Abstract
Rationale: Intense exercise in elite athletes is associated with increased left ventricular (LV) and right ventricular (RV) mass and volumes. However, the effect of physical activity on the RV in an older community-based population is unknown.
Objectives: We studied the association between levels of physical activity in adults and RV mass and volumes.
Methods: The Multi-Ethnic Study of Atherosclerosis (MESA) performed cardiac magnetic resonance imaging on community-based participants without clinical cardiovascular disease. RV volumes were determined from manually contoured endocardial margins. RV mass was determined from the difference between epicardial and endocardial volumes multiplied by the specific gravity of myocardium. Metabolic equivalent–minutes/day were calculated from the self-reported frequency, duration, and intensity of physical activity.
Measurements and Main Results: The study sample (n = 1,867) was aged 61.8 ± 10 years, 48% male, 44% white, 27% African American, 20% Hispanic, and 9% Chinese. Higher levels of moderate and vigorous physical activity were linearly associated with higher RV mass (P = 0.02) after adjusting for demographics, anthropometrics, smoking, cholesterol, diabetes mellitus, hypertension, and LV mass. Higher levels of intentional exercise (physical activity done for the sole purpose of conditioning or fitness) were nonlinearly associated with RV mass independent of LV mass (P = 0.03). There were similar associations between higher levels of physical activity and larger RV volumes.
Conclusions: Higher levels of physical activity in adults were associated with greater RV mass independent of the associations with LV mass; similar results were found for RV volumes. Exercise-associated RV remodeling may have important clinical implications.
Keywords: exercise, pulmonary heart disease, pulmonary hypertension, magnetic resonance imaging
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
There is an association between long-term high-intensity physical activity and changes in the cardiac ventricles, often referred to as the “athlete's heart.” Most studies have focused on the left ventricular morphology in small cohorts of young male endurance athletes.
What This Study Adds to the Field
We studied a large multi-ethnic population of adults using magnetic resonance imaging to evaluate the association between exercise and the right ventricle. We found that increasing levels of self-reported physical activity have an independent association with larger right ventricular mass and volumes.
Physical activity has many physiologic effects on the cardiovascular system, both acutely and chronically. Exercise acutely raises cardiac output through tachycardia, augmented stroke volume, and increased ejection fraction (1). Systemic and pulmonary arterial pressures increase and vascular resistances decrease. Long-term high-intensity physical activity is associated with increased left ventricle (LV) mass, volume, and wall thickness (2–6), a constellation of changes known as the “athlete's heart.” Most studies of the cardiac effects of physical activity focus on LV morphology in small cohorts of young male endurance athletes.
The substantial differences in embryology, morphology, perfusion, workload, and downstream vascular beds (and the diseases which affect them) make extrapolation of findings from the LV to the right ventricle (RV) difficult. The small number of studies performed have shown significant effects of intense exercise on RV mass and volumes (3–6). However, because RV structure and function are difficult to measure with standard transthoracic echocardiography, little is known about the relationship between physical activity and RV structure and function in large cohorts of adults who are not elite athletes. We hypothesized that high levels of self-reported physical activity would be associated with increased RV mass and volumes by cardiac magnetic resonance imaging (MRI) in an older, multiethnic adult population without clinical cardiovascular disease. Some of the results of this study have been previously reported in the form of an abstract (7).
METHODS
The Multi-Ethnic Study of Atherosclerosis
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter prospective cohort study designed to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease in whites, African Americans, Hispanics, and Chinese without clinical cardiovascular disease at baseline (8). In 2000 to 2002, MESA recruited 6,814 men and women aged 45 to 84 years old from six U.S. communities: Forsyth County, NC; Northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St. Paul, MN; Chicago, IL; and Los Angeles, CA. Exclusion criteria included clinical cardiovascular disease (physician diagnosis of heart attack, stroke, transient ischemic attack, heart failure, angina, current atrial fibrillation, any cardiovascular procedure), weight greater than 136 kg (300 lb), pregnancy, or impediment to long-term participation. The protocols of MESA and all studies described herein were approved by the institutional review boards of all collaborating institutions and the NHLBI. NHLBI staff participated in the design of the MESA study. The MESA-Right Ventricle Study is an ancillary study that measured RV morphology in 4,204 MESA participants with interpretable cardiac MRIs at the baseline examination.
Cardiac MRI Measures
The cardiac MRI protocol has been previously described (9, 10). All imaging was performed on 1.5 T magnets with a four-element phased-array surface coil positioned anteriorly and posteriorly and electrocardiographic gating. Imaging consisted of fast gradient echo cine images with temporal resolution 50 ms or less. Methods for interpretation of LV and RV parameters have been previously reported (11). Briefly, RV image analysis was performed by two independent analysts on Windows workstations using QMASS software (Medis, Leiden, The Netherlands). The endocardial and epicardial borders of the RV were traced manually on the short axis cine images at the end-systolic and end-diastolic phase. Papillary muscles and trabeculae were included in the RV volumes (12) and excluded from RV mass. RV end-diastolic volume (RVEDV) and RV end-systolic volume (RVESV) were calculated using Simpson's rule by summation of areas on each slice multiplied by the sum of slice thickness and image gap. RV mass was determined at the end-diastole phase as the difference between end-diastolic epicardial and endocardial volumes multiplied by the specific gravity of myocardium (1.05 g/ml) (9). RV stroke volume (RVSV) was calculated by subtracting RVESV from RVEDV. RV ejection fraction (RVEF) was calculated by dividing RVSV by RVEDV. The intrareader intraclass correlation coefficients were 0.99 for RVEDV, 0.89 for RVEF (both n = 230), and 0.94 for RV mass (n = 229). The inter-reader intraclass correlation coefficients from random, blinded re-reads of 240 scans for RV mass and RVEDV were 0.89 and 0.96, respectively, and 0.80 for RVEF.
