Abstract
Background
The mechanisms and consequences of the observed association between obesity and childhood asthma are unclear.
Objectives
To determine the effect of obesity on treatment responses to inhaled corticosteroids in asthmatic children.
Methods
We performed a post hoc analysis to evaluate the interaction between body mass index (BMI) and treatment with inhaled budesonide on lung function in the Childhood Asthma Management Program (CAMP) trial. Participants were then stratified into overweight/obese and non-overweight, and their response to inhaled budesonide was analyzed longitudinally over the 4 years of the trial.
Results
There was a significant interaction between BMI and budesonide for pre-BD FEV1/FVC (P=0.0007) and bronchodilator response (BDR) (P=0.049), and a non-significant trend for an interaction between BMI and budesonide on pre-BD FEV1 (P=0.15). Non-overweight children showed significant improvement with inhaled budesonide in lung function (FEV1, FEV1/FVC, and BDR) during the early (years 1–2) and late stages (years 3–4) of the trial. Overweight/obese children had improved FEV1 and BDR during the early but not the late stage of the trial, and showed no improvement in FEV1/FVC. When comparing time points where both groups showed significant response, the degree of improvement among non-overweight children was significantly greater than in overweight/obese children at most visits. Non-overweight children had a 44% reduction in the risk of ER visits or hospitalizations throughout the trial (P=0.001); there was no reduction in risk among overweight/obese (P=0.97).
Conclusions
Compared to children of normal weight, overweight/obese children in CAMP showed a decreased response to inhaled budesonide on measures of lung function and ER visits/hospitalizations for asthma.
Keywords: Asthma, obesity, pediatric asthma, childhood obesity, budesonide
INTRODUCTION
Asthma and obesity are major public health concerns. Over the last few decades, the prevalence of both diseases has increased worldwide (1–4). In the U.S., the prevalence of overweight increased from 6.5% to ~19% in school-aged children between 1976–1980 and 2003–2004 (5,6).
There is ample evidence of an association between obesity and asthma in children and adults (7–15). A recent meta-analysis of 12 longitudinal studies found that children with high birth weight and/or high body mass index (BMI) had an increased risk of developing asthma (7). The mechanisms underlying the association between obesity and asthma are incompletely understood but may include genetic predisposition, abnormal immune-modulation and/or a pro-inflammatory state in obese individuals, hormonal influences, and mechanical effects (e.g. certain studies have reported decreased FEV1 and FVC in morbidly obese adult asthmatics, a restrictive deficit likely caused by the increased amount of adipose tissue on the chest wall and in the abdominal cavity)(9,16).
Little is known about treatment responses in children with asthma and overweight or obesity. Because obesity may promote inflammation, we hypothesized that overweight/obese asthmatics would have suboptimal response to anti-inflammatory medications compared to non-overweight/non-obese asthmatics. In this report, we demonstrate that the effect of inhaled budesonide on lung function and clinical outcomes is reduced in overweight or obese asthmatic children compared to non-overweight asthmatics.
METHODS
Study population
The Childhood Asthma Management Program (CAMP) study is a randomized clinical trial that enrolled 1,041 children with asthma between 1993 and 1995. A detailed description of the trial has been previously published (17). Inclusion criteria were age 5–12 years, a history of asthma for at least 6 months in the previous year, mild to moderate asthma severity, and airway responsiveness to <12.5 mg/ml of methacholine. Subjects were randomly assigned to one of three inhaled treatment arms (budesonide 200 mcg twice daily, nedocromil 8mg twice daily, or placebo twice daily) and were followed every 4 months for 4 years. The institutional review board at each of the participating centers approved the study, and parents or guardians of the participating children gave informed consent. The present study is a post hoc analysis using data from CAMP.
Pulmonary function tests (PFTs)
Spirometry testing was performed according to American Thoracic Society (ATS) criteria (17). The completion rate for lung function measures during the trial was ~94%.
Serum 25-Hydroxy Vitamin D3 (vitamin D)
Measurement of serum 25-hydroxyvitamin D (“vitamin D”) was performed on all subjects using serum banked at the start of the trial (18). Levels were log10-transformed for analysis.
Outcome measures
Our main outcomes were pre-bronchodilator FEV1 and FEV1/FVC, and bronchodilator response (BDR). BDR was defined as the percentage change in FEV1 from baseline [(post FEV1 – pre FEV1)/pre FEV1]. Secondary asthma-related outcomes included the number of prednisone bursts and the number of ER/urgent care visits and hospitalizations reported for each visit interval during the trial.
Overweight/obese status
The Centers for Disease Control and Prevention (CDC) defines “overweight” as having a BMI ≥ 85 percentile (pct) for age and gender, and “obesity” as a BMI ≥ 95pct (19). To attain maximal power and due to clinical considerations, we grouped both categories into one: participants were classified as “overweight/obese” if their randomization BMI was ≥ 85pct, and as “non-overweight” if it was <85pct. BMI data were available for 1027 (98.7%) participants.
Statistical analysis
We analyzed data for each outcome from randomization through month 48 post-randomization. As previously done (12), the placebo and nedocromil treatment arms were combined into one because of lack of effect of nedocromil on lung function and to maximize statistical power. All multivariate analyses were adjusted for age and height at randomization, gender, race/ethnicity, duration of asthma (age at randomization – self-reported age of onset of asthma symptoms), environmental tobacco exposure (ETS) in early life (parental report of ETS in the child’s household during the first ~5 years of life, from birth to first grade), vitamin D level (at randomization), and study center.
To assess the longitudinal effect of inhaled budesonide on lung function over the 4-year course of the trial, we used mixed-effects regression models incorporating all available measurements. Residual maximum likelihood estimation with a spatial-exponential covariance structure was used, since measurements were obtained at different intervals. Fixed-effects test statistics were adjusted using the “sandwich” error estimator. P-values for the overall effect of treatment arm are from χ2 tests with n-1 degrees of freedom, where n is the number of measurements for each outcome; the overall longitudinal effect was divided into an early stage (months 0–20) and a late stage (months 24–48) of the trial. When reported, P-values at each time point are from t-tests within the mixed effects regression model. For count data (prednisone bursts) and binary outcomes (ER visits/hospitalizations) we used marginal logistic regression models with Poisson distribution and marginal log-linear regression models, respectively.
To assess the interaction between BMI and budesonide beyond their main effects, the initial longitudinal analysis included the main effects for BMI (as a continuous variable) and treatment arm (budesonide vs. placebo/nedocromil), as well as an interaction term (BMI*budesonide). Once the significance of the interaction was established, all subsequent analyses were performed by stratifying children according to their BMI, as above, in order to maximize power and to present data based on a clinically relevant definition. All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics of the study population are presented in Table I. As expected from randomization, there were no differences in most subject characteristics among treatment arms at baseline within each BMI group (overweight/obese vs. non-overweight). However, serum vitamin D levels were slightly higher in children on inhaled budesonide than in those on inhaled placebo or nedocromil, regardless of BMI group. The mean age of the 1,041 participating children was 8.9 years, with a mean duration of asthma of ~6 years and a mean BMI percentile of 63.2. Of these 1,041 children, ~60% were male and ~68% were white. As expected in a study of children with mild to moderate asthma, the mean pre-bronchodilator FEV1 was normal (93.7% of predicted) but the mean FEV1/FVC was slightly reduced (79.6%); the mean BDR was 10.8% of baseline FEV1.
