0.001 | 141 (22) | 137 (22) | 0.834 |
| Current smoker | 248 (20) | 214 (15) | <0.001 | 106 (17) | 108 (17) | 0.937 |
| Body mass index, kg/m2 | 35 (10) | 35 (10) | 0.525 | 35 (10) | 36 (10) | 0.567 |
| Past medical history | ||||||
| Months of heart failure | 33 (53) | 39 (61) | <0.001 | 36 (60) | 33 (61) | 0.799 |
| Coronary artery disease | 733 (60) | 824 (58) | 0.300 | 365 (58) | 380 (60) | 0.383 |
| Coronary bypass surgery | 347 (28) | 415 (29) | 0.646 | 183 (29) | 183 (29) | 1.000 |
| Percutaneous coronary interventions | 192 (16) | 221 (16) | 0.911 | 101 (16) | 95 (15) | 0.701 |
| Angina pectoris | 664 (54) | 698 (49) | 0.008 | 309 (49) | 331 (53) | 0.230 |
| Hypertension | 670 (55) | 893 (63) | <0.001 | 362 (58) | 369 (59) | 0.739 |
| Diabetes mellitus | 419 (34) | 524 (37) | 0.166 | 221 (35) | 223 (35) | 0.953 |
| Hyperlipidaemia | 560 (46) | 582 (41) | 0.012 | 272 (43) | 276 (44) | 0.863 |
| Atrial fibrillation | 267 (22) | 372 (26) | 0.010 | 142 (23) | 150 (24) | 0.646 |
| Peripheral arterial disease | 183 (15) | 244 (17) | 0.126 | 93 (15) | 107 (17) | 0.319 |
| Chronic kidney disease | 289 (24) | 693 (49) | <0.001 | 197 (31) | 200 (32) | 0.898 |
| Medications | ||||||
| Bucindolol | 614 (50) | 710 (50) | 0.888 | 308 (49) | 314 (50) | 0.781 |
| ACE inhibitors | 1176 (96) | 1375 (97) | 0.456 | 607 (96) | 607 (96) | 1.000 |
| Digitalis | 1117 (91) | 1319 (93) | 0.176 | 575 (91) | 580(92) | 0.691 |
| Diuretics | 1068 (87) | 1399 (98) | <0.001 | 612 (97) | 611 (97) | 1.000 |
| Vasodilators | 499 (41) | 657 (46) | 0.005 | 276 (44) | 265 (42) | 0.576 |
| Anti-arrhythmic drugs | 36 (3) | 37 (3) | 0.593 | 17 (3) | 14 (2) | 0.711 |
| Anti-coagulants | 695 (57) | 834 (59) | 0.344 | 347 (55) | 358 (57) | 0.578 |
| Allopurinol | 148 (12) | 86 (6) | <0.001 | 60 (10) | 62 (10) | 0.922 |
| Heart rate per minute | 80 (16) | 80 (20) | <0.001 | 80 (16) | 80 (17) | 0.795 |
| Systolic blood pressure, mmHg | 118 (24) | 112 (24) | <0.001 | 116 (24) | 115 (26) | 0.655 |
| Diastolic blood pressure, mmHg | 70 (16) | 70 (17) | <0.001 | 70 (16) | 70 (16) | 0.615 |
| New York Heart Association class III | 1142 (93) | 1285 (90) | 0.005 | 584 (93) | 579 (92) | 0.791 |
| Clinical findings | ||||||
| Elevated jugular venous pressure | 498 (41) | 712 (50) | <0.001 | 272 (43) | 294 (47) | 0.240 |
| Pulmonary rales | 147 (12) | 207 (15) | 0.056 | 84 (13) | 86 (14) | 0.934 |
| S3 gallop | 457 (37) | 691 (49) | <0.001 | 263 (42) | 273 (43) | 0.598 |
| Lower extremity oedema | 292 (24) | 423 (30) | 0.001 | 160 (25) | 163 (26) | 0.897 |
| Pulmonary oedema by chest X-ray | 113 (9) | 184 (13) | 0.003 | 69 (11) | 81 (13) | 0.342 |
| Cardiomegaly by chest X-ray | 831 (68) | 1167 (44) | <0.001 | 468 (74) | 472 (75) | 0.838 |
| Laboratory values | ||||||
| Uric acid, mg/dL | 6.3 (2.0) | 9.3 (2.4) | <0.001 | 6.5 (1.9) | 9.3 (2.4) | <0.001 |
| Creatinine, mg/dL | 1.1 (0.4) | 1.3 (0.6) | <0.001 | 1.1 (0.5) | 1.2 (0.4) | 0.791 |
| Potassium, mEq/L | 4.3 (0.5) | 4.3 (0.7) | <0.001 | 4.3 (0.6) | 4.3 (0.6) | 0.644 |
| Magnesium, mg/dL | 1.7 (0.2) | 1.8 (0.3) | 0.001 | 1.8 (0.3) | 1.8 (0.2) | 0.285 |