Physical Activity
The MESA Typical Week Physical Activity Survey (TWPAS), adapted from the Cross-Cultural Activity Participation Study, was designed to identify the time spent in and frequency of various physical activities during a typical week in the past month (13). The time frame was designed to capture typical activity patterns in daily life. The survey has 28 items in categories of household chores, yard work and gardening, care of children/adults, transportation, walking (not at work), dancing and sport activities, conditioning activities, leisure activities, occupational activities, and volunteer activities. Where appropriate, questions differentiated between light-, moderate-, and heavy-intensity activities. Respondents were asked whether they participated in these activities, and if applicable they answered questions regarding the average number of days per week and time per day engaged in each activity. Minutes of activity were summed for each discrete activity type and multiplied by metabolic equivalent (MET) level.
The MESA TWPAS by design had the following summary measures: total hours/week and total MET-minutes/week for the nine physical activity categories, three intensity levels (light, moderate, vigorous), total physical activity (sum of light, moderate, and vigorous activity), moderate and vigorous physical activity, and intentional exercise (sum of walking for exercise, sports/dancing, and conditioning). We used two summary variables as our independent variables, as previously described (14). The first was the sum of moderate and vigorous physical activity (MVPA) in MET-minutes/day. The second was total intentional exercise (physical activity performed for the sole purpose of conditioning or fitness) also in MET-minutes/day, which captures activities typically recommended by physician guidelines.
Covariates
Race/ethnicity was self-reported during the baseline MESA examination according to 2000 U.S. Census criteria as race (white, African American, etc.) and ethnicity (Hispanic or non-Hispanic). Participants self-identifying as Hispanic were categorized as Hispanic. Standard questionnaires were used to ascertain smoking status (classified as never, former, or current) and pack-years. Resting blood pressure was measured three times using the Dinamap Monitor PRO 100 (Critikon, Tampa, FL) automated oscillometric device, and the average of the last two measurements was used. Hypertension was defined as systolic blood pressure 140 mm Hg or greater, diastolic blood pressure 90 mm Hg or greater, or self-reported hypertension and current use of antihypertensive medication. Presence of diabetes mellitus was based on self-reported physician diagnosis, use of medication for hyperglycemia, or a fasting glucose value 126 mg/dl or higher, the latter measured by rate reflectance spectrophotometry (Johnson and Johnson Clinical Diagnostics, Inc., Rochester, NY). Fasting glucose between 100 and 125 mg/dl was considered impaired fasting glucose. Fasting blood samples were drawn and sent to a central laboratory for measurement of glucose and lipids. Total cholesterol was assessed using standard methods. Spirometry measures of acceptable quality were available for a subset of 1,285 participants. Detailed methods for these measures are published elsewhere (15).
Statistical Analysis
Descriptive characteristics are summarized by quintiles of MVPA and intentional exercise. Continuous variables are expressed as means and standard deviations or ranges. Categorical variables are expressed as a percentage. Multivariable linear regression was used to characterize the relationship between RV parameters (dependent variables) and physical activity (independent variables). Adjustment for height and weight in all analyses avoided the assumptions made in indexing the RV measures to certain parameters of body size (e.g., body surface area), while achieving the same end of accounting for differences in body size between participants. Covariates were chosen based on known associations with ventricular size and heart disease, including demographics and anthropometric variables, as well as variables reflecting comorbidities, such as smoking, hypertension, cholesterol levels, and diabetes mellitus. Adjustment for LV parameters was performed (1) to account for the contribution of LV abnormalities to RV changes (for example, increased LV mass causing elevated LV end-diastolic pressure leading to pulmonary venous hypertension and increased RV mass), (2) to better account for differences in body size by “indexing” to this other chamber, and (3) to examine RV-specific associations (rather than more general associations with biventricular morphology). RVSV was not adjusted for LV stroke volume considering the significant interdependence of these measures.
Primary analyses were performed using continuous physical activity variables. Least square means adjusted for covariates were calculated using multivariate linear regression with physical activity variables by quintile. Generalized additive models were used if there was significant nonlinearity of the association between physical activity and RV morphology. We show the results of the generalized additive models after standardization (subtracting the mean from each value and dividing by the standard deviation) to compare these results with the associations of exercise with LV morphology, published previously (16). Sensitivity analyses were performed using the subset of participants with available spirometry after excluding individuals with obstructive (FEV1/FVC ratio < 0.70) or restrictive (FVC < lower limit of normal with FEV1/FVC > 0.70) ventilatory defects (17).
RESULTS
MESA included 6,814 participants (Figure 1). Of these, 5,098 had available cardiac MRIs, of which 5,004 were interpretable for the LV. Of the 6,814, reasons for not completing the cardiac MRI included ineligibility (7%) (usually because of metallic fragment, implant, or device, or weight > 136 kg), being unable to tolerate the scan (14%) (usually because of claustrophobia), refusal (3%), or mechanical problem with the scanner or unknown (both ≤ 1%). Individuals who had an interpretable cardiac MRI were similar in age and sex to those who did not (61.5 vs. 63.9 yr and 52% vs. 54% female, respectively). Participants with MRI were somewhat lighter than those without MRI (body mass index [BMI] 28 vs. 30 kg/m2), were more likely to be Chinese, and were less likely to be African American (MRI: 13% Chinese and 26% African American, vs. no MRI: 8% Chinese and 34% African American).
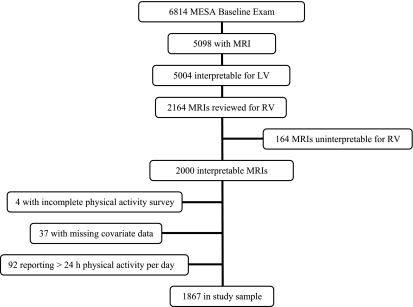
Figure 1.
Flow of subjects. LV = left ventricle; MESA = Multi-Ethnic Study of Atherosclerosis; MRI = magnetic resonance imaging; RV = right ventricle.
