Abstract
Many behavioral and physiological processes, including locomotor activity, blood pressure, body temperature, sleep(fasting)/wake(feeding) cycles as well as metabolic regulation display diurnal rhythms. The biological clock ensures proper metabolic alignment of energy substrate availability and processing. Studies in animals and humans highlight a strong link between circadian disorders and altered metabolic responses and cardiovascular events. Shiftwork, for instance, increases the risk to develop metabolic abnormalities resembling the Metabolic Syndrome. Nuclear receptors have long been known as metabolic regulators. Several of them (ie. Rev-erbα, RORα, PPARs) are subjected to circadian variations and are integral components of the molecular clock machinery. In turn, these nuclear receptors regulate downstream target genes in a circadian manner, acting to properly gate metabolic events to the appropriate circadian time window.
Keywords: Adipose Tissue; metabolism; Animals; Cardiovascular Agents; therapeutic use; Cardiovascular Diseases; drug therapy; genetics; metabolism; physiopathology; Cardiovascular System; drug effects; metabolism; Circadian Rhythm; drug effects; genetics; Energy Metabolism; drug effects; genetics; Humans; Receptors, Cytoplasmic and Nuclear; drug effects; genetics; metabolism; Risk Factors; Signal Transduction; drug effects; genetics
Keywords: circadian rhythm, cardiometabolic disorders, nuclear receptors, Rev-erbalpha, RORalpha, PPAR, PGC1alpha, biological clock, metabolic syndrome
I. Introduction: circadian rhythms in physiology
Circadian rhythms are variations which occur with a period of approximately one day (‘circa diem’) and allow the organism to anticipate and optimize its metabolic, hormonal and locomotor activity to predictable environmental daily changes 1. In mammals, a central clock resides in the suprachiasmatic nuclei (SCN) of the hypothalamus and synchronizes physiology to day/night alternances. In metabolic organs, output signals from the SCN clock conveyed by peripheral oscillators are combined to additional circadian cues such as food availability and information concerning local fuel availability and the hormonal milieu to drive circadian rhythms in intermediary metabolites (such as the AMP/ATP or NAD+/NADH ratios) and enzymes involved in local physiology. Consequently, many metabolic functions, including lipid and carbohydrate metabolism, and hormone secretion follow circadian variations 2. The circadian clock also synchronizes the cardio-vascular system. The heart and vasculature have an autonomous circadian pacemaker to anticipate physiological demand in heart fuel utilization and contractile function 3. For instance, blood pressure displays marked circadian variations, rising in the morning hours and decreasing at night. Heart beat and blood flow, vascular tone, fibrinolytic activity and endothelial function are all naturally subjected to diurnal variations 4. Interestingly, the incidence of acute myocardial infarction, sudden cardiac death and ischemic stroke is highest early in the morning.
II. Adverse cardiometabolic consequences of altered circadian rhythms: clinical evidences
The metabolic syndrome comprises a constellation of abnormalities including dyslipidemia, high fasting blood glucose and hypertension 5. It is precipitated by central obesity and increases the risk for type 2 diabetes and cardiovascular complications. Beside genetic risk factors, numerous environmental factors such as increased food intake and physical inactivity, contribute to the etiology of the metabolic syndrome. Chronic circadian derangement experienced by shift workers also increases the risk to develop features of the metabolic syndrome (Figure 1) 6,7. Interestingly, in humans subjected to a progressive forced desynchrony, circadian misalignment increased blood glucose despite increased insulin, suggestive of decreased insulin sensitivity, and increased blood pressure with a maximal disturbance during maximal misalignment (ie 180° phase shift)8. A decrease in sleep duration and poor-quality sleep, although not circadian disorders per se, are often seen in nightshift workers, travellers and patients suffering from obstructive sleep apnea. Sleep curtailment results in reduced glucose tolerance and insulin sensitivity, and increased hunger and appetite 9. These data suggest that long-term sleep restriction may have deleterious effects on glucose homeostasis and body weight. Indeed, the prevalence of type 2 diabetes and higher BMI is increased in self-reported short sleepers 10.
Figure 1.