| BUN, mg/dL | 18 (8) | 24 (17) | <0.001 | 19 (11) | 20 (11) | 0.600 |
| Plasma norepinephrine | 459 (249) | 492 (240) | <0.001 | 459 (276) | 481 (238) | 0.423 |
| Haemoglobin, g/dL | 14 (3) | 14 (2) | <0.001 | 14 (3) | 14 (2) | 0.832 |
| MUGA scan | ||||||
| LVEF, % | 24 (11) | 22 (11) | <0.001 | 23 (11) | 24 (11) | 0.654 |
| RVEF, % | 35 (13) | 34 (13) | <0.001 | 34 (12) | 34 (13) | 0.423 |
aNormal uric acid is defined as serum uric acid of ≤6 mg/dL for women and ≤8 mg/dL for men.
bHigh uric acid is defined as serum uric acid of >6 mg/dL for women and >8 mg/dL for men.
ACE, angiotensin converting enzyme; BUN, blood urea nitrogen; LVEF, left ventricular ejection fraction; MUGA, multi gated acquisition scan; RVEF, right ventricular ejection fraction.
Figure 1.
Absolute standardized differences before and after propensity score matching comparing covariate values for patients with normal and high levels of uric acid. ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; NYHA, New York Heart Association.
Statistical analysis
Baseline characteristics of patients with and without high serum uric acid were compared using Pearson's χ2 test and Mann–Whitney test (pre-match) and McNemar and paired-sample t-test (post-match) as appropriate. We used Kaplan–Meier and matched Cox regression analyses to determine association of hyperuricaemia with all-cause mortality and HF hospitalization. Formal sensitivity analysis was performed to quantify the degree of a hidden bias that would need to be present to invalidate those associations.16 To determine homogeneity of associations of hyperuricaemia with outcomes we repeated our analysis in subgroup of patients including those with and without CKD, defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2.17,18 Overall, 982 patients had CKD, who had a mean eGFR of 46 mL/min/1.73 m2. The mean eGFR for the 1662 patients without CKD was 83 mL/min/1.73 m2. All data analyses were performed using SPSS-15 for Windows.19 All P-values were two-sided and P < 0.05 was regarded as statistically significant.
Results
Patient characteristics
Patients (n = 1260) had a mean (±SD) age of 60 (±12) years, 19% were women, and 22% were African Americans. Before matching, patients with hyperuricaemia were more likely to be women and African-American. However, overall those with hyperuricaemia had longer mean duration of HF with a higher burden of HF symptoms and lower mean left and right ventricular ejection fraction (Table 1). They also had higher prevalence of comorbidities such as hypertension and CKD. These and other pre-match imbalances in baseline characteristics were balanced after matching (Table 1 and Figure 1). The mean (±SD) serum uric acid levels for matched patients with and without hyperuricaemia were 9.2 (±1.6) and 6.3 (±1.2) mg/dL, respectively (P < 0.001). The median (interquartile range) of serum uric acid for matched patients with and without hyperuricaemia were 8.9 (8.3–10.1) and 6.5 (5.5–7.4), respectively. All post-match absolute standardized differences were <10% and were <5% for 67 of the 75 baseline characteristics, suggesting substantial bias reduction (Figure 1).