Table I.
Characteristics of study participants at randomization
| Non-overweight | Overweight/obese | |||||
|---|---|---|---|---|---|---|
| Budesonide | Placebo/nedocromil | All | Budesonide | Placebo/nedocromil | All | |
| N | 205 | 500 | 705 | 103 | 219 | 322 |
| Age (yrs) | 9.0 (2.04) | 8.7 (2.12) | 8.8 (2.10)* | 9.1 (2.11) | 9.3 (2.12) | 9.2 (2.11) |
| Duration of asthma (yrs) | 5.9 (2.5) | 5.7 (2.6) | 5.8 (2.6)* | 6.1 (2.6) | 6.2 (2.7) | 6.1 (2.7) |
| Male gender | 59% | 61% | 60% | 58% | 59% | 59% |
| Race: White | 70% | 73% | 72%* | 55% | 62% | 60% |
| African-American | 11% | 11% | 11%* | 20% | 17% | 18% |
| Hispanic/Other | 19% | 16% | 17% | 24% | 21% | 22% |
| Tobacco exposure | 38% | 35% | 36% | 36% | 43% | 41% |
| BMI (percentile) | 50.4 (22.8) | 48.8 (23.7) | 49.3 (23.5)* | 93.9 (4.1) | 93.5 (4.3) | 93.6 (4.2) |
| BMI (absolute) | 16.5 (1.5) | 16.3 (1.6) | 16.4 (1.6)* | 22.2 (3.7) | 22.1 (3.3) | 22.1 (3.4) |
| Height (cm) | 132.5 (12.8) | 131.2 (13.5) | 131.5 (13.3)* | 137.3 (14.4) | 138.1 (13.6) | 137.8 (13.8) |
| Pre FEV1 (%pred) | 93.2 (14.7) | 93.7 (13.9) | 93.6 (14.1) | 94.2 (13.8) | 93.9 (15.0) | 94.0 (14.6) |
| Pre FEV1/FVC (%) | 79.9 (8.8) | 80.0 (8.3) | 80.0 (8.4)* | 78.3 (7.7) | 79.1 (8.3) | 78.9 (8.1) |
| BDR (FEV1 % change) | 11.9 (11.4) | 10.8 (10.0) | 11.1 (10.4) | 9.8 (9.2) | 10.1 (10.1) | 10.0 (9.8) |
| Vitamin D (log10) | 1.58 (.17)† | 1.54 (.19) | 1.55 (.18)* | 1.55 (.19)† | 1.49 (.19) | 1.51 (.19) |
| Total IgE (IU/mL)1 | 398 (145–1072) | 452 (186–1259) | 427 (178–1175) | 490 (191–1047) | 468 (162–1445) | 468 (174–1259) |
| Eosinophils (cell/mm3)1 | 398 (200–653) | 407 (200–646) | 398 (200–647) | 339 (180–543) | 390 (200–676) | 355 (200–617) |
| Compliance2 | 1.05 (.26) | 1.08 (.33) | 1.07 (.32) | 1.03 (.17) | 1.05 (.26) | 1.04 (.24) |
| Household income3 | 3.1 (.98) | 3.1 (.90) | 3.1 (.92) | 3.0 (1.03) | 3.2 (.88) | 3.1 (.94) |
| Parental education level4 | 5.2 (.80) | 5.2 (.86) | 5.2 (.84) | 5.1 (1.03) | 5.2 (.75) | 5.2 (.85) |
Numbers shown are mean (SD) for continuous variables, and percentage of subjects in group for categorical variables. BDR: Bronchodilator responsiveness.
P<0.05 for the comparison by BMI group (all subjects).
P<0.05 for the comparison by treatment arm (budesonide vs. placebo/nedocromil) within each BMI group.
Presented as median (IQR) and analyzed as log10. Categorical variables:
Subjective report of compliance by the treating physician (most of the time; some of the time; or rarely).
Yearly combined household income (<$15,000; $15,000–$30,000; $30,000–$50,000; or >$40,000).
Highest parental education level (<8th grade; completed 8th; some high school; completed high school; some college or post-high school training; or completed college).
Of the 1,027 participating children who had data for BMI at randomization, 322 (31.4%) were overweight/obese (176 overweight and 146 obese). Compared to non-overweight children, overweight/obese children were more likely to be African American (p=0.002), to be older (by ~0.4 years, p=0.002) and taller (by ~6 cm, p <0.0001), and to have a longer duration of asthma symptoms (by ~0.3 years, p=0.02), a lower FEV1/FVC ratio (~1.1% lower, p=0.046), and a lower vit.D level. There were no differences in total IgE level or eosinophil count between the BMI groups or the treatment arms.
Table II summarizes the adjusted longitudinal analysis of the relations among BMI, treatment with inhaled budesonide, and lung function. Both BMI (as a continuous variable) and treatment arm (budesonide vs. placebo/nedocromil) had significant effects on lung function. Beyond those effects, there was a significant interaction between BMI and budesonide (BMI*budesonide) on FEV1/FVC and BDR, and a non-statistically significant trend on percent-predicted FEV1 (p=0.15). This means that, among children treated with budesonide, each 1% increase in BMI would diminish their response to budesonide treatment by ~0.04% in FEV1/FVC (95% CI = 0.02–0.06), and by ~0.025% in BDR (95% CI = 0.0001–0.05).
Table II.
Longitudinal Analysis of the Relation Among BMI, Use of Inhaled Budesonide, and Measures of Lung Function
|
P-values from adjusted longitudinal analysisll |
|||
|---|---|---|---|
| FEV1 (%pred)* | FEV1/FVC* | BDR | |
| Time† | <0.0001 | <0.0001 | <0.0001 |
| Budesonide‡ | <0.0001 | <0.0001 | <0.0001 |
| BMI (percentile) | <0.0001 | 0.001 | 0.04 |
| BMI* budesonide | 0.15 | 0.0007 | 0.049 |
| Gender | <0.0001 | <0.0001 | 0.36 |
| Age (years) | 0.02 | 0.29 | 0.29 |
| Height (cm) | 0.002 | 0.003 | 0.4 |
| Duration of asthma | 0.07 | 0.004 | 0.005 |
| Tobacco exposure | 0.02 | 0.91 | 0.6 |
| Race§: African-American | 0.56 | 0.19 | 0.01 |
| Hispanic/Other | 0.009 | 0.96 | 0.43 |
| Vitamin D (log10) | 0.03 | 0.92 | 0.17 |
Shown are the P-values for each variable from the adjusted longitudinal analysis. The interaction between BMI (as a continuous variable) and treatment with budesonide is highlighted in grey. Both budesonide and BMI had significant effects on all three lung function measures (P<0.05), as do some of the covariates. There was a significant interaction between budesonide treatment and BMI (budesonide*BMI) for FEV1/FVC and for BDR.