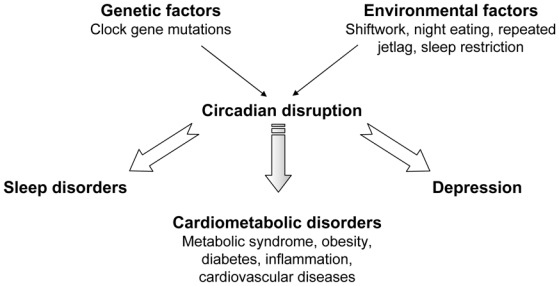
Circadian disruption may arise from genetic (clock gene mutations) or environmental (shiftwork,..) factors, and contributes to the development of behavioural and cardio-metabolic disorders.
In humans, polymorphisms in different genes belonging to the clock machinery are linked to the development of features of the metabolic syndrome. Indeed, Bmal1 is associated to type 2 diabetes in humans 11 and several polymorphisms in the clock gene are associated with body weight and increased susceptibility to obesity 12,13, and weight loss in response to dietary intervention 14. Similarly, polymorphisms in per2 and npas genes are linked to high fasting blood glucose and hypertension, respectively 15.
III. The biological circadian clock
A. Molecular organization of the clock
In mammals, Circadian Locomotor Output Cycles Kaput (CLOCK), Brain and Muscle ARNT like protein 1 (Bmal1), and the CLOCK paralog NPAS2 form the positive limb which activates the transcription of target genes including the per (Period) and cry (Cryptochrome) genes (Figure 2). In turn, the proteins Per and Cry repress CLOCK/Bmal1-mediated gene transactivation 2. The nuclear receptors Rev-erbα and RORα form an additional regulatory loop. Rev-erbα gene transcription is activated by CLOCK/Bmal1 resulting in daily fluctuations of Rev-erbα, which, in turn, represses Bmal1. RORα competes with Rev-erbα for the binding to the Bmal1 promoter through a common RORE/RevRE site, and activates its transcription 2. The nuclear receptors Peroxisome Proliferator-Activated Receptor (PPAR)α and γ bind to the Rev-erbα and Bmal1 promoters and up-regulate their expression. Finally, PPARγ co-activator (PGC)1α potentiates RORα transcriptional activity and enhances Rev-erbα and Bmal1 transcription.
Figure 2.
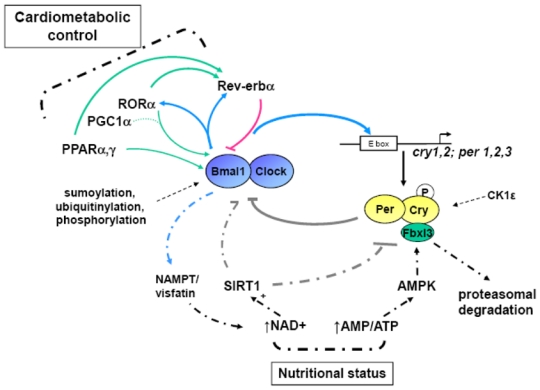
The core clock machinery consists of a series of interlocked transcriptional/translational loops which generate and maintain circadian rhythms, and post-translational modifications (dashed lines) ensure the proper timing of the clock. Intracellular metabolism (through NAD+/SIRT1 and ATP/AMP-AMPK) and nutrients (through binding to nuclear receptors) impinge on the clock machinery (doted lines).
Moreover, (post)translational modifications of the clock components, via phosphorylation, sumoylation, ubiquitination and acetylation, dictate the clock components’ stability and thus the appropriate timing of the circadian period to nearly 24h.