Hyperuricaemia and mortality
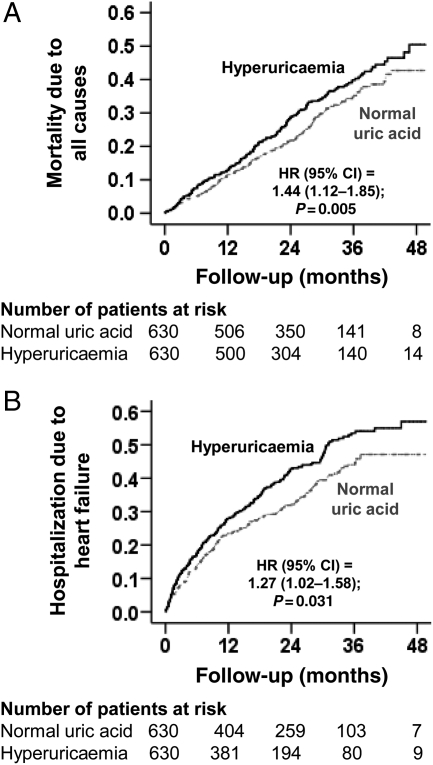
All-cause mortality occurred in 33 and 28% of patients with and without hyperuricaemia [hazard ratio (HR) when hyperuricaemia was compared with normouricemia, 1.44; 95% confidence interval (CI), 1.12–1.85; P = 0.005; Table 2 and Figure 2A]. In the absence of a hidden bias, a sign-score test for matched data with censoring provides evidence (P = 0.005) that patients without hyperuricaemia clearly outlived those with hyperuricaemia. An unmeasured covariate that is a near-perfect predictor of mortality could potentially explain away this association if it would increase the odds of hyperuricaemia by 1.1%. Pre-match association between hyperuricaemia and all-cause mortality is displayed in Table 2. The associations of hyperuricaemia with cause-specific mortalities are displayed in Table 3.
Table 2.
Association of hyperuricaemia with all-cause mortality and heart failure hospitalization, before and after propensity-score matching
| Events (%) |
Absolute risk difference (%)a | Hazard ratio (95% confidence interval) | P-value | ||
|---|---|---|---|---|---|
| Normal uric acidb | High uric acidc | ||||
| Before matching | n = 1223 | n = 1422 | |||
| All-cause mortality | 312 (26%) | 530 (37%) | +11 | 1.65 (1.44–1.90) | <0.001 |
| Heart failure hospitalization | 390 (32%) | 634 (45%) | +13 | 1.68 (1.48–1.91) | <0.001 |
| After matching | n = 630 | n = 630 | |||
| All-cause mortality | 179 (28%) | 209 (33%) | +5 | 1.44 (1.12–1.85) | 0.005 |
| Heart failure hospitalization | 220 (35%) | 264 (42%) | +7 | 1.27 (1.02–1.58) | 0.031 |
aAbsolute differences in event rates were calculated by subtracting the event rates in the high uric acid group from the event rates in the normal uric acid group (before values were rounded).
bNormal uric acid is defined as serum uric acid of ≤6 mg/dL for women and ≤8 mg/dL for men.
cHigh uric acid is defined as serum uric acid of >6 mg/dL for women and >8 mg/dL for men.
Figure 2.
Kaplan–Meier plots for (A) all-cause mortality and (B) heart failure hospitalization. CI, confidence interval; HR, hazard ratio.
Table 3.
Association of hyperuricaemia with other outcomes in propensity-matched patients
| Events (%) |
Absolute risk difference (%)a | Hazard ratio (95% confidence interval) | P-value | ||
|---|---|---|---|---|---|
| Normal uric acidb (n = 630) | High uric acidc (n = 630) | ||||
| Cardiovascular mortality | 153 (24%) | 178 (28%) | +4 | 1.48 (1.13–1.95) | 0.005 |
| Heart failure mortality | 53 (8%) | 61 (10%) | +2 | 1.41 (0.86–2.31) | 0.175 |
| Sudden cardiac death | 84 (13%) | 96 (15%) | +2 | 1.44 (1.01–2.05) | 0.042 |
| All-cause hospitalization | 380 (60%) | 415 (66%) | +6 | 1.23 (1.03–1.48) | 0.023 |
aAbsolute differences in event rates were calculated by subtracting the event rates in the high uric acid group from the event rates in the normal uric acid group (before values were rounded).
bNormal uric acid is defined as serum uric acid of ≤6 mg/dL for women and ≤8 mg/dL for men.
cHigh uric acid is defined as serum uric acid of >6 mg/dL for women and >8 mg/dL for men.