Pre-bronchodilator FEV1 and FEV1/FVC.
As months of follow-up during CAMP.
Effect of budesonide compared to placebo/nedocromil.
Compared to non-Hispanic whites.
All models were adjusted for all of the variables listed in the first column.
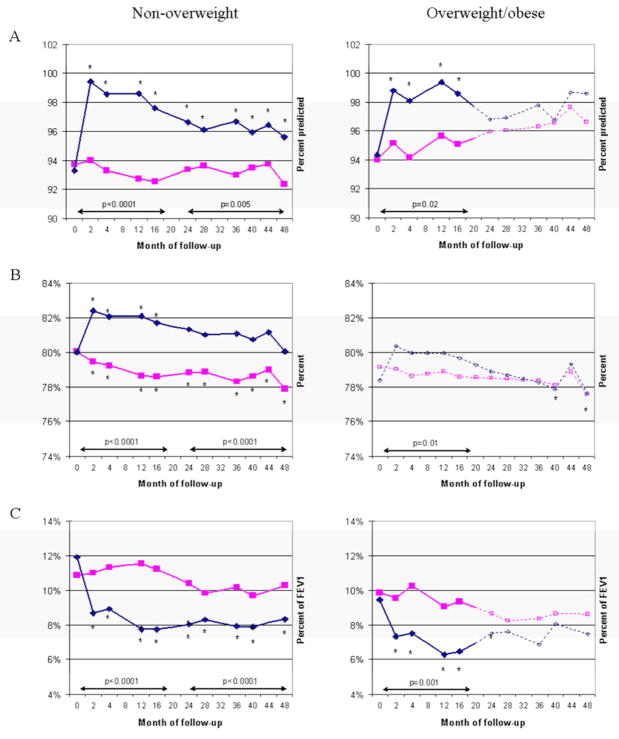
Figure 1 depicts the mean responses to inhaled budesonide (assessed by percent-predicted FEV1, FEV1/FVC, and BDR) from randomization to month 48 of the trial, stratified by BMI group (overweight/obese vs. non-overweight). Non-overweight children showed a significant improvement in all of the outcomes (FEV1, FEV1/FVC and BDR) during the early (months 0–20) and late (months 24–48) stages of the trial. Among overweight/obese children, there was significant improvement in FEV1 and BDR during the early stage of the trial but not thereafter. Overweight/obese children had no improvement in FEV1/FVC at any point during follow-up.
Figure 1. Effect of budesonide on measures of lung function and bronchodilator response by BMI group.
A. Pre FEV1 (% of predicted); B. Pre FEV1FVC; C. BDR. Budesonide in blue/diamonds; placebo/nedocromil in red/squares. Solid lines indicate time points where difference between treatment arms was significant; non-significant visits in dotted lines. Stars show points where there was a significant difference between arms and from baseline. Arrows and p-values are for overall longitudinal effect of budesonide over months 0–20 or 24–48, compared to baseline.
We then analyzed the magnitude of improvement with budesonide at each individual time point (Table III). During the early stage of the trial (0–20 months), non-overweight children had a significant improvement with inhaled budesonide: FEV1 improved by 5.1–5.8% of predicted, FEV1/FVC improved by 2.8–3.5%, and BDR decreased by 2.3–3.8%. Among overweight/obese children, inhaled budesonide produced a significant improvement in FEV1 (~3.5%–3.9% of predicted) but not in FEV1/FVC or BDR during the early stage of the trial. During the late stage of the trial (24–48 months), non-overweight children continued to show a significant improvement: FEV1 improved by 2.5–3.7% of predicted, FEV1/FVC improved by 2.1–2.8%, and BDR decreased by 1.5–2.4%. In contrast, overweight/obese children showed no significant response to inhaled budesonide at any point in the late stage of the trial. At most time points, the improvement in the non-overweight group was significantly greater than the improvement among the overweight/obese.
Table III.
Improvement in lung function in the budesonide arm, at each visit
| Month of follow-up | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 12 | 16 | 24 | 28 | 36 | 40 | 48 | |
| FEV1 (%pred) | ||||||||||
| Non-overweight | ns | 5.4* | 5.3* | 5.8 | 5.1 | 3.3* | 2.5* | 3.7 | 2.5* | 3.3* |
| Overweight | ns | 3.6 | 3.9 | 3.7 | 3.5 | ns | ns | ns | ns | ns |
| FEV1/FVC (%) | ||||||||||
| Non-overweight | ns | 2.9* | 2.8* | 3.5* | 3.1* | 2.5* | 2.1* | 2.8* | 2.1* | 2.1* |
| Overweight | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| BDR (%FEV1) | ||||||||||
| Non-overweight | ns | −2.3 | −2.4 | −3.8* | −3.5* | −2.4* | −1.5* | −2.3* | −1.8* | −1.9* |
| Overweight | ns | −2.4 | −2.9 | −2.9 | −3.1 | ns | ns | ns | ns | ns |
Predicted improvement in lung function with budesonide (compared to placebo/nedocromil) at each individual visit, based on means from multivariate mixed effects regression models. ns: No statistically significant improvement with budesonide at that time point.
p<0.05 for comparison between the improvement in the overweight vs the non-overweight group (t-test for the difference of the means from the longitudinal models, assuming all other covariates equal).
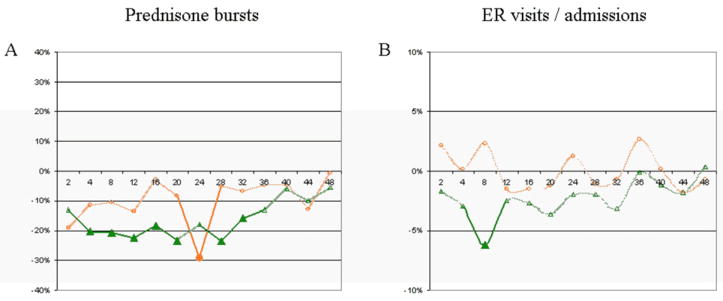
The effect of inhaled budesonide on asthma-related outcomes after stratification by BMI group is shown in Figure 2. Non-overweight children on inhaled budesonide had a significant overall reduction in the number of prednisone bursts reported at each visit (p<0.0001). When evaluating individual visits by non-overweight children during the trial, there was a significant decrease in the number of prednisone bursts in the budesonide arm on months 4, 8, 12, 16, 20, 28, and 32: non-overweight children on inhaled budesonide received 32–55% fewer prednisone bursts than children on inhaled placebo/nedocromil across these visits (95% CI = 0.5%–74%). Among overweight/obese children, the overall effect of inhaled budesonide on reducing prednisone bursts was also significant (p=0.001). When evaluating individual visits by overweight/obese children during the trial, children on inhaled budesonide reported a 58% (95% CI = 14–79%) reduction in prednisone bursts at month 2, and a 77% (95% CI = 47–90%) reduction at month 24, compared to children on inhaled nedocromil or placebo; there was no significant difference in prednisone bursts between the treatment arms at the other time points.
