Abstract
Inflammation is considered the underlying cause of numerous disorders, and the practice of taking antiinflammatories as diet supplements has become increasingly prevalent. This study addresses the bioavailablity of a well-established dietary antiinflammatory, curcumin, and examines its effect on adaptive immunity. Visceral leishmaniasis is a major parasitic disease which protection relies on cell-mediated immunity and production of nitric oxide. We found that long-term, low-dose, oral consumption of curcumin activates peroxisome proliferator-activated receptor-γ, deactivates type 1 response, inhibits inducible nitric oxide synthase, and interferes with adaptive immunity to exacerbate the pathogenesis of Leishmania donovani infection in vivo. These in vivo effects can be correlated to activities on infected residential macrophages in vitro. Therefore, when reactive radicals generated from inflammation play the dominant role in elimination of pathogens, excessive use of the antioxidative supplements may compromise microbial defense. Nonetheless, it should be noted with equal importance that our finding, conversely, also strengthens the prospect that curcumin may alleviate type 1 response disorders.
Keywords: antiinflammatories, curcumin, Leishmania donovani, nitric oxide, peroxisome proliferator-activated receptor-γ, T helper 1 immunity
Inflammation and the associated oxidative stress have been recognized as risk factors for many chronic disorders that threaten the nation’s population; for example, chronic heart diseases, diabetes, and cancer.1 The aging population is at risk for many of these disorders. Antioxidants and antiin-flammatories are explored as cancer chemopreventive agents, and have yielded convincing evidence in animal models.2–4 Nowadays, laymen as well as professionals accept long-term use of antiinflammatories. According to CDC’s national health and nutrition examination survey, many individuals have been taking them daily for more than a 2-year period.5 The easier access for consumers following the Dietary Supplement Health and Education Act of 1994 boosted the popularity of dietary supplements, also known as nu-traceuticals.4,5 However, to date, even though there are many reports that described the fact that dietary compounds attenuate pathological damages, few have illustrated how oral intake of these antiinflammatories and/or antioxidants modulates the immune pathways that underlie disorders.
Inflammation is essential for initiation of immune defense, even though it is etiological to certain disorders. The immune system manages the response by a balance of pro- (type 1) and anti- (type 2) inflammation factors. In a type I response, production of cytokines, such as interleukin-12 (IL-12), tumor-necrosis factor-α (TNFα), directs the launching of specific immunity, leading to the production of interferon-γ (IFNγ) and nitric oxide for protection against microbial infections, including many opportunistic microbial infections of public health concern—tuberculosis, hepatitis, herpes, and cytomegalovirus infections. Conversely in a type 2 response, IL-10 keeps inflammation in check. Suppression of inflammation in rheumatoid arthritis with infliximab and etanercept, the antibodies that block TNFα, and anakinra, the IL-1 receptor antagonist, increases the likelihood of tuberculosis and leishmaniasis.6,7 The general objective of this work is to evaluate whether long-term use of anti-oxidants may compromise the immune defense to exacerbate microbial infections, a concern that has not been critically examined. From the multitude of antiinflammatory compounds and chronic infections, we chose to study the effect of curcumin on leishmaniasis as our investigation model.
Curcumin is an antioxidant, antiinflammatory active principal ingredient of the curry spice turmeric.8 For the past two decades, the compound has attracted interest as a cancer preventive agent.8 The compound is generally regarded as safe in a phase I clinical trial of cancer patients and marketed as a dietary supplement.9 It is well known that curcumin preventing the onset of inflammation by inhibiting the activation of nuclear factor-κB (NFκB), the production of TNFα, IFNγ, and nitric oxide, and gene expression of inducible nitric oxide synthase (iNOS).10–15 More recently, it was found to activate the nuclear receptor peroxisome proliferator-activated receptor-γ (PPARγ), which has a promising target for switching off inflammation. It acts by transrepressing NFκB, activating protein-1, and signal transducer and activator of transcription-1.16,17 Many anti-inflammatory natural products, especially dietary lipids like linoleic acids, activate PPARs.18
Defense against Leishmania donovani depends upon T helper 1 response in the murine model. The etiological agent is an insect-borne protozoan parasite that invades human macrophages. In both humans and mice, the liver and spleen harbor the parasite, and curcumin is effective in these organs. The mechanisms of defense for this chronic infection have been extensively investigated. Proinflammatory factors, including TNFα and IFNγ are crucial for controlling leishmaniasis. The balance between type 1, cell-mediated immunity, and type 2, humoral immunity, underlies resistance and susceptibility.19,20 This model has further significance, as leishmaniasis is endemic in India where turmeric, containing 1–5% curcumin, is copiously used in cooking, cosmetics, household remedies for insect bites, swelling, minor wounds, and in Ayurveda medicine for liver disorders, inflammatory diseases, and diabetes.2,8,21 We anticipate results from this study will provide some practical insight towards treatment of this disease, from which 0.5 million people suffer annually and which has created a heavy social and economic burden around India.22,23
This report shows that curcumin when taken orally below the recommended dietary supplement dose, activates PPARs in mice, modulates the type 1/type 2 immune balance, and influences adaptive immunity to L. donovani. It also suggests that curcumin has the potential to attenuate or exacerbate inflammatory diseases of the liver and spleen depending on whether inflammation and type 1 immune response are protective or detrimental.
MATERIALS AND METHODS
Experimental Design for In Vivo Studies
Promastigotes that are cultured in vitro were enumerated under the microscope and intravenously injected to lessen variability that may arise from using amastigotes in organ extracts of infected animals. Mice were infected with 107 stationary-phase promastigotes of L. donovani, and divided into groups. Before feeding of the mice, curcumin was dissolved to a solution of 7.52 mg/ml in 0.1 N NaOH and immediately brought to pH 7.2 by diluting to a concentration of 11.1 μg/ml in PBS. The integrity of the curcumin was checked by TLC analysis as described in Chan et al.11 The experimental group received, by gavage, 0.2 ml of the curcumin solution three times a week, on every other day except for the weekends, throughout the course of the study. Food pellets were removed for 4 h, and then returned 3 h after feeding to maximize absorption of the compound. The control group was sham fed with PBS in the same manner. The mice received regular, non-fortified food pellets. At the peak of hepatic infection (4 weeks) livers and spleens were harvested.
