Abstract
The objective of the study is to test whether circulating proteasomes are increased in burn patients and to assess whether possible alterations are associated with severity of injury, organ failure, and/or clinically relevant outcomes. In this study, plasma was obtained from burn patients on days 0 (admission, n = 50), 1 (n = 36), 3 (n = 35), 5 (n = 28), 7 (n=34), and 30 (n = 10) (controls: 40 volunteers). The 20S/26S proteasome levels were measured by enzyme-linked immunosorbent assay. Proteasome peptidase activity was assessed using a chymotryptic-like peptide substrate in combination with epoxomicin (specific proteasome inhibitor). Percentage of TBSA burned, presence of inhalation injury, development of sepsis/multiple organ failure, and sequential organ failure assessment scores were documented. On admission, plasma proteasome activity was higher in patients than in controls (P = .011). 26S proteasomes were not detectable. The 20S proteasome concentrations (median [25th/75th percentile]) peaked on day 0 (673 [399/1566] ng/mL; control: 195 [149/249] ng/mL, P < .001), gradually declined within 7 days, and fully returned to baseline at day 30 (116.5 [78/196] ng/mL). Elevated 20S proteasomes were associated with the presence of inhalation injury and correlated linearly with %TBSA in patients without inhalation injury. Initial 20S proteasome concentrations discriminated the presence of inhalation injury in patients with (sensitivity 0.88 and specificity 0.71) and without (sensitivity 0.83 and specificity 0.97) cutaneous burns but did not discriminate sepsis/multiple organ failure development or survival. Circulating 20S proteasome is a biomarker of tissue damage. The 20S proteasome plasma concentrations in patients with burns and/or inhalation injury are unlikely to predict outcomes but may be useful for the diagnosis of inhalation injury.
Proteasomes are important proteolytic machineries in all eukaryotic cells and regulate a variety of essential intracellular functions.1,2 They consist of a cylinder-shaped multimeric protein complex (20S proteasome core particle, 20S proteasome) that can be singly or doubly capped by a 19S regulator complex when ATP/Mg2+ is present and is then termed 26S proteasome. While the 26S proteasome degrades proteins that have been covalently linked to ubiquitin (=ubiquitylation or ubiquitination), the 20S proteasome alone is involved in the removal of misfolded and damaged proteins.1–4 More recently, 20S proteasomes have also been detected in normal serum and plasma, and elevated circulating 20S proteasome concentrations have been described in patients with various pathological conditions, such as hematologic malignancies, autoimmune diseases, or critical illness.5–9 Although circulating 20S proteasomes have been shown to possess enzymatic activity,10 evidence for a functional role of circulating 20S proteasomes has not yet been demonstrated. Nevertheless, earlier findings suggested that systemic 20S proteasome concentrations reflect cellular damage independent of the underlying cause of the disease and that circulating 20S proteasomes might be a useful biomarker to assess disease severity and/or progression.9,11,12
Elevated 20S proteasome plasma concentrations have been detected previously in patients within 24 hours after severe trauma.8 However, the time course and possible clinical associations of circulating 20S proteasome concentrations after trauma are unknown. Systemic proteasome concentrations in patients after burns have not been studied.
Based on the foregoing, we hypothesized that circulating proteasomes are also increased in patients with burns and that its systemic concentrations are associated with the size of the burn injury. Therefore, we conducted a prospective observational study in burn patients to test these hypotheses and to further evaluate whether possible changes in circulating proteasome levels are associated with organ failure and/or clinically relevant outcomes.
METHODS
Patients and Participants
This study was approved by the Internal Review Boards of the Loyola University Medical Center and the University of Munich. Informed consent was obtained from all participants. The study population comprised 69 patients with burns and/or inhalation injury who were admitted to the emergency room of the participating hospitals and 40 healthy blood donors. Exclusion criteria were age younger than 18 years, hospital admission later than 6 hours after injury, and severe preexisting infectious, immunological or cardiovascular diseases that required long-term medication. Thirty-six burn patients were recruited in Loyola and 33 patients in Munich. Healthy blood donors were recruited in Loyola (n = 10) and Munich (n = 30). Blood donors (age: 43 ± 11 years [mean ± SD]; 68% men) had no signs of infection at least 4 weeks before the blood draw.
All patients requiring surgical intervention received standard surgical care and postoperative intensive care unit treatment according to the standard treatment protocols of the individual hospitals. Injury severity was assessed by the percentage of at least second-degree burned TBSA and the presence of inhalation injury, as documented in the patient records.