Figure 2. Effect of budesonide on asthma-related outcomes, by overweight status.
Lines represent the difference between treatment arms (budesonide – placebo/nedocromil; negative numbers indicate an improvement in the budesonide arm compared to the non-budesonide arm). Overweight/obese group in orange/circles; non-overweight in green/triangles. A: Average number of prednisone courses per patient since the previous visit. B: Percentage of children reporting any urgent care visits or hospital admissions. Note: Solid lines/symbols represent visits where difference was significant; non-significant time points in dotted lines.
For the interval incidence of ER visits/hospitalizations, the effect of inhaled budesonide among non-overweight children was significant: the risk of requiring an ER visit or hospital admission between visits throughout the trial was reduced by ~44% (95% CI = 20.7–60.9%, p=0.001). In contrast, among overweight/obese children there was no significant difference in ER visits or hospital admissions between the treatment arms (p=0.97).
DISCUSSION
To our knowledge, this is the first report of modification of the effect of inhaled corticosteroids (ICS) on pediatric asthma control by overweight/obesity status. Among children in a large multi-center clinical trial, non-overweight children had more consistent and significant effects of inhaled budesonide on measures of lung function and asthma morbidity and severity than overweight/obese children.
FEV1 and FEV1/FVC are widely used in clinical practice and constitute one of the components in the Guidelines for the Diagnosis and Management of Asthma from the National Heart, Lung and Blood Institute (NHLBI) to assess asthma severity and asthma control, and to adjust management (20). Decreased ICS response in FEV1 and FEV1/FVC has been reported in adults followed for 6–12 months (21). In our study, children of normal weight on inhaled budesonide showed a significant improvement in FEV1, FEV1/FVC and bronchodilator response throughout the 4 years of the trial. Overweight/obese children had an improvement in FEV1 that was of lesser magnitude, and it was limited to the first half of the trial; they also showed no improvement in their FEV1/FVC. By the latter half of the trial, overweight/obese children showed no improvement in any of the measures of lung function. A secondary analysis using BMI z-scores instead of BMI percentiles yielded similar results (data not shown).
Moreover, we performed an exploratory analysis including only children who belonged to the same BMI category at all time points, and excluding all children whose BMI crossed the 85th percentile at any time during the trial: the effects seen on FEV1 in the overweight/obese group at months 2, 4, 12, and 16 became non-significant, while all time points remained significant and of the same magnitude for the non-overweight (data not shown). Although this exploratory analysis needs to be interpreted with caution, it suggests that the initial response seen for FEV1 in overweight/obese asthmatics may have been driven by a subgroup of children who were “incorrectly” classified as overweight/obese but who had normal BMI after randomization.
Many mechanisms have been suggested to explain the relationship between asthma and obesity. Studies in morbidly obese adult asthmatics have reported symmetrically reduced FEV1 and FVC with a normal FEV1/FVC (22,23), pointing towards a restrictive pattern that could explain increased dyspnea and other symptoms by mechanical effects (24). However, some pediatric studies have found that overweight asthmatic children have low FEV1/FVC, compatible with an obstructive deficit (12,25). We found a similar pattern, with overweight/obese children in CAMP showing low FEV1/FVC. More importantly, inhaled budesonide failed to improve the obstructive deficit observed in these children.
There is increasing evidence of shared genetic determinants of asthma and obesity. The genes for the β2-adrenergic (ADRB2) and the glucocorticoid (NR3C1) receptors are located on chromosome 5q, and have been implicated in pathways related to both asthma and obesity (15,26,27). Tumor necrosis factor α (TNF-α) haplotypes have been associated with asthma and airway hyper-responsiveness (28) and with obesity (29). Recently the gene for protein kinase Cα (PRKCA) was reported to be associated with both asthma and BMI (30). Similarly, different genetic polymorphisms could reduce the efficacy of inhaled steroids by conferring obese asthmatics higher resistance, lower receptor binding, and/or lower retention of the medication in the lung.
There also is evidence of a generalized pro-inflammatory state in obesity, with several cytokines and chemokines increased in obese individuals (31). TNF-α and interleukin 6 (IL-6) are produced by adipocytes and correlate with total body fat (32); TNF-α increases production of IL- 6 and IL-1b, which are all elevated in both obesity and asthma (33). Adipose tissue can express other pro-inflammatory molecules such as transforming growth factor β1 (TGF-β1)(34), which has also been linked to asthma and asthma exacerbations (35). Similarly, polymorphysms of the fractalkine CX3CR1 receptor have been linked with asthma, atopy, and obesity (36,37).
Inhaled steroids may be less effective in overweight and obese asthmatics, in whom the inflammatory state might have a systemic component rather than be confined to the airways. Peters-Golden et al. reported decreased effect of inhaled beclomethasone on asthma control days and night-time awakenings for asthma with increasing BMI, whereas the response to oral montelukast was not affected (38). The β-isoform of the glucocorticoid receptor (GRβ) has been associated with steroid resistance in asthma (39): GRβ does not activate glucocorticoid- responsive genes, but it strongly inhibits the activation of such genes by GRα, which is the active isoform. Cytokines associated with obesity such as TNF-α, IL-6, etc., regulate GR expression with accumulation of GRβ (40). Sutherland et al. demonstrated that increasing BMI in asthmatics produced a decreased in vitro response to glucocorticoids (e.g. blunted inhibition of mitogen-activated protein kinase phosphatase-1, MKP1, and a consequent increase in TNF-α) both in blood mononuclear cells and in bronchoalveolar lavage cells, with no such effect in non- asthmatic controls (41).
Other mechanisms postulated include differences in the hormonal milieu between obese and lean individuals. Adipocytes secrete a number of substances that may influence airway inflammation and reactivity. Shore et al. have shown that leptin can increase IgE and airway responsiveness after allergen exposure in murine models (42). Adiponectin is the only adipokine reduced in obesity, and it has been shown that exogenous adiponectin reduces or abolishes airway inflammation and responsiveness in mice exposed to inhaled allergens (43); recently, Kattan et al. reported that adiponectin was associated with increasing FEV1/FVC and decreasing asthma symptoms and exacerbations in asthmatic teenage males (44). Our group has reported that reduced Vit.D levels are associated with asthma severity (45) and Vit.D levels differ between lean and obese subjects. Accordingly, we showed decreased serum levels of Vit.D among overweight/obese participants, and these levels were significantly associated with some of our outcomes. However, the interaction between BMI and budesonide remained significant after adjustment for Vit.D levels.
