Abstract
SCH-23390, a selective D-1 dopamine antagonist, was found to antagonize the locomotor stimulation induced by LY-171555, an action similar to that for haloperidol in control animals. However, this action of SCH-23390 was prevented in rats treated with 6-hydroxydopamine (6-OHDA) or with reserpine plus α-methyl-tyrosine pretreatment. These results indicate that the action of SCH-23390 to antagonize D-2 dopamine receptor actions is dependent upon functional catecholamine-containing neurons. In contrast to the lack of action of SCH-23390 to antagonize LY-171555 in 6-OHDA-treated rats, SCH-23390 blocked the locomotor stimulation induced by SKF-39393, a D-1 dopamine agonist, after this treatment. Thus, D-1 dopamine receptors are distinct from D-2 receptor sites and can exhibit a behavior similar to that observed when D-2 receptors are stimulated. These data suggest that D-1 receptor sites modulate D-2 dopamine receptor function through a mechanism dependent upon functionally intact catecholamine-containing neurons.
Keywords: D-1 dopamine receptors; 6-Hydroxydopamine, neonatal; Tyrosine hydroxylase inhibition; SCH-23390; D-2 dopamine receptors; 6-Hydroxydopamine, adult; Locomotor activity; LY-171555; SKF-38393; Reserpine
1. Introduction
SCH-23390 is a compound that antagonizes dopamine stimulation of adenylate cyclase (Iorio et al., 1983) and does not displace radiolabelled butyrophenones from brain membranes (Hyttel and Christensen, 1983). Further, [3H]SCH-23390 is not displaced from brain membranes by butyrophenones, phenothizines or other types of neuroleptics (Billard et al., 1984). Thus, this benzazepine derivative is suggested to be a specific D-1 dopamine antagonist (Hyttel, 1983). In support of this view, SCH-23390 has been shown to antagonize the effects of the selective D-1 agonist, SKF-38393 (Setler et al., 1978), which induces turning after unilateral lesions to the nigrostriatal pathway (Arnt and Hyttel, 1984) and increases locomotor activity after 6-OHDA treatment (Breese et al., 1985).
In spite of this demonstrated specificity for the D-1 receptor (Billard et al., 1984; Molloy and Waddington, 1984; Breese et al., 1985), SCH-23390 also antagonizes the effects of directly acting dopamine agonists that are also antagonized by compounds classified as D-2 dopamine receptor antagonists (Iorio et al., 1983; Christensen et al., 1984a,b; Mailman et al., 1984; Breese et al., 1985). However, several investigators have demonstrated that the blocking action of SCH-23390 against D-2 dopamine agonists is reduced in 6-OHDA-treated rats (Christensen et al., 1984a,b; Breese et al., 1985). Since SCH-23390 effectively blocks the D-1 agonist, SKF-38393, in 6-OHDA-treated rats, the data suggest that in control rats SCH-23390 is not acting directly on the D-2 receptor site to antagonize the effects of dopamine agonists (Breese et al., 1985).
The present study examined the action of SCH-23390 against a specific D-2 dopamine agonist, LY-171555 (Tsuruta et al., 1981), in control rats and in 6-OHDA-treated rats (Breese et al., 1985). This investigation provides additional confirmation that SCH-23390 is a weaker inhibitor of D-2 agonist activity than of D-1 agonist effect in 6-OHDA-lesioned rats (Arnt and Hyttel, 1984). In addition, this work permitted us to establish that SCH-23390 would antagonize a specific D-2 agonist in control rats as it does other less specific dopamine agonists. Further studies were performed to test whether catecholamine-containing fibers must be destroyed to reduce the dopamine-antagonist action of SCH-23390 and if nondestructive disruption of catecholaminergic function would have a similar inhibitory effect on the action of SCH-23390 to antagonize D-2 receptor function in control rats.