Experimental Design for In Vitro Studies
Nonelicited peritoneal exudates were collected by aspiration and cultured in DMEM medium, which does not contain sodium nitrite but was supplemented as described in Chan et al.24 The cells received stationary-phase promastigotes at a 1:10 ratio for a period of 16–20 h, and then curcumin and 100 U/ml of IFNγ and 10 ng/ml of TNFα or primed lymphocytes obtained from spleen of mice that had been infected with L. donovani for 4 weeks. Curcumin was prepared by dissolving the powder to 20 mM in acetone and then the solution was diluted to the desired concentrations in supplemented DMEM containing 0.1% acetone. Supernatants were harvested at 72 h for analysis of cytokine production by ELISA and on day 5 for quantifying nitric oxide production with Griess reagent. On the fifth day, macrophages were stained with propidium iodide and infectivity was determined by enumerating the number of amastigotes per macrophage as well as the percent of infected macrophages under a fluorescence microscope.
Limiting Dilution Analysis
Parasite load was estimated according to the limiting dilution analyses procedure of Titus et al.25 Briefly, liver and spleen single-cell suspensions were plated into wells of 96-well plates at a range of concentrations, and parasite were allowed to transform into promastigotes and proliferate by incubation at 27°C. After 2–3 weeks, the number of wells that were positive for growth was scored under a microscope, and the L-Calc™ software for limiting dilution analysis (provided by Stem Cell Technology, Vancouver, Canada) was used to determine the frequency of parasite.
RT–PCR and PCR
DNA and RNA extractions were performed using TRI reagent (Invitrogen). For determining parasite load, the real-time PCR procedure was that of Nicolas et al,26 except the reaction occurred in SYBR Green I PCR master mix from Superarray according to the manufacture’s instruction. DNA at 40 ng per reaction was denatured at 95°C for 10 min, and then Leishmania kinetoplast DNA (kDNA) was amplified in a thermal cycler, Rotor-gene 6.0 from Corbett Research. The number of copies of kDNA per μg of DNA was determined using a standard curve that was established with the cloned PCR product. For levels of gene expression, first-strand cDNA was synthesized by reverse transcribing 1 μg of RNA, in a 20-μl volume with 100 U MMLV reverse transcriptase, 20 U RNase inhibitor, 0.6 mM of dNTP, and 0.4 mM of oli-go(dT16). Real-time PCR employed commercial primers and SYBR Green I PCR master mix from Superarray. The housekeeping gene 18S RNA was reverse transcribed with random hexamer, and its cDNA solution was diluted to attain an amplification efficiency that was comparable to that of the experimental gene. Gene expression was normalized to number per copy of 18S RNA. The primers and conditions for end-point PCR analysis have been described in Chan et al.11 The PCR products were stained with SYBR Green I dye (Invitrogen) and separated on 2% agarose gel for kDNA analysis and 1.6% agarose gel for iNOS and GAPDH. The intensities of the bands were determined with the Kodak 1D image analysis system. The degree of modulation was calculated as a percent by the following formula: percent change = 100 × (untreated Leishmania-infected sample–Leishmania-infected and curcumin-treated sample)/(untreated Leishmania-infected sample).
Ex Vivo Analysis for IFNγ
Spleen cells from infected mice that had been fed curcumin or PBS, and from uninfected normal mice were plated at 3 × 106 per ml in 24-well plates with L. donovani promastigotes. Then, supernatants were collected at 72 h of incubation and IFNγ was quantified by ELISA.
ELISA
Measurement of cytokine production employed OptEIA ELISA kits form BD Pharmingen and was performed according to the manufacturer’s protocols.
Nitrite Assay
Nitrite production was measured by Griess reaction as described in Chan et al.13
PI Staining
Cover slips with attached cells were washed twice with PBS, and the cells were fixed using 4% paraformaldehyde in monobasic sodium phosphate for 20 min. Following another two washes with PBS, the cover slips were blocked with 2% BSA and 0.1% Tween-20 for 20 min. Then the cells were stained with 100 ng/ml of propidium iodide in the dark, and the number of infected macrophages and amastigotes were counted under a fluorescent microscope.
Statistical Analysis
For in vitro experiments to study the effect of curcumin on Leishmania-infected peritoneal macrophages, lymph node cells, and splenocytes, a statistical analysis was performed using ANOVA. For in vivo experiments, cytokine production employed a qualitative, nonparametric, Mann–Whitney U-test, whereas parasite load was analyzed by ANOVA after the data underwent natural log transformation.
RESULTS
Consumption of Curcumin Increased Parasite Burden in Liver and Spleen
Leishmania is transmitted by sandflies, which inject the promastigote form of the parasite into the mammalian host, where they transform into amastigotes within the macrophages. Visceral targets of Leishmania include liver, spleen, and bone marrow of man, and infections can be fatal if untreated. In mouse, infection of the liver is controlled by the fourth week, whereas the parasite establishes nonfatal chronic residence in the spleen. The C3H/He strain of mice, which controls the parasite, is considered resistant, whereas the BALB/c strain, which allows the parasite to proliferate, is considered susceptible.19
We examined the effect of curcumin on pathogenesis of leishmaniasis in both the BALB/c and C3H/He strains (Figure 1; Table 1). In multiple trials, the mice were infected with stationary-phase promastigotes and divided into two groups of 10 each. The experimental group was fed 0.2 ml of 30 μM curcumin in PBS per mouse. The dose was chosen based on extrapolation from the dose for human consumption. As a dietary supplement, the recommended dose is 300 mg 2–3 times daily.8 In Indian diet, 38–190 mg of turmeric is consumed daily, which translates to 440 μg–2 mg/kg (44 ng–2 μg/g) curcumin for an average individual of 85 kg, assuming that curcumin constitutes 1–5% of turmeric.8 Rural Indian diet provides as high as 3.8 g of turmeric.23 At 4 weeks after infection, the parasite load was quantified by two complementary methods, limiting dilution analysis25 and real-time PCR detection,26 and compared by the parametric test ANOVA after data transformation.