B. Peripheral clocks
Circadian variations are observed in the expression of 10–20% of the transcriptome in metabolic tissues. Mice harboring a dysfunctional hepatic molecular clock display a nearly complete dampening of circadian variations of the hepatic transcriptome 16, suggesting that local peripheral pacemakers are able to elicit and sustain local circadian variations. Feeding time is a dominant ‘zeitgeber’ for peripheral clocks and changes in the time of food availability entrain a new schedule in peripheral rhytms of body temperature, behavior (locomotor activity), and clock gene expression independently of the master SCN clock 17–19. In Cry1−/−cry2−/− mice, restricted feeding partially restored oscillations of certain nutrient-regulated genes which were blunted when fed ad libitum 20. Thus the ‘nutritional’ and ‘circadian’ network somehow superimpose at the regulatory level. Recent reports have revealed that several ‘nutrient sensors’ and intermediary metabolites couple metabolic and circadian regulation (Figure 2). Adenosine monophosphate-activated protein kinase (AMPK) is a nutrient sensor which is activated upon food deprivation and phosphorylates and destabilizes CRY1 21. This results in an increased circadian period and derepression of CLOCK/BMAL1 target genes as evidenced by an increased Rev-erbα circadian amplitude. Interestingly, substrate phosphorylation by AMPK follows a diurnal rhythm, linking nutrient status and the clock machinery. The cellular NAD(P)+/NAD(P)H ratio is another marker of the metabolic status. Fasting, by increasing the cellular content of NAD+, stimulates the activity of the NAD+-dependent histone deacetylase sirtuin (SIRT)1, which then interacts with PGC1α to enhance the gluconeogenic pathway (Figure 2). SIRT1 counter-regulates the histone acetyl transferase activity of CLOCK and drives cyclic expression of Bmal1, Per2 and Cry1 22,23. In turn, CLOCK/BMAL1 regulates the expression of nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme of the NAD+ synthetic pathway also known as visfatin 24,25. Interestingly, SIRT1 activity and NAD+ metabolism are modulated by AMPK, and concerted action of AMPK and SIRT1 through PGC1α likely connect cellular energy status and the circadian clock.
IV. Circadian control of energy homeostasis
A. Circadian control of glucose and lipid metabolism by clock genes and nuclear receptors
Blood concentrations of glucose and many hormones (insulin, ghrelin and leptin) exhibit circadian variations in animals and in humans. Daily fluctuations are also observed in insulin sensitivity 26. For instance, glucose tolerance decreases during the course of the day, whereas the glucose-stimulated increase in insulin is higher in the morning. These variations are lost in obese subjects and in type 2 diabetic patients 26,27.
Clock genes impinge on metabolic pathways and body weight control. Indeed, clockΔ19 mutant mice are hyperphagic, become obese and develop hyperlipidemia and hyperglycemia 28. By contrast, others have reported that clock mutant mice on a ICR genetic background are protected against diet-induced obesity because of a reduced intestinal fat absorption 29. In addition, a clock mutation specifically in the liver and muscle results in a modest, sex-dependent effect on glucose tolerance and insulin sensitivity 30. Whole-body deletion of Bmal1 results in blunted gluconeogenesis as revealed by a pyruvate tolerance test 31. A more detailed comparison of total versus liver-specific Bmal1 deletion revealed that total Bmal1 deficiency leads to increased fat mass, impaired glucose tolerance and decreased insulin sensitivity and secretion, but normal resting glycemia, whereas deletion of this gene specifically in the liver results in hypoglycemia during the inactive phase, as well as altered hepatic circadian expression of genes involved in glucose metabolism 32. Thus, although some discrepancies exist between the different reports, these data suggest that whole-body and/or organ specific alterations in the clock machinery result in compromised energy homeostasis. In the same line, over-expression of a mutant CRY1 in mice results in altered glucose homeostasis 33. In vitro, 7α-hydroxycholesterol modulates glucose output and G6Pase and PEPCK expression in a RORα-dependent manner 34. Rev-erbα, whose activity is modulated by heme 35,36, also regulates de novo glucose synthesis in human HepG2 cells 36, although the expression of gluconeogenic genes remains unaltered, and glucose tolerance appears normal in Rev-erbα-deficient and Rev-erbα over-expressing mice. As mentioned earlier, PGC1α enhances Rev-erbα transcription through enhanced RORα transcriptional activity 37. PGC1α also regulates the expression of heme/δ-aminolevulinic acid synthase (ALAS)-1, the rate limiting enzyme in the heme synthesis patway, indicating a cross-talk between PGC1α and Rev-erbα 38. Conversely, heme binding to Rev-erbα results in a repression of PGC1α and ALAS1 gene expression in vitro 39. The in vivo physiological meaning of these observations remains to be determined. Rev-erbα and RORα play also a crucial role in vivo in the control of lipid metabolism by regulating the expression of liver apolipoproteins 40, sterol regulatory element binding protein (SREBP) 41,42 and the fatty acid elongase elovl3 43, and both Rev-erbα-deficient and staggerer mice, which harbor a natural non-functional mutation in the RORα gene, are dyslipidemic. Rev-erbα also regulates bile acid metabolism by down-regulating Cyp7A1 expression through indirect mechanisms 41,44. Whereas CYP7A1 expression was not affected in staggerer mice, RORα regulates the expression of the oxysterol 7α-hydroxylase (CYP7B1), an enzyme of the alternative bile acid synthesis pathway 45.