2. Materials and methods
2.1. General
Male rats were supplied by Charles River Laboratories (Wilmington, Mass.). Rats were housed in environmentally controlled rooms with constant light-dark cycle (7:00-19:00 h), temperature maintained at 23-25°C, and given access to Wayne Blox Laboratory chow and water. Control rats were housed 4 per cage. Some adult rats (225 g) were given 6-hydroxydopamine (6-OHDA) hydrobromide (200 μg free base) intracisternally 30 min after 50 mg/kg pargyline; 1 week later an additional 200 μg dose of 6-OHDA was administered (Breese and Traylor, 1970). These rats were not screened for supersensitivity for at least 2 weeks after recovery from any disturbances in ingestion of food and water (i.e., total time equals approximately 4 weeks from second injection until testing). Only those adult-6-OHDA-treated rats that exhibited activity counts that were 3 times those observed in control rats after 1 mg/kg apomorphine were used for further investigations. In addition, neonates 5 days of age were given 100 μg 6-OHDA intracisternally under ether anesthesia (Smith et al., 1973; Breese et al., 1984). These rats needed no further care and were screened with L-dihydroxyphenylalanine (L-DOPA; 100 mg/kg after 50 mg/kg RO-4-4602) when only 40-50 days of age. Only those treated with 6-OHDA neonatally that were positive for self-biting were used in this investigation. The screening procedure assured that all rats were supersensitive as regards the behavioral effects of dopaminergic agonists (Breese et al., 1984). Another group of rats were treated with 2.5 mg/kg reserpine 24 h before receiving α-methyltyrosine (50 mg/kg). One hour later animals were given LY-171555 (1.0 mg/kg). Some of these animals received either 0.3 mg/kg of SCH-23390 (30 min) or 0.3 mg/kg of haloperidol (60 min) before the D-2 agonist. The 6-OHDA-treated rats received haloperidol (0.3 mg/kg) and SCH-23390 before 0.1 and 0.3 mg/kg of LY-171555 or 3 mg/kg of SKF-38393.
2.2. Locomotor activity determinations
Locomotor activity was measured in circular activity monitors with six sensors about the periphery of the doughnut shaped units (Hollister et al., 1974). Rats were habituated to the chambers for 1 h before receiving dopamine agonists. LY-171555 was administered to adult-6-OHDA-treated rats and not neonatally-6-OHDA-treated rats because the response to this agonist is much greater in the rats lesioned as adults. SKF-38393 was administered to the rats treated neonatally with 6-OHDA because a greater response is seen in these animals than those treated as adults, in keeping with the relatively specific increase in D-1 receptor supersensitivity in the neonatal-6-OHDA-treated rats (Breese et al., 1984, 1985). SCH-23390 was administered 30 min and haloperidol 60 min before agonists. Counts were accumulated every 10 min for 2.5 h.
2.3. Biochemical investigations
Dopamine was determined in striatum by the HPLC-electrochemical detection procedure described by Kilts et al. (1981). Norepinephrine and dopamine were determined on brain remaining after dissection (6-OHDA-treated rats) and on whole brain (animals treated with reserpine) utilizing the fluorometric procedure described by Breese and Traylor (1970).
2.4. Drugs
The 6-OHDA hydrobromide was purchased from Regis Chemical Company (Chicago, Illinois). The L-α-methyltyrosine was a gift of Merck, Sharpe and Dohme (West Point, Pa.). The L-DOPA and RO-4-4602 used for screening animals were provided by Hoffman-LaRoche Co. (Nutley, N.J.). The SCH-23390 was provided by Schering Corporation (Bloomfield, N.J.) and the SKF-38393 by Smith, Kline, and French Lab (Philadelphia, PA). The LY-171555 was provided by Lilly Laboratories (Indianapolis, Ind.). The reserpine (2.5 mg/ml) used was the commercial product sold by Ciba Pharmaceutical Co. (Summit, N.J.). Haloperidol was provided by McNeil Pharmaceutical (Spring House, Pa.).