Hyperuricaemia and hospitalization
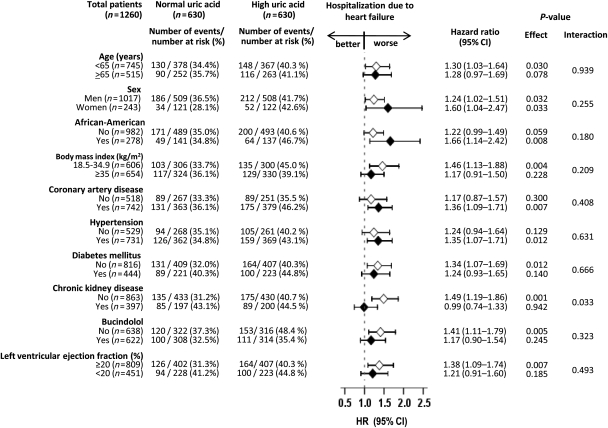
Hospitalization due to worsening HF occurred in 42 and 35% of patients with and without hyperuricaemia, respectively (HR when hyperuricaemia is compared with normouricaemia, 1.27; 95% CI, 1.02–1.58; P = 0.031; Table 2 and Figure 2B). In the absence of a hidden bias, a sign-score test for matched data with censoring provided strong evidence (P = 0.031) that patients without hyperuricaemia clearly had fewer HF hospitalization than those with hyperuricaemia. An unmeasured covariate that is a near-perfect predictor of HF hospitalization could potentially explain away this association if it would increase the odds of hyperuricaemia by 1.00%. The association between hyperuricaemia and HF hospitalization was homogenous across other subgroups of patients (Figure 3). Pre-match association between hyperuricaemia and HF hospitalization is displayed in Table 2. The association of hyperuricaemia with all-cause hospitalization is displayed in Table 3.
Figure 3.
Association of hyperuricaemia and heart failure hospitalization in subgroups of matched patients. CI, confidence interval; HR, hazard ratio.
Effect modification by chronic kidney disease
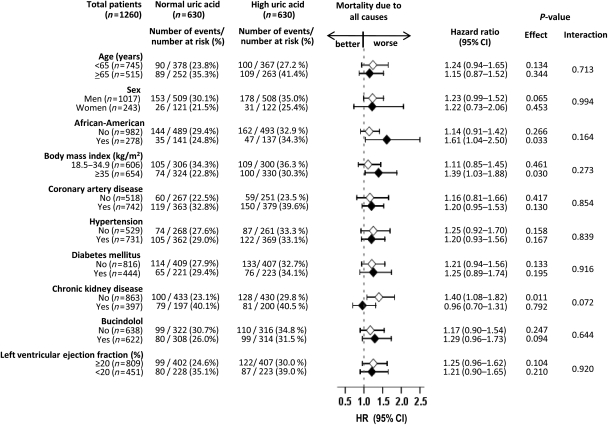
Overall, 397 (32%) patients had CKD. The mean (±SD) serum uric acid levels for matched patients with and without CKD were 8.0 (±2.0) and 7.7 (±2.0) mg/dL, respectively (P = 0.005). Among patients without CKD, all-cause mortality occurred in 30 and 23% of those with and without hyperuricaemia (HR for hyperuricaemia, 1.40; 95% CI, 1.08–1.82; P = 0.011; Figure 4). Among those with CKD, 41 and 40% of patients with and without hyperuricaemia died, respectively (HR for hyperuricaemia, 0.96; 95% CI, 0.70–1.31; P = 0.792; P for interaction = 0.072; Figure 4). Similar associations were noted for cardiovascular mortality (data not shown).