PPARα is also rhythmically expressed in liver and regulates diurnal variations in the expression of fatty acid synthase (FAS) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoAR), two enzymes involved in lipid and cholesterol synthesis 46. In addition, PPARα participates in the circadian variations of fibroblast growth factor (FGF)-21 and administration of the PPARα ligand bezafibrate at night, compared to day time injection, has a more pronounced effect on FGF21 expression. In addition, PPARα plays a role in the entrainment by food of peripheral pacemakers 47.
These data indicate that the clock components and nuclear receptors integrate signals from both intermediary metabolism and the circadian clock to optimize fuel utilization or storage across the light/dark cycle.
B. Circadian control of adipose tissue physiology
Adipose tissue physiology demonstrates circadian variations. For instance, genes encoding proteins involved in lipid storage are highly expressed at the feeding time. In addition, expression of adipokines (such as adiponectin) and plasminogen activator inhibitor (PAI)-1 display diurnal variations. These circadian variations are however blunted in obese and diabetic animals 48 and obese humans 49,50.
As mentioned earlier, clockΔ19 mutant mice become obese upon feeding a high fat diet, at least in part due to increased food intake and an altered circadian pattern in locomotor activity 28. Other reports have highlighted the role of Bmal1 in adipogenesis and the development of diet-induced obesity. Indeed, Bmal1-deficient mice display increased fat content 32, although adipogenesis is impaired in vitro in Bmal1-deficient embryonic fibroblasts 51. Although these data appear contradictory, such a difference between the in vivo and in vitro situation has been frequently observed for other adipogenic factors. RORα over-expression in 3T3L1 cells also blocks the adipogenic process 52. Interestingly, REV-ERBα regulates this process in a very subtle manner since the REV-ERBα protein must increase and then fall to allow proper differentiation of fibroblasts into mature adipocytes 53,54.
Human data are still scarce but a few studies demonstrate that clock genes, incl. Rev-erbα and RORα, are expressed in human adipose depots. A study conducted in lean, overweight or obese subjects demonstrates that the expression of Rev-erbα, RORα, as well as Bmal1, NPAS2, Cry1, PGC1α, and PPARγ in subcutaneous tissue correlates with BMI in young subjects 55, suggesting they may interfere with adipocyte function, thereby affecting the timing of alternance of diverse processes such as lipid storage/lipolysis, which may participate in the long-term deleterious effects of circadian disorders on BMI control.
V. Circadian control of the cardio-vascular system
A. Animal studies identifying clock genes as major players in cardiovascular physiology
The clock machinery directly influences risk factors predisposing to vascular diseases and cardiac dysfuntion. RORα modulates plasma lipids, and low plasma HDL-cholesterol levels contribute to the atherosclerosis susceptibility of staggerer mice 56. PAI-1 is an important inhibitor of the fibrinolysis cascade that may promote the development of atherothrombosis. Its expression oscillates in a circadian manner with a zenith in the early morning in humans, a time which coincides with acute thrombotic and cardiovascular events such as myocardial infarction. Rev-erbα dampens PAI-1 oscillations suggesting it may affect the expression and rhythmicity of PAI-1, and affect the fibrinolysis cascade in a circadian manner 57. Rev-erbα and RORα are present in vascular wall cells including macrophages where they influence the inflammatory response 58,59. In rat vascular smooth muscle cells, Rev-erbα up-regulates the expression of interleukin (IL)-6 and cyclooxygenase-2 58. In human macrophages, it represses the induction of toll like receptor (TLR)-4, the receptor of lipopolysaccharide (LPS), thereby diminishing the production of cytokines in response to LPS 59.