2.5. Statistical analysis
Analysis of variance was applied to data. Group means were compared utilizing the Dunnett's t-test (Dunnett, 1955) when multiple comparisons among groups were required. When only the experimental and a control group were compared, Student's t-test was used.
3. Results
3.1. Effect of SCH-23390 on LY-171555-induced locomotion in control rats
Administration of SCH-23390 was found to antagonize the locomotor stimulation induced by 0.3 and 1.0 mg/kg of LY-171555, a specific D-2 receptor agonist, similar to the action of haloperidol in control rats (fig. 1).
Fig. 1.
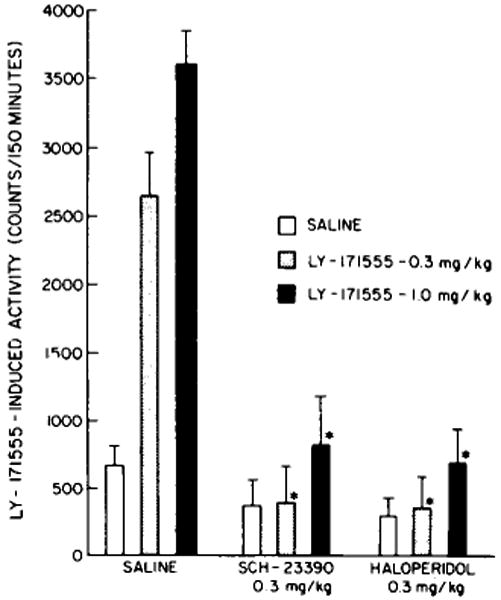
Effects of SCH-23390 and haloperidol on the locomotor stimulation induced by LY-171555 in normal rats. SCH-23390 (0.3 mg/kg i.p.) was administered 30 min and haloperidol (0.3 mg/kg i.p.) 60 min before the doses of LY-171555, i.p. The bars refer to the mean ±S.E.M. of at least 6 rats. * P < 0.001 when compared to corresponding response in saline-treated rats.
3.2. Effect of SCH-23390 on the locomotor stimulation induced by LY-171555 and SKF-38393 in 6-OHDA-treated rats
As previously described (Breese et al., 1985), LY-171555 and SKF-38393 produced a major increase in locomotor activity in adult and neonatally 6-OHDA-treated rats, respectively. In contrast to results in control rats (fig. 1), the action of LY-171555 was not antagonized by SCH-23390 in adult 6-OHDA-treated rats (fig. 2A). However, the locomotor stimulant action of SKF-383913 was blocked by SCH-23390 pretreatment in the neonatally 6-OHDA-treated rats (fig. 2B).
Fig. 2.
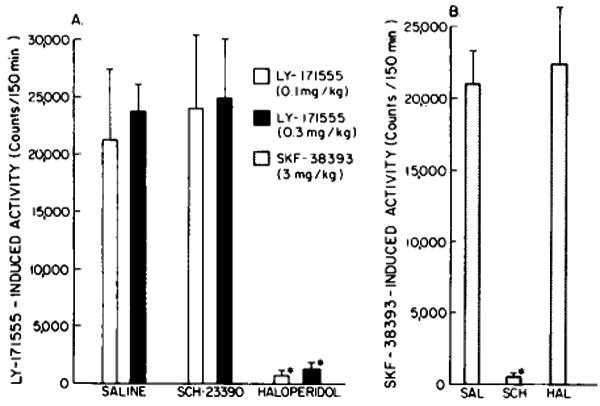
Effects of SCH-23390 (0.3 mg/kg) and haloperidol (0.3 mg/kg) on the locomotor responses induced by LY-171555 (panel A) and SKF-38393 (panel B) in 6-OHDA-treated rats. SCH = SCH-23390 and HAL = haloperidol. The LY-171555 was administered to adult-6-OHDA-treated rats and the SKF-38393 was administered to neonatal 6-OHDA-treated rats (Breese et al., 1984). There are at least 6 animals in each group. Bars refer to mean ± S.E.M. Striatal dopamine content in control animals was 101.8 ± 2.2 ng/mg protein. In neonatal 6-OHDA-treated rats (panel B), the dopamine content was 0.3 ± 0.1 ng/mg protein and in the adult 6-OHDA-treated rats was 6.9 ± 1.5 ng/mg protein. Norepinephrine was 229 ± 40 mg/g tissue in brain remaining after removal of the striatum in controls; content in adult and neonatal 6-OHDA-treated rats was 20.2 ± 3.2 and 14.1 ± 2.1 respectively. * P < 0.001 when compared to response in saline-treated rats.