All patients were prospectively observed for the development of severe sepsis, septic shock, or multiple organ failure (sepsis/MOF). Severe sepsis and septic shock were diagnosed according to the American College of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM) consensus conference criteria.13 MOF was diagnosed using a scoring system modified according to Goris et al.14,15 In brief, failure of a single organ was assumed when the following criteria were present for at least 3 consecutive days: lung, mechanical ventilation with positive end-expiratory pressure >10 cm H2O and/or FiO2 >0.4; heart, dopamine >700 μg or catecholamine treatment; kidney, hemofiltration or hemodialysis; liver, bilirubin >6 mg/dL or glutamic oxaloacetic transaminase (GOT) >100 U/L; blood, hemorrhagic diathesis or leukocytes >60 × 109/L or <2.5×109/L; gastrointestinal, parenteral nutrition; brain, treatment for increased intracranial pressure. MOF was diagnosed when these criteria were fulfilled for two or more organs. Because MOF without positive criteria of severe sepsis or septic shock after burns was expected to be rare, these posttraumatic complications were combined and documented as sepsis/MOF positive or negative.
The sepsis-related organ failure/Sequential Organ Failure Assessment (SOFA) scores16,17 were calculated daily in all patients recruited in Munich. Survival was documented based on the final patient records. The epidemiological and clinical characteristics are shown in Table 1.
Table 1.
Clinical and epidemiological characteristics of the patient population
| All | Burn and Inhalation Injury | Burn Alone | Inhalation Injury Alone | |
|---|---|---|---|---|
| No. patients | 68 | 34 | 28 | 6 |
| Age (yr) | 51 ± 19 | 54 ± 18 | 47 ± 19 | 53 ± 23 |
| Sex (female/male) | 19/49 | 4/30 | 10/18 | 5/1 |
| %TBSA | 33 ± 20 | 37 ± 23 | 34 ± 10 | 0 |
| Inhalation injury (%) | 59 | 100 | 0 | 100 |
| Sepsis/MOF (%) | 40 | 59 | 21 | 17 |
| Mortality (%) | 19 | 26 | 11 | 17 |
Data are mean ± standard deviation, percentages or number of patients.
Venous blood samples (1–2 mL) were drawn after admission to the emergency room (day 0) and in the morning of the subsequent days 1, 3, 5, and 7 in Munich and on days 0, 7, and 30 at Loyola along with the routine laboratory work-up. The timing of the blood collection was different in Loyola and Munich because the plasma samples derived from ongoing prospective biomarker studies with Internal Review Boards preapproved time points of blood collection in both centers. Because blood samples could not be obtained from each patient at each individual time point, the exact number of analyzed blood samples is provided for each time point in the “Results” section. Blood samples were collected in plasma test tubes that were in routine use in the participating hospitals (K3 ethylenediaminetetraacetic acid tubes [Sarstedt, Numbrecht, Germany] and 9NC Na citrate tubes [BD, Franklin Lakes, NJ]), plasma prepared according to the standard hospital procedures and stored at −70°C until further analysis. After all blood samples were collected and patient data recorded, proteasome plasma concentrations were determined with the investigators blinded to the patient-related data.
20S and 26S Proteasome Enzyme-Linked Immunosorbent Assay
The 20S and 26S proteasome plasma concentrations were quantified by enzyme-linked immunosorbent assay (ELISA), as described in detail previously.18 In brief, microtiter plates (Nunc Maxisorp, Nalge Nunc International, Rochester, NY) were coated with the capture antibodies (20S: anti-20S subunit α6 [PW8100, Biomol, Plymouth Meeting, PA]; 26S: anti-19S subunit Rpn2 [AP-104, Boston Biochem, Boston, MA]) diluted in phosphate-buffered saline (PBS), pH 7.4 over night at 4°C. After coating, plates were blocked with PBS and 1% bovine serum albumin (BSA) (Sigma, PBS-BSA). Standard curves were prepared using highly purified 20S (PW8270) and 26S proteasomes (PW 9310, both from Biomol) diluted in PBS-BSA. One hundred microliters of standards and samples diluted 1:5 in PBS-BSA were placed in the wells and incubated for 2 hours. After washing the plates, the secondary antibodies {20S: anti-20S “core subunits” [α5, α7, β1, β5, β5i, β7] (PW8155, Biomol); 26S: anti-20S subunit α6 (PW8100, Biomol)} were added to the wells and incubated for 1 hour at room temperature. The plates were washed again and horseradish peroxidase-labeled anti-rabbit or anti-mouse (NA934V and NA931V, GE Healthcare, Piscataway, NJ) were added. After 1 hour of incubation, plates were washed again and the bound antibodies were detected using tetramethylbenzidine (Sigma-Aldrich, St. Louis, MO). The reaction was stopped by addition of 50 μL 2N HCL and the optical densities were determined at 450/540 nm in a microplate reader (Synergy 2, Biotek Instruments, Winooski, VT). For the 26S ELISA, all buffers contained 5 mM ATP and 5 mM Mg2+ (Sigma). Although the 20S proteasome ELISA detects free 20S proteasomes and 20S proteasomes within the 26S proteasome complex, the 26S proteasome ELISA shows no cross-reactivity with free 20S proteasomes. The lower detection limits were 1.5 ng/mL and 11.7 ng/mL for the 20S and 26S proteasome ELISA, respectively.