Medication compliance plays an important role in asthma control (46). Gamble et al. reported that up to 35% of patients with severe asthma filled less than 50% of their inhaled medication prescriptions (47). While they did not find a difference in compliance between obese and non-obese asthmatics, obesity is associated with depression and other factors (48,49) that could decrease compliance with asthma medications. However, our results were unchanged by further adjustment for medication compliance (as reported by the child or the CAMP physician) or indicators of socioeconomic status.
Despite our incomplete understanding of the mechanisms involved, overweight and obese asthmatics report a higher prevalence and severity of symptoms than non-overweight asthmatics (7,50). In our analysis, non-overweight children receiving budesonide had a significant decrease in the number of prednisone courses between visits, when looking at either the overall effect over time or at individual visits. Overweight/obese children had an overall accumulated improvement over time, but only 1 visit (at 24 months) showed significant interval improvement. Finally, children of normal weight had a significant reduction in the incidence of ER visits and hospitalizations during the trial, while there was no improvement in the overweight/obese group.
Of interest, overweight/obese children receiving placebo had a steady increase in FEV1 as percent of predicted during the 4 years of the trial (i.e., FEV1 increased ~0.5%/year from ~94% to ~96%, whereas children of normal weight had stable levels at ~93–94%). Rather than an “improvement” in overweight/obese asthmatics, this might represent residual confounding by BMI in the equations used to calculate predicted values. Because current pediatric reference values may thus underestimate the effect of high BMI on lung function.
There are several limitations to this study. First, this is a post hoc analysis of a randomized clinical trial. Bias could thus be present that we are not aware of (e.g., children were not randomized based on their BMI status; there may be other unmeasured characteristics which account for our findings). The probability of a Type I error in subgroup analysis increases significantly with the number of subgroups tested (51). Second, CAMP excluded children with severe asthma, and thus we had limited ability to evaluate modification of the effect of inhaled budesonide by BMI in these children. . Similarly, given that the budesonide doses were predetermined in the trial, we could not assess whether higher doses would have been effective in overweight children. Although frequent systemic steroid use in poorly controlled asthmatics can lead to overweight, this is an unlikely explanation for our findings, as there was no difference in the number of days on oral corticosteroids in the 6 months prior to the study between overweight and non-overweight children (2.6 +/− 4.9 vs. 2.9 +/−5.1, P=0.43). Finally, our power to assess small differences in the overweight/obese subgroup may have been suboptimal, particularly for binary and count data such as hospitalizations or number of prednisone courses.
In summary, we found that the effect of budesonide on measures of lung function and clinical outcomes among overweight/obese asthmatic children was of lesser significance and/or magnitude than in non-overweight children with asthma. The treatment of asthma in overweight/obese children may require new approaches such as a simultaneous management of obesity and/or treatment of systemic inflammation.
Acknowledgments
Sources of funding: The Childhood Asthma Management Program trial and CAMP Continuation Study were supported by contracts NO1-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052 with the National Heart, Lung, and Blood Institute and General Clinical Research Center grants M01RR00051, M01RR0099718-24, M01RR02719-14, and RR00036 from the National Center for Research Resources.
We thank all participants and their families for their invaluable participation in the Childhood Asthma Management Program (CAMP) study. We acknowledge the CAMP investigators and research team for their help in data collection.
Abbreviations
- BDR
Bronchodilator reactivity
- BMI
Body mass index
- CAMP
Childhood Asthma Management Program
- ER
Emergency room
- ETS
Environmental tobacco exposure
- FEV1
Forced expiratory volume in the first second
- FVC
Forced vital capacity
- ICS
Inhaled corticosteroids
- IgE
Immuneglobulin E
- Pct
Percentile
- PFT
Pulmonary function test
CAMP Credit Roster
Source of funding
The Childhood Asthma Management Program trial and CAMP Continuation Study were supported by contracts NO1-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052 with the National Heart, Lung, and Blood Institute and General Clinical Research Center grants M01RR00051, M01RR0099718-24, M01RR02719-14, and RR00036 from the National Center for Research Resources.
Members of the CAMP Research Group:
Clinical centers
ASTHMA, Inc, Seattle, WA: Paul Williams, MD (Principal Investigator); Mary V. Lasley, MD (Co-Director); Tamara Chinn, MSN, ARNP (Coordinator). Michele Hinatsu, MSN, ARNP; Clifton T. Furukawa, MD; Leonard C. Altman, MD; Frank S. Virant, MD; Michael S. Kennedy, MD; Jonathan W. Becker, MD; Stephen Tilles, MD; Miranda MacLaren. C. Warren Bierman, MD (1992–1997); Dan Crawford, RN (1996–2002); Thomas DuHamel (1991–2004); Heather Eliassen, BA (1996–1999); Babi Hammond (1996–1999); Dominick A. Minotti, MD (1992–2003); Chris Reagan (1992–2003); Gail Shapiro (1991–2006, Principal Investigator); Marian Sharpe, RN (1992–1994); Ashley Tatum, MD (2004–2007); Grace White (1991–2007). Timothy G. Wighton, PhD (1994–1998).
Brigham & Women's Hospital, Boston, MA: Anne Fuhlbrigge, MD (Principal Investigator); Anne Plunkett, NP, MS (Coordinator). Nancy Madden, RN, BSN; Mark Boehnert, MD; Christine Darcy; Anita Feins, MD; Natalia Kandror, MD; Kelly MacAulay, MD; Scott Weiss MD. Walter Torda, MD (Co-Investigator Director, 1993–2003); Martha Tata, RN (1993–2002); Sally Babigian, RN (1997–1999); Peter Barrant, MD (2004–2007); Linda Benson (1998–2004); Jose Caicedo (1998–1999); Tatum Calder (1998–2001); Anthony DeFilippo (1994–2000); Cindy Dorsainvil (1998–2001); Julie Erickson (1998–1999); Phoebe Fulton (1997); Mary Grace, RN (1994–1996); Jennifer Gilbert (1997–1998); Dirk Greineder, MD (1993–2000); Stephanie Haynes (1993–1998); Margaret Higham, MD (1996–1998); Deborah Jakubowski (1999); Susan Kelleher (1993–1997); Jay Koslof, PhD (1993–1995); Dana Mandel (1996–1998); Patricia Martin (2001–2003); Agnes Martinez (1994–1997); Jean McAuliffe (1994–1995); Erika Nakamoto (2002–2004); Paola Pacella (1993–1998); Paula Parks (1993–1995); Johanna Sagarin (1998–1999); Kay Seligsohn, PhD (1995–2004); Susan Swords (2003–2005); Meghan Syring (1998–2001); June Traylor, MSN, RN (1996–1998); Melissa Van Horn, PhD (1996–1999); Carolyn Wells, RN (1993–1995); Ann Whitman, RN (1994–1996).