3.3. Effect of reserpine and α-methyltyrosine pretreatment on the action of SCH-23390 to antagonize LY-171555-induced locomotion
Administration of reserpine and α-methyltyrosine is known to reduce drastically the amount of catecholamines in tissue (Breese et al., 1973). Therefore, in order to assess whether it was the loss of catecholamine-containing fibers or simply disruption of catecholamine availability that was crucial for the SCH-23390 antagonism of LY-171555 induced locomotion in control rats (fig. 1), the antagonism was examined in rats given the reserpine-α-methyltyrosine combination.
As shown in fig. 3, the locomotion-induced by LY-171555 in rats given the reserpine-α-methyltyrosine regimen was not antagonized by SCH-23390 as it was in control rats that received this antagonist. Haloperidol reduced LY-171555-induced activity in reserpine-treated rats as observed in the saline treated rats. It was also demonstrated that reserpine treatment alone would block the action of SCH-23390 to antagonize LY-171555 (data not shown). An unexpected aspect of this investigation was that locomotion induced by LY-171555 was significantly lower in the reserpine-α-methyltyrosine-treated rats than in control (untreated) animals (table 1).
Fig. 3.
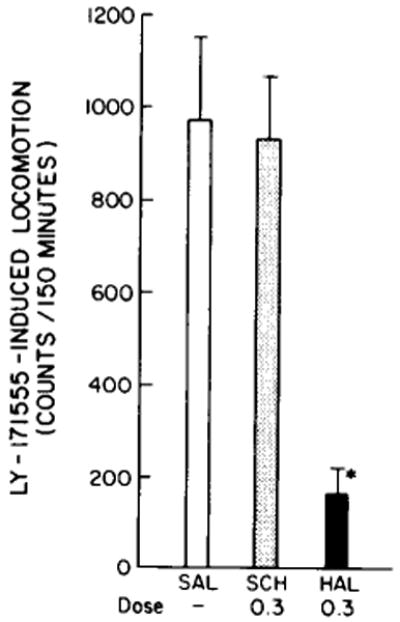
Effect of reserpine-α-methyltyrosine treatment on the action of SCH-23390 (SCH, 0.3 mg/kg) and haloperidol (HAL, 0.3 mg/kg) to antagonize LY-171555 (1 mg/kg) S = saline. The reserpine (2.5 mg/kg) was administered subcutaneously 24 h prior to the i.p. injection of α-methyltyrosine (50 mg/kg). The latter drug was injected 1 h prior to the injection of the dopamine agonists. The SCH-23390 was administered 30 min and the haloperidol 60 min before the LY-171555. There are at least 7 animals in each group. Bars refer to mean ± S.E.M. levels for dopamine and norepinephrine in whole brain were: control norepinephrine = 267 ± 11 ng/g of tissue and reserpine + α-methyltyrosine = 3.8 ± 0.7 ng/g tissue. Control dopamine = 852.5 ± 54 ng/g and reserpine + α-methyltyrosine = 23.6 ± 5.0 ng/g of brain tissue. * P < 0.01 when compared to saline.
TABLE 1.
