Abstract
BACKGROUND
Post-infectious autoimmunity and immune deficiency have been implicated in the pathogenesis of Tourette syndrome (TS). We asked here whether B cell immunity of patients with TS differs from healthy subjects.
METHODS
In two independent cross-sectional samples, we compared serum levels of IgG1, IgG2, IgG3, IgG4, IgM, IgA, and IgE in 21 patients with TS from Yale University (17 males, 4 females, 8–16 years) versus 21 healthy controls (13 males, 8 females, 7–17 years); and in 53 patients with TS from Groningen University (45 males, 8 females, 6–18 years) versus 53 healthy controls (22 males, 31 females, 6–18 years), respectively. We also investigated correlations between Ig concentrations and symptom severity. In 13 additional patients (9 males, 4 females, age range 9–14), we established Ig profiles at time points before, during, and after symptom exacerbations.
RESULTS
IgG3 levels were significantly lower in Yale patients compared to healthy children (medians 0.28 versus 0.49 mg/ml, p = .04), while levels of IgG2, IgG4, and IgM in patients were lower at trend-level significance (p ≤ .10). Decreased IgG3 (medians 0.45 versus 0.52 mg/ml; p = .05) and IgM (medians 0.30 versus 0.38 mg/ml; p = .04) levels were replicated in the Groningen patients. Ig levels did not correlate with symptom severity. There was a trend-level elevation of IgG1 during symptom exacerbations (p = .09).
CONCLUSION
These pilot data indicate that at least some patients with TS have decreased serum IgG3, and possibly also IgM levels, though only few subjects had fully expressed Ig immunodeficiency. Whether these changes are related to TS pathogenesis needs to be investigated.
Search terms: Tic disorder, Tourette syndrome, symptom exacerbation, immunoglobulin
INTRODUCTION
Tourette syndrome (TS) is a chronic neuropsychiatric disorder characterized by the presence of involuntary, rapid, recurrent, non-rhythmic movements, and/or vocalizations, often accompanied by obsessions and/or compulsions. The onset of symptoms occurs mostly between 5 to 7 years of age, symptoms typically wax and wane over time, and the disorder is often outgrown by young adulthood, except in a minority of cases (Leckman, 2002; Lombroso and Scahill, 2008).
Strong evidence indicates that the disorder is hereditary, but the exact mechanisms involved in the genetic transmission are not resolved (Hoekstra et al., 2004). It appears that TS is genetically heterogeneous and that environmental factors may influence the onset, course, and severity of symptoms. Among multiple environmental factors, infections with group A beta hemolytic streptococcus (GABHS) have been proposed to play a key role in the pathogenesis of TS in at least some patients. This has received significant attention after a wave of infections with GABHS in Rhode Island was followed by increased frequency of tic disorders in pediatric practice (Kiessling et al., 1993). A subgroup of patients with TS with a medical history of GABHS infection prior to the onset of TS symptoms was designated as PANDAS (pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections ((Swedo et al., 1998). In addition, other infections were implicated in TS by studies demonstrating that common cold preceded TS symptom exacerbations (Hoekstra et al., 2005) and that antibodies against mycoplasma pneumoniae are elevated in patients with TS (Muller et al., 2004), and by case reports suggesting relations of TS with Lyme disease (Riedel et al., 1998) or toxoplasmosis (Brynska et al., 2001). Establishing evidence for the role of post-infectious autoimmunity in the pathogenesis of TS has proven quite challenging and the concept remains controversial (reviewed in (Giovannoni, 2006; Martino et al., 2009). This is mainly due to the complexity of neuro-immune interactions and the limited accessibility to the site of pathology, particularly in children. Nevertheless, this line of investigation is important as it may prove relevant not only to TS itself, but also to other pediatric neuropsychiatric diseases, for example autism spectrum disorders, where a role of the immune system was implicated and which has a well-established clinical link with TS (Baron-Cohen et al., 1999; Ivarsson and Melin, 2008).
Why children with TS would be prone to post-infectious autoimmune disorders remains unclear. Two recent epidemiological studies brought new insight into possible immune mechanisms in these patients by revealing that children with a newly diagnosed tic disorder or obsessive-compulsive disorder (OCD) were significantly more likely to have had a GABHS infection in the previous year than unaffected control subjects (Leslie et al., 2008; Mell et al., 2005). A higher vulnerability to infections may be due to some form of immune deficiency. Patients with immune deficiencies (even the isolated deficiencies that were originally considered to be benign, such as isolated immunoglobulin (Ig) A deficiency) are now known to have increased occurrence of autoimmune disorders (Bussone and Mouthon, 2009; Woof and Kerr, 2006). Previous studies have suggested that patients with TS may have enhanced activity of T cell immunity (Gabbay et al., 2009; Leckman et al., 2005) (Moller et al., 2008), implying that the deficient immune mechanisms may concern humoral branch of the immunity. In our first pilot study, we found decreased plasma IgA levels in patients with TS and/or OCD, particularly in the subgroup fulfilling the PANDAS criteria, implying that mucosal immunity may be compromised in these patients (Kawikova et al., 2010).
In this second pilot study, we continued to investigate the hypothesis that humoral immune deficiency may be present in patients with TS. We used existing archived samples and included a larger number of subjects from two clinical sites, focused on patients with mostly non-PANDAS TS and established detailed serum Ig profiles (including IgG subclasses and IgE). We chose to not specifically enrich the sample for PANDAS cases as we did in an earlier pilot study (Kawikova et al., 2010), since other microorganisms than GABHS have been implicated in TS (Hoekstra et al., 2005; Muller et al., 2004; Muller et al., 2000).