clock mutant and Bmal1-deficient mice display impaired vascular remodelling, pronounced intimal hyperplasia, and thrombosis associated to increased expression of PAI-1 after surgical ligation of the left carotid artery 60. Both models exhibit endothelial dysfunction as revealed by an impaired relaxation in response to acetylcholine. In addition, Bmal1−/− mice are hypotensive and have blunted circadian rhythms in blood pressure 61, whereas Cry−/− mice suffer from hypertension 62. Staggerer mice also display lower mean arterial blood pressure, altered vascular function in mesenteric arteries and attenuated response to vasoconstrictors indicating a role for RORα in normal contractile function of smooth muscle cells 63. In the same line, PPARγ ablation in either endothelial or smooth muscle cells results in attenuations of circadian variations in blood pressure and higher heart rate, and blunted circadian variations in the aortic expression of clock genes 64. A cardiomyocyte clock mutant (CCM) mouse model in which the clockΔ19 gene is expressed specifically in cardiomyocytes, displays an altered circadian response to epinephrine, attenuated circadian variations in heart rate with a decrease during the dark phase and signs of bradycardia in isolated hearts 65. In wild-type mice, the infarct size after experimental ischemia is greatly influenced by the time of the day of ischemia infliction. A 3.5-fold increase in infarct size, fibrosis and adverse remodelling was observed in mice subjected to ischemia at the sleep-to-wake transition compared to the wake-to-sleep transition 66. CCM mice exhibited attenuated time-of-day variations in these different parameters, and significantly reduced infarct size irrespective of the time of the day, indicating that the cardiomyocyte circadian machinery plays an important role in the response to ischemic injury.
B. Clock genes and nuclear receptors control the circadian control of cardiometabolism
Fatty acids are the major source of energy for the heart and disruption in their circadian utilization may alter cardiac function. Cardiomyocytes display circadian oscillations in numerous transcriptional programs involved, for instance, in glycogen and triglyceride metabolism 65,67. However, these variations are lost in the CCM heart, and fatty acid oxidation remains constant and abnormally high. PPARα which plays an important role in fatty acid utilization by the heart, intervenes in its timing. Indeed, expression of pyruvate dehydrogenase kinase (PDK)-4, a PPARα target gene, peaks in the middle of the night, and PPARα activation induces PDK-4 gene expression to a larger extent during the night 68. PPARα is also necessary for food entrainment of the clock in the mouse heart 47. One can hypothesize that altered cardiac fatty acid utilization and cardiac function may result from perturbed circadian PPARα signalling.
VI. Modulation of the circadian control of metabolism
Together these data indicate that a tight temporal control is required for normal cardiometabolic function. They also suggest that metabolic abnormalities resulting from circadian disorders may be modulated by pharmacologically manipulating the activity and expression of clock genes and nuclear receptors. Interestingly, administration of the PPARα ligand bezafibrate during the night phase increases FGF21 and PDK4 to a larger extent as compared with daytime administration 69. In humans, the PPARα ligand fenofibrate only lowers blood pressure during sleep 70. Moreover, dexamethasone, a glucocorticoid receptor (GR) ligand potently induces a phase shift in fibroblasts in vitro as well as in peripheral mouse tissues in vivo 71. Glucocorticoids inhibit the phase adjustment of the peripheral clock in response to a restricted feeding to the light phase 72. By contrast, GR-deficient mice adapt more rapidly to food restriction. Similarly, a synthetic Rev-erbα ligand induces a phase resetting in primary lung fibroblasts and lung slices 73, and the resulting shift (advance vs delay) depends on the rhythmic expression profile of Rev-erbα. In addition, GSK3β-mediated stabilization of Rev-erbα appears a crucial event for circadian rhythm initiation, maintenance and synchronisation after serum shock 74. Thus, it is likely that the response to Rev-erbα ligands will ultimately depend on cyclic Rev-erbα abundance, and might be affected by the individual chronotype.