Figure 4.
Association of hyperuricaemia and all-cause mortality in subgroups of matched patients. CI, confidence interval; HR, hazard ratio.
Heart failure hospitalization occurred in 41 and 31% of non-CKD patients with and without hyperuricaemia (HR for hyperuricaemia, 1.49; 95% CI, 1.19–1.86; P = 0.001) and 45 and 43% of CKD patients with and without hyperuricaemia (HR for hyperuricaemia, 0.99; 95% CI, 0.74–1.33; P = 0.942; P for interaction = 0.033; Figure 3). The combined endpoint of HF hospitalization or all-cause mortality occurred in 54 and 43% of non-CKD patients with and without hyperuricaemia (HR for hyperuricaemia, 1.44; 95% CI, 1.18–1.74; P < 0.0001) and 62 and 59% of CKD patients with and without hyperuricaemia (HR for hyperuricaemia, 0.99; 95% CI, 0.77–1.28; P = 0.955; P for interaction = 0.024; data not shown). The association between hyperuricaemia and outcomes were generally homogeneous across other subgroups of patients (Figures 3 and 4).
Discussion
Findings from the current analysis demonstrate that hyperuricaemia was common in patients with advanced chronic systolic HF and was associated with increased mortality and hospitalization in these patients. However, these associations were only observed in patients without CKD but not in those with CKD, despite a higher mean serum uric acid level among the latter group. Because patients without CKD are expected to have normal renal clearance of uric acid, hyperuricaemia in those patients is likely primarily due to increased production and thus a marker of increased xanthine oxidase activity. In patients with CKD, on the other hand, hyperuricaemia could be both due to impaired renal clearance and increased production. The significant association of hyperuricaemia with poor outcomes, when hyperuricaemia is primarily due to increased production, suggests that hyperuricaemia may predict poor outcomes only when it is a marker of increased xanthine oxidase activity. These findings are important as they may provide important insights into the complex association between elevated serum uric acid levels and poor outcomes in HF.
As the association between hyperuricaemia and poor outcomes was observed in a cohort of propensity-matched patients who were balanced on 75 baseline characteristics, it is tempting to conclude that hyperuricaemia may have an intrinsic association with poor outcomes. However, the observed association of hyperuricaemia with poor outcomes in those without CKD, but not in those with CKD, suggests a more complex relationship and may provide possible mechanistic insights into the effect of uric acid. Uric acid is produced as an end product of purine catabolism by xanthine oxidase and is excreted through the kidneys.2,6 Therefore, serum uric acid levels can be elevated as a result of either an increased production, or an impaired renal excretion, or a combination thereof. Because uric acid excretion involves renal blood flow, glomerular filtration, and tubular secretion and reabsorption, it is possible that renal excretion of uric acid may be impaired even in the presence of normal GFR. However, findings from our pre-match cohort suggest that CKD was significantly associated with both higher prevalence and greater severity of hyperuricaemia. The prevalence of hyperuricaemia was higher (71 vs. 44% for those without CKD; P < 0.001) and the mean serum uric acid level was also higher (9.0 vs. 7.6 mg/dL for those without CKD; P < 0.001) among those with CKD. Therefore, patients with CKD had a higher degree of impairment in renal excretion of uric acid.