Proteasome Peptidase Activities
Proteasome peptidase activities were measured using the fluorogenic chymotryptic-like peptide substrate N-Suc-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Biomol), as described.18–20 Reaction mixtures contained 10 mM Tris/HCl, pH 7.5, 100 μM peptide substrate, and 35 μL of plasma. Mixtures were incubated for 60 minutes at 37°C. Ethanol (2:1; volume: volume) was added, mixtures placed on ice for 10 minutes, and centrifuged at 16,000 g, 5°C for 6 minutes. Supernatants were transferred into microplates (Corning, Acton, MA) and free 7-amino-4-methylcoumarin cleaved from the substrates measured in a microplate reader (Synergy 2, Biotek, λexcitation/emission = 340/440 nm) against standard curves of 7-amino-4-methylcoumarin (Sigma). To differentiate the proteasome from other peptidase activities, the epoxomicin (specific proteasome inhibitor)-sensitive proportion was determined by addition of 7 μM epoxomicin (Boston Biochem) to the mixtures.21 Proteasome peptidase activity (total peptidase activity minus peptidase activity in the presence of epoxomicin) was calculated as mol of 7-amino-4-methylcoumarin released from the peptide substrate per milliliter of plasma and hour.
Statistics
Proteasome concentrations are described as median with 25th and 75th percentiles (in parenthesis). All other variables are described as mean ± standard deviation, when applicable. All data are presented along with the exact number of measurements. Normal distribution was assessed with the Kolmogorov-Smirnov test. Because the determined 20S proteasome plasma concentrations in burn patients did not pass the normality test (alpha = 0.05) on each time point, the Mann-Whitney U test and the Kruskal-Wallis H test with Dunn's post hoc correction to control for multiple testing were used to compare differences between groups. Fisher's exact test was used for dichotomic categorical variables. Correlations were assessed using bivariate Spearman correlation coefficients (rs) and partial correlation coefficients (rp) controlling for the effects of one or more additional variables. Receiver operator characteristic (ROC) analyses were used to evaluate the diagnostic or prognostic value of 20S proteasome concentrations (null hypothesis [no diagnostic/prognostic value] = area under the ROC curve of 0.5). Statistical analyses were calculated with the SPSS for Windows release 16.0 program (SPSS Inc., Chicago, IL). Regression analyses were calculated with the GraphPad Prism program (GraphPad Software Inc., La Jolla, CA). A two-tailed P < .05 was considered significant.
RESULTS
The 20S proteasomes were detectable by ELISA in all plasma specimens from volunteers and patients. Measurements of 26S proteasome plasma concentrations in randomly selected specimens (n = 21) from volunteers and patients did not result in signals above the detection limit of the ELISA (data not shown). Although the determined 20S proteasome plasma concentrations were normally distributed in volunteers and in burn patients on day 7 and on day 30, the distribution of 20S proteasome plasma concentrations in burn patients did not pass the normality test between day 0 and day 5.
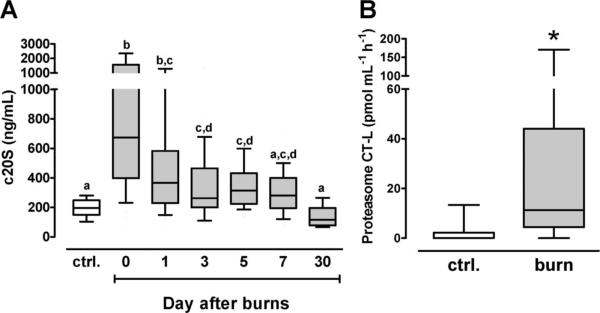
The 20S proteasome concentrations in plasma from healthy volunteers and the time course of 20S proteasome plasma concentrations in patients are shown in Figure 1A. When compared with volunteers, 20S proteasome plasma levels peaked on the day of admission (volunteers [n = 40] − 195 [149/249] ng/mL; patients day 0 [n = 50]: 673 [399/1566] ng/mL, P < .001), gradually declined within the first week after burn injury, and fully returned to baseline levels after 30 days (n = 10; 116.5 [78/196] ng/mL).