METHODS
Subjects
The study involved samples of convenience from cross-sectional collections that were obtained and archived at two distinct clinical sites. Additionally, we assessed changes during symptom exacerbations in serum samples collected longitudinally in one of the sites. Table 1 shows demographic and clinical characteristics of all subjects.
Table 1.
Demographic and clinical characteristics of patients with TS and healthy subjects
| Yale University Cross-sectional sample | Groningen University Cross-sectional sample | Yale University Longitudinal sample | |||
|---|---|---|---|---|---|
| Patients n = 21 | Healthy subjects n = 21 | Patients n = 53 | Healthy subjects n = 53 | n = 13 | |
| Male, n (%) | 17 (81.0) | 13 (61.9) | 45 (84.9) | 22 (42) | 9 (69.0) |
| Age, Mean (SD), range (years) | 11.9 (2.6), 8–16 | 12.2 (2.7), 7–17 | 12.3 (3.2), 6–18 | 12.2 (3.0), 6–18 | 10.6 (1.7), 9–14 |
| n (%) | n (%) | n (%) | |||
| Type of tic disorder | |||||
| Tourette’s disorder | 18 (85.7) | 52 (98.1) | 12 (92.3) | ||
| Chronic motor tic disorder | 3 (14.3) | 1 (1.9) | 1 (7.7) | ||
| Patients fulfilling PANDAS criteria | 3 (14.3) | 0 | 5 (38.5) | ||
| Psychotropic medication use | |||||
| Antipsychotic agents | 1 (4.8) | 9 (17.0) | 0 | ||
| Antidepressive agents | 2 (9.5) | 1 (1.9) | 1 (7.7) | ||
| Clonidine | 5 (23.8) | 3 (5.7) | 5 (3.8) | ||
| Psychostimulants | 0 | 4 (7.5) | 0 | ||
| Atomoxetine | 0 | 4 (7.5) | 0 | ||
| Combination of two or more agents | 8 (38.1) | 12 (22.6) | 6 (4.6) | ||
| No psychotropic medication | 5 (23.8) | 20 (37.8) | 1 (7.7) | ||
| Antibiotics | 0 | 0 | 4(33.1) | ||
| Mean (SD), range (n=21) | Mean (SD), range (n=21) | Mean (SD), Range (n=13) | |||
| YGTSS ratings | |||||
| Total | 18.5 (7.6), 5–32 | 22.1 (8.1), 3–39 | |||
| Motor | 10.3 (4.5), 0–18 | 13.7 (4.2), 0–22 | |||
| Vocal | 8.2 (4.8), 0–15 | 8.5 (5.5), 0–19 | |||
| Δ YGTSS before – during exacerbation | 15.6 (7.5), 9–31 | ||||
| Δ YGTSS after – during exacerbation | 10.4 (5.5), 2–20 | ||||
| Mean (SD), range (n=12) | Mean (SD), range (n=30) | ||||
| CY-BOCS ratings | |||||
| Total | 14.8 (8.0) 5–30 | 11.1 (6.7) 3–28 | |||
| Obsessive | 7.2 (3.9) 0–15 | 4.2(4.9) 0–16 | |||
| Compulsive | 7.7 (5.2) 0–15 | 6.9 (3.7) 0–14 | |||
PANDAS Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections; YGTSS Yale Global Tic Severity Scale; CY-BOCS Children’s Yale-Brown Obsessive-Compulsive Scale.
Selection of samples from these collections was performed based on the diagnosis of TS (TS patients with and without OCD symptoms were included, while patients who had OCD but no TS symptoms were excluded), on the severity of clinical symptoms (patients with moderate and severe symptoms were included), and on the availability of age-matched control subjects.
Cross-sectional samples
The first sample consisted of 21 patients with TS and 21 age-matched healthy control subjects recruited at the Child Study Center of Yale University New Haven, CT. Patients fulfilled Diagnostic and Statistical Manual of Mental Disorders fourth edition, text revised (DSM-IV-TR) criteria for TS or another chronic tic disorder. Information concerning the patients, including assessment of PANDAS status according to criteria proposed by Swedo et all (Swedo et al., 1998), was collected in a two-stage process as previously described (Leckman et al., 2005). Healthy volunteers were recruited by use of telemarketing lists of telephone numbers. Exclusion criteria included an intelligence quotient of < 75, presence of a serious medical illness, major sensory handicaps (e.g., blindness, deafness), major neurological diseases (including a seizure disorder), head trauma resulting in loss of consciousness, or a current (past 6 months) psychiatric disorder that could interfere with participation, such as major depression, psychosis, or an autism spectrum disorder (Leckman et al., 2005). Serum samples of these subjects were collected between the years 2000 and 2003, and were since stored unthawed in a –80°C freezer.
The second sample involved 53 pediatric patients who also fulfilled DSM-IV-TR criteria for TS or another chronic tic disorder, and 53 age-matched healthy volunteers. Patients had either been recruited through the Groningen University Child and Adolescent Psychiatry outpatient clinic in the Netherlands (n = 40) or through the Dutch TS Association (n = 13); healthy volunteers had been recruited through advertisements in local newspapers. Patients and parents were asked whether the onset or exacerbations of tic symptoms were temporarily associated with GABHS infections. None of the patients reported such an association. To prevent inclusion of patients with acute inflammatory disease, patients with clinical signs of an active infection and/or patients with C-reactive protein (acute phase protein) above its physiological levels of 10 mg/l were excluded from the study (this was the case in 2 patients). Further exclusion criteria were presence of an atopic condition, autoimmune disorder, or autism. These samples were collected in 2007, and since then were stored in a −20°C freezer.