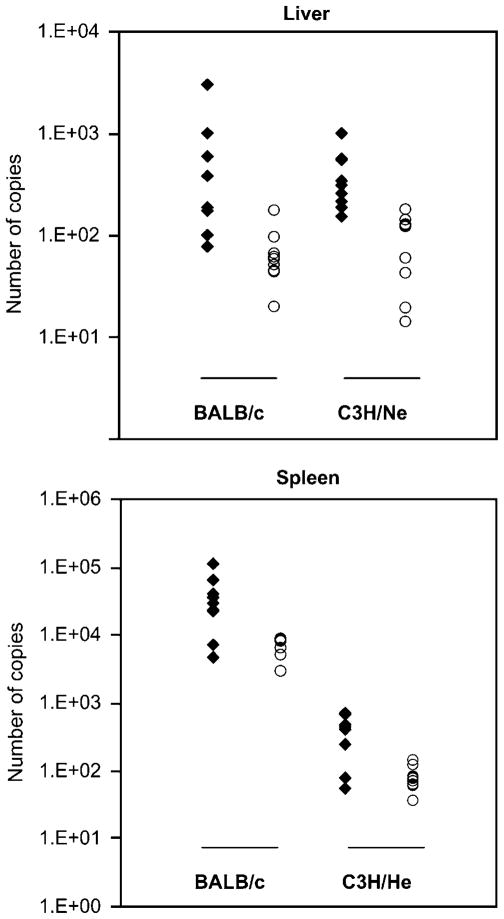
Figure 1.
Comparison of parasite burden in curcumin-fed and PBS-fed mice. DNA samples from curcumin-fed, L. donovani-infected mice are represented in filled diamonds (N = 10) and those from PBS-fed mice by open circles (N = 10). Samples were quantified by real-time PCR, amplifying a 116-bp product of kDNA. The result shown is a plot of a representative experiment from one of the multiple PCR analysis trials used in computing the statistical analysis in Table 1.
Table 1.
Effect of curcumin on parasite load in liver and spleen
| N | Mean | s.d. | PBS vs curcumin significance | |||||
|---|---|---|---|---|---|---|---|---|
| LDA | Liver | BALB/c | PBS | 40 | 6.853 | 1.133 |  |
P < 0.001 |
| Curcumin | 40 | 8.171 | 2.016 | |||||
| C3H/He | PBS | 31 | 4.052 | 1.988 | ||||
| Curcumin | 31 | 5.300 | 2.17 | |||||
| Spleen | BALB/c | PBS | 40 | 4.931 | 1.225 |  |
P < 0.05 | |
| Curcumin | 40 | 5.872 | 1.259 | |||||
| C3H/He | PBS | 31 | 3.746 | 1.316 | ||||
| Curcumin | 31 | 4.361 | 1.171 | |||||
| PCR | Liver | BALB/c | PBS | 28 | 8.258 | 0.770 | P < 0.001 | |
| Curcumin | 30 | 9.692 | 1.021 | |||||
| C3H/He | PBS | 21 | 4.762 | 1.212 | P < 0.05 | |||
| Curcumin | 21 | 5.494 | 1.0007 | |||||
| Spleen | BALB/c | PBS | 30 | 8.673 | 0.572 |  |
P < 0.001 | |
| Curcumin | 30 | 10.018 | 1.102 | |||||
| C3H/He | PBS | 21 | 5.086 | 0.988 | ||||
| Curcumin | 21 | 5.543 | 0.999 |
Mice were intravenously infected with the 107 stationary-phase promastigotes and divided into two groups. One group was fed with 0.2 ml of 30 μM solution of curcumin by oral gavage three times a week throughout the course of the study. The second group received PBS in the same manner. Parasite load in the liver and spleen of BALB/c and C3H/He strains of mice was determined by limiting dilution and real-time PCR analyses at 4 weeks, the peak of hepatic infection. DNA was extracted with TRI reagent, and real-time PCR analysis was performed using SYBR Green master mix and primers from Superarray Bioscience (Frederick, MD, USA) in a Rotorgene 3000 (Corbett) thermocycler. The data were subjected to natural log transformation and then ANOVA was performed to compare the parasite load in the two groups. ‘N’ indicates the number of mice used in the statistic analyses after exclusion of outliers. The experimental treatment (independent variables) groups compared in a statistical analysis are bracketed and the degrees of confidence are listed in the column to the right as P values. Where the BALB/c and C3H/He strains of mice did not differ in their response to curcumin, the two strains were combined in the analysis (large brackets). By PCR, an interaction between mouse strain and treatment was found when total parasite counts were compared, hence, the BALB/c and C3H/He strains were analyzed separately (small brackets).
Oral administration of curcumin exacerbated L. donovani (WHOM/SD/00/1S) infection in both the resistant and the susceptible strains as assessed by both limiting dilution analysis and PCR analysis. The former method largely reflects the percent of infected macrophages, as each infected macrophage results in a positive well regardless of whether it is singly or multiple infected. The latter measures the copies of kDNA, and therefore reflects the number of parasites in the organs (Figure 1; Table 1). The two strains of mice did not differ in their response to curcumin, except the increase in parasite load was worse in the liver of the BALB/c than C3H/He strain. The increase in frequency of infected macrophages and number of parasites in the liver of the susceptible BALB/c mice can be concluded with 99.9% confidence. We can also conclude with 95 or 99% confidence that the same effects occurred in the spleen and liver of the C3H/He mice, respectively.
Increased in Parasite Burden is Associated with Decrease in Production of ProInflammatory Cytokines and Nitric Oxide
Early after infection, resistant mice produce inflammatory cytokines (IFNγ, TNFα, and IL-12) that lead to cell-mediated immunity that confers protection,27,28 and these cytokines are associated with production of nitric oxide. Many studies29–31 have demonstrated that the nitric oxide radical and its reactive metabolites are crucial for killing of Leishmania; for example, Murray and Nathan32 have clearly shown that defense against L. donovani infection is compromised in iNOS knockout mice. Our laboratory10,11,13,15 and others’12,14 have shown that curcumin suppresses the gene expression of TNF and iNOS as well as production of nitric oxide, in vitro and in vivo. Furthermore, it impedes elimination of Leishmania by nitric oxide donors.24 Therefore, the level of IFNγ, TNFα, and iNOS gene expression in the liver and spleen of the mice whose parasite load had increased was examined (Figure 2).
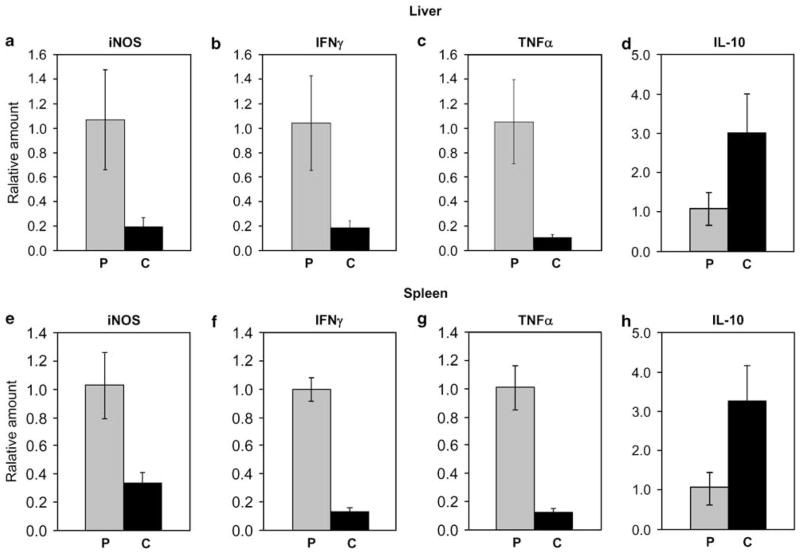
Figure 2.