Figure 1.
Boxes extend from the 25th to 75th percentile, the horizontal line shows the median. Error bars show the 10th/90th percentile. A, The 20S proteasome plasma concentrations (c20S). Ctrl.: volunteers, n = 40; patients: day 0: n = 50, day 1: n = 36, day 3: n = 35, day 5: n = 28, day 7: n = 34, day 30: n = 10. Boxes not sharing the same letter are significantly different (P < .05). B, Chymotryptic-like (CT-L) proteasome plasma peptidase activity. Ctrl.: volunteers, n = 9. Burn: burn patients, n = 9. *P < .05.
To assess whether the circulating 20S proteasomes are enzymatically active, we randomly selected each nine plasma specimens from volunteers and burn patients from the day of admission and tested for chymotryptic-like proteasome peptidase activity, the main peptidase activity of the 20S proteasome. In plasma from volunteers, chymotryptic-like proteasome peptidase activities were detectable in only three specimens. In contrast, proteasome peptidase activity was detectable in eight of the nine plasma specimens from burn patients (P = .0198 vs ctrl.). Plasma chymotryptic-like proteasome peptidase activity was 11.27 (4.4/44.05) pmol/mL/hr in patients and 0 (0/2.2) pmol/mL/hr in volunteers (P = .011 vs patients; Figure 1B).
On the day of admission, 20S proteasome plasma concentrations were significantly associated with the presence of inhalation injury but did not correlate with age, sex, or %TBSA (Table 2). The 20S proteasome concentrations in plasma from patients with burns without inhalation injury were significantly higher (n = 18, 390 [264/585] ng/mL) than in plasma from volunteers, but significantly lower than in plasma from patients with burns and inhalation injury (n = 26, 1064 [508/1803] ng/mL) and in plasma from patients with inhalation injury only (n = 6, 774 [407/1870] ng/mL; Figure 2).
Table 2.
Correlation of 20S proteasome plasma concentrations on hospital admission with clinical and epidemiological parameters
| rs (P) | All (n = 50) | Burn and Inhalation Injury (n = 32) | Burn Alone (n = 18) |
|---|---|---|---|
| Age | .175 (.235) | .033 (0.86) | −.087 (.74) |
| Sex | .076 (.60) | .14 (0.44) | −.252 (.33) |
| %TBSA | −.086 (.56) | .033 (0.86) | .71 (.0014) |
| Inhalation injury | .581 (<.0001) | — | — |
rs, Spearman's correlation coefficient; P, level of statistical significance (in parentheses).
Figure 2.
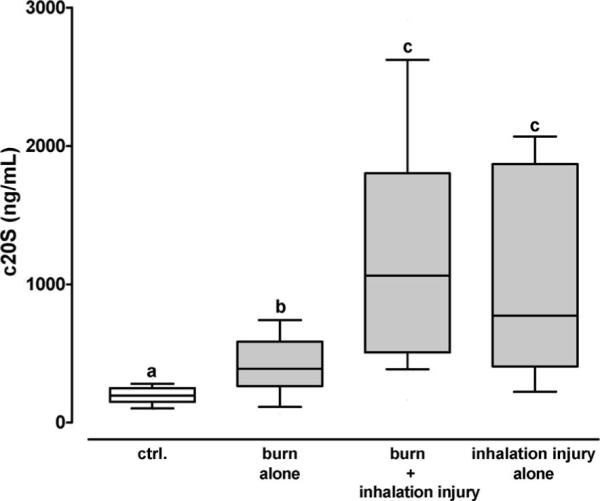
The 20S proteasome plasma concentrations (c20S) from the day of hospital admission in patients with burn alone (n = 18), burn and inhalation injury (n = 26), and inhalation injury alone (n = 6). Ctrl.: volunteers (n = 40). Boxes extend from the 25th to 75th percentile, the horizontal line shows the median. Error bars show the 10th/90th percentile. Boxes not sharing the same letter are significantly different (P < .05).