In both samples, tic severity was assessed by using the Yale Global Tic Severity Scale (YGTSS; (Scahill et al., 1996). This is a well-validated semi-structured interview which records the number, frequency, intensity, complexity, and interference of motor and vocal tics separately (Leckman et al., 1989). Moreover, we used the Children’s Yale-Brown Obsessive Compulsive Scale (CYBOCS, a semi-structured interview measuring obsessive-compulsive symptom severity (Scahill et al., 1997).
Longitudinal sample
Serum samples of 13 additional patients with TS were investigated to evaluate possible changes in Ig profiles during tic exacerbations. The samples were collected in the course of a 24-month prospective longitudinal study at the Yale Child Study Center during which children were evaluated every 3 months, or earlier if an exacerbation of symptoms occurred (Leckman et al., 2005). This enabled us to select serum samples obtained when patients had well-defined tic exacerbations and to compare serum Ig profiles obtained at visits before and after an exacerbation of tics. An exacerbation was defined as a total YGTSS score exceeding the score established at the previous visit by nine points and the total YGTSS score exceeding 19 points (Leckman et al., 2005). Yale University is one of the few sites where prospective longitudinal studies have been performed. Similar samples were not available at Groningen University at this time.
Informed consent from parents and children of 12 years or older, and assent from children between 6 and 12 years had been obtained. The study had been approved by the relevant Institutional Review Boards.
Analysis of serum samples
All samples were analyzed at the Yale site. Samples of TS cases and control subjects of all three groups (Yale cross-sectional, Groningen cross-sectional, and Yale longitudinal) were proportionally represented on each plate of the assay. The person who performed the assays was blinded to the clinical status of samples.
Ig concentrations were measured in archived sera using a Luminex based Ig Isotyping reagent kit for measurement of IgG1, IgG2, IgG3, and IgG4, and IgM, IgA, and IgE (Bio-Rad Laboratories, Hercules, CA, catalog number 171-A3001M) in 10,000 fold diluted serum samples according to manufacturer’s instructions. Briefly, anti-isotype-conjugated beads were pipetted in a 96-well pre-wet filtration plate (Catalog number MSBVN1210) and incubated with 50 μl of 10,000 times diluted sample on a microplate shaker (IKA NTS 2/4 digital microtiter, catalog number 3208000), followed by three washes using multiscreen resist vacuum manifold (Millipore, Billerica, MA, catalog number MSVMHTS00). Then, biotinylated detection antibody solution was added, incubated for 30 minutes on a microplate shaker, and washed three times. Subsequently, streptavidin-phycoerythrin was added and incubated for 10 minutes while shaking and then washed three times. The fluorescence signal was analyzed using a Bioplex 200 suspension array system (Bio-Rad Laboratories, Hercules, CA, catalog number 171-000201) and Bioplex manager software (Bio-Rad Laboratories, Hercules, CA). To establish inter-assay variability, three samples were measured on each of the assay-days. The values obtained on the two following assay-days differed less than 9% from those obtained on the first assay. The intra-assay variability was established by comparing values of duplicates and the differences were less than 11%.
Statistical analyses
The Mann-Whitney test was used to compare difference in median Ig concentrations between groups of subjects (healthy controls versus patients, patients with medication versus patients without medication, or female subjects versus male subjects). Spearman’s correlation coefficient was used to determine relationships between serum Ig levels and YGTSS and CYBOCS ratings. Repeated-measures analysis of variance (ANOVA) was employed in the longitudinal analysis of Ig levels in patients with symptom exacerbation to assess differences in Ig profiles before, during, and after a tic exacerbation. If serum levels yielded at least trend-level statistically significant differences between time points (defined as a p value ≤ 0.1), Bonferroni Post Hoc analyses were performed.
This pilot study was hypothesis driven and values of p ≤ .05 were considered as significant. We did not perform correction of multi-comparisons in this study considering that immune disturbances could be present in only a subset of TS patients and too stringent criteria in this first evaluation of complete Ig profiles in TS patients could disguise the existence of such a subgroup. In order to validate our data using this approach, we included samples from two distinct clinical sites and asked whether similar changes can be observed at both sides. To avoid type II (false negative) errors, we also report trend-level significant findings. All tests were two-sided.
RESULTS
Cross-sectional analyses
Ig serum levels in control subjects from Yale University versus those from Groningen University significantly differed. This could be due to differences in environmental factors to which the subjects were exposed at the different sites, as well as due to differences in temperature and length of sample storage. The existence of these differences prevented pooling the samples from the two clinical sites. We therefore compared patients with versus age-matched healthy controls at each clinical site separately.
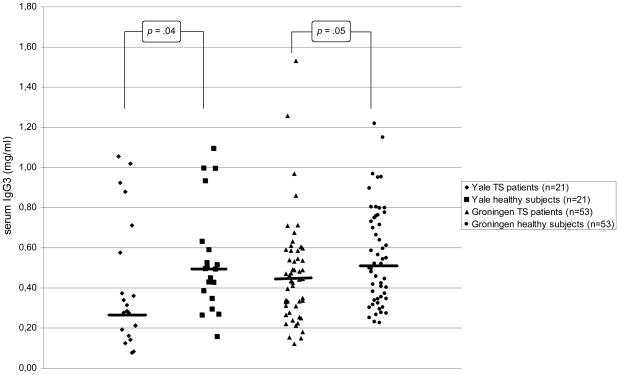
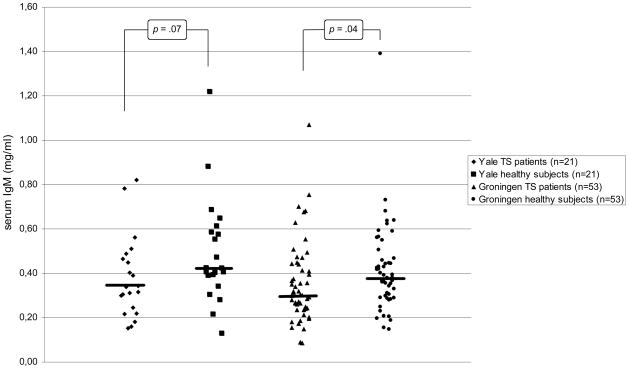
In the Yale sample, serum IgG3 levels were significantly lower in the patients with TS than in healthy control subjects, and there were trend-level decreased serum IgG2, Ig4, and IgM concentrations in patients (see Table 2a and Figures 1A and 1B). In the Groningen sample that involved a larger number of subjects, the decrease in serum IgG3 and IgM levels was replicated (Table 2b and Figures 1A and 1B). The findings of decreased IgG2 and IgG4 levels in the Yale patients were not confirmed. In addition, significantly increased IgG4 levels and trend-level decreased IgE levels were found.