Effect of curcumin on gene expression of proinflammatory and antiinflammatory mediators in Leishmania-infected (a–d) liver and (e–h) spleen. Sample of organs from BALB/c strain with parasite load more than one standard deviation from the average load in the PBS group in the same trial were selected to identify correlation between the gene expression of iNOS, IFNγ, TNFα, IL-10, and outcome of infection. The levels of mRNA in liver and spleen of PBS-fed mice (P, N = 10), and curcumin-fed (C, N = 10) Leishmania-infected mice were determined by RT–PCR, each gene was normalized to 18S RNA, and then the level of expression was expressed relative to that of the PBS control using the ΔΔCt method. Statistical analysis performed using Mann–Whitney U-test indicated that there were significant differences between the curcumin-fed and PBS-fed groups (P < 0.001 for iNOS, IFNγ and TNFα and P < 0.05 for IL-10). The level of mRNA expression for IFNγ, TNFα, and iNOS in uninfected mice was not detectable after 40 cycles of amplifications.
Exacerbation of infection was most prominent in the susceptible BALB/c mice, hence subjected to detailed study. Real-time PCR analyses revealed that gene expression for TNFα, IFNγ, and iNOS in the liver and spleen were activated by Leishmania infection. Curcumin-fed mice that had exacerbated infection expressed about a log less of all these genes than did the PBS-fed counterpart. As for normal, uninfected mice, they expressed very low levels of these genes in the liver and spleen, below accurate detection by the real-time PCR assay. In the liver, curcumin reduced the expression of iNOS, IFNγ, and TNFα by 80, 81, and 90%, respectively (Figure 2a and c). In the spleen, curcumin also decreased the expression of IFNγ and iNOS expression by 86 and 68%, respectively (Figure 2e and f). The copy of TNFα RNA in the spleen was about a tenth of iNOS or IFNγ, yet curcumin feeding reduced the relative copy number by 90% (Figure 2g). Statistic analysis by nonparametric Mann–Whitney U-test indicated that the difference between the curcumin and PBS groups is significant for each of the genes in both organs (P < 0.001).
Ex vivo assays further established that curcumin reduced the production of IFNγ in the L. donovani-infected mice at the protein level. As shown in Table 2, sensitized spleen cells from L. donovani-infected mice produced IFNγ at about 2110 pg/ml upon restimulation with parasites. In contrast, equal amount of splenocytes from mice that were fed with curcumin produced only 1430 pg/ml, a decrease of 32%. The specificity of the T helper cells were verified in that when spleen cells were not restimulated with parasite or when naive splenocytes from uninfected mice were used, the amount of IFNγ was produced a log lower, at a basal level of 100–300 pg/ml.
Table 2.
Ex vivo analysis of IFNγ production
| Source of splenocytes | Ex vivo restimulation with L. donovani | IFNγ |
|
|---|---|---|---|
| pg/ml | s.d. | ||
| Vehicle control mice | + | 2110.5* | 96.5 |
| − | 123.2 | 9.9 | |
| Curcumin-fed mice | + | 1430.0** | 89.1 |
| − | 161.7 | 11.8 | |
| Normal mice | + | 296.5 | 19.7 |
| − | 213.0 | 17.8 | |
IFNγ, interferon-γ.
Splenocytes obtained from curcumin or PBS-fed (vehicle control) mice were coincubated with L. donovani 1S promastigotes ex vivo for 72 h, then supernatants were collected for IFNγ assays. Student’s t-test showed that the difference between * and ** is with a P-value of < 0.05.
Increased in Parasite Burden is Associated with Activation of IL-10 and PPARγ
As oppose to type 1, the type 2 antiinflammatory cytokine, IL-10, is considered deleterious in Leishmania pathogenesis. Detectable in the plasma and lymphocytes of L. donovani patients, IL-10 inhibits the production of IL-12 and IFNγ in peripheral blood mononuclear leukocytes.33,34 When PCR analysis was performed on IL-10, curcumin feeding increased its gene expression by threefold in contrast to the decrease in proinflammatory cytokines (Figure 2d and h). Thus, infection of BALB/c mice led to an upregulation of IL-10, and curcumin further upregulated this detrimental cytokine.
PPARγ transrepresses NFκB to reduce inflammation in thioglycolate-elicited peritoneal macrophages, leading to decreased expression of iNOS.16 Liver expresses the PPARα isoform predominantly, and cells of the immune system, monocytes, macrophages and lymphocytes express both the α and γ isoforms. Chen and Xu35 reported that curcumin activates the nuclear receptor PPARγ in Moser cells, a human colon cancer cell line. Siddiqui et al36 have also claimed that intravenous administration of curcumin at 2 μM suppresses sepsis through PPARγ, and the antagonist, GW9662, blocks the effect. In fact, Zheng and Chen37 have suggested that a curcumin-responsive element resides in the regulatory region of the PPARγ gene.