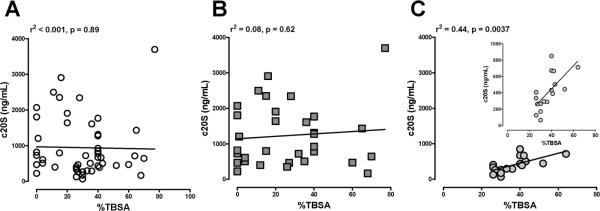
When patients were grouped into patients with and without inhalation injury, 20S proteasome plasma concentrations on the day of admission correlated positively with %TBSA in patients without inhalation injury but not in patients with inhalation injury (Table 2). Regression analyses showed that the association between 20S plasma concentrations and %TBSA in patients with cutaneous burns only was linear (r2: .44, P = .0037; Figure 3).
Figure 3.
Correlation of 20S proteasome plasma concentrations (c20S) from the day of admission with total burn size (%TBSA) in (A) all patients (n = 50), (B) patients with inhalation injury (n = 32), and (C) patients with burn alone (n = 18). The insert to C displays the same data on a different scale to demonstrate the linear relationship. The squared correlation coefficients of the regression curves and the level of statistical significance are displayed in the graphs.
There were no differences in proteasome plasma concentrations between patients with and without development of sepsis/MOF and between survivors and nonsurvivors on any day after injury (data not shown).
In bivariate correlation analyses, 20S proteasome plasma concentrations correlated positive with SOFA scores from the corresponding time points (Table 3) and increased from 327 (239/480) ng/mL in patients with SOFA scores of 0–5 to 387 (255/644) ng/mL with SOFA scores of 5–8 and to 745 (391/1993) ng/mL with SOFA scores of 9 and higher (P = .0036; Figure 4).
Table 3.
Correlation of SOFA scores with circulating 20S proteasome levels, patient characteristics, and outcomes
| SOFA Scores vs | rs (P, n) | rp(P, n) |
|---|---|---|
| 20S proteasome (ng/mL) | .233 (.007, 135) | .134 (.123, 131) |
| Age (yr) | .256 (.002, 148) | .376 (<.001, 131) |
| Male gender | .214 (.009, 149) | .067 (.444, 131) |
| %TBSA | .306(<.001, 149) | .302(<.001, 131) |
| Inhalation injury | .350 (<.001, 149) | .256 (.03, 131) |
| Sepsis/MOF | .626 (<.001, 149) | .426 (<.001, 131) |
| Nonsurvival | .485 (<.001, 149) | .252 (.004, 131) |
rs, Spearman's correlation coefficient; rp, partial correlation coefficient controlling for the effects of all other parameters excluding the outcome measures sepsis/MOF and non-survival; P, level of statistical significance.
Figure 4.
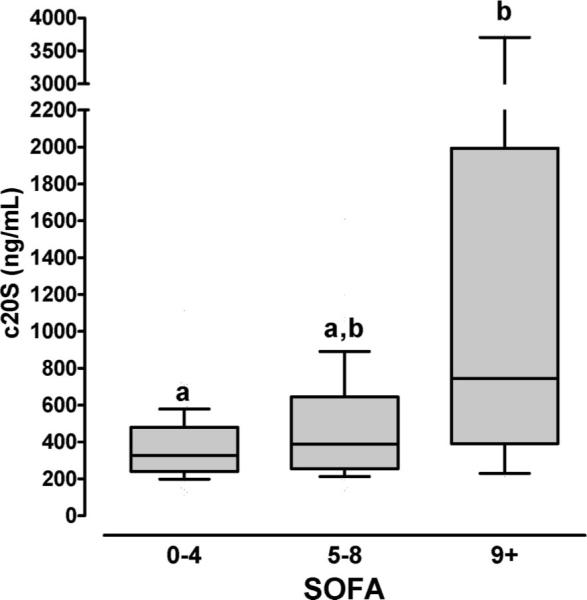
The 20S proteasome plasma concentrations (c20S) and SOFA scores in burn patients. Boxes extend from the 25th to 75th percentile, the horizontal line shows the median. Error bars show the 10th/90th percentile. SOFA 0–4: n = 77; SOFA 5–8: n = 47; SOFA 9+:n = 11. Boxes not sharing the same letter are significantly different (P < .05).
Because age, male gender, total burn size, and presence of inhalation injury also correlated significantly positive with SOFA scores, we calculated partial correlation coefficients to control for the effects of these variables. Although SOFA scores correlated independently with age, %TBSA, presence of inhalation injury, and measured development of sepsis/MOF and survival, 20S proteasome plasma levels as well as male gender were not independently associated with SOFA scores (Table 3).