Because hyperuricaemia in HF patients without CKD is likely to be primarily due to an increased production of uric acid, any effect of hyperuricaemia in these patients might be either a direct effect of uric acid or that of xanthine oxidase. Hyperuricaemia in HF patients with CKD, on the other hand, is additionally caused by an impaired renal excretion of uric acid. Yet, despite having a significantly higher mean serum uric acid level, hyperuricaemia had no association with outcomes in those with CKD. However, when hyperuricaemia is a marker of increased xanthine oxidase activity, it predicts poor outcomes, thus highlighting a potential mechanistic role of xanthine oxidase. Xanthine oxidase is known to generate free oxygen radicals and cause inflammation and oxidative stress.20–23 Although the role of xanthine oxidase in HF is not clearly established, its inhibition has been shown to improve myocardial energetics and left ventricular function in both animal models and human HF.24–27 On the other hand, a reduction in the serum uric acid level without also reducing the xanthine oxidase activity has not been shown to improve haemodynamic impairment in HF.28 Interestingly, xanthine oxidase inhibition has not been shown to improve outcomes in HF patients.29
The findings of the current study are consistent with our recent findings from the Cardiovascular Health Study in community-dwelling older adults.30 In that study, hyperuricaemia-associated increased risk of incident HF was significant only in those without CKD, but not in those with CKD.30 Although this difference was not statistically significant, in that study, the hyperuricaemia-associated increase in incident HF was only observed in those with normal serum insulin levels, but not in those with hyperinsulinaemia, a difference that was statistically significant.31 As in CKD, renal excretion of uric acid has been shown to be impaired in those with hyperinsulinaemia.32–37 These findings are also consistent with findings from the Atherosclerosis Risk In Communities study, in which hyperuricaemia was associated with poor outcomes among those without CKD but not in those with CKD.38 Cumulative data from these studies suggest that hyperuricaemia may be associated with adverse cardiovascular outcomes when it is a marker of increased xanthine oxidase activity but not when it is caused by impaired renal elimination of uric acid.
To the best of our knowledge, this is the first report of interactions between serum uric acid and CKD on major natural history endpoints in a relatively large propensity-matched population of advanced chronic systolic HF patients, who were well-balanced in 75 measured baseline covariates. These findings have several important clinical and public health implications. Given the high prevalence of CKD in HF, serum uric acid may not be an efficient predictor of poor outcomes in an unselected population of HF patients. However, our data suggests that serum uric acid may be used to risk-stratify HF patients without CKD. While the use of inhibitors of xanthine oxidase may improve endothelial function and peripheral vasodilator capacity, currently there is no evidence that their use improves outcomes in HF.24,26,29,39,40 One potential explanation for this may be that CKD is common in HF and hyperuricaemia in the presence of CKD may not represent enhanced xanthine oxidase activity. Whether the use of inhibitors of xanthine oxidase in HF patients without CKD will improve outcomes remains to be seen. In the absence of such evidence, drugs such as allopurinol should not be routinely used to treat asymptomatic hyperuricaemia in unselected HF patients.
Our study has several limitations. Participants in the BEST trial were relatively young, predominantly male patients with advanced HF which may limit generalizability. Loss of patients in the matching process may have compromised external validity. However, it is likely to have enhanced internal validity as these patients were balanced on 75 demographic, clinical, subclinical, and biochemical variables. Further, we were able to replicate our key findings in all patients using traditional multivariable and propensity score adjustments. Our sensitivity analysis demonstrated that the association of high serum uric acid and HF hospitalization in our matched cohort may be modestly sensitive to an unmeasured binary covariate. However, sensitivity analysis cannot determine if any such covariate exists or not. Further, for any unmeasured covariate to be a confounder it must be a near-perfect predictor of outcome and not be strongly correlated with any of the covariates used in the propensity model. Patients with normal serum uric acid at baseline may have developed hyperuricaemia during follow-up and those with hyperuricaemia may have received therapy with allopurinol. However, this regression dilution is known to underestimate true associations.41
In conclusion, although hyperuricaemia had unadjusted associations with poor outcomes in patients with advanced chronic systolic HF, these associations did not appear to be intrinsic in nature. Although hyperuricaemia was greater in those with CKD than in those without, it had no association with outcomes in those with CKD but was associated with poor outcomes in those without CKD. These findings suggest that hyperuricaemia may predict poor outcomes when it is primarily a marker of increased xanthine oxidase activity, but not when it is primarily due to impaired renal excretion of uric acid. Future studies need to determine if inhibiting xanthine oxidase activity may improve outcomes in HF patients without CKD.
Funding
A.A. is supported by the National Institutes of Health through grants (R01-HL085561 and R01-HL097047) from the National Heart, Lung, and Blood Institute and a generous gift from Ms Jean B. Morris of Birmingham, Alabama.
Conflict of interest: none declared.
Acknowledgement
The BEST is conducted and supported by the NHLBI in collaboration with the BEST Study Investigators. This Manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the BEST or the NHLBI.