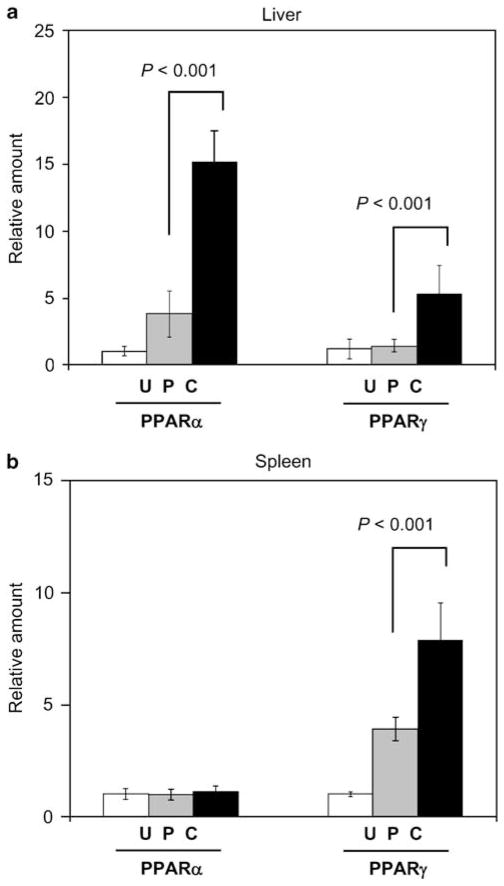
Interestingly, we found that Leishmania infection upregulated the expression of PPARs in mice (Figure 3). In an isotype- and organ-specific manner, infection upregulated the expression of PPARs in the liver and the spleen by 3.5-fold, and curcumin enhanced their expression further in the infected liver (Figure 3a) and spleen (Figure 3b). The effect is most prominent in the liver, where 40-fold excess was observed with PPARα. Even though PPARγ expression in the liver was very low, a fourfold increase was seen. In the spleen, curcumin feeding also heightened the expression of PPARγ by twofold. Thus, there is an association between increase in PPAR expression and increase in parasite load.
Figure 3.
Upregulation of PPAR gene expression by Leishmania infection and curcumin in vivo. Levels of PPARα and PPARγ mRNA in liver (a) and spleen (b) of uninfected mice (U, N = 5), Leishmania-infected and PBS-fed mice (P, N = 10), and Leishmania-infected and curcumin-fed mice (C, N = 10) were determined by RT–PCR as described in the legend to Figure 2. Statistic analysis was performed using the nonparametric Mann–Whitney U-test.
The In Vivo Effect of Curcumin Corresponds with Its Actions on Infected Residential Macrophages
In parallel to the in vivo studies, the mechanisms of action of curcumin on infected residential macrophages and primed lymphocytes were examined in vitro. Corresponding to the feeding studies, we found that curcumin increased PPARs, and decreased iNOS gene expression in infected macrophages, as well as downregulated IFNγ production by primed lymphocytes. Furthermore, these effects can be correlated with increase of parasite within the macrophages. Huang et al38 have shown that IL-4 induces the expression of PPARγ in macrophages and IL-4 is associated with susceptibility to Leishmania major.39 As we have observed that Leishmania infection led to upregulation of PPARγ in the liver and spleen, we proceed to investigate whether PPARγ expression is related to susceptibility and resistance to Leishmania. To date, whether PPAR expression may influence microbial pathogenesis has not been explored. We employed nonelicited, uninfected peritoneal exudate cells from the BALB/c susceptible strain. As shown in Figure 4, infection with Leishmania-induced PPARγ expression, and the level was increased by 2.3-fold in a typical experiment. Exogenous addition of IL-4 to the infected macrophages did not further upregulate the expression with statistical significance, whereas curcumin increased PPARγ gene expression beyond what is induced by the parasite. The combination of curcumin and IL-4 synergistically induced PPARγ expression by 7.5-fold in excess of the level produced by macrophages that were infected but not given IL-4 nor curcumin. Conversely, the protective cytokine, IFNγ, suppressed the parasite induction of PPARγ gene expression in macrophages. Curcumin, however, was able to restore the IFNγ-deactivated PPARγ expression to a level similar to that of the infected macrophages. Henceforth, there is an association between PPARγ gene expression and susceptibility to Leishmania at three sites of infection, the macrophage, liver, and spleen. Both the in vitro and in vivo experiments indicate an association between PPARγ gene expression and susceptibility to Leishmania infection, and the in vitro indicated that curcumin increased PPARγ gene expression irrespectively of whether IL-4 or IFNγ was present.
Figure 4.
Upregulation of PPARγ gene expression by Leishmania infection and curcumin in vitro. Nonelicited peritoneal exudate cells were infected as described in the legend to Table 2, except IL-4, at 5 ng/ml, or IFNγ, at 100 U/ml, or curcumin at 10 μM were added 30 min after addition of parasites, and the cultures were incubated for 16–20 h before isolation of total RNA. Levels of gene expression in each sample were normalized with 18S RNA. Then modulation was expressed relative to the uninfected control using the ΔΔCt method. The X-axis intercepts the Y-axis at ‘1’ to illustrate activation and deactivation of PPARγ. A two-tailed Student’s t-test was performed and the differences between the indicated groups were significant.
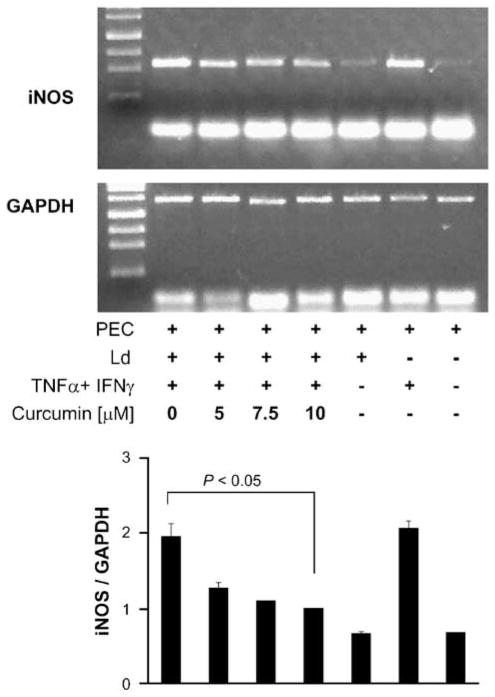
PPARγ activation leads to suppression of iNOS gene expression.17 The effect of curcumin on nitric oxide production by Leishmania-infected peritoneal exudate cells was examined, using IFNγ and TNFα to activate iNOS gene expression. Studies on cells from the resistant C3H/He strain (Table 3) revealed a reduction of 18, 39.3, and 61.4% in nitric oxide levels at 5, 7.5, and 10 μM of curcumin, respectively. The production of nitric oxide in the susceptible BALB/c strain was lower, but the degrees of reduction were more prominent at 21, 32, and 54%, for 5, 7.5, and 10 μM of curcumin, respectively. The decrease is associated inversely with an increase in proportion of infected macrophages and number of parasite within the cells. Investigation at the molecular level showed that the inhibition had occurred at the level of iNOS gene expression. At 10 μM, curcumin reduced the level of steady-state RNA by 70% (Figure 5). Corresponding to the decrease in nitric oxide production, microscopic enumeration with propidium iodide revealed a dose-dependent increase in the number of infected macrophages and the number of parasites in cultures of the peritoneal exudate cells, whether they were from the susceptible BALB/c or resistant C3H/He strain (Table 3 and Figure 6).