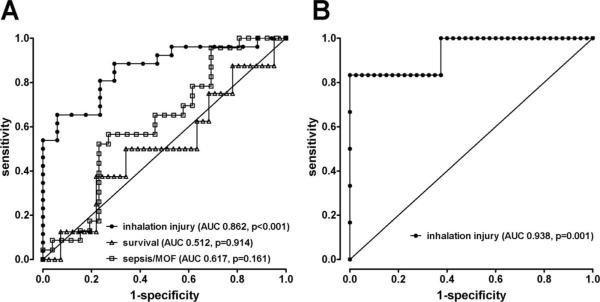
To evaluate the prognostic or diagnostic value of 20S proteasome concentrations on the day of admission, we performed ROC analyses for the presence of inhalation injury, sepsis/MOF development, and survival (Figure 5A). The 20S proteasome concentrations did not discriminate sepsis/MOF development (area under the ROC curve: 0.617, P = .161) or survival (area under the ROC curve: 0.512, P = .914). The area under the ROC curve for the presence of inhalation injury in patients with cutaneous burn injuries was 0.862 (95% confidence interval: 0.754–0.970; P <.001). To diagnose inhalation injury in burn patients, a 20S proteasome plasma concentration on the day of admission of ≥455 ng/mL had a positive predictive value of 88% and a negative predictive value of 71%.
Figure 5.
Receiver operator characteristic (ROC) curves for 20S proteasome plasma concentrations on the day of admission. The diagonal lines represent the reference curves (area under the ROC curve of 0.5 = no diagnostic/prognostic value). Each symbol represents a 20S proteasome plasma concentration. A, Patients with cutaneous burns. ROC curves for the presence of inhalation injury (●), sepsis/MOF development (□]) and survival (△). B, Patients with inhalation injury only and volunteers. ROC curve for the presence of inhalation injury in the absence of cutaneous burns.
The 20S proteasome plasma concentrations on the day of admission were above the range of concentrations measured in volunteers in five of six patients with inhalation injury only. To obtain a preliminary estimate on the possible value of 20S proteasome plasma levels for the diagnosis of inhalation injury in the absence of cutaneous burns, we also calculated a ROC curve from the 20S proteasome concentrations measured in plasma from volunteers and patients with inhalation injury only (Figure 5B). This resulted in an area under the ROC curve of 0.938 (95% confidence interval: 0.821–1.054, P = .001). For the diagnosis of inhalation injury in the absence of cutaneous burns, a plasma 20S proteasome concentration of ≥300 ng/mL had a positive predictive value of 83% and a negative predictive value of 95%.
DISCUSSION
This is the first description of systemic proteasome concentrations after burn and inhalation injury. The finding that 20S proteasomes were detectable in all plasma specimens is consistent with previous observations in healthy individuals and other patient populations.5–9 Because assays to quantify 26S proteasomes have not been available until recently,18 data on the presence of circulating 26S proteasomes are sparse. The initial assessment of 26S plasma levels with a specific and sensitive ELISA18 in this study did not result in measurable concentrations. Although we cannot exclude that circulating 26S proteasome concentrations were below the detection limit of the ELISA, our observation along with the known requirement of ATP for 26S proteasome assembly and stability rather suggests that only 20S proteasomes are present in the systemic circulation.18,22
The determined circulating 20S proteasome concentrations in healthy volunteers in this study were comparable with previously reported normal concentrations.7,9,12 As expected and similar to patients with severe mechanical injuries,8 circulating 20S proteasome levels were also significantly elevated within 24 hours after admission in patients with thermal injuries. The finding that increased proteasome peptidase activity was detectable in plasma specimens from patients on the day of admission further supports the assumption that circulating 20S proteasomes are intact and enzymatically active.10 Although the exact origin of circulating 20S proteasomes is currently unknown, its time-related changes after burns and inhalation injury with peak concentrations on admission, its linear correlation with burn size in patients without inhalation injury, and the observation that initial 20S proteasome concentrations did not correlate with age or sex suggest that 20S proteasome is released from damaged tissues in response to the initial injury.
Although a direct comparison between tissue proteasome levels in the skin and lung is currently not available, we showed previously that the lung is among the organs with significantly higher proteasome tissue levels than skeletal muscle.20 Along with the observation that circulating 20S proteasome concentrations in patients with pulmonary manifestation of systemic lupus erythematosus were also significantly higher than in patients without pulmonary disease manifestation,9 the finding that inhalation injury was associated with significantly elevated 20S proteasome levels in this study suggests its release form the damaged lung after inhalation injury and further points to damaged tissues as the origin of circulating 20S proteasomes.