Table 2a.
Serum Ig levels of patients with TS and healthy subjects of the Yale sample
| Serum Ig level | Patients n = 21 | Healthy subjects n = 21 | U | z | p |
|---|---|---|---|---|---|
| Median (p25/p75) | Median (p25/p75) | ||||
| IgG11 | 8.46 (6.14/13.1) | 9.61 (8.18/13.8) | 175 | −1.145 | .25 |
| IgG21 | 2.30 (1.07/3.69) | 3.22 (2.05/6.24) | 150 | −1.786 | .07 |
| IgG31 | 0.28 (0.18/0.64) | 0.49 (0.37/0.61) | 137 | −2.101 | .04 |
| IgG41 | 0.23 (0.09/0.48) | 0.45 (0.17/0.63) | 152 | −1.723 | .09 |
| IgM1 | 0.34 (0.23/0.48) | 0.42 (0.37/0.60) | 149 | −1.799 | .07 |
| IgA1 | 1.11 (0.70/1.98) | 1.32 (0.96/1.79) | 196 | −0.616 | .54 |
| IgE2 | 270 (163/803) | 314 (207/731) | 193 | −0.692 | .49 |
Ig Immunoglobulin.
mg/ml;
ng/ml
Figure 1.
Serum IgG3 levels in children with TS and healthy subjects of the Yale and Groningen samples. The bars represent the medians in each group.
Table 2b.
Serum Ig levels of patients with TS and healthy subjects of the Groningen sample
| Serum Ig level | Patients n = 53 | Healthy subjects n = 53 | U | z | p |
|---|---|---|---|---|---|
| Median (p25/p75) | Median (p25/p75) | ||||
| IgG11 | 7.84 (5.95/11.1) | 8.44 (7.01/11.8) | 1381 | −0.148 | .88 |
| IgG21 | 1.76 (1.12/2.75) | 1.88 (1.13/3.34) | 1313 | −0.578 | .56 |
| IgG31 | 0.45 (0.31/0.59) | 0.52 (0.34/0.75) | 1095 | −1.956 | .05 |
| IgG41 | 0.25 (0.14/0.54) | 0.18 (0.06/0.32) | 1038 | −2.313 | .02 |
| IgM1 | 0.30 (0.24/0.44) | 0.38 (0.29/0.46) | 1083 | −2.031 | .04 |
| IgA1 | 1.02 (0.85/1.77) | 1.27 (0.80/1.52) | 1380 | −0.152 | .88 |
| IgE2 | 400 (205/753) | 639 (279/1184) | 1101 | −1.918 | .06 |
Ig Immunoglobulin.
mg/ml;
ng/ml
In the Groningen sample, patients and control subjects were not fully gender-matched. To address the possibility of gender effect on Ig levels, we compared levels in the healthy control females with those of the healthy control males at both sites. In the Yale sample, no differences were identified between the healthy male (n= 13) and female (n=8) subjects. In the Groningen sample, however, serum IgM levels were significantly lower in the healthy male (n=22) compared to the healthy female (n=31) children (respective medians 0.32 mg/ml versus 0.40 mg/ml, U = 232.0, z = −1.968, p = .05). No other significant or trend-level differences were found. Since TS affects mainly boys and there was a preponderance of males in the Groningen patient groups (45 males versus 8 females), it is possible that the differences in IgM between the Groningen patient and control subjects might have been influenced by gender.
To address whether medication (Table 1) affected Ig levels, we compared Ig profiles in patients with or without medication. In the Yale sample, no differences were present. In the Groningen sample, patients with psychotropic medication (n=33) had lower serum levels of IgG4 compared to those who were medication free (n=20) (respective medians 0.22 mg/ml versus 0.32 mg/ml, U = 224.0, z = −1.945, p = .05), but no other effects were observed on remaining Ig isotypes and subclasses.
In both the Yale and Groningen samples, no statistically significant, nor trend-level significant, correlations were present between any of the serum Ig levels and YGTSS or CYBOCS ratings.
In the earlier pilot study, we observed a decrease of IgA, mainly in PANDAS cases. This was not revealed here. For the purpose of assessing the consistency in IgA findings between these two studies, we pooled all PANDAS patients in the current study (three patients in the Yale University cross-sectional sample and five patients in the Yale University longitudinal sample) and compared them to values of the Yale non-PANDAS subjects (n=23). The mean IgA level of PANDAS cases was 13.4 mg/dl, while in the non-PANDAS cases this was 18.8 mg/dl. This is implies similar decrease in IgA in PANDAS cases as in our earlier study. The difference, however, was not statistically significant, emphasizing the importance of reproducing the data with larger groups of age- and gender matched PANDAS and non-PANDAS subjects.