Table 3.
Effect of curcumin on production of nitric oxide and parasite load in peritoneal macrophages
| PEC | Ld | TNFα IFNγ | Curcumin (μM) | Nitrite (μM) | % Infection | Amastigotes/infected macrophage | |||
|---|---|---|---|---|---|---|---|---|---|
| BALB/c | |||||||||
| + | − | − | 0 | Undetectable | NA | NA | |||
| + | + | − | 0 | Undetectable | 84.0 ± 3.5 | 8.0 ± 0.3 | |||
| + | − | + | 0 | 47.9 ± 0.8 | NA | NA | |||
| + | + | + | 0 | 54.5 ± 0.8 |  |
50.3 ± 3.1 |  |
4.5 ± 0.6 |  |
| + | + | + | 5 | 45.0 ± 1.9 | 67.3 ± 2.3 | 6.3 ± 0.3 | |||
| + | + | + | 7.5 | 39.1 ± 0.5 | 76.0 ± 3.6 | 7.2 ± 0.4 | |||
| + | + | + | 10 | 32.2 ± 2.6 | 80.7 ± 2.9 | 7.8 ± 0.2 | |||
| C3H/He | |||||||||
| + | − | − | 0 | Undetectable | NA | NA | |||
| + | + | − | 0 | Undetectable | 66.6 ± 1.2 | 5.2 ± 0.2 | |||
| + | − | + | 0 | 66.0 ± 0.2 | NA | NA | |||
| + | + | + | 0 | 77.4 ± 1.3 |  |
33.3 ± 4.2 |  |
1.8 ± 0.1 |  |
| + | + | + | 5 | 61.4 ± 0.3 | 42.3 ± 3.8 | 2.5 ± 0.2 | |||
| + | + | + | 7.5 | 53.0 ± 0.4 | 52.3 ± 2.1 | 2.9 ± 0.3 | |||
| + | + | + | 10 | 45.6 ± 1.0 | 56.6 ± 2.1 | 4.7 ± 0.7 | |||
IFNγ, interferon-γ; TNFα, tumor-necrosis factor-α.
Nonelicited PEC were cultured with L. donovani (Ld) promastigotes in wells that contained cover slips for a period of 16–20 h; then curcumin and IFNγ and TNFα were added. On day 5, the amount of nitric oxide was determined with Griess reagent, and the cover slips were stained with propidium iodide to enumerate the percent of infected macrophages and number of amastigotes per macrophage under a fluorescence microscope.
Figure 5.
Effect of curcumin on iNOS gene expression and Leishmania infection in macrophages. The gel shows the end-point PCR-amplified iNOS (496 bp) and GAPDH (996 bp) from RNA isolated from the described samples. The culture conditions for each sample have been described in ‘Materials and methods’ section. Amplification occurred for 35 cycles for iNOS and 23 cycles for GAPDH. At the bottom, a plot shows the levels of iNOS gene expression normalized to GAPDH. ANOVA showed that the effect of curcumin was significant (P < 0.001).
Figure 6.
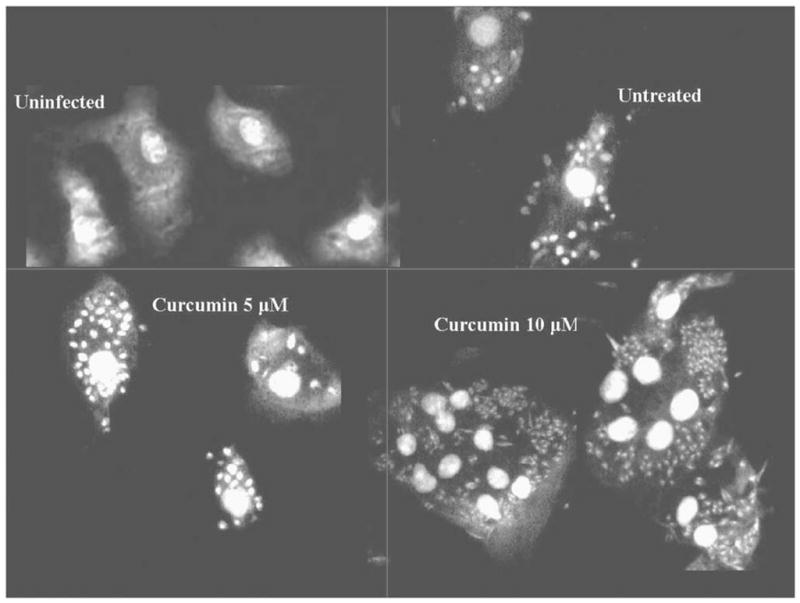
Microscopic visualization of Leishmania in macrophages. The number of parasites increased in a dose-dependent manner with increasing concentrations of curcumin.
IFNγ is a product of T helper 1 lymphocytes and Gao et al40 have shown that curcumin decreases the production of IFNγ in mixed lymphocyte cultures. We examined the effect of curcumin on IFNγ using splenocytes of infected mice as a source of Leishmania-specific, primed T helper 1 lymphocytes, and found that production was reduced, in a dose-dependent manner (Table 4). For example, adding 10 μM of curcumin decreased IFNγ production in the cultured C3H/He spleen cells by 60%. Associated with this modulation in cytokines was a decrease in the levels of nitric oxide and, inversely, an increase in parasite burden. Therefore, overall, the in vitro mechanism corroborates with the in vivo observation.
Table 4.