In conclusion, modulating nuclear receptor activity is an interesting possibility to affect physiological processes altered by a circadian challenge. However, more studies are necessary to better understand the influence of the time of administration of a ligand and its formula (rapid vs extended release,..), as well as the rhythmic abundance of the targeted nuclear receptor to obtain a maximal efficacy of the drug.
Acknowledgments
The authors acknowledge funding supports from INSERM, Contrat Plan Etat Région (CPER), the Région Nord Pas-de-Calais/FEDER, COST-action BM0602. This research was supported by a Marie Curie International Reintegration Grant within the 7th European Community Framework Programme.
References
- 1.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106:447–462. doi: 10.1161/CIRCRESAHA.109.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durgan DJ, Young ME. The cardiomyocyte circadian clock: emerging roles in health and disease. Circ Res. 2010;106:647–658. doi: 10.1161/CIRCRESAHA.109.209957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruger M, Scheer FA. Effects of circadian disruption on the cardiometabolic system. Rev Endocr Metab Disord. 2009;10:245–260. doi: 10.1007/s11154-009-9122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 6.Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Nogawa K. Shift work is a risk factor for increased blood pressure in Japanese men: a 14-year historical cohort study. Hypertension. 2008;52:581–586. doi: 10.1161/HYPERTENSIONAHA.108.114553. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 13.Sookoian S, Gemma C, Gianotti TF, Burgueno A, Castano G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 14.Garaulet M, Corbalan MD, Madrid JA, Morales E, Baraza JC, Lee YC, Ordovas JM. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int J Obes (Lond) 2010;34:516–523. doi: 10.1038/ijo.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Englund A, Kovanen L, Saarikoski ST, Haukka J, Reunanen A, Aromaa A, Lonnqvist J, Partonen T. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms. 2009;7:5. doi: 10.1186/1740-3391-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
- 18.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 19.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vollmers C, Gill S, Ditacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A. 2009;106:21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamia KA, Sachdeva UM, Ditacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 23.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai SI, Bass J. Circadian Clock Feedback Cycle Through NAMPT-Mediated NAD+ Biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanCauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocrine Reviews. 1997;18:716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 27.Boden G, Chen XH, Polansky M. Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes. 1999;48:2182–2188. doi: 10.2337/diabetes.48.11.2182. [DOI] [PubMed] [Google Scholar]
- 28.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oishi K, Atsumi G, Sugiyama S, Kodomari I, Kasamatsu M, Machida K, Ishida N. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett. 2006;580:127–130. doi: 10.1016/j.febslet.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 30.Kennaway DJ, Owens JA, Voultsios A, Boden MJ, Varcoe TJ. Metabolic homeostasis in mice with disrupted Clock gene expression in peripheral tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1528–R1537. doi: 10.1152/ajpregu.00018.2007. [DOI] [PubMed] [Google Scholar]