The Hospital for Sick Children, Toronto, Ontario, Canada: Hartmut Grasemann, MD (Principal Investigator); Melody Miki, RN, BSN (Coordinator); Melinda Solomon, MD; Padmaja Subbarao, MD. Ian MacLusky, MD, FRCP (Director 1999–2007); Joe Reisman, MD, FRCP(C), MBA (Director, 1996–1999); Henry Levison, MD, FRCP(C) (Director, 1992–1996); Anita Hall, RN (Coordinator, 1993–2007). Yola Benedet (1994–1999); Susan Carpenter, RN (1998–2001); Jennifer Chay (2004); Michelle Collinson, RN (1994–1998); Jane Finlayson-Kulchin, RN (1994–1998); Kenneth Gore, MA (1993–1999); Nina Hipolito, RN (2003–2004); Noreen Holmes, RRT (1998–1999); Erica Hoorntje, RN (2002–2003); Sharon Klassen, MA(1999–2000); Joseé Quenneville, MSc (1993–1995); Renée Sananes, PhD (1993–2004); Christine Wasson, PhD (1999); Margaret Wilson, RN (2001–2002).
Johns Hopkins Asthma & Allergy Center, Baltimore, MD: N. Franklin Adkinson, Jr, MD (Director); Deborah Bull, LPN (Coordinator); Stephanie Philips, RN. Peyton Eggleston, MD (Co-Director, 1991–2004); Karen Huss, DNSc (Co-Investigator, 1991–2004); Leslie Plotnick, MD (Co-Investigator, 1991–1999); Margaret Pulsifer, PhD (Co-Investigator, 1993–2004); Cynthia Rand, PhD (Co-Investigator, 1991–2004). Elizabeth Aylward, PhD (1991–2004), Nancy Bollers, RN (Coordinator, 1993–2004); Kathy Pessaro (2004–2007); Barbara Wheeler, RN, BSN (Coordinator, 1991–1999).
National Jewish Health, Denver, CO: Stanley Szefler, MD (Director); Harold S. Nelson, MD (Co-Director); Bruce Bender, PhD (Co-Investigator); Ronina Covar, MD (Co-Investigator); Andrew Liu, MD (Co-Investigator); Joseph Spahn, MD (Co-Investigator); D Sundström (Coordinator); Melanie Phillips; Michael P. White; Melanie Gleason, PA-C; Marzena Krawiec, MD; Gary Larsen, MD; Gayle Spears, PA-C. Kristin Brelsford (1997–1999); Jessyca Bridges (1995–1997); Jody Ciacco (1993–1996); Michael Eltz (1994–1995); Jeryl Feeley, MA (Coordinator, 1992–1995); Michael Flynn (1995–1996); Tara Junk-Blanchard (1997–2000); Joseph Hassell (1992–1998); Marcia Hefner (1992–1994); Caroline Hendrickson, RN (1995–1998; Coordinator, 1995–1997); Daniel Hettleman, MA (1995–1996); Charles G. Irvin, PhD (1992–1998); Alan Kamada, PharmD (1994–1997); Sai Nimmagadda, MD (1993–1996); Kendra Sandoval (1995–1997); Jessica Sheridan (1994–1995); Trella Washington (1993–1997); Eric Willcutt, MA (1996–1997). We also thank the pediatric allergy/immunology and pulmonary fellows for their participation (Ivan Cardona, MD; Kirstin Carel, MD; Jayna Doshi, MD; Rich Hendershot, MD; Jeffrey Jacobs, MD; Neal Jain, MD; June-ku Brian Kang, MD; Tracy Kruzick, MD; Harvey Leo, MD; Beth Macomber, MD; Jonathan Malka, MD; Chris Mjaanes, MD; John Prpich, MD; Lora Stewart, MD; Ben Song, MD; Grace Tamesis, MD).
University of California, San Diego and Kaiser Permanente Southern California Region, San Diego, CA: Robert S. Zeiger, MD, PhD (Director); Noah Friedman, MD (Co-Investigator); Michael H. Mellon, MD (Co-Investigator); Michael Schatz, MD (Co-Investigator); Kathleen Harden, RN (Coordinator). Terrie Long, RN; Travis Macaraeg; Elsa Rodriguez; Eva Rodriguez, RRT. Sandra Christensen, MD (2004–2007); James G. Easton, MD (Co-Director, 1993–1994); M. Feinberg (1997–1998); Linda L. Galbreath (1991–2002); Jennifer Gulczynski (1998–1999); Ellen Hansen (1995–1997); Al Jalowayski, PhD (Co-Investigator, 1991–2005); Elaine Jenson (2004–2007); Alan Lincoln, PhD (Co-Investigator, 1991–2003); Jennie Kaufman (1994); Shirley King, MSW (1992–1999); Brian Lopez (1997–1998); Michaela Magiari-Ene, MA (1994–1998); Kathleen Mostafa, RN (1994–1995); Avraham Moscona (1994–1996); Catherine A. Nelle, RN (1991–2005); Jennifer Powers (2001–2003); Karen Sandoval (1995–1996); Nevin W. Wilson, MD (Co-Director, 1991–1993).
University of New Mexico, Albuquerque, NM: H. William Kelly, PharmD (Director); Aaron Jacobs (Co-Investigator); Hengameh H. Raissy, PharmD, PhC (Co-Investigator); Mary Spicher, RN (Coordinator). Christina Batson. Robert Annett, PhD (Co-Investigator, 1993–2004); Teresa Archibeque (1994–1999); Naim Bashir, MD (Co-Investigator, 1998–2005); H. Selda Bereket (1995–1998); Marisa Braun (1996–1999); Carrie Bush (1995–1999); Shannon C. Bush (2002–2007); Michael Clayton, MD (Co-Investigator, 1999–2001); Angel Colon-Semidey, MD (Co-Investigator, 1997–2000); Sara Devault (1993–1997); Anna Esparham (2004–2007); Roni Grad, MD (Co-Investigator, 1993–1995); David Hunt, RRT (1995–2004); Jeanne Larsson, RN (1995–1996); Katie McCallum (2009); Sandra McClelland, RN (Coordinator, 1993–1995); Bennie McWilliams, MD (Co-Investigator, Director, 1992–1998); Elisha Montoya (1997–2000); Margaret Moreshead (1996–1999); Shirley Murphy, MD (Co-Investigator, 1992–1994); Barbara Ortega, RRT (1993–1999); David Weers (1997–1998); Jose Zayas (1995–1996).
Washington University, St. Louis, MO: Robert C. Strunk, MD (Director); Leonard Bacharier, MD (Co-Investigator); Gordon R. Bloomberg, MD (Co-Investigator); Denise Rodgers, RPFT (Coordinator). Ellen Albers (1999–2003); James M. Corry, MD (Co-Investigator, 1995–2004); Karen DeMuth (2006–2007); Lila Kertz, MSN, RN, CPNP (2005–2007); Valerie Morgan, RRT (2004–2007); Cynthia Moseid (2007); Tina Oliver-Welker, CRTT (1993–2007); Deborah K. White, RPFT, RRT (1993–2007).