Because of the wide distribution of 20S proteasome concentrations in patients, the small patient population and the obvious influence of inhalation injury on its systemic concentrations, it was not surprising that 20S proteasome levels did not discriminate patients with or without sepsis/MOF development or survivors and nonsurvivors. On the basis of the distribution of 20S proteasome concentrations and the observed incidence of sepsis/MOF development and death, we calculate that our study had a power of 0.8 to detect a 60% difference in 20S proteasome levels between patients with and without sepsis/MOF development on a P = .05 level and to detect a difference of 72% between survivors and non-survivors, respectively.
Although 20S proteasome levels correlated significantly positive with the corresponding SOFA scores in univariate analyses, partial correlation analyses showed that this association was not independent of its association with other and well-established risk factors for organ failure development after burns.23–25 The finding that sepsis/MOF development and mortality correlated independently with SOFA scores validates the correlation analyses.23,24
Although our previous observations on proteasomes in the extracellular bronchoalveolar space point toward a possible functional role in the lung after blunt trauma or burns,26,27 evidence for a pathophysiologic role of circulating 20S proteasomes has not been provided to date. Therefore, patient group differences in circulating 20S proteasome levels appear less important than the distribution of the individual concentrations, as the latter indicate whether they could be useful as a disease biomarker. Because the diagnosis of burns and the assessment of %TBSA are determined precisely by clinical examination, a biomarker for the assessment of %TBSA would have little practical relevance for the hospital burn surgeon. In contrast, the presence of inhalation injury is not always obvious, pulmonary symptoms usually appear delayed, and inhalation injury significantly affects the prognosis of the patient. Thus, a biomarker for the diagnosis of inhalation injury could improve the treatment of the burn patient, especially when a diagnostic bronchoscopy is not possible. Our previous9 and present observations suggest that any acute lung damage is likely to increase circulating 20S proteasome concentrations, independent of the etiology. Thus, if other lung pathologies can be excluded, the distribution of 20S proteasome plasma concentrations in this study may suggest that they could be a useful diagnostic tool to predict the presence of inhalation injury in burn patients and to diagnose or exclude inhalation injury in patients without cutaneous burns.
In conclusion, this study shows that intact circulating 20S proteasomes are elevated after burn and inhalation injury and implies that systemic 20S proteasome concentrations are a biomarker of tissue damage.
Measurements of circulating 20S proteasome concentrations in patients with burns and/or inhalation are unlikely to predict outcomes. Nevertheless, this study indicates that measurements of circulating 20S proteasome concentrations on hospital admission may be useful for the diagnosis of inhalation injury. Because the patient population of this study was small, confirmation of our initial observations in a larger cohort of patients is mandatory.
Acknowledgments
Supported by Deutsche Forschungsgemeinschaft (DFG) MA 2474/2-2 (to M.M.), USAMRAA #6123-1035-00-B contract #W81XWH-05-1-0585 (to M.M.), NIH R01 AA012034 and AG018859 (to E.J.K.), NIH T32 GM008750 (to R.L.G.), International Association of Fire Fighters Burn Fund (to J.M.A. and R.L.G.) and Dr. Ralph and Marian Falk Medical Research Trust (to R.L.G.).
REFERENCES
- 1.Baumeister W, Walz J, Zuhl F, Seemüller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–80. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 2.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 3.Hershko A, Ciechanover A, Varshavsky A. Basic medical research award. The ubiquitin system. Nat Med. 2000;6:1073–81. doi: 10.1038/80384. [DOI] [PubMed] [Google Scholar]
- 4.Groll M, Huber R. Inhibitors of the eukaryotic 20S proteasome core particle: a structural approach. Biochim Biophys Acta. 2004;1695:33–44. doi: 10.1016/j.bbamcr.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Dutaud D, Aubry L, Henry L, et al. Development and evaluation of a sandwich ELISA for quantification of the 20S proteasome in human plasma. J Immunol Methods. 2002;260:183–93. doi: 10.1016/s0022-1759(01)00555-5. [DOI] [PubMed] [Google Scholar]