Longitudinal analysis
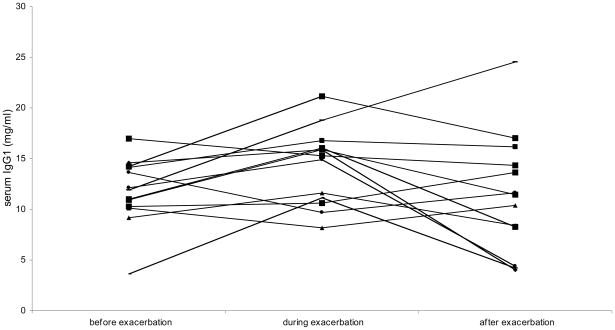
Comparison of serum Ig levels before, during, and after a tic exacerbation by repeated-measures ANOVA revealed a trend-level increase in IgA and IgG1 during exacerbations. The trend to an increase of IgG1 remained present after post hoc analyses (Table 3 and Figure 2).
Table 3.
Serum Ig levels before, during, and after a tic exacerbation in 13 patients with TS.
| Serum Ig levels | Before exacerbation | During exacerbation | After exacerbation | F | df | p |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||||
| IgG11 | 11.8 (3.29) | 14.3 (3.79) | 11.4 (5.88) | 2.63 | 2, 24 | .09b |
| IgG21 | 2.82 (0.99) | 3.48 (1.69) | 3.07 (2.11) | 1.09 | 2, 23 | .35 |
| IgG31 | 0.65 (0.36) | 0.79 (0.47) | 0.65 (0.44) | 2.94 | 2, 24 | .17 |
| IgG41 | 0.39 (0.35) | 0.42 (0.27) | 0.35 (0.27) | 0.93 | 2, 24 | .41 |
| IgM1 | 0.42 (0.20) | 0.50 (0.24) | 0.46 (0.32) | 1.33 | 2, 24 | .28 |
| IgE2 | 221 (123) | 235 (150) | 204 (136) | 1.18 | 2, 18 | .33 |
| IgA1 | 1.34 (0.66) | 1.74 (0.93) | 1.38 (0.69) | 2.86 | 2, 24 | .08a |
Ig Immunoglobulin; df Degrees of Freedom
mg/ml;
ng/ml
Bonferroni Post Hoc test:
Mean difference between before and during exacerbation: −0.40 (SE 0.2), p=.193
Mean difference between during and after exacerbation: 0.36 (SE 0.2), p=.343
Bonferroni Post Hoc test:
Mean difference between before and during exacerbation: −2.56 (SE 1.0), p=.082
Mean difference between during and after exacerbation: 2.88 (SE 1.5), p=.236
Figure 2.
Serum IgM levels in children with TS and healthy subjects of the Yale and Groningen samples. The bars represent the medians in each group.
DISCUSSION
This pilot study represents the first comparison of detailed serum Ig profiles in children and adolescents with TS versus healthy age-matched subjects. The data were obtained by analysis of two independent cross-sectional samples and indicate that at least some patients with TS have lower serum levels of IgG3, and possibly also IgM, than control subjects. Similar decreases in IgG3 and IgM serum levels were recently also reported in patients with autism by a study involving 116 patients with autistic disorder versus several relevant control groups (Heuer et al., 2008).
IgG3 and pentameric IgM are the most potent activators of the classical complement cascade that leads to the formation of membrane attack complexes and the destruction of microbial pathogens. In patients with TS, there has been no report about the activity of complement cascade. In children with autism, however, a decrease in plasma levels of complement protein C4 has been reported (Warren et al., 1994). Both IgG3 and monomeric IgM also play a role in phagocytosis (Meyts et al., 2006; Shibuya et al., 2000). Since IgG3 and IgM are involved in immune responses against multiple microorganisms (Goldstein et al., 2008; Noyes et al., 1986; Oxelius et al., 1986), decreased levels may compromise the immune defense to diverse pathogens.
The levels of Ig isotypes in healthy control subjects are within the range of previously reported results (Ritchie et al., 1998). The decrease in IgG3 and IgM that we observed in patients with TS does not correspond to fully expressed immunoglobulin deficiencies (defined as a decrease of two standard deviations below the appropriate age mean levels). Only four Yale University patients (19%) and one Groningen University patient (1.9%) fulfilled these criteria for Ig deficiency. Also, only one patient had low levels of both IgG3 and IgM, suggesting that different TS patients may suffer different types of dysgammaglobulinemia. Individuals with isolated IgG3 and IgM deficiencies are known to have recurrent infections affecting mainly the upper respiratory tract (pharyngitis, rhinitis, or sinusitis) (Meyts et al., 2006; Yel et al., 2009). Similarly, patients with TS suffer more frequently from upper respiratory infection (Leslie et al., 2008; Mell et al., 2005). These findings point to the need to investigate further whether patients with TS who have Ig abnormalities suffer infectious diseases more often, and whether these infections are causally related to the onset, course, and/or severity of TS symptoms.
The observations in the present cross-sectional analyses differ somewhat from the results of our recent pilot study. Previously we measured total and antigen specific levels of IgG, IgA, and IgM in patients with TS. We observed a decrease in IgA levels, but not in IgG and IgM levels (Kawikova et al., 2007), . The earlier study significantly differs from the current study in several aspects, however. First, in the previous study, the patient group involved 39% PANDAS cases, whereas in the current study, the Yale University group involved only 14% and the Groningen University had no PANDAS cases. The decrease in IgA in the pilot study was observed predominantly in PANDAS cases. In the present study, we observed a tendency to a similar decrease in PANDAS cases compared to non-PANDAS cases. The number of PANDAS cases, however, was low which could be the reason for lack of statisticaly significant difference. Second, in the earlier pilot study, we measured total IgG and observed no differences. In the current study, we measured IgG subclasses and found a decrease in IgG3 levels. IgG3 represents only 4–8% of total IgG and thus it is understandable that the differences could not be revealed in the pilot study where total IgG, but not subclasses, were measured. Third, in our previous study, we did not observe changes in IgM levels, similarly to Libbey at all (Libbey et al., 2008). With larger numbers of subjects in the current study, we found a decrease in both the Yale and Groningen groups, but the possibility of a confounding effect of gender cannot be excluded in the Groningen group. Future studies will need to resolve the uncertainty.