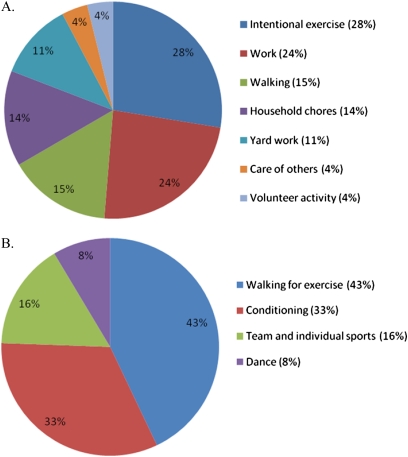
The goal of The MESA-Right Ventricle Study was to interpret 4,204 scans with interpretable LV measures. This study was conducted after the first 2,164 scans were reviewed, of which 2,000 had interpretable RV measures. We excluded participants who did not complete the physical activity survey (n = 4), those with missing data for covariates (n = 37), and those who reported more than 24 hours per day of physical activity (n = 92), leaving 1,867 participants in the final study sample (Figure 1). The mean age was 61.8 ± 10 years, and 48.2% were men. Forty-four percent self-identified as white, 26.6% African American, 19.7% Hispanic, and 9.6% Chinese. Weight was normal in 28.7% (BMI < 25 kg/m2), 41.7% were overweight (BMI 25–29.9 kg/m2), and almost 30% were obese (BMI ≥ 30 kg/m2). A majority of MVPA was spent in intentional exercise, work, household chores, and walking (Figure 2A). Intentional exercise was mostly composed of walking for exercise, conditioning, and sports (Figure 2B).
Figure 2.
Proportion of metabolic equivalent-minutes/day of (A) moderate and vigorous physical activity, and (B) intentional exercise.
Moderate and Vigorous Physical Activity
The mean daily time spent performing MVPA among the participants was 761 MET-minutes (equivalent to approximately 7.4 h of running per week), and the median daily time was 574 MET-minutes (equivalent to approximately 5.6 h of running per week). Participants with higher levels of MVPA were somewhat younger, more likely to be male, and less likely to be Chinese (Table 1). There was a lower prevalence of hypertension, hypercholesterolemia, and impaired glucose handling in higher quintiles of physical activity.
TABLE 1.
CHARACTERISTICS BY QUINTILE OF MODERATE AND VIGOROUS PHYSICAL ACTIVITY
| Quintile |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | All | |
| No. of participants | 376 | 371 | 373 | 374 | 373 | 1867 |
| Moderate and vigorous physical activity | ||||||
| MET-minutes/day, mean | 138 | 342 | 581 | 932 | 1815 | 761 |
| Running-hour equivalents/wk | 1.3 | 3.3 | 5.6 | 9.1 | 17.7 | 7.4 |
| Demographics | ||||||
| Age, yr | 62.7 | 62.8 | 62.2 | 61.4 | 59.7 | 61.8 |
| Male, % | 42.8 | 42.3 | 44.0 | 49.7 | 62.2 | 48.2 |
| Race/ethnicity, % | ||||||
| White | 37.2 | 43.7 | 49.3 | 47.6 | 42.9 | 44.1 |
| African American | 25.3 | 27.2 | 25.6 | 27.8 | 27.1 | 26.6 |
| Hispanic | 22.9 | 12.9 | 16.9 | 20.1 | 25.7 | 19.7 |
| Chinese | 14.6 | 16.2 | 8.3 | 4.6 | 4.3 | 9.6 |
| Education, % | ||||||
| < High school | 21.8 | 11.3 | 12.3 | 15.0 | 13.9 | 14.9 |
| High school | 22.6 | 18.9 | 13.9 | 18.2 | 20.6 | 18.9 |
| < College, > high school | 23.1 | 23.2 | 28.1 | 28.9 | 34.9 | 27.6 |
| ≥ College | 32.5 | 46.6 | 45.6 | 38.0 | 30.6 | 38.6 |
| Anthropometrics | ||||||
| Height, cm | 164.7 | 165.9 | 167.0 | 167.4 | 168.8 | 166.8 |
| Weight, kg | 77.1 | 75.8 | 78.1 | 77.9 | 80.7 | 77.9 |
| BMI, kg/m2 | 28.3 | 27.4 | 27.9 | 27.7 | 28.2 | 27.9 |
| Weight status, % | ||||||
| Normal (BMI < 25 kg/m2) | 26.9 | 33.7 | 27.1 | 30.2 | 25.5 | 28.7 |
| Overweight (BMI 25–29.9 kg/m2) | 39.6 | 38.0 | 42.9 | 41.7 | 46.1 | 41.7 |
| Obese (BMI 30–39.9 kg/m2) | 31.7 | 27.0 | 29.0 | 27.3 | 26.5 | 28.3 |
| Morbidly obese (BMI > 40 kg/m2) | 1.9 | 1.4 | 1.1 | 0.8 | 1.9 | 1.4 |
| Disease prevalence | ||||||
| Hypertension, % | 50.8 | 46.6 | 45.3 | 42.8 | 39.4 | 45.0 |
| Systolic blood pressure, mm Hg | 129.0 | 127.2 | 126.0 | 127.2 | 124.9 | 126.9 |
| Diastolic blood pressure, mm Hg | 71.6 | 71.8 | 70.9 | 73.4 | 73.1 | 72.2 |
| Diabetes mellitus, % | ||||||
| Normal glucose tolerance | 71.5 | 78.2 | 79.9 | 77.3 | 77.8 | 76.9 |
| Impaired fasting glucose | 15.4 | 11.3 | 9.7 | 13.1 | 9.4 | 11.8 |
| Untreated diabetes | 2.1 | 1.1 | 2.7 | 2.7 | 3.0 | 2.3 |
| Treated diabetes | 10.9 | 9.4 | 7.8 | 7.0 | 9.9 | 9.0 |
| Total cholesterol, mg/dl | 194.0 | 192.9 | 194.1 | 195.2 | 197.1 | 194.6 |
| Statin use, % | 16.5 | 14.3 | 16.9 | 14.4 | 13.4 | 15.1 |
| Smoking status | ||||||
| Nonsmoker, % | 54.5 | 52.6 | 46.1 | 51.6 | 46.1 | 50.2 |
| Former smoker, % | 30.6 | 39.4 | 41.6 | 37.2 | 37.3 | 37.2 |
| Current smoker, % | 14.9 | 8.1 | 12.3 | 11.2 | 16.6 | 12.6 |
| Pack-years, among ever-smokers | 25.9 | 20.7 | 22.1 | 22.8 | 22.5 | 22.8 |
Definition of abbreviations: BMI = body mass index; MET = metabolic equivalent.