Effect of reserpine and α-methyltyrosine on activity induced by LY-171555a.
| Treatment | N | LY-171555-induced activity (counts/150 min) |
|---|---|---|
| Saline | 7 | 2669 ± 349 |
| Reserpine + α-methyltyrosine | 6 | 972 ± 178b |
Rats were habituated to the chamber for 1 h before LY-171555 (1 mg/kg) administration. The α-methyltyrosine (40 mg/kg) was given just prior to the habituation period and 23 h after reserpine (2.5 mg/kg, subcutaneously). See fig. 3 for effects of dopamine antagonists.
P < 0.01 when compared to saline.
4. Discussion
Several investigators have reported that SCH-23390 reduces actions of dopamine agonists whose effects are also antagonized by compounds believed to interact with D-2 dopamine receptors (Iorio et al., 1983; Mailman et al., 1984; Christensen et al., 1984a,b; Breese et al., 1985). Recently, O'Boyle et al., (1984) showed that SCH-23390 antagonized the effects of RU-24213, a selective D-2 dopamine agonist. In accord with this latter result, the present study clearly demonstrates that in control rats SCH-23390 will antagonize the locomotion induced by LY-171555, another D-2 dopamine agonist (Tsuruta et al., 1981).
Several observations indicate that dopamine receptor sites designated as D-2 are distinct separate sites from those classified as D-1 dopamine receptors. For example, in 6-OHDA-treated rats, SCH-23390 antagonized the locomotor function induced by SKF-38393 but not that induced by LY-171555, consistent with our previous results (Breese et al., 1985). The locomotor stimulant effect observed here after SKF-38393 in neonatally 6-OHDA-treated rats confirms that D-1 receptors can contribute to a dopamine mediated behavior (Breese et al., 1985). Further, Arnt and Hyttel (1984) found that SKF-38393 induced turning was antagonized by SCH-23390 but not other antagonists that displace [3H]haloperidol from brain tissue. They also reported that SCH-23390 had little effect on the action of Pergolide (Arnt and Hyttel, 1984). Because of the specificity of SKF-38393 and SCH-23390, one must conclude that the site acted upon by these drugs to influence locomotor activity has properties of the D-1 dopamine receptor, as classified by Kebabian and Calne (1979). However, it is not presently known whether the site being explored here is associated with dopamine stimulated adenylate cyclase or if it is a site with similar properties to the adenylate cyclase site but not linked to this enzyme.
The present work confirms that SCH-23390 acts to antagonize locomotion induced by compounds acting on D-2 dopamine receptor sites in control rats, but not in rats treated with 6-OHDA (Breese et al., 1985; Christensen et al., 1984ab; Arnt and Hyttel, 1984). This apparent paradoxical finding has been proposed to be due to a loss of functional catecholamine-containing neurons (Breese et al., 1985). Since binding of [3H]-flupentixol and [3H]SCH-23390 is not altered in striatum of 6-OHDA-treated rats (Breese et al., 1985; unpublished data), it does not appear that removal of D-1 receptor sites is responsible for the loss of action of SCH-23390 against a D-2 agonist in 6-OHDA-treated rats (Breese et al., 1985; unpublished data). For this reason, an additional experiment was undertaken to determine whether disruption of catecholamine-containing neural function with reserpine and α-methyltyrosine would have an effect similar to 6-OHDA-treatment (Breese et al., 1973; Hollister et al., 1974). In this study, the response to LY-171555 was not antagonized by SCH-23390 in the rats treated with reserpine, as was accomplished by haloperidol administration. This experiment provides evidence that reduced catecholamine neuronal function, not the loss of the fibers, is responsible for the absence of action of SCH-23390 in 6-OHDA-treated rats to antagonize a D-2 dopamine receptor agonist. Future work will determine whether norepinephrine or dopamine is essential for the influence of SCH-23390 on D-2 receptor function. Regardless, these investigations suggest that D-1 receptors interact with D-2 dopamine receptors via a circuit that involves catecholamine-containing neurons.