To determine whether worsening of TS symptoms is associated with changes in Ig profiles, we employed an additional group of patients with well-defined symptom exacerbations and compared their serum Ig profiles obtained at pre-exacerbation, during exacerbation, and at post-exacerbation time points (Leckman et al., 2005). This preliminary analysis revealed a trend towards an increase of IgG1, which might be interesting since anti-basal ganglia IgG1 and IgG3 antibodies levels were elevated in cerebrospinal fluid of patients with Sydenham’s chorea (a neuropsychiatric component of post-streptococcal rheumatic fever that clinically resembles some aspects of TS) and IgG1 levels were also increased in serum of mice involved in an experimental model of PANDAS where Ig was shown to be necessary for the elicitation of symptoms (Yaddanapudi et al., 2009).
We observed here deviations in B cell immunity of patients with TS that appear relatively mild. However, the existence of subtle changes in such a gross parameter as is a concentrations of total Igs warrants further investigation of antigen-specific Ig profiles in the context of total Igs. A frequently raised concern is that changes in immune parameters in subjects with neuropsychiatric disorders may not be the cause of the disease, but rather a consequence of stress induced by the presence of the chronic disorder. Patients with TS do have enhanced responsiveness to acute stress (Chappell et al., 1994; Corbett et al., 2008), psychosocial stress negatively impacts their symptoms (Lin et al., 2007), and basal levels of cortisol negatively correlates with TS symptoms (Corbett et al., 2008). The immune changes that were previously reported in subjects with TS, including enhanced activity of T lymphocytes (Gabbay et al., 2009; Leckman et al., 2005; Moller et al., 2008), decreased numbers of regulatory T cells (Kawikova et al., 2007), and altered activity of natural killer cells (Du et al., 2006; Lit et al., 2007) are also present in patients with chronic stress conditions, such as post-traumatic stress disorder (Gill et al., 2009; Gotovac et al., 2010; Inoue-Sakurai et al., 2000; Sommershof et al., 2009). However, to our knowledge, no changes in IgG3 or IgM levels have been reported in chronic stress conditions. Instead, rather elevated levels of Igs were found in chronic stress (Boscarino, 2004; Kosor Krnic et al., 2007). In light of this, it seems relevant to consider that the decrease in IgG3 and possibly IgM in the present study might reflect primary dysgammaglobulinemias in TS subjects which should be confirmed in the future by testing B lymphocyte functions in patients with TS.
Several limitations of our study need to be acknowledged. First, the patients of the Groningen sample were age- but not gender-matched. Second, an important possible confounding factor is the effect of medications. Although in our study, serum levels of IgG3 and IgM did not differ between patients with and without medication use, other studies reported various effects of psychiatric medications on multiple immune parameters. Thus, future studies should ideally involve medication free patients. Lastly, the analysis of Ig profiles during exacerbations involved a relatively small number of subjects. Future studies involving larger numbers of patients are required to replicate and solidify these longitudinal data.
In summary, this study demonstrates patients with TS may have abnormalities in their humoral immunity, though only few subjects had fully expressed isolated Ig immunodeficiency. These data also imply that even non-PANDAS patients may have immune disturbances, which was previously neglected. Future studies need to address whether the abnormalities represent a primary dysgammaglobulinemia and whether they are related to more frequent infections that were previously reported in patients with TS and whether these infections are involved in the pathogenesis of TS symptoms. Future studies should also address whether there is any relationship between stress, cortisol levels, and alterations of immune parameters.
Figure 3.
Serum IgG1 levels of 13 children with TS before, during, and after an exacerbation of tics. Patients showed increased IgG1 levels during exacerbation: F(2, 24) = 2.63, p = .09.
Acknowledgments
The research was supported by Tourette’s Syndrome Association (IK), National Institute of Health Grant Nos. MH066187, P01MH049351 (JFL), R01MH061940 (JFL), R01NS42240, MH014235, K05 MH076273 (JFL), M01RR006022, and RR00125. Dr. Olieman was supported during her stay at Yale University by Groningen University Funds, Marco Polo Scholarship and Tourette’s Syndrome Association. Dr. Tobiasova was supported by Tourette’s Syndrome Association and by the Czech Ministry of Education (MSM 00211620812). Support of James F. Leckman for the past three years: NIH (salary and research funding), Tourette Syndrome Association (research funding), Klingenstein Third Generation Foundation (medical student fellowship program), John Wiley and Sons (book royalties), McGraw Hill (book royalties), Oxford University Press (book royalties). Other authors do not claim any conflict of interest.
Contributor Information
Netty G.P. Bos-Veneman, Email: N.Bos-Veneman@Accare.nl.
Renske Olieman, Email: renskeolieman@hotmail.com.
Zuzana Tobiasova, Email: zuzana.tobiasova@yale.edu.
Pieter J. Hoekstra, Email: p.hoekstra@accare.nl.
Lily Katsovitch, Email: lily.katsovich@yale.edu.
Alfred L. M. Bothwell, Email: alfred.bothwell@yale.edu.
James F. Leckman, Email: james.leckman@yale.edu.