Resource centers
Data Coordinating Center, The Johns Hopkins University, Baltimore, MD: James Tonascia, PhD (Director). Patricia Belt; Karen Collins; Betty Collison; Ryan Colvin, MPH; John Dodge; Michele Donithan, MHS; Cathleen Ewing; Rosetta Jackson; Hope Livingston; Jill Meinert; Girlie Reyes; Michael Smith; Alice L. Sternberg, ScM; Mark L. Van Natta, MHS; Annette Wagoner; Laura Wilson, ScM; Robert Wise, MD; Katherine Yates, ScM.
Project Office, National Heart, Lung, and Blood Institute, Bethesda, MD: Virginia Taggart, MPH (Project Officer); Lois Eggers; James Kiley, PhD; Howard Moore; Gang Zheng, PhD. Paul Albert, PhD (1991–1999); Suzanne Hurd, PhD (1991–1999); Sydney Parker, PhD (1991–1994); Pamela Randall (1992–2003); Margaret Wu, PhD (1991–2001).
Committees
Data and Safety Monitoring Board: Michelle Cloutier, MD; John Connett, PhD; Leona Cuttler, MD; Frank Gilliland, MD, PhD. Clarence E. Davis, PhD (1993–2003); Howard Eigen, MD (1993–2009, Chair); David Evans, PhD (1993–2007); Meyer Kattan, MD (1993–2007); Rogelio Menendez, MD (1993–2007); F. Estelle R. Simons, MD (1993–2007); Sanford Leikin, MD (1993–1999).
Steering Committee: Robert Strunk, MD (Study Chair); N. Franklin Adkinson, MD; Robert Annett, PhD (1992–1995, 1997–1999); Bruce Bender, PhD; Mary Caesar, MHS (1994–1996); Reuben Cherniack, MD (Study Chair 1993–2007); Thomas R. DuHamel, PhD (1992–1994, 1996–1999); Anne Fuhlbrigge, MD; Hartmut Grasemann, MD; H. William Kelly, PharmD; Henry Levison, MD (1992–1996); Alan Lincoln, PhD (1994–1995); Ian MacLusky, MD (1999–2006); Bennie McWilliams, MD (1992–1998); Curtis L. Meinert, PhD; Sydney Parker, PhD (1991–1994); Joe Reisman, MD, FRCP(C), MBA (1991–1999); Denise Rodgers; Kay Seligsohn, PhD (1996–1997); Gail G. Shapiro, MD (1991–2006); Marian Sharpe (1993–1994); D Sundström (1998–1999); Stanley Szefler, MD; Virginia Taggart, MPH; Martha Tata, RN (1996–1998); James Tonascia, PhD; Scott Weiss, MD, MS; Barbara Wheeler, RN, BSN (1993–1994); Paul Williams, MD; Robert Wise, MD; Robert Zeiger, MD, PhD.
Footnotes
Clinical implications
Overweight/obese asthmatic children have a decreased response to inhaled steroids. Management of these children may require other treatment approaches, such as weight management.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kotseva K, Wood D, De Backer G, De Bacquer D, Pyorala K, Keil U. Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countries. Lancet. 2009 Mar 14;373(9667):929–40. doi: 10.1016/S0140-6736(09)60330-5. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1(1):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 3.Patel SP, Jarvelin MR, Little MP. Systematic review of worldwide variations of the prevalence of wheezing symptoms in children. Environ Health. 2008;7:57. doi: 10.1186/1476-069X-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akinbami L. The state of childhood asthma, United States, 1980–2005. Adv Data. 2006 Dec;12(381):1–24. [PubMed] [Google Scholar]
- 5.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002 Oct 9;288(14):1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006 Apr 5;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 7.Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch Dis Child. 2006 Apr;91(4):334–9. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mamun AA, Lawlor DA, Alati R, O’Callaghan MJ, Williams GM, Najman JM. Increasing body mass index from age 5 to 14 years predicts asthma among adolescents: evidence from a birth cohort study. Int J Obes (Lond) 2007 Apr;31(4):578–83. doi: 10.1038/sj.ijo.0803571. [DOI] [PubMed] [Google Scholar]
- 9.Matricardi PM, Gruber C, Wahn U, Lau S. The asthma-obesity link in childhood: open questions, complex evidence, a few answers only. Clin Exp Allergy. 2007 Apr;37(4):476–84. doi: 10.1111/j.1365-2222.2007.02664.x. [DOI] [PubMed] [Google Scholar]
- 10.Taveras EM, Rifas-Shiman S, Camargo CA, Jr, Gold DR, Litonjua AA, Oken E, et al. Higher adiposity in infancy associated with recurrent wheeze in a prospective cohort of children. J All Clin Immun. 2008;121(5):1161–6. doi: 10.1016/j.jaci.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Marcos L, Valverde-Molina J, Ortega ML, Sanchez-Solis M, Martinez-Torres AE, Castro-Rodriguez JA. Percent body fat, skinfold thickness or body mass index for defining obesity or overweight, as a risk factor for asthma in schoolchildren: which one to use in epidemiological studies? Matern Child Nutr. 2008 Oct;4(4):304–10. doi: 10.1111/j.1740-8709.2008.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003 Dec;58(12):1036–41. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rönmark E, Andersson C, Nyström L, Forsberg B, Järvholm B, Lundbäck B. Obesity increases the risk of incident asthma among adults. Eur Respir J. 2005 Feb;25(2):282–8. doi: 10.1183/09031936.05.00054304. [DOI] [PubMed] [Google Scholar]
- 14.Michelson PH, Williams LW, Benjamin DK, Barnato AE. Obesity, inflammation, and asthma severity in childhood: data from the National Health and Nutrition Examination Survey 2001–2004. Ann Allergy Asthma Immunol. 2009 Nov;103(5):381–5. doi: 10.1016/S1081-1206(10)60356-0. [DOI] [PubMed] [Google Scholar]
- 15.Ma J, Xiao L, Knowles SB. Obesity, insulin resistance and the prevalence of atopy and asthma in US adults. Allergy. 2010 May 7; doi: 10.1111/j.1398-9995.2010.02402.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006 Jul 15;174(2):112–9. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Childhood Asthma Management Program Research Group. Control Clin Trials. 1999 Feb;20(1):91–120. [PubMed] [Google Scholar]
- 18.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- 19.Defining childhood overweight and obesity [homepage on the Internet] Atlanta, GA: CDC; 2009. Aug 19, Available from: http://www.cdc.gov/obesity/childhood/defining.html. [Google Scholar]