The action of LY-171555 was attenuated in rats treated with reserpine + α-methyltyrosine, but potentiated in rats in which catecholamine-containing neurons were destroyed. Reserpine has been reported to attenuate bromocriptine-stimulated locomotion and stereotyped behavior (Corrodi et al., 1973; Silbergeld and Pfeiffer, 1977; Kilts et al., 1979) and the stereotyped behavior induced by Piribedil (Costall and Naylor, 1973; Kilts et al., 1979). These findings are suggestive that the actions of these compounds are dependent upon intact catecholamine stores (Kilts et al., 1979). However, like LY-171555, behavior mediated by these compounds are also potentiated by 6-OHDA treatment (Hollister et al., 1979; Kilts et al., 1979). Thus these dopamine agonists are unlike d-amphetamine which has its locomotor stimulant action blocked by 6-OHDA-treatment (Hollister et al., 1974), suggesting a different underlying neural mechanism from that induced by d-amphetamine. Whether the pharmacological profile of LY-171555 is in some way linked to the pharmacology of SCH-23390 is at present unknown. The answer to this question will have to be explored in future investigations.
Acknowledgments
The authors acknowledge the excellent assistance of Marcine Garrison and Edna Edward for biochemical work and Sue Ellis and Jeanette Carney for typing the manuscript.
References
- Arnt J, Hyttel J. Differential inhibition by dopamine D-1 and D-2 antagonists of circling behaviour induced by dopamine agonists in rats with unilateral 6-hydroxydopamine lesions. European J Pharmacol. 1984;102:349. doi: 10.1016/0014-2999(84)90267-x. [DOI] [PubMed] [Google Scholar]
- Billard W, Ruperto V, Crosby G, Iorio CS, Barnett A. Characterization of the binding of 3H-SCH-23390, a selective D-1 receptor antagonist ligand, in rat striatum. Life Sci. 1984;35:1985. doi: 10.1016/0024-3205(84)90540-x. [DOI] [PubMed] [Google Scholar]
- Breese GR, Baumeister AA, McCown TJ, Emerick SG, Frye GD, Crotty K, Mueller RA. Behavioral differences between neonatal and adult-6-hydroxydopamine treated rats to dopamine agonists: Relevance to neurological symptoms in clinical syndromes with reduced brain dopamine. J Pharmacol Exp Ther. 1984;231:343. [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Cooper BR, Smith RA. Biochemical and behavioral alterations following 6-hydroxydopamine administration into brain. In: Usdin E, Snyder S, editors. Frontiers in Catecholamine Research. Pergamon Press; New York: 1973. p. 701. [Google Scholar]
- Breese GR, Napier TC, Bondy SC, Mueller RA. Relatively selective D-1 dopamine receptor supersensitivity after neonatal-6-OHDA-treatment. Fed Proc. 1985 in press. [Google Scholar]
- Breese GR, Taylor TD. Effects of 6-hydroxydopamine on brain norepinephrine and dopamine: Evidence for selective degeneration of catecholamine neurons. J Pharmacol Exp Ther. 1970;174:413. [PMC free article] [PubMed] [Google Scholar]
- Christensen AV, Arnt J, Hyttel J, Larsen JJ, Svendsen O. Pharmacological effects of a specific dopamine D-1 antagonist SCH-23390 in comparison with neuroleptics. Life Sci. 1985b;34:1529. doi: 10.1016/0024-3205(84)90607-6. [DOI] [PubMed] [Google Scholar]
- Christensen AV, Arnt J, Svendsen O. Animal models for neuroleptic-induced neurological dysfunction. In: Usdin E, Carlsson A, Dahlstrom A, Engel J, loss R, editors. Catecholamine: Part C: Neuro-pharmacology and Central Nervous System – Therapeutic Aspects. New York: 1984a. p. 99. [Google Scholar]