Ivana Kawikova, Email: ivana.kawikova@yale.edu.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revison (DSM-IV-TR) [Google Scholar]
- Baron-Cohen S, Scahill VL, Izaguirre J, Hornsey H, Robertson MM. The prevalence of Gilles de la Tourette syndrome in children and adolescents with autism: a large scale study. Psychol Med. 1999;29:1151–1159. doi: 10.1017/s003329179900896x. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Annals of the New York Academy of Sciences. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- Brynska A, Tomaszewicz-Libudzic E, Wolanczyk T. Obsessive-compulsive disorder and acquired toxoplasmosis in two children. European child & adolescent psychiatry. 2001;10:200–204. doi: 10.1007/s007870170027. [DOI] [PubMed] [Google Scholar]
- Bussone G, Mouthon L. Autoimmune manifestations in primary immune deficiencies. Autoimmun Rev. 2009;8:332–336. doi: 10.1016/j.autrev.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Chappell P, Riddle M, Anderson G, Scahill L, Hardin M, Walker D, Cohen D, Leckman J. Enhanced stress responsivity of Tourette syndrome patients undergoing lumbar puncture. Biol Psychiatry. 1994;36:35–43. doi: 10.1016/0006-3223(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza SP, Baym CL, Bunge SA, Levine S. Examining cortisol rhythmicity and responsivity to stress in children with Tourette syndrome. Psychoneuroendocrinology. 2008;33:810–820. doi: 10.1016/j.psyneuen.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Tang Y, Xu H, Lit L, Walker W, Ashwood P, Gregg JP, Sharp FR. Genomic profiles for human peripheral blood T cells, B cells, natural killer cells, monocytes, and polymorphonuclear cells: comparisons to ischemic stroke, migraine, and Tourette syndrome. Genomics. 2006;87:693–703. doi: 10.1016/j.ygeno.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Coffey BJ, Guttman LE, Gottlieb L, Katz Y, Babb JS, Hamamoto MM, Gonzalez CJ. A cytokine study in children and adolescents with Tourette’s disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33:967–971. doi: 10.1016/j.pnpbp.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspectives in psychiatric care. 2009;45:262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- Giovannoni G. PANDAS: overview of the hypothesis. Adv Neurol. 2006;99:159–165. [PubMed] [Google Scholar]
- Goldstein MF, Goldstein AL, Dunsky EH, Dvorin DJ, Belecanech GA, Shamir K. Pediatric selective IgM immunodeficiency. Clin Dev Immunol. 2008;2008:624850. doi: 10.1155/2008/624850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotovac K, Vidovic A, Vukusic H, Krcmar T, Sabioncello A, Rabatic S, Dekaris D. Natural killer cell cytotoxicity and lymphocyte perforin expression in veterans with posttraumatic stress disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2010;34:597–604. doi: 10.1016/j.pnpbp.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Heuer L, Ashwood P, Schauer J, Goines P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008;1:275–283. doi: 10.1002/aur.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra PJ, Anderson GM, Limburg PC, Korf J, Kallenberg CG, Minderaa RB. Neurobiology and neuroimmunology of Tourette’s syndrome: an update. Cell Mol Life Sci. 2004;61:886–898. doi: 10.1007/s00018-003-3320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra PJ, Manson WL, Steenhuis MP, Kallenberg CG, Minderaa RB. Association of common cold with exacerbations in pediatric but not adult patients with tic disorder: a prospective longitudinal study. J Child Adolesc Psychopharmacol. 2005;15:285–292. doi: 10.1089/cap.2005.15.285. [DOI] [PubMed] [Google Scholar]
- Inoue-Sakurai C, Maruyama S, Morimoto K. Posttraumatic stress and lifestyles are associated with natural killer cell activity in victims of the Hanshin-Awaji earthquake in Japan. Preventive medicine. 2000;31:467–473. doi: 10.1006/pmed.2000.0744. [DOI] [PubMed] [Google Scholar]
- Ivarsson T, Melin K. Autism spectrum traits in children and adolescents with obsessive-compulsive disorder (OCD) J Anxiety Disord. 2008;22:969–978. doi: 10.1016/j.janxdis.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Kawikova I, Grady P, Tobiasova Z, Zhang Y, Vojdani A, Katsovich L, TWP, Bothwell A, Leckman J. Children with Tourette’s sydnrome may suffer IgA dysgammaglobulinemia: preliminary report. Biological Psychiatry. 2010;67:596. doi: 10.1016/j.biopsych.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawikova I, Leckman JF, Kronig H, Katsovich L, Bessen DE, Ghebremichael M, Bothwell AL. Decreased numbers of regulatory T cells suggest impaired immune tolerance in children with tourette syndrome: a preliminary study. Biol Psychiatry. 2007;61:273–278. doi: 10.1016/j.biopsych.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies in movement disorders. Pediatrics. 1993;92:39–43. [PubMed] [Google Scholar]
- Kosor Krnic E, Gagro A, Kozaric-Kovacic D, Vilibic M, Grubisic-Ilic M, Folnegovic-Smalc V, Drazenovic V, Cecuk-Jelicic E, Gjenero-Margan I, Kuzman I, Jeren T, Sabioncello A, Kerhin-Brkljacic V, Kaic B, Markotic A, Gotovac K, Rabatic S, Mlinaric-Galinovic G, Dekaris D. Outcome of influenza vaccination in combat-related post-traumatic stress disorder (PTSD) patients. Clinical and experimental immunology. 2007;149:303–310. doi: 10.1111/j.1365-2249.2007.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF. Tourette’s syndrome. Lancet. 2002;360:1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Katsovich L, Kawikova I, Lin H, Zhang H, Kronig H, Morshed S, Parveen S, Grantz H, Lombroso PJ, King RA. Increased serum levels of interleukin-12 and tumor necrosis factor-alpha in Tourette’s syndrome. Biol Psychiatry. 2005;57:667–673. doi: 10.1016/j.biopsych.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Leslie DL, Kozma L, Martin A, Landeros A, Katsovich L, King RA, Leckman JF. Neuropsychiatric Disorders Associated With Streptococcal Infection: A Case-Control Study Among Privately Insured Children. J Am Acad Child Adolesc Psychiatry. 2008 doi: 10.1097/CHI.0b013e3181825a3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Coon HH, Kirkman NJ, Sweeten TL, Miller JN, Stevenson EK, Lainhart JE, McMahon WM, Fujinami RS. Are there enhanced MBP autoantibodies in autism? J Autism Dev Disord. 2008;38:324–332. doi: 10.1007/s10803-007-0400-6. [DOI] [PubMed] [Google Scholar]