- 6.Lavabre-Bertrand T, Henry L, Carillo S, et al. Plasma proteasome level is a potential marker in patients with solid tumors and hemopoietic malignancies. Cancer. 2001;92:2493–500. doi: 10.1002/1097-0142(20011115)92:10<2493::aid-cncr1599>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 7.Wada M, Kosaka M, Saito S, Sano T, Tanaka K, Ichihara A. Serum concentration and localization in tumor cells of proteasomes in patients with hematologic malignancy and their pathophysiologic significance. J Lab Clin Med. 1993;121:215–23. [PubMed] [Google Scholar]
- 8.Roth GA, Moser B, Krenn C, et al. Heightened levels of circulating 20S proteasome in critically ill patients. Eur J Clin Invest. 2005;35:399–403. doi: 10.1111/j.1365-2362.2005.01508.x. [DOI] [PubMed] [Google Scholar]
- 9.Majetschak M, Perez M, Sorell LT, Lam J, Maldonado ME, Hoffman RW. Circulating 20S proteasome levels in patients with mixed connective tissue disease and systemic lupus erythematosus. Clin Vaccine Immunol. 2008;15:1489–93. doi: 10.1128/CVI.00187-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoeger A, Blau M, Egerer K, Feist E, Dahlmann B. Circulating proteasomes are functional and have a subtype pattern distinct from 20S proteasomes in major blood cells. Clin Chem. 2006;52:2079–86. doi: 10.1373/clinchem.2006.072496. [DOI] [PubMed] [Google Scholar]
- 11.Jakob C, Egerer K, Liebisch P, et al. Circulating proteasome levels are an independent prognostic factor for survival in multiple myeloma. Blood. 2007;109:2100–5. doi: 10.1182/blood-2006-04-016360. [DOI] [PubMed] [Google Scholar]
- 12.Egerer K, Kuckelkorn U, Rudolph PE, et al. Circulating proteasomes are markers of cell damage and immunologic activity in autoimmune diseases. J Rheumatol. 2002;29:2045–52. [PubMed] [Google Scholar]
- 13.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 14.Goris RJ, te Boekhorst TP, Nuytinck JK, Gimbrère JS. Multiple-organ failure. Generalized autodestructive inflammation? Arch Surg. 1985;120:1109–15. doi: 10.1001/archsurg.1985.01390340007001. [DOI] [PubMed] [Google Scholar]
- 15.Ertel W, Keel M, Marty D, et al. Significance of systemic inflammation in 1,278 trauma patients. Unfallchirurg. 1998;101:520–6. doi: 10.1007/s001130050304. [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 17.Antonelli M, Moreno R, Vincent JL, et al. Application of SOFA score to trauma patients. Sequential Organ Failure Assessment. Intensive Care Med. 1999;25:389–94. doi: 10.1007/s001340050863. [DOI] [PubMed] [Google Scholar]
- 18.Majetschak M, Sorell LT. Immunological methods to quantify and characterize proteasome complexes: development and application. J Immunol Methods. 2008;334:91–103. doi: 10.1016/j.jim.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Majetschak M, Patel MB, Sorell LT, Liotta C, Li S, Pham SM. Cardiac proteasome dysfunction during cold ischemic storage and reperfusion in a murine heart transplantation model. Biochem Biophys Res Commun. 2008;365:882–8. doi: 10.1016/j.bbrc.2007.11.092. [DOI] [PubMed] [Google Scholar]
- 20.Patel MB, Majetschak M. Distribution and interrelationship of ubiquitin proteasome pathway component activities and ubiquitin pools in various porcine tissues. Physiol Res. 2007;56:341–50. doi: 10.33549/physiolres.931005. [DOI] [PubMed] [Google Scholar]
- 21.Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, Crews CM. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci USA. 1999;96:10403–8. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eytan E, Armon T, Heller H, Beck S, Hershko A. Ubiquitin C-terminal hydrolase activity associated with the 26 S protease complex. J Biol Chem. 1993;268:4668–74. [PubMed] [Google Scholar]
- 23.Klein MB, Hayden D, Elson C, et al. The association between fluid administration and outcome following major burn: a multicenter study. Ann Surg. 2007;245:622–8. doi: 10.1097/01.sla.0000252572.50684.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorente JA, Vallejo A, Galeiras R, et al. Organ dysfunction as estimated by the sequential organ failure assessment score is related to outcome in critically ill burn patients. Shock. 2009;31:125–31. doi: 10.1097/SHK.0b013e31817fc3ef. [DOI] [PubMed] [Google Scholar]
- 25.Lundgren RS, Kramer CB, Rivara FP, et al. Influence of comorbidities and age on outcome following burn injury in older adults. J Burn Care Res. 2009;30:307–14. doi: 10.1097/BCR.0b013e318198a416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majetschak M, Sorell LT, Patricelli T, Seitz DH, Knoferl MW. Detection and possible role of proteasomes in the bronchoalveolar space of the injured lung. Physiol Res. 2009;58:363–72. doi: 10.33549/physiolres.931526. [DOI] [PubMed] [Google Scholar]
- 27.Albright JM, Romero J, Saini V, et al. Proteasomes in human bronchoalveolar lavage fluid after burn and inhalation injury. J Burn Care Res. 2009;30:948–56. doi: 10.1097/BCR.0b013e3181c07f37. [DOI] [PMC free article] [PubMed] [Google Scholar]