Effect of curcumin on production of IFNγ and parasite load in peritoneal macrophages
| PEC | Ld | Spleen cells | Curcumin (μM) | IFNγ (pg/ml) | Nitrite (μM) | % Infection | Amastigotes/infected macrophage | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BALB/c | |||||||||||
| + | − | − | 0 | 67.0±2.5 | 2.3±0.1 | NA | NA | ||||
| + | + | − | 0 | 104.5±2.5 | 2.2±0.1 | 90.6±1.2 | 10.0±0.8 | ||||
| − | − | + | 0 | 318.0±113.6 | 0.00±0.2 | NA | NA | ||||
| − | + | + | 0 | 724.7±32.2 | 4.7±1.7 | NA | NA | ||||
| + | + | + | 0 | 1268.2±56.3 |  |
38.3±1.4 |  |
36.3±3.1 |  |
2.3±0.0 |  |
| + | + | + | 5 | 1134.0±17.5 | 17.9±1.0 | 51.0±1.0 | 3.9±0.1 | ||||
| + | + | + | 7.5 | 848.2±86.4 | 8.2±0.6 | 65.3±1.5 | 7.8±0.4 | ||||
| + | + | + | 10 | 632.8±72.9 | 2.5±0.2 | 81.0±2.6 | 8.8±0.4 | ||||
| C3H/He | |||||||||||
| + | − | − | 0 | 96.2±0.1 | 1.7±0.0 | NA | NA | ||||
| + | + | − | 0 | 114.5±0.1 | 0.9±0.1 | 87.0±2.0 | 7.5±0.4 | ||||
| − | − | + | 0 | 358.0±10.0 | 0.2±0.1 | NA | NA | ||||
| − | + | + | 0 | 1271.3±0.1 | 4.9±0.2 | NA | NA | ||||
| + | + | + | 0 | 2225.0±208.8 |  |
41.4±0.2 |  |
26.6±2.1 |  |
2.1±0.1 |  |
| + | + | + | 5 | 1682.3±89.8 | 20.2±1.5 | 44.3±2.1 | 3.7±0.2 | ||||
| + | + | + | 7.5 | 1606.5±24.8 | 16.2±0.8 | 65.3±3.1 | 5.2±0.4 | ||||
| + | + | + | 10 | 679.0±30.3 | 5.6±0.8 | 80.3±2.1 | 7.1±0.2 | ||||
IFNγ, interferon-γ.
Nonelicited PEC were cultured with L. donovani (Ld) promastigotes in wells that contained cover slips for a period of 16–20 h; then curcumin and splenocytes from mice that had been infected with Leishmania were added. On day 5, the amount of nitric oxide was determined with Griess reagent, and the cover slips were stained with propidium iodide to enumerate the percent of infected macrophages and number of amastigotes per macrophage under a fluorescence microscope. The amount of IFNγ in the supernatant was determined by ELISA 72 h after addition of curcumin and primed splenocytes. The brackets indicate the groups compared in the ANOVA, which showed that the effect of curcumin was significant (P < 0.001).
DISCUSSION
Although many have considered dietary phytochemicals as modifiers for diseases in various organs, much skepticism remains over their bioefficacy. Our findings showed that consumption of curcumin in doses below what is recommended for dietary supplement activated expression of the inflammation suppressive nuclear transactivating receptor PPARs, downregulated type 1 responses, and affected antimicrobial immunity in two major organs, the liver and spleen. Thus, curcumin has the potential to be beneficial or detrimental, depending on whether inflammation is needed, or merely destructive to the host. Curcumin exacerbated nonfatal, murine leishmaniasis. In cases where the immune response is causing more tissue destruction than the pathogens, attenuating inflammation might extend survival of the host.
This study, in vitro and in vivo, is one of the first to investigate the participation of PPAR in microbial infection. The leishmaniasis exacerbating cytokine, IL-4, enhances PPARγ activation. Conversely, the protective cytokine IFNγ suppresses its activation in infected peritoneal macrophages. Our study showed a correlation between increase in parasite load and curcumin-induced enhancement of PPAR expression in Leishmania-infected spleen, liver, and macrophages. This finding warrants future investigation of whether PPARγ expression is causal to susceptibility and/or pivotal in modulating adaptive immunity.
Leishmaniasis is a parasitic disease that threatens many developing countries, often as devastating epidemics. In Sudan, several epidemics of visceral leishmaniasis since 1988 claimed more than 100 000, a third of the population in the Western Upper Nile province.22,41 To protect against infection, the host immune response emanates reactive oxygen and nitrogen species, whereas parasites defend their integrity by their ability to resist oxidative stress. Our experimental model bears resemblance to the clinical condition where patients are heavily infected towards the later stage of the disease, even though we cannot project the effect of curcumin at the initial stage of infection, where sandflies inject a low dose of the parasite intradermally. This report suggests that when treating leishmaniasis, physicians may want to consider the patients’ diet. Conceivably, in rural Indian, one may consider whether the high level of turmeric in the diet of the populations may interfere with therapy.23 Likewise, one must be careful when consuming botanical and dietary supplements with antiinflammatory and antioxidant properties when at risk for microbial infections.
Furthermore, this study warns against oversimplifications in antimicrobial agent discovery and development. Many natural compounds with redox potential inhibit microbial growth in vitro, and several laboratories have found curcumin to inhibit replication in Leishmania cultures.42 Our study shows that the in vivo effect of curcumin is very different from the in vitro effect. In vivo, the outcome of an infection is determined by a balance between the means of the host immune defense vs that of the parasite, and antioxidants and antiinflammatories may weaken the immune defense and exacerbate infection. Corroborating our previous publication, we have shown that in the presence of oxidative stress from nitric oxide and superoxide, curcumin neutralizes the toxicity of free radicals to enhance growth of the parasites.24
Acknowledgments
This study was supported by NIH Grant R01 AI 45555, and fundings from American Institute for Cancer Research and CAFT of Rutgers University to Marion Chan. Experimental protocols used in this study were approved by the Temple University Institutional Animal Care and Use Committee (IACUC). We thank Dr Joe Shih, Division of Biometrics, The Cancer Institute of New Jersey, for performing statistic analyses, Dr Samuel Spadone, Dr Hyung Hee Kim, Ms Andrea Rossi, and Mr Rodger Brown for various assistance and Mr Gregory Harvey for editing the manuscript. Above all, the intellectual support from Dr Dunne Fong is greatly appreciated.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare that they have no competing financial interests.