- Lin H, Katsovich L, Ghebremichael M, Findley DB, Grantz H, Lombroso PJ, King RA, Zhang H, Leckman JF. Psychosocial stress predicts future symptom severities in children and adolescents with Tourette syndrome and/or obsessive-compulsive disorder. J Child Psychol Psychiatry. 2007;48:157–166. doi: 10.1111/j.1469-7610.2006.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lit L, Gilbert DL, Walker W, Sharp FR. A subgroup of Tourette’s patients overexpress specific natural killer cell genes in blood: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:958–963. doi: 10.1002/ajmg.b.30550. [DOI] [PubMed] [Google Scholar]
- Lombroso PJ, Scahill L. Tourette syndrome and obsessive-compulsive disorder. Brain Dev. 2008;30:231–237. doi: 10.1016/j.braindev.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino D, Dale RC, Gilbert DL, Giovannoni G, Leckman JF. Immunopathogenic mechanisms in tourette syndrome: A critical review. Mov Disord. 2009;24:1267–1279. doi: 10.1002/mds.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mell LK, Davis RL, Owens D. Association between streptococcal infection and obsessive-compulsive disorder, Tourette’s syndrome, and tic disorder. Pediatrics. 2005;116:56–60. doi: 10.1542/peds.2004-2058. [DOI] [PubMed] [Google Scholar]
- Meyts I, Bossuyt X, Proesmans M, De B. Isolated IgG3 deficiency in children: to treat or not to treat? Case presentation and review of the literature. Pediatr Allergy Immunol. 2006;17:544–550. doi: 10.1111/j.1399-3038.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- Moller JC, Tackenberg B, Heinzel-Gutenbrunner M, Burmester R, Oertel WH, Bandmann O, Muller-Vahl KR. Immunophenotyping in Tourette syndrome--a pilot study. Eur J Neurol. 2008;15:749–753. doi: 10.1111/j.1468-1331.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- Muller N, Riedel M, Blendinger C, Oberle K, Jacobs E, Abele-Horn M. Mycoplasma pneumoniae infection and Tourette’s syndrome. Psychiatry Res. 2004;129:119–125. doi: 10.1016/j.psychres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Muller N, Riedel M, Forderreuther S, Blendinger C, Abele-Horn M. Tourette’s syndrome and mycoplasma pneumoniae infection. Am J Psychiatry. 2000;157:481–482. doi: 10.1176/appi.ajp.157.3.481-a. [DOI] [PubMed] [Google Scholar]
- Noyes J, Woodmansee D, Chervinsky P. IgG subtype abnormalities with normal total IgG in a clinical allergy practice. Ann Allergy. 1986;57:273–275. [PubMed] [Google Scholar]
- Oxelius VA, Hanson LA, Bjorkander J, Hammarstrom L, Sjoholm A. IgG3 deficiency: common in obstructive lung disease. Hereditary in families with immunodeficiency and autoimmune disease. Monogr Allergy. 1986;20:106–115. [PubMed] [Google Scholar]
- Riedel M, Straube A, Schwarz MJ, Wilske B, Muller N. Lyme disease presenting as Tourette’s syndrome. Lancet. 1998;351:418–419. doi: 10.1016/S0140-6736(05)78357-4. [DOI] [PubMed] [Google Scholar]
- Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O. Reference distributions for immunoglobulins A, G, and M: a comparison of a large cohort to the world’s literature. Journal of clinical laboratory analysis. 1998;12:371–377. doi: 10.1002/(SICI)1098-2825(1998)12:6<371::AID-JCLA7>3.0.CO;2-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Scahill L, Vitulano LA, Brenner EM, Lynch KA, King RA. Behavioral therapy in children and adolescents with obsessive-compulsive disorder: a pilot study. J Child Adolesc Psychopharmacol. 1996;6:191–202. doi: 10.1089/cap.1996.6.191. [DOI] [PubMed] [Google Scholar]
- Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, Eyre HJ, Sutherland GR, Endo Y, Fujita T, Miyabayashi T, Sakano S, Tsuji T, Nakayama E, Phillips JH, Lanier LL, Nakauchi H. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol. 2000;1:441–446. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg EM, Elbert T, Groettrup M, Kolassa IT. Substantial reduction of naive and regulatory T cells following traumatic stress. Brain, behavior, and immunity. 2009;23:1117–1124. doi: 10.1016/j.bbi.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Lougee L, Dow S, Zamkoff J, Dubbert BK. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155:264–271. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- Warren RP, Burger RA, Odell D, Torres AR, Warren WL. Decreased plasma concentrations of the C4B complement protein in autism. Arch Pediatr Adolesc Med. 1994;148:180–183. doi: 10.1001/archpedi.1994.02170020066011. [DOI] [PubMed] [Google Scholar]
- Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006;208:270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- Yaddanapudi K, Hornig M, Serge R, De Miranda J, Baghban A, Villar G, Lipkin WI. Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.77. [DOI] [PubMed] [Google Scholar]
- Yel L, Ramanuja S, Gupta S. Clinical and Immunological Features in IgM Deficiency. Int Arch Allergy Immunol. 2009;150:291–298. doi: 10.1159/000222682. [DOI] [PubMed] [Google Scholar]