- Corrodi H, Fuxe K, Hökfelt T, Lidbrink P, Ungerstedt U. Effect of ergot drugs on central catecholamine neurons: evidence for a stimulation of central dopamine neurons. J Pharm Pharmacol. 1973;25:409. doi: 10.1111/j.2042-7158.1973.tb10037.x. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. The site and mode of action of ET 495 for the mediation of stereotyped behavior in the rat. Naunyn-Schmiedeb Arch Pharmacol. 1973;278:117. doi: 10.1007/BF00500645. [DOI] [PubMed] [Google Scholar]
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Statist Assoc. 1955;50:1096. [Google Scholar]
- Hollister AS, Breese GR, Cooper BR. Comparison of tryosine hydroxylase and dopamine-β-hydroxylase inhibition with the effects of various 6-hydroxydopamine treatments on d-amphetamine induced motor activity. Psychopharmacologia. 1974;36:1. doi: 10.1007/BF00441377. [DOI] [PubMed] [Google Scholar]
- Hyttel J. SCH-23390 – The first selective dopamine D-1 antagonist. European J Pharmacol. 1983;91:153. doi: 10.1016/0014-2999(83)90381-3. [DOI] [PubMed] [Google Scholar]
- Hyttel J, Christensen AV. Biochemical and pharmacological differentiation of neuroleptic effects on dopamine D-1 and D-2 receptors. J Neural Transm Suppl. 1983;18:157. [PubMed] [Google Scholar]
- Iorio LC, Barnett A, Leitz FH, Houser VP, Korduba A. SCH-23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983;226:462. [PubMed] [Google Scholar]
- Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature (London) 1979;277:93. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Breese GR, Mailman RB. Simultaneous quantification of dopamine, 5-hyroxytryptamine and four metabolically related compounds by means of reverse phase HPLC with electrochemical detection. J Chromatogr Biol Med Appl. 1981;225:347. doi: 10.1016/s0378-4347(00)80283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Smith DA, Ondrusek MG, Mailman RB, Mueller RA, Breese GR. Differential effects of ‘dopaminergic agonists’ on measures of dopaminergic function. Soc Neurosci Abst. 1979;5:562. [Google Scholar]
- Mailman RB, Schulz DW, Lewis MG, Staples L, Rollema H, DeHaven DL. SCH-23390: a selective D-1 dopamine antagonist with potent D-2 behavioral actions. European J Pharmacol. 1984;101:159. doi: 10.1016/0014-2999(84)90044-x. [DOI] [PubMed] [Google Scholar]
- Molloy AG, Waddington TL. Dopaminergic behaviour stereospecifically promoted by the D-1 agonist R-SK&F 38393 and selectively blocked by the D-1 antagonist SCH-23390. Psychopharmacology. 1984;82:409. doi: 10.1007/BF00427697. [DOI] [PubMed] [Google Scholar]
- O'Boyle KM, Pugh M, Waddington JL. Stereotypy induced by the D2 antagonist R022-2586 and the D1 antagonist SCH 23390. Br J Pharmacol. 1984;82:242P. [Google Scholar]
- Setler PE, Sarau HM, Zirkle CL, Saunders HL. The central effects of a novel dopamine agonist. European J Pharmacol. 1978;50:419. doi: 10.1016/0014-2999(78)90148-6. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Pfeiffer RF. Differential effects of three dopamine agonists: apomorphine, bromocriptine and lergotrile. J Neurochem. 1977;28:1323. doi: 10.1111/j.1471-4159.1977.tb12327.x. [DOI] [PubMed] [Google Scholar]
- Smith RD, Cooper BR, Breese GR. Growth and behavioral changes in developing rats treated intracisternally with 6-hydroxydopamine: evidence for involvement of brain dopamine. J Pharmacol Exp Ther. 1973;185:609. [PubMed] [Google Scholar]
- Tsuruta K, Frey EA, Grewe CW, Cote TE, Eskay RL, Kebabian TW. Evidence that LY-171555 specifically stimulates the D-2 dopamine receptor. Nature (London) 1981;292:463. doi: 10.1038/292463a0. [DOI] [PubMed] [Google Scholar]