Table 2 shows the regression coefficients (βs) for MVPA as a continuous variable and the least square means of RV parameters shown across quintiles of MVPA. Higher levels of MVPA were associated with greater RV mass after adjustment for age, sex, race/ethnicity, and body size and after additional adjustment for other covariates (P < 0.001) with a 1-g (∼ 5%) difference in RV mass from the lowest to highest quintiles of MVPA. Further adjustment for LV mass slightly weakened the association, but it was still statistically significant (P = 0.02). Similarly, higher levels of MVPA were associated with larger age-, sex-, race-, and body-size adjusted RVEDV (P < 0.001), with an approximately 7% difference between the lowest and highest quintiles of MVPA. The association remained significant after adjustment for all covariates (P < 0.001) and LV mass (P = 0.03). Higher MVPA was also associated with larger RVSV (P < 0.001). There were no significant associations between MVPA and RVEF. Generalized additive models were not consistent with a nonlinear relationship for the associations between MVPA and RV parameters (data not shown).
TABLE 2.
ESTIMATED MEAN VALUES BY QUINTILE OF MODERATE AND VIGOROUS PHYSICAL ACTIVITY
| Quintile |
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | β* | P Value* | |
| RV end-diastolic mass, g | |||||||
| Model including age, sex, race/ethnicity | 20.5 | 21.2 | 21.3 | 21.5 | 21.5 | 0.04 | <0.001 |
| Full model† | 20.5 | 21.2 | 21.3 | 21.5 | 21.5 | 0.05 | <0.001 |
| Full model + LV end-diastolic mass | 20.6 | 21.2 | 21.3 | 21.5 | 21.3 | 0.03 | 0.02 |
| RV end-diastolic volume, ml | |||||||
| Model including age, sex, race/ethnicity | 120.8 | 125.4 | 125.7 | 126.2 | 128.6 | 0.39 | <0.001 |
| Full model† | 120.8 | 125.2 | 125.4 | 126.5 | 128.9 | 0.42 | <0.001 |
| Full model + LV end-diastolic volume | 123.3 | 124.9 | 126.4 | 126.3 | 125.8 | 0.12 | 0.03 |
| RV stroke volume, ml | |||||||
| Model including age, sex, race/ethnicity | 83.4 | 86.8 | 86.1 | 86.4 | 87.8 | 0.21 | <0.001 |
| Full model† | 83.4 | 86.6 | 85.8 | 86.6 | 88.0 | 0.24 | <0.001 |
| RV ejection fraction, % | |||||||
| Model including age, sex, race/ethnicity | 69.5 | 69.6 | 68.9 | 69.0 | 68.8 | −0.04 | 0.09 |
| Full model† | 69.5 | 69.6 | 68.9 | 69.0 | 68.8 | −0.03 | 0.18 |
| Full model + LV ejection fraction | 69.5 | 69.4 | 68.9 | 69.1 | 68.9 | −0.02 | 0.35 |
Definition of abbreviations: LV = left ventricular; MET = metabolic equivalent; RV = right ventricular.
All models adjusted for height and weight.
β and P value derived from multivariate linear regression with physical activity as a continuous variable. β reflects 100 MET-minutes/day increments.
Full model includes: age, sex, race/ethnicity, education, hypertension, systolic blood pressure, diastolic blood pressure, diabetes, total cholesterol, statin use, smoking status, and pack-years.
Intentional Exercise
The mean daily time spent performing intentional exercise among the participants was 210 MET-minutes (equivalent to approximately 2 h of running per week), and the median daily time was 120 MET-minutes (equivalent to approximately 1.2 h of running per week). Participants performing greater amounts of exercise were more likely to be white and less likely to be Chinese or Hispanic (Table 3). There was a higher level of education, lower weight and BMI, and lower prevalence of hypertension and hypercholesterolemia in higher quintiles of intentional exercise.
TABLE 3.
CHARACTERISTICS BY QUINTILE OF INTENTIONAL EXERCISE
| Quintile |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | All | |
| No. of participants | 382 | 371 | 367 | 374 | 373 | 1867 |
| Intentional exercise | ||||||
| MET-minutes/day, mean | 0 | 49 | 123 | 244 | 636 | 210 |
| Running-hour equivalents/wk | 0.0 | 0.5 | 1.2 | 2.4 | 6.2 | 2.0 |
| Demographics | ||||||
| Age, yr | 61.3 | 60.6 | 62.2 | 62.0 | 62.8 | 61.8 |
| Male, % | 45.3 | 42.1 | 43.3 | 51.1 | 59.3 | 48.2 |
| Race/ethnicity, % | ||||||
| White | 30.6 | 45.8 | 43.6 | 49.5 | 51.5 | 44.1 |
| African American | 28.3 | 28.8 | 24.3 | 25.1 | 26.3 | 26.6 |
| Hispanic | 27.8 | 17.5 | 22.3 | 13.6 | 17.2 | 19.7 |
| Chinese | 13.4 | 7.8 | 9.8 | 11.8 | 5.1 | 9.6 |
| Education, % | ||||||
| < High school | 25.1 | 11.3 | 13.6 | 13.1 | 11.0 | 14.9 |