References
- 1.Leyva F, Anker S, Swan JW, Godsland IF, Wingrove CS, Chua TP, Stevenson JC, Coats AJ. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. Eur Heart J. 1997;18:858–865. doi: 10.1093/oxfordjournals.eurheartj.a015352. [DOI] [PubMed] [Google Scholar]
- 2.Doehner W, Rauchhaus M, Florea VG, Sharma R, Bolger AP, Davos CH, Coats AJ, Anker SD. Uric acid in cachectic and noncachectic patients with chronic heart failure: relationship to leg vascular resistance. Am Heart J. 2001;141:792–799. doi: 10.1067/mhj.2001.114367. [DOI] [PubMed] [Google Scholar]
- 3.Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107:1991–1997. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- 4.Jankowska EA, Ponikowska B, Majda J, Zymlinski R, Trzaska M, Reczuch K, Borodulin-Nadzieja L, Banasiak W, Ponikowski P. Hyperuricaemia predicts poor outcome in patients with mild to moderate chronic heart failure. Int J Cardiol. 2007;115:151–155. doi: 10.1016/j.ijcard.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 5.Pascual-Figal DA, Hurtado-Martinez JA, Redondo B, Antolinos MJ, Ruiperez JA, Valdes M. Hyperuricaemia and long-term outcome after hospital discharge in acute heart failure patients. Eur J Heart Fail. 2007;9:518–524. doi: 10.1016/j.ejheart.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Nugent CA, Tyler FH. The renal excretion of uric acid in patients with gout and in nongouty subjects. J Clin Invest. 1959;38:1890–1898. doi: 10.1172/JCI103966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49:298–303. doi: 10.1161/01.HYP.0000254480.64564.b6. [DOI] [PubMed] [Google Scholar]
- 9.Akgul A, Bilgic A, Ibis A, Ozdemir FN, Arat Z, Haberal M. Is uric acid a predictive factor for graft dysfunction in renal transplant recipients? Transplant Proc. 2007;39:1023–1026. doi: 10.1016/j.transproceed.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Kawada T, Amezawa M. Effects of exercise and serum uric acid on the metabolic syndrome for Japanese workers. Metab Syndr Relat Disord. 2008;6:137–141. doi: 10.1089/met.2008.0011. [DOI] [PubMed] [Google Scholar]
- 11.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 13.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 14.Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am J Cardiol. 2007;99:549–553. doi: 10.1016/j.amjcard.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor. Observational Studies. 2nd ed. New York: Springer-Verlag; 2002. pp. 110–124. [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 19.SPSS. SPSS for Windows, Rel. 15. Chicago, IL: SPSS Inc.; 2008. [Google Scholar]
- 20.Chambers DE, Parks DA, Patterson G, Roy R, McCord JM, Yoshida S, Parmley LF, Downey JM. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol. 1985;17:145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- 21.de Jong JW, Schoemaker RG, de Jonge R, Bernocchi P, Keijzer E, Harrison R, Sharma HS, Ceconi C. Enhanced expression and activity of xanthine oxidoreductase in the failing heart. J Mol Cell Cardiol. 2000;32:2083–2089. doi: 10.1006/jmcc.2000.1240. [DOI] [PubMed] [Google Scholar]
- 22.Terada LS, Guidot DM, Leff JA, Willingham IR, Hanley ME, Piermattei D, Repine JE. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc Natl Acad Sci USA. 1992;89:3362–3366. doi: 10.1073/pnas.89.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed MI, Gladden JD, Litovsky SH, Lloyd SG, Gupta H, Inusah S, Denney T, Jr, Powell P, McGiffin DC, Dell'Italia LJ. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction >60% J Am Coll Cardiol. 2010;55:671–679. doi: 10.1016/j.jacc.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekelund UE, Harrison RW, Shokek O, Thakkar RN, Tunin RS, Senzaki H, Kass DA, Marban E, Hare JM. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ Res. 1999;85:437–445. doi: 10.1161/01.res.85.5.437. [DOI] [PubMed] [Google Scholar]