References
- 1.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Kumar A, Aggarwal MS, et al. Curcumin derived from Turmeric (Curcuma longa): a spice for all seasons. In: Preuss H, editor. Phytopharmaceuticals in Cancer Chemoprevention. CRC Press; Boca Raton: 2005. pp. 349–387. [Google Scholar]
- 3.Rapaka RS, Coates PM. Dietary supplements and related products: a brief summary. Life Sci. 2006;78:2026–2032. doi: 10.1016/j.lfs.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Radimer K, Bindewald B, Hughes J, et al. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160:339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 5.Goldrosen MH, Straus SE. Complementary and alternative medicine; assessing the evidence for immunological benefits. Nature. 2004;4:912–921. doi: 10.1038/nri1486. [DOI] [PubMed] [Google Scholar]
- 6.Giles JT, Bathon JM. Serious infections associated with anticytokine therapies in the rheumatic diseases. J Intensive Care Med. 2004;19:320–334. doi: 10.1177/0885066604267854. [DOI] [PubMed] [Google Scholar]
- 7.Bagalas V, Kioumis I, Argyropoulou P, et al. Visceral leishmaniasis infection in a patient with rheumatoid arthritis treated with etanercept. Clin Rheumatol. 2007;26:1344–1345. doi: 10.1007/s10067-006-0356-5. [DOI] [PubMed] [Google Scholar]
- 8.Kelloff GJ, Boone CW, Crowell JA, et al. Chemopreventive drug development: perspectives and progress. Cancer Epidemiol Biomarkers Prev. 1994;3:85–89. [PubMed] [Google Scholar]
- 9.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 10.Chan MM. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem Pharmacol. 1995;49:1551–1556. doi: 10.1016/0006-2952(95)00171-u. [DOI] [PubMed] [Google Scholar]
- 11.Chan MM, Huang H, Fenton MR, et al. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol. 1998;55:1955–1962. doi: 10.1016/s0006-2952(98)00114-2. [DOI] [PubMed] [Google Scholar]
- 12.Brouet I, Ohshima H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun. 1995;206:533–540. doi: 10.1006/bbrc.1995.1076. [DOI] [PubMed] [Google Scholar]
- 13.Chan MM, Ho CT, Huang HI. Effects of three dietary phytochemicals from tea, rosemary and turmeric on inflammation-induced nitrite production. Cancer Lett. 1995;96:23. doi: 10.1016/0304-3835(95)03913-h. [DOI] [PubMed] [Google Scholar]
- 14.Kang BY, Song YJ, Kim KM, et al. Curcumin inhibits Th1 cytokine profile in CD4+ T cells by suppressing interleukin-12 production in macrophages. Br J Pharmacol. 1999;128:380–384. doi: 10.1038/sj.bjp.0702803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan MM, Huang HI, Mattiacci JA, et al. Modulation of cytokine gene expression by curcumin. In: Shahidi F, Ho CT, Watanabe S, editors. Food Factors for Disease Prevention and Health Promotion. American Chemical Society Press; Washington, DC: 2003. pp. 86–99. [Google Scholar]
- 16.Zheng S, Chen A. Disruption of transforming growth factor-beta signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-gamma in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G113–G123. doi: 10.1152/ajpgi.00200.2006. [DOI] [PubMed] [Google Scholar]
- 17.Welch JS, Ricote M, Akiyama TE, et al. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Natl Acad Sci USA. 2003;100:6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rau O, Wurglics M, Paulke A, et al. Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med. 2006;72:881–887. doi: 10.1055/s-2006-946680. [DOI] [PubMed] [Google Scholar]
- 19.Wilson ME, Jeronimo SM, Pearson RD. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb Pathog. 2005;38:147–160. doi: 10.1016/j.micpath.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Engwerda CR, Ato M, Kaye PM. Macrophages, pathology and parasite persistence in experimental visceral leishmaniasis. Trends Parasitol. 2004;20:524–530. doi: 10.1016/j.pt.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Gruenwald J, Brendler T, Jaenicke C. PDR for Herbal Medicines. Medical Economics Company; Montvale, NJ: 1998. pp. 786–788. [Google Scholar]
- 22.Bora D. Epidemiology of visceral leishmaniasis in India. Natl Med J India. 1999;12:62–68. [PubMed] [Google Scholar]
- 23.Krishnaswamy K. Indian functional foods: role in prevention of cancer. Nutr Rev. 1996;54:S127–S131. doi: 10.1111/j.1753-4887.1996.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 24.Chan MM, Adapala NS, Fong D. Curcumin overcomes the inhibitory effect of nitric oxide on Leishmania. Parasitol Res. 2005;1:49–56. doi: 10.1007/s00436-005-1323-9. [DOI] [PubMed] [Google Scholar]
- 25.Titus RG, Marchand M, Boon T, et al. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 26.Nicolas L, Prina E, Lang T, et al. Real-time PCR for detection and quantitation of Leishmania in mouse tissues. J Clin Microbiol. 2002;40:1666–1669. doi: 10.1128/JCM.40.5.1666-1669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiner S, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 28.Trinchieri G, Scott P. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res Immunol. 1995;146:423–431. doi: 10.1016/0923-2494(96)83011-2. [DOI] [PubMed] [Google Scholar]
- 29.MacMicking J, Xie Q, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 30.Green SJ, Crawford RM, Hockmeyer JT, et al. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-γ-stimulated macrophages by induction of tumor necrosis factor-α. J Immunol. 1990;145:4290–4297. [PubMed] [Google Scholar]
- 31.Liew FY, Li Y, Millott S. Tumor necrosis factor-α synergizes with IFN-γ in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;145:4306–4310. [PubMed] [Google Scholar]
- 32.Murray HW, Nathan CF. Macrophage microbicidal mechanisms in vivo: reactive nitrogen vs oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J Exp Med. 1999;189:741–746. doi: 10.1084/jem.189.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy ML, Wille U, Villegas EN, et al. IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol. 2001;31:2848–2856. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 34.Murray HW, Lu CM, Mauze S, et al. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect Immun. 2002;70:6284–6293. doi: 10.1128/IAI.70.11.6284-6293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen A, Xu J. Activation of PPAR{gamma} by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am J Physiol Gastrointest Liver Physiol. 2005;288:G447–G456. doi: 10.1152/ajpgi.00209.2004. [DOI] [PubMed] [Google Scholar]
- 36.Siddiqui AM, Cui X, Wu R, et al. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-gamma. Crit Care Med. 2006;34:1874–1882. doi: 10.1097/01.CCM.0000221921.71300.BF. [DOI] [PubMed] [Google Scholar]
- 37.Zheng S, Chen A. Disruption of transforming growth factor-beta signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-gamma in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G113–G123. doi: 10.1152/ajpgi.00200.2006. [DOI] [PubMed] [Google Scholar]
- 38.Huang JT, Welch JS, Ricote M, et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 39.Heinzel FP, Sadick MD, Holaday BJ, et al. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao X, Kuo J, Jiang H, et al. Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem Pharmacol. 2004;68:51–61. doi: 10.1016/j.bcp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Zijlstra EE, el-Hassan AM. Leishmaniasis in Sudan. Visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2001;95:S27–S58. doi: 10.1016/s0035-9203(01)90218-4. [DOI] [PubMed] [Google Scholar]
- 42.Saleheen D, Ali SA, Ashfaq K, et al. Latent activity of curcumin against leishmaniasis in vitro. Biol Pharm Bull. 2002;25:386–389. doi: 10.1248/bpb.25.386. [DOI] [PubMed] [Google Scholar]