| High school | 24.4 | 24.0 | 16.1 | 13.1 | 16.6 | 18.9 |
| < College, > high school | 29.6 | 27.2 | 24.3 | 30.8 | 26.3 | 27.6 |
| ≥ College | 21.0 | 37.5 | 46.1 | 43.1 | 46.1 | 38.6 |
| Anthropometrics | ||||||
| Height, cm | 165.0 | 166.1 | 165.8 | 167.9 | 169.0 | 166.8 |
| Weight, kg | 78.0 | 79.2 | 76.8 | 77.2 | 78.4 | 77.9 |
| BMI, kg/m2 | 28.5 | 28.6 | 27.8 | 27.3 | 27.3 | 27.9 |
| Weight status, % | ||||||
| Normal (BMI < 25 kg/m2) | 27.2 | 22.6 | 28.1 | 31.8 | 33.5 | 28.7 |
| Overweight (BMI 25–29.9 kg/m2) | 36.9 | 41.5 | 44.4 | 40.9 | 44.8 | 41.7 |
| Obese (BMI 30–39.9 kg/m2) | 33.8 | 34.2 | 25.3 | 27.0 | 20.9 | 28.3 |
| Morbidly obese (BMI > 40 kg/m2) | 2.1 | 1.6 | 2.2 | 0.3 | 0.8 | 1.4 |
| Disease prevalence | ||||||
| Hypertension, % | 46.1 | 45.8 | 48.0 | 44.7 | 40.5 | 45.0 |
| Systolic blood pressure, mm Hg | 128.4 | 126.1 | 127.3 | 127.0 | 125.6 | 126.9 |
| Diastolic blood pressure, mm Hg | 72.4 | 71.8 | 72.2 | 72.4 | 72.1 | 72.2 |
| Diabetes mellitus, % | ||||||
| Normal glucose tolerance | 77.0 | 74.4 | 75.5 | 78.9 | 78.8 | 76.9 |
| Impaired fasting glucose | 12.6 | 12.9 | 12.8 | 10.7 | 9.9 | 11.8 |
| Untreated diabetes | 2.1 | 2.4 | 1.4 | 2.4 | 3.2 | 2.3 |
| Treated diabetes | 8.4 | 10.2 | 10.4 | 8.0 | 8.0 | 9.0 |
| Total cholesterol, mg/dl | 194.5 | 194.2 | 195.5 | 196.7 | 192.3 | 194.6 |
| Statin use, % | 15.2 | 12.7 | 16.1 | 15.2 | 16.4 | 15.1 |
| Smoking status | ||||||
| Nonsmoker, % | 47.4 | 53.9 | 51.8 | 52.7 | 45.3 | 50.2 |
| Former smoker, % | 34.3 | 34.0 | 38.2 | 37.4 | 42.1 | 37.2 |
| Current smoker, % | 18.3 | 12.1 | 10.1 | 9.9 | 12.6 | 12.6 |
| Pack-years, among ever-smokers | 18.8 | 21.5 | 25.8 | 24.9 | 23.4 | 22.8 |
For definition of abbreviations, see Table 1.
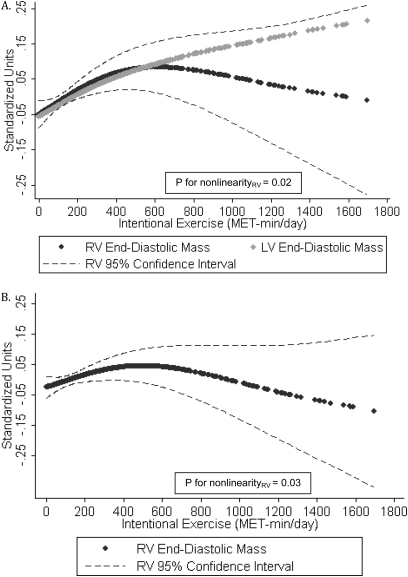
Higher levels of intentional exercise were associated with greater RV mass before and after adjustment for all covariates as well as after adjustment for LV mass (Table 4). Figure 3A shows the association of intentional exercise with standardized RV mass and LV mass in fully adjusted generalized additive models. There was a strong direct association between intentional exercise and RV mass at lower levels of exercise (with a magnitude similar to that of the association of exercise with LV mass) that plateaued at higher levels of exercise (P for nonlinearity = 0.02). The apparent negative association at the extreme of intentional exercise was limited by very few subjects and very wide confidence intervals, making it difficult to draw definitive conclusions from this part of the figure. Figure 3B shows that the relationship between intentional exercise and RV mass remained significant even after adjustment for LV mass (P for nonlinearity = 0.03).
TABLE 4.
ESTIMATED MEAN VALUES BY QUINTILE OF INTENTIONAL EXERCISE
| Quintile |
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | β* | P Value* | |
| RV end-diastolic mass, g | |||||||
| Model including age, sex, race/ethnicity | — | — | — | — | — | — | 0.03† |
| Full model‡ | — | — | — | — | — | — | 0.02† |
| Full model + LV end-diastolic mass | — | — | — | — | — | — | 0.03† |
| RV end-diastolic volume, ml | |||||||
| Model including age, sex, race/ethnicity | — | — | — | — | — | — | 0.009† |
| Full model‡ | — | — | — | — | — | — | 0.01† |
| Full model + LV end-diastolic volume | — | — | — | — | — | — | 0.25† |
| RV stroke volume, ml | |||||||
| Model including age, sex, race/ethnicity | 85.4 | 84.1 | 84.3 | 88.9 | 87.8 | 0.39 | 0.004 |
| Full model‡ | 85.5 | 84.1 | 84.3 | 88.8 | 87.7 | 0.37 | 0.006 |
| RV ejection fraction, % | |||||||
| Model including age, sex, race/ethnicity | 69.4 | 69.2 | 69.0 | 69.5 | 68.8 | 0.01 | 0.80 |
| Full model‡ | 69.4 | 69.2 | 68.9 | 69.4 | 68.8 | 0.01 | 0.80 |
| Full model + LV ejection fraction | 69.3 | 69.2 | 69.0 | 69.4 | 69.0 | 0.03 | 0.47 |
For definition of abbreviations, see Table 2. All models adjusted for height and weight.
β and P value derived from multivariate linear regression with physical activity as a continuous variable. β reflects 100 MET-minutes/day increments.
Generalized additive models with P values for nonlinearity.
Full model includes: age, sex, race/ethnicity, education, hypertension, systolic blood pressure, diastolic blood pressure, diabetes, total cholesterol, statin use, smoking status, and pack-years.
Figure 3.