- 20.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007 Nov;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland ER, Lehman EB, Teodorescu M, Wechsler ME. National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Body mass index and phenotype in subjects with mild-to-moderate persistent asthma. J Allergy Clin Immunol. 2009 Jun;123(6):1328–34.e1. doi: 10.1016/j.jaci.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thyagarajan B, Jacobs DR, Jr, Apostol GG, Smith LJ, Jensen RL, Crapo RO, et al. Longitudinal association of body mass index with lung function: the CARDIA study. Respir Res. 2008;9:31. doi: 10.1186/1465-9921-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Horne SL, Dosman JA. Body weight and weight gain related to pulmonary function decline in adults: a six year follow up study. Thorax. 1993 Apr;48(4):375–80. doi: 10.1136/thx.48.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002 Jul 8;162(13):1477–81. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- 25.Chu YT, Chen WY, Wang TN, Tseng HI, Wu JR, Ko YC. Extreme BMI predicts higher asthma prevalence and is associated with lung function impairment in school-aged children. Pediatr Pulmonol. 2009 May;44(5):472–9. doi: 10.1002/ppul.21023. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins GA, Amelung PJ, Smith RS, Jongepier H, Howard TD, Koppelman GH, et al. Identification of polymorphisms in the human glucocorticoid receptor gene (NR3C1) in a multi-racial asthma case and control screening panel. DNA Seq. 2004 Jun;15(3):167–73. doi: 10.1080/10425170410001704517. [DOI] [PubMed] [Google Scholar]
- 27.Buemann B, Black E, Holst C, Toubro S, Echwald S, Pedersen O, et al. The N363S polymorphism of the glucocorticoid receptor and metabolic syndrome factors in men. Obes Res. 2005 May;13(5):862–7. doi: 10.1038/oby.2005.99. [DOI] [PubMed] [Google Scholar]
- 28.Choi IW, Sun K, Kim YS, Ko HM, Im SY, Kim JH, et al. TNF-alpha induces the late-phase airway hyperresponsiveness and airway inflammation through cytosolic phospholipase A(2) activation. J Allergy Clin Immunol. 2005 Sep;116(3):537–43. doi: 10.1016/j.jaci.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 29.Castro-Giner F, Kogevinas M, Imboden M, de Cid R, Jarvis D, Machler M, et al. Joint effect of obesity and TNFA variability on asthma: two international cohort studies. Eur Respir J. 2009 May;33(5):1003–9. doi: 10.1183/09031936.00140608. [DOI] [PubMed] [Google Scholar]
- 30.Murphy A, Tantisira KG, Soto-Quiros ME, Avila L, Klanderman BJ, Lake S, Weiss ST, Celedon JC. PRKCA: a positional candidate gene for body mass index and asthma. Am J Hum Genet. 2009;85:87–96. doi: 10.1016/j.ajhg.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warnberg J, Marcos A. Low-grade inflammation and the metabolic syndrome in children and adolescents. Curr Opin Lipidol. 2008 Feb;19(1):11–5. doi: 10.1097/MOL.0b013e3282f4096b. [DOI] [PubMed] [Google Scholar]
- 32.Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J Parenter Enteral Nutr. 2004 Nov-Dec;28(6):410–5. doi: 10.1177/0148607104028006410. [DOI] [PubMed] [Google Scholar]
- 33.Tillie-Leblond I, Pugin J, Marquette CH, Lamblin C, Saulnier F, Brichet A, et al. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med. 1999 Feb;159(2):487–94. doi: 10.1164/ajrccm.159.2.9805115. [DOI] [PubMed] [Google Scholar]
- 34.Alessi MC, Bastelica D, Morange P, Berthet B, Leduc I, Verdier M, et al. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes. 2000 Aug;49(8):1374–80. doi: 10.2337/diabetes.49.8.1374. [DOI] [PubMed] [Google Scholar]
- 35.Sharma S, Raby BA, Hunninghake GM, Soto-Quiros M, Avila L, Murphy AJ, et al. Variants in TGFB1, dust mite exposure, and disease severity in children with asthma. Am J Respir Crit Care Med. 2009 Mar 1;179(5):356–62. doi: 10.1164/rccm.200808-1268OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tremblay K, Lemire M, Provost V, Pastinen T, Renaud Y, Sandford AJ, Laviolette M, Hudson TJ, Laprise C. Association study between the CX3CR1 gene and asthma. Genes Immun. 2006;7:632–639. doi: 10.1038/sj.gene.6364340. [DOI] [PubMed] [Google Scholar]
- 37.Sirois-Gagnon D, Chamberland A, Perron S, Brisson D, Gaudet D, Laprise C. Association of Common Polymorphisms in the Fractalkine Receptor (CX3CR1) With Obesity. Obesity (Silver Spring) 2010 doi: 10.1038/oby.2010.125. [DOI] [PubMed] [Google Scholar]
- 38.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006 Mar;27(3):495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 39.Sousa AR, Lane SJ, Cidlowski JA, Staynov DZ, Lee TH. Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. J Allergy Clin Immunol. 2000;105:943–950. doi: 10.1067/mai.2000.106486. [DOI] [PubMed] [Google Scholar]
- 40.Webster JC, Oakley RH, Jewell CM, Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci U S A. 2001;98:6865–6870. doi: 10.1073/pnas.121455098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178:682–687. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005 Jan;115(1):103–9. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006 Aug;118(2):389–95. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 44.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010 Mar;125(3):584–592. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009 May 1;179(9):765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams LK, Joseph CL, Peterson EL, Wells K, Wang M, Chowdhry VK, et al. Patients with asthma who do not fill their inhaled corticosteroids: A study of primary nonadherence. J Allergy Clin Immunol. 2007 Nov;120(5):1153–9. doi: 10.1016/j.jaci.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 47.Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2009 Nov 1;180(9):817–22. doi: 10.1164/rccm.200902-0166OC. [DOI] [PubMed] [Google Scholar]
- 48.Strine TW, Mokdad AH, Balluz LS, Gonzalez O, Crider R, Berry JT, et al. Depression and anxiety in the united states: Findings from the 2006 behavioral risk factor surveillance system. Psychiatr Serv. 2008 Dec;59(12):1383–90. doi: 10.1176/ps.2008.59.12.1383. [DOI] [PubMed] [Google Scholar]
- 49.Forno E, Celedon JC. Asthma and ethnic minorities: Socioeconomic status and beyond. Curr Opin Allergy Clin Immunol. 2009 Apr;9(2):154–60. doi: 10.1097/aci.0b013e3283292207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Gent R, van der Ent CK, Rovers MM, Kimpen JL, van Essen-Zandvliet LE, de Meer G. Excessive body weight is associated with additional loss of quality of life in children with asthma. J Allergy Clin Immunol. 2007 Mar;119(3):591–6. doi: 10.1016/j.jaci.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Lagakos SW. The challenge of subgroup analyses – Reporting without distorting. N Engl J Med. 2006 Apr 20;354(16):1667–9. doi: 10.1056/NEJMp068070. [DOI] [PubMed] [Google Scholar]