- 25.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 26.Saliaris AP, Amado LC, Minhas KM, Schuleri KH, Lehrke S, St John M, Fitton T, Barreiro C, Berry C, Zheng M, Kozielski K, Eneboe V, Brawn J, Hare JM. Chronic allopurinol administration ameliorates maladaptive alterations in Ca2+ cycling proteins and beta-adrenergic hyporesponsiveness in heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H1328–1335. doi: 10.1152/ajpheart.00461.2006. [DOI] [PubMed] [Google Scholar]
- 27.Ukai T, Cheng CP, Tachibana H, Igawa A, Zhang ZS, Cheng HJ, Little WC. Allopurinol enhances the contractile response to dobutamine and exercise in dogs with pacing-induced heart failure. Circulation. 2001;103:750–755. doi: 10.1161/01.cir.103.5.750. [DOI] [PubMed] [Google Scholar]
- 28.Ogino K, Kato M, Furuse Y, Kinugasa Y, Ishida K, Osaki S, Kinugawa T, Igawa O, Hisatome I, Shigemasa C, Anker SD, Doehner W. Uric acid lowering treatment with benzbromarone in patients with heart failure: a double-blind placebo-controlled cross-over preliminary study. Circ Heart Fail. 2010;3:73–81. doi: 10.1161/CIRCHEARTFAILURE.109.868604. [DOI] [PubMed] [Google Scholar]
- 29.Hare JM, Mangal B, Brown J, Fisher C, Jr, Freudenberger R, Colucci WS, Mann DL, Liu P, Givertz MM, Schwarz RP. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J Am Coll Cardiol. 2008;51:2301–2309. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 30.Ekundayo OJ, Dell'italia LJ, Sanders PW, Arnett D, Aban I, Love TE, Filippatos G, Anker SD, Lloyd-Jones DM, Bakris G, Mujib M, Ahmed A. Association between hyperuricemia and incident heart failure among older adults: a propensity-matched study. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.01.010. Epub ahead of print; doi:10.1016/j.ijcard.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai RV, Ahmed MI, Fonarow GC, Filippatos GS, White M, Aban IB, Aronow WS, Ahmed A. Effect of serum insulin on the association between hyperuricemia and incident heart failure. Am J Cardiol. 2010;106:1134–1138. doi: 10.1016/j.amjcard.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. J Am Med Assoc. 1991;266:3008–3011. [PubMed] [Google Scholar]
- 33.Ochiai ME, Barretto AC, Oliveira MT, Jr, Munhoz RT, Morgado PC, Ramires JA. Uric acid renal excretion and renal insufficiency in decompensated severe heart failure. Eur J Heart Fail. 2005;7:468–474. doi: 10.1016/j.ejheart.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Chonchol M, Shlipak MG, Katz R, Sarnak MJ, Newman AB, Siscovick DS, Kestenbaum B, Carney JK, Fried LF. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50:239–247. doi: 10.1053/j.ajkd.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Quinones-Galvan A, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, Ferrannini E. Effect of insulin on uric acid excretion in humans. Am J Physiol. 1995;268:E1–5. doi: 10.1152/ajpendo.1995.268.1.E1. [DOI] [PubMed] [Google Scholar]
- 36.Langford HG, Blaufox MD, Borhani NO, Curb JD, Molteni A, Schneider KA, Pressel S. Is thiazide-produced uric acid elevation harmful? Analysis of data from the Hypertension Detection and Follow-up Program. Arch Intern Med. 1987;147:645–649. doi: 10.1001/archinte.147.4.645. [DOI] [PubMed] [Google Scholar]
- 37.Tykarski A. Evaluation of renal handling of uric acid in essential hypertension: hyperuricemia related to decreased urate secretion. Nephron. 1991;59:364–368. doi: 10.1159/000186593. [DOI] [PubMed] [Google Scholar]
- 38.Navaneethan SD, Beddhu S. Associations of serum uric acid with cardiovascular events and mortality in moderate chronic kidney disease. Nephrol Dial Transplant. 2009;24:1260–1266. doi: 10.1093/ndt/gfn621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 40.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 41.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]