Generalized additive models for intentional exercise and (A) standardized values of right ventricular (RV) end-diastolic mass and left ventricular (LV) end-diastolic mass, fully adjusted model; and (B) standardized RV end-diastolic mass, fully adjusted model with LV end-diastolic mass. MET = metabolic equivalent.
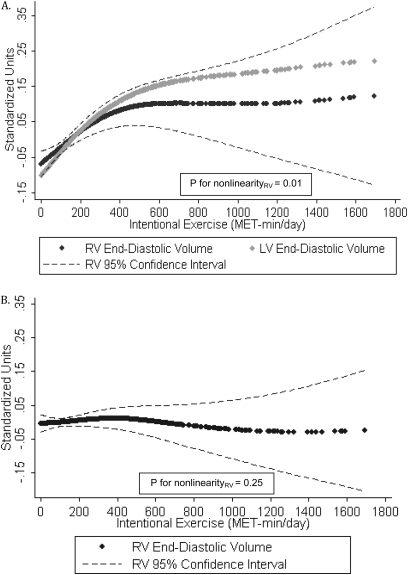
Similarly, a greater amount of intentional exercise was associated with larger RVEDV in a nonlinear fashion after adjustment for all covariates (P for nonlinearity = 0.01) (Figure 4A). The magnitude of the association was similar to that of LV end-diastolic volume, with a plateau at higher levels of exercise. Figure 4B shows that the association was no longer significant after adjustment for LV end-diastolic volume (P for nonlinearity = 0.25), consistent with a biventricular effect. We found higher levels of intentional exercise to be linearly associated with larger RVSV after full adjustment (P = 0.006), but there was no association between intentional exercise and RVEF (Table 4).
Figure 4.
Generalized additive models for intentional exercise and (A) standardized values of right ventricular (RV) end-diastolic volume and left ventricular (LV) end-diastolic volume, fully adjusted model; and (B) standardized RV end-diastolic volume, fully adjusted model with LV end-diastolic volume.
There were no interactions between MVPA or intentional exercise and age, sex, or race. Subset analyses including those participants in whom spirometry was available (n = 1,285) showed no differences in the effect estimates after excluding those with obstructive (23%) or restrictive (7%) ventilatory defects (data not shown).
DISCUSSION
We have shown that higher self-reported levels of physical activity are associated with greater RV mass and larger RV volumes in cardiovascular disease–free individuals in a multiethnic community-based adult population. MVPA (including both intentional exercise as well as other activities) was directly associated with RV mass and volumes, independent of the LV. Intentional exercise was also associated with an increase in RV mass and volumes at lower levels of intentional exercise, which plateaued at more extreme levels of exercise. Higher levels of both MVPA and intentional exercise were associated with larger RVSV without change in RVEF. These findings are consistent with those seen in the “athlete's heart,” where both ventricular mass and volumes increase while ejection fraction is preserved. Our findings were unchanged after limiting our analyses to participants with available spirometry and without obstructive or restrictive ventilatory defects. This is the first documentation of RV morphologic differences associated with physical activity in a large, multi-ethnic sample of untrained adults.
Prior studies have shown that endurance athletes demonstrate physiologic cardiac hypertrophy in response to the episodic volume load imposed on the ventricles by periods of physical activity. Physiologic LV hypertrophy due to an episodic volume load, such as that caused by physical activity, results from the addition of sarcomeres in series accounting for the lengthening of cardiac myocytes and increase in mass, a process distinct from the concentric hypertrophy that results from a pressure load (e.g., high blood pressure or weight-lifting) (18, 19). Studies of the hearts of exercised animals compared with those of sedentary animals show hypertrophy of the RV out of proportion to the LV, with a 21 to 31% increase in RV mass and 7 to 12% increase in LV mass, as well as an increase in RV and septal myocyte length of up to 26% (20–23). A recent study in rats with stable pulmonary hypertension suggested that physical activity helped to improve RV function and remodeling, but in those with progressive pulmonary hypertension physical activity accelerated RV failure (24). Capillary density increased in the exercise-trained RV. Other studies have demonstrated cellular differences between the two types of cardiac remodeling, with physiologic cardiac hypertrophy characterized by synthesis of normal contractile proteins, metabolic enzymes, and mitochondria, and pathologic cardiac hypertrophy associated with increased fetal isoform contractile proteins, collagen production, and glycolysis (18, 19, 25).
Observational studies of young male endurance athletes have shown a balanced physiologic hypertrophy of the RV and LV in response to high levels of training (2–6). The two studies that used MRI included 44 athletes and 31 control subjects (mean age, 25 yr) and found a 22 to 37% increase in RV mass and a 3 to 25% increase in RVEDV in athletes compared with control subjects (3, 4). A study of 10 obese adults (mean age, 32 yr) who were untrained at baseline found a 12% increase in RV mass and 4% increase in RVEDV after 8 weeks of training (26). A study of competitive athletes before and after intense training using echocardiography showed similar results (27). Limited by a small number of individuals with homogeneity of age, sex, and race/ethnicity participating in intense physical activity, these studies are likely not generalizable to older adults in the general population. Recent studies of patients with pulmonary arterial hypertension have shown that exercise training is safe and can improve exercise endurance, 6-minute walk distance, and quality of life (28, 29). Our results suggest that adaptive changes in RV mass and volumes may be a mechanism by which exercise improves functional status in pulmonary disease, such as chronic obstructive pulmonary disease and pulmonary arterial hypertension.
Our results reveal differently shaped relationships between MVPA and intentional exercise and the RV. More MET-minutes of MVPA each day were linearly associated with greater RV mass and volumes throughout the spectrum of MVPA. Intentional exercise was also directly associated with RV mass and volumes, but plateaued at higher levels (> 500 MET-minutes/day or approximately 4.9 h of running each week) of intentional exercise. Although these results suggest adaptive RV changes in response to increased activity (to a certain level for intentional exercise), there are possible explanations for the differences in the relationships. The pattern, duration, and distribution of activity throughout the average day may determine the pattern of remodeling; shorter episodes of intentional exercise rather than longer periods of MVPA could lead to distinct ventricular phenotypes. In addition, the absolute differences in the amount of physical activity between MVPA and intentional exercise are attributable to occupational, household, and yard work activities, which may impact the RV in different ways.