- 31.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, FitzGerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okano S, Akashi M, Hayasaka K, Nakajima O. Unusual circadian locomotor activity and pathophysiology in mutant CRY1 transgenic mice. Neurosci Lett. 2009;451:246–251. doi: 10.1016/j.neulet.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Kumar N, Solt LA, Richardson TI, Helvering LM, Crumbley C, Garcia-Ordonez RA, Stayrook KR, Zhang X, Novick S, Chalmers MJ, Griffin PR, Burris TP. Modulation of ROR{alpha} and ROR{gamma} activity by 7-oxygenated sterol ligands. J Biol Chem. 2009;285:5013–5025. doi: 10.1074/jbc.M109.080614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Li SM, Liu TH, Borjigin J, Lin JD. Transcriptional coactivator PGC-1a integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–4U4. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 38.Estall JL, Ruas JL, Choi CS, Laznik D, Badman M, Maratos-Flier E, Shulman GI, Spiegelman BM. PGC-1{alpha} negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erb{alpha} axis. Proc Natl Acad Sci U S A. 2009;106:22510–22515. doi: 10.1073/pnas.0912533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu N, Yin L, Hanniman EA, Joshi S, Lazar MA. Negative feedback maintenance of heme homeostasis by its receptor, Rev-erbalpha. Genes Dev. 2009;23:2201–2209. doi: 10.1101/gad.1825809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duez H, Staels B. The nuclear receptors Rev-erbs and RORs integrate circadian rhythms and metabolism. Diab Vasc Dis Res. 2008;5:82–88. doi: 10.3132/dvdr.2008.0014. [DOI] [PubMed] [Google Scholar]
- 41.Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Sasso GL, Moschetta A, Schibler U. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7:e1000181. doi: 10.1371/journal.pbio.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lau P, Fitzsimmons RL, Raichur S, Wang SC, Lechtken A, Muscat GE. The orphan nuclear receptor, RORalpha, regulates gene expression that controls lipid metabolism: staggerer (SG/SG) mice are resistant to diet-induced obesity. J Biol Chem. 2008;283:18411–18421. doi: 10.1074/jbc.M710526200. [DOI] [PubMed] [Google Scholar]
- 43.Anzulovich A, Mir A, Brewer M, Ferreyra G, Vinson C, Baler R. Elovl3: a model gene to dissect homeostatic links between the circadian clock and nutritional status. J Lipid Res. 2006;47:2690–2700. doi: 10.1194/jlr.M600230-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Duez H, van der Veen JN, Duhem C, Pourcet B, Touvier T, Fontaine C, Derudas B, Bauge E, Havinga R, Bloks VW, Wolters H, van der Sluijs FH, Vennstrom B, Kuipers F, Staels B. Regulation of bile acid synthesis by the nuclear receptor Rev-erbalpha. Gastroenterology. 2008;135:689–698. doi: 10.1053/j.gastro.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 45.Wada T, Kang HS, Angers M, Gong H, Bhatia S, Khadem S, Ren S, Ellis E, Strom SC, Jetten AM, Xie W. Identification of oxysterol 7alpha-hydroxylase (Cyp7b1) as a novel retinoid-related orphan receptor alpha (RORalpha) (NR1F1) target gene and a functional cross-talk between RORalpha and liver X receptor (NR1H3) Mol Pharmacol. 2008;73:891–899. doi: 10.1124/mol.107.040741. [DOI] [PubMed] [Google Scholar]
- 46.Patel DD, Knight BL, Wiggins D, Humphreys SM, Gibbons GF. Disturbances in the normal regulation of SREBP-sensitive genes in PPAR alpha-deficient mice. J Lipid Res. 2001;42:328–337. [PubMed] [Google Scholar]
- 47.Goh BC, Wu X, Evans AE, Johnson ML, Hill MR, Gimble JM. Food entrainment of circadian gene expression altered in PPARalpha−/− brown fat and heart. Biochem Biophys Res Commun. 2007;360:828–833. doi: 10.1016/j.bbrc.2007.06.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146:5631–5636. doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 49.Calvani M, Scarfone A, Granato L, Mora EV, Nanni G, Castagneto M, Greco AV, Manco M, Mingrone G. Restoration of adiponectin pulsatility in severely obese subjects after weight loss. Diabetes. 2004;53:939–947. doi: 10.2337/diabetes.53.4.939. [DOI] [PubMed] [Google Scholar]
- 50.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A. 2004;101:10434–10439. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci U S A. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duez H, Duhem C, Laitinen S, Patole PS, Abdelkarim M, Bois-Joyeux B, Danan JL, Staels B. Inhibition of adipocyte differentiation by RORalpha. FEBS Lett. 2009;583:2031–2036. doi: 10.1016/j.febslet.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 53.Fontaine C, Dubois G, Duguay Y, Helledie T, Vu-Dac N, Gervois P, Soncin F, Mandrup S, Fruchart JC, Fruchart-Najib J, Staels B. The orphan nuclear receptor Rev-Erbalpha is a peroxisome proliferator-activated receptor (PPAR) gamma target gene and promotes PPARgamma-induced adipocyte differentiation. J Biol Chem. 2003;278:37672–37680. doi: 10.1074/jbc.M304664200. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Lazar MA. Bifunctional role of Rev-erbalpha in adipocyte differentiation. Mol Cell Biol. 2008;28:2213–2220. doi: 10.1128/MCB.01608-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu X, Xie H, Yu G, Hebert T, Goh BC, Smith SR, Gimble JM. Expression profile of mRNAs encoding core circadian regulatory proteins in human subcutaneous adipose tissue: correlation with age and body mass index. Int J Obes (Lond) 2009;33:971–977. doi: 10.1038/ijo.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mamontova A, Seguret-Mace S, Esposito B, Chaniale C, Bouly M, Delhaye-Bouchaud N, Luc G, Staels B, Duverger N, Mariani J, Tedgui A. Severe atherosclerosis and hypoalphalipoproteinemia in the staggerer mouse, a mutant of the nuclear receptor RORalpha. Circulation. 1998;98:2738–2743. doi: 10.1161/01.cir.98.24.2738. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Yin L, Lazar MA. The orphan nuclear receptor Rev-erb alpha regulates circadian expression of plasminogen activator inhibitor type 1. J Biol Chem. 2006;281:33842–33848. doi: 10.1074/jbc.M607873200. [DOI] [PubMed] [Google Scholar]
- 58.Migita H, Morser J, Kawai K. Rev-erbalpha upregulates NF-kappaB-responsive genes in vascular smooth muscle cells. FEBS Lett. 2004;561:69–74. doi: 10.1016/S0014-5793(04)00118-8. [DOI] [PubMed] [Google Scholar]
- 59.Fontaine C, Rigamonti E, Pourcet B, Duez H, Duhem C, Fruchart JC, Chinetti-Gbaguidi G, Staels B. The nuclear receptor Rev-erbalpha is a liver X receptor (LXR) target gene driving a negative feedback loop on select LXR-induced pathways in human macrophages. Mol Endocrinol. 2008;22:1797–1811. doi: 10.1210/me.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, FitzGerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- 63.Besnard S, Bakouche J, Lemaigre-Dubreuil Y, Mariani J, Tedgui A, Henrion D. Smooth muscle dysfunction in resistance arteries of the staggerer mouse, a mutant of the nuclear receptor ROR alpha. Circulation Research. 2002;90:820–825. doi: 10.1161/01.res.0000014489.24705.71. [DOI] [PubMed] [Google Scholar]
- 64.Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE, Yang T. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 2008;8:482–491. doi: 10.1016/j.cmet.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, Young ME. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–H1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 66.Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C, Jahoor A, Gonzalez R, Garvey ME, Boland B, Blasier Z, McElfresh TA, Nannegari V, Chow CW, Heird WC, Chandler MP, Dyck JR, Bray MS, Young ME. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem. 2009 doi: 10.1074/jbc.M109.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stavinoha MA, RaySpellicy JW, Hart-Sailors ML, Mersmann HJ, Bray MS, Young ME. Diurnal variations in the responsiveness of cardiac and skeletal muscle to fatty acids. Am J Physiol Endocrinol Metab. 2004;287:E878–E887. doi: 10.1152/ajpendo.00189.2004. [DOI] [PubMed] [Google Scholar]
- 69.Oishi K, Uchida D, Ishida N. Circadian expression of FGF21 is induced by PPARalpha activation in the mouse liver. FEBS Lett. 2008;582:3639–3642. doi: 10.1016/j.febslet.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 70.Chew GT, Watts GF, Davis TM, Stuckey BG, Beilin LJ, Thompson PL, Burke V, Currie PJ. Hemodynamic effects of fenofibrate and coenzyme Q10 in type 2 diabetic subjects with left ventricular diastolic dysfunction. Diabetes Care. 2008;31:1502–1509. doi: 10.2337/dc08-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 72.Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meng QJ, McMaster A, Beesley S, Lu WQ, Gibbs J, Parks D, Collins J, Farrow S, Donn R, Ray D, Loudon A. Ligand modulation of REV-ERBalpha function resets the peripheral circadian clock in a phasic manner. J Cell Sci. 2008;121:3629–3635. doi: 10.1242/jcs.035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]