RV afterload could also account for the RV-specific effect seen with MVPA (for RV mass and volumes) as well as with intentional exercise (for RV mass). LV diastolic dysfunction at early stages is often undiagnosed and can cause elevated pulmonary venous pressures, increasing RV afterload. One study found that almost half of a sample of 91 healthy volunteers over the age of 50 met the definition of exercise-induced pulmonary hypertension (30), likely due to clinically occult LV diastolic dysfunction. Another study demonstrated decreased distensibility of the pulmonary vasculature in men over the age of 60, similar to the reduced vascular compliance seen in the systemic vasculature (31). Increased RV afterload in addition to the volume load of exercise may lead to the observed increases in RV mass and size.
The lowest and highest quintiles of physical activity in this study were associated with 5 to 7% differences in RV mass and volumes, equal to or greater than the relative magnitude of the effects of physical activity on LV morphology in MESA (32). Active smoking and diabetes mellitus have impacts of similar magnitude on LV mass (33), indicating that this degree of RV adaptation likely has clinical significance. Although the implications of these differences in normal older adults are unclear, even small absolute changes in the very thin-walled RV in disease-free participants may have significant long-term effects. Such exercise-induced RV remodeling would be presumed beneficial, with potentially important implications for diseases characterized by pulmonary hypertension and RV dysfunction. Our findings suggest that the current recommendations for aerobic physical activity in older adults (150 min of moderate-vigorous physical activity per week, approximately 250 MET-minutes/day) may have RV effects in healthy individuals. Thus, physical activity may have potential benefit for those with (or at risk for) RV dysfunction, including pulmonary hypertension, chronic obstructive pulmonary disease, diffuse parenchymal lung disease, sleep-disordered breathing, and congestive heart failure (34). These data may be useful in studying the RV-specific benefits of exercise in at-risk populations.
There are several potential limitations of this study. We performed a cross-sectional observational study of activity level and RV morphology, making it difficult to prove cause and effect. Survey data were used to quantify the amount of physical activity performed, relying on the participants to remember their typical activities within the last month. Error in the recall of physical activity data is inevitable; however, we would expect such error (if nondifferential) to bias to the null. Therefore, the impact of exercise on the RV may be even greater than our data suggest. Confounding by unmeasured variables or imprecisely measured variables could explain our findings. However, we adjusted for an extensive list of covariates with persistence of our findings. Although subjects could have significant pulmonary hypertension, this is unlikely in a cohort of healthy cardiovascular disease–free participants. Even if present, significant pulmonary hypertension would likely be associated with exercise limitation and less self-reported physical activity and more pronounced RV changes, negatively confounding our results, so that the actual associations between exercise and RV morphology would be even stronger than shown. There were some missing data; however, this affected relatively few participants in this large cohort and was unlikely to cause significant bias.
In summary, we have demonstrated an association between higher levels of MVPA and greater RV mass and volumes that is linear and independent of the LV. Higher levels of intentional exercise have a nonlinear association with increased RV mass and volumes, and the association with RV mass is independent of LV mass. We believe that these may be adaptive morphologic changes in response to exercise, but further studies on the mechanisms and implications of these changes in RV morphology are needed.
Acknowledgments
This manuscript has been reviewed by the MESA Investigators for scientific content and consistency of data interpretation with previous MESA publications and significant comments have been incorporated before submission for publication. The authors thank the other investigators, staff, and participants of the MESA and MESA-Lung Studies for their valuable contributions. A full list of participating MESA Investigators and institutions can be found at http://www.mesa-nhlbi.org.
Supported by National Institute of Health grants R01-HL086719 (S.M.K.), R01-HL077612 (R.G.B.), N01-HC95159 through HC95165; and N01-HC95169.
Originally Published in Press as DOI: 10.1164/rccm.201003-0469OC on September 2, 2010
Author Disclosure: C.P.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.G.B. received a sponsored grant from National Institutes of Health (NIH) for more than $100,001. W.C.J. received a sponsored grant from NIH for $50,001–$100,000. E.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.K. received a sponsored grant from NIH for more than $100,001. A.B. received consultancy fees from Tethys Bioscience for $1,001–$5,000 and a sponsored grant for $50,001–$100,000, both for diabetic research. A.B. also received a sponsored research grant from NIH for more than $100,001. J.A.C.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.A.B. is a full-time employee of NIH Clinical Center. S.M.K. received consultancy fees from Gilead and Novartis for $1,001–$5,000 each and served on the advisory board of Bayer for $1,001–$5,000, Gilead for $1,001–$5,000 and (twice) for $10,001–$50,000, and Pfizer for $5001–$10,000. S.M.K. also received lecture fees from Gilead for $1,001–$5,000 and Actelion for $1,001–$5,000. S.M.K. received sponsored grants from Actelion, Gilead, United Therapeutics, Lung Rx, Merck, and Pfizer, all for $10,001–$50,000 each, and from Bayer for $5,001–$10,000 and Pfizer for $50,001–$100,000. S.M.K. served on the advisory board of NIH for less than $1,000, and the American Lung Association for $1,001–$5,000. S.M.K. received a sponsored grant from NIH for more than $100,001.
References
- 1.Rerych SK, Scholz PM, Sabiston DC Jr, Jones RH. Effects of exercise training on left ventricular function in normal subjects: a longitudinal study by radionuclide angiography. Am J Cardiol 1980;45:244–252. [DOI] [PubMed] [Google Scholar]
- 2.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete's heart. A meta-analysis of cardiac structure and function. Circulation 2000;101:336–344. [DOI] [PubMed] [Google Scholar]
- 3.Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W. Athlete's heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol 2002;40:1856–1863. [DOI] [PubMed] [Google Scholar]
- 4.Perseghin G, De Cobelli F, Esposito A, Lattuada G, Terruzzi I, La Torre A, Belloni E, Canu T, Scifo P, Del Maschio A, et al. Effect of the sporting discipline on the right and left ventricular morphology and function of elite male track runners: a magnetic resonance imaging and phosphorus 31 spectroscopy study. Am Heart J 2007;154:937–942. [DOI] [PubMed] [Google Scholar]
- 5.Kasikcioglu E, Oflaz H, Akhan H, Kayserilioglu A. Right ventricular myocardial performance index and exercise capacity in athletes. Heart Vessels 2005;20:147–152. [DOI] [PubMed] [Google Scholar]
- 6.Erol MK, Karakelleoglu S. Assessment of right heart function in the athlete's heart. Heart Vessels 2002;16:175–180. [DOI] [PubMed] [Google Scholar]
- 7.Aaron CP, Tandri H, Barr RG, Johnson C, Bagiella E, Chahal H, Jain A, Kizer J, Lima JA, Bluemke DA, et al. Physical activity and right ventricular structure and function: The MESA-Right Ventricle Study [abstract]. Presented at the International Conference of the American Thoracic Society, San Diego, California, May 15–20, 2009.
- 8.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 9.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, et al. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 2006;186:S357–S365. [DOI] [PubMed] [Google Scholar]
- 10.Chahal H, Johnson W, Tandri H, Jain A, Hundley W, Barr R, Kawut S, Lima JA, Bluemke DA. Relation of cardiovascular risk factors to right ventricular structure and function as determined by magnetic resonance imaging (results from The Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 2010;106:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel-Claussen J, Finn JP, Gomes AS, Hundley GW, Jerosch-Herold M, Pearson G, Sinha S, Lima JA, Bluemke DA. Left ventricular papillary muscle mass: relationship to left ventricular mass and volumes by magnetic resonance imaging. J Comput Assist Tomogr 2006;30:426–432. [DOI] [PubMed] [Google Scholar]
- 13.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the cross-cultural activity participation study. J Womens Health Gend Based Med 1999;8:805–813. [DOI] [PubMed] [Google Scholar]
- 14.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2009;169:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multi-ethnic sample of adults: the Multi-Ethnic Study of Atherosclerosis (MESA)-Lung Study. Chest 2010;137:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turkbey EB, Jorgensen NW, Johnson WC, Bertoni AG, Polak JF, Roux AV, Tracy RP, Lima JA, Bluemke DA. Physical activity and physiological cardiac remodelling in a community setting: the Multi-Ethnic Study of Atherosclerosis (MESA). Heart 2010;96:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, Austin JH, Jiang R, Lovasi GS, Barr RG. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-Lung Study. Am J Respir Crit Care Med 2009;180:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorn GW II, Robbins J, Sugden PH. Phenotyping hypertrophy: eschew obfuscation. Circ Res 2003;92:1171–1175. [DOI] [PubMed] [Google Scholar]
- 19.Dorn GW II. The fuzzy logic of physiological cardiac hypertrophy. Hypertension 2007;49:962–970. [DOI] [PubMed] [Google Scholar]
- 20.Anversa P, Beghi C, Levicky V, McDonald SL, Kikkawa Y. Morphometry of right ventricular hypertrophy induced by strenuous exercise in rat. Am J Physiol 1982;243:H856–H861. [DOI] [PubMed] [Google Scholar]
- 21.Anversa P, Levicky V, Beghi C, McDonald SL, Kikkawa Y. Morphometry of exercise-induced right ventricular hypertrophy in the rat. Circ Res 1983;52:57–64. [DOI] [PubMed] [Google Scholar]
- 22.Anversa P, Beghi C, Levicky V, McDonald SL, Kikkawa Y, Olivetti G. Effects of strenuous exercise on the quantitative morphology of left ventricular myocardium in the rat. J Mol Cell Cardiol 1985;17:587–595. [DOI] [PubMed] [Google Scholar]
- 23.Wisloff U, Loennechen JP, Falck G, Beisvag V, Currie S, Smith G, Ellingsen O. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res 2001;50:495–508. [DOI] [PubMed] [Google Scholar]
- 24.Handoko ML, de Man FS, Happe CM, Schalij I, Musters RJ, Westerhof N, Postmus PE, Paulus WJ, van der Laarse WJ, Vonk-Noordegraaf A. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 2009;120:42–49. [DOI] [PubMed] [Google Scholar]
- 25.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med 2008;358:1370–1380. [DOI] [PubMed] [Google Scholar]
- 26.Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Picard MH, Hutter AM Jr, Wood MJ. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol 2008;104:1121–1128. [DOI] [PubMed] [Google Scholar]
- 27.Vogelsang TW, Hanel B, Kristoffersen US, Petersen CL, Mehlsen J, Holmquist N, Larsson B, Kjaer A. Effect of eight weeks of endurance exercise training on right and left ventricular volume and mass in untrained obese subjects: a longitudinal MRI study. Scand J Med Sci Sports 2008;18:354–359. [DOI] [PubMed] [Google Scholar]
- 28.de Man FS, Handoko ML, Groepenhoff H, van 't Hul AJ, Abbink J, Koppers RJ, Grotjohan HP, Twisk JW, Bogaard HJ, Boonstra A, et al. Effects of exercise training in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2009;34:669–675. [DOI] [PubMed] [Google Scholar]
- 29.Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, Meyer FJ, Karger G, Buss J, Juenger J, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 2006;114:1482–1489. [DOI] [PubMed] [Google Scholar]
- 30.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009;34:888–894. [DOI] [PubMed] [Google Scholar]
- 31.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol 2005;288:L419–L425. [DOI] [PubMed] [Google Scholar]
- 32.Turkbey B, Ozer C, Akinci D, Akpinar E. Abdominal chyloma: CT findings and percutaneous drainage. Cardiovasc Intervent Radiol 2009;32:601–602. [DOI] [PubMed] [Google Scholar]
- 33.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol 2006;48:2285–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Physical Activity Guidelines Advisory Committee. Physical activity guidelines advisory committee report. Washington, D.C.: US Department of Health and Human Services; 2008.