Abstract
Bet3p, a component of a large novel complex called TRAPP, acts upstream of endoplasmic reticulum (ER)–Golgi SNAREs. Unlike the SNAREs, which reside on multiple compartments, Bet3p is localized exclusively to Golgi membranes. While other proteins recycle from the Golgi to the ER, Bet3p and other TRAPP subunits remain associated with this membrane under conditions that block anterograde traffic. We propose that the persistent localization of TRAPP to the Golgi may be important for its role in docking vesicles to this membrane. Consistent with this proposal, we find that transport vesicles fail to bind to Golgi membranes in vitro in the absence of Bet3p. Binding is restored by the addition of cytosol containing Bet3p. These findings indicate that TRAPP stably associates with the Golgi and is required for vesicle docking.
Keywords: Bet3p/ER–Golgi transport/membrane traffic/TRAPP/vesicle docking
Introduction
Each step of the secretory pathway is thought to be mediated by a conserved mechanism of vesicle budding and fusion, which ensures that vesicles dock and fuse with their correct target membrane. Explaining exactly how this fidelity is achieved continues to be a major challenge facing researchers in this field. One model, known as the SNARE hypothesis, was put forward by Rothman and colleagues a number of years ago (Söllner et al., 1993; Rothman, 1994). In this model, vesicles that bud from donor membranes contain transmembrane proteins specific to this compartment. These proteins, called v-SNAREs (vesicle SNAREs), only interact with compartment-specific proteins on the target membrane (t–SNAREs). The ability of vesicles to dock and fuse thus depends on the ability of the v-SNAREs on vesicles to match their corresponding t-SNAREs on the target membrane. Once v-SNARE–t-SNARE pairing has occurred, the soluble proteins NSF and SNAP assemble on the SNAREs to disassemble the complex, an event originally thought to initiate membrane fusion (Söllner et al., 1993). Work on vacuolar fusion has demonstrated that disassembly of the SNARE complexes serves as a priming step, freeing the SNAREs to interact in a subsequent docking and fusion event (Mayer et al., 1996). Other studies have shown that while SNARE proteins are essential for membrane fusion (Weber et al., 1998), they are not likely to provide the sole layer of specificity in the vesicle docking step (Brennwald et al., 1994; Garcia et al., 1995; Holthius et al., 1998; Ungermann et al., 1998).
Additionally, in endoplasmic reticulum (ER) to Golgi transport, soluble and membrane-bound components (Uso1p, Sec35p/Sec34p and the TRAPP complex), whose action may be regulated by the GTPase Ypt1p, have been identified (Sapperstein et al., 1996; Sacher et al., 1998; Kim et al., 1999; VanRheenen et al., 1999). These components act upstream of SNARE complex formation and are implicated in the vesicle docking event (Sapperstein et al., 1996; Barlowe, 1997; VanRheenen et al., 1999). Uso1p, a homodimer with two N-terminal globular heads and a long coiled-coil tail (Sapperstein et al., 1995), is a soluble component that has been shown to be required to tether vesicles to Golgi membranes in vitro (Barlowe, 1997). Sec35p and Sec34p, both non-essential gene products, are components of a large complex (Kim et al., 1999), and are required for the docking of vesicles in the in vitro tethering assay (VanRheenen et al., 1999).
We previously described the identification of TRAPP, a large novel highly conserved protein complex that acts in the late stages of ER to Golgi transport in yeast (Rossi et al., 1995; Sacher et al., 1998). TRAPP is composed of 10 subunits (Sacher et al., 2000), two of which (Bet3p and Bet5p) were identified in genetic screens (Rossi et al., 1995; Jiang et al., 1998). Here we show that Bet3p plays a pivotal role in docking ER to Golgi transport vesicles to their acceptor compartment.
Results
Bet3p co-fractionates with Golgi markers Sed5p and Och1p
We previously demonstrated that Bet3p, one of the 10 subunits of TRAPP, co-localizes with the t-SNARE Sed5p and fractionates away from the more distal enzymes GDPase and Kex2p (medial/trans) on sucrose density gradients (Sacher et al., 1998). By fluorescence, Sed5p resides predominantly on early Golgi cisternae (Hardwick and Pelham, 1992; Wooding and Pelham, 1998); however, recent findings indicate that it recycles between the ER and Golgi and, as a consequence, may be present on more than one membrane (Wooding and Pelham, 1998). In mammalian cells, there are two forms of the Sed5p homolog syntaxin 5: a long (42 kDa) and short (35 kDa) form. The long form appears to be localized more predominantly in peripheral punctate structures than in the Golgi and contains an ER retrieval signal (Hui et al., 1997).
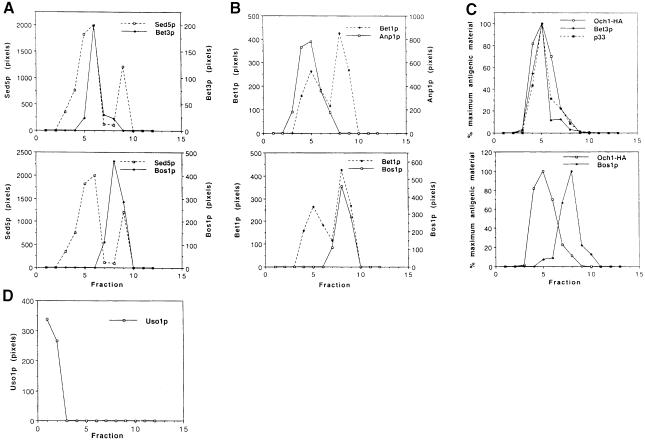
To address the localization of Bet3p and its relationship to Sed5p in more detail, we performed subcellular fractionation studies using a sucrose velocity gradient that previously has been shown to resolve the ER from Golgi membranes (Antebi and Fink, 1992). On this gradient, Sed5p is found in two peaks. The major peak co-fractionates with Bet3p (Figure 1A), while the smaller one cofractionates with Bos1p, an ER marker. Bos1p also co–fractionates with a second ER marker, Sec61p (data not shown). The localization of Sed5p to multiple compartments indicates that this t-SNARE has a dynamic localization. This finding prompted us to examine the localization of other SNAREs on this gradient.
Fig. 1. Steady-state localization of TRAPP subunits, SNAREs and Uso1p on sucrose velocity gradients. Data in (C) and (D) are from separate gradients. (A) Top panel: Bet3p (solid line) co-fractionates with the Golgi peak of Sed5p (broken line). Bottom panel: the second peak of Sed5p (broken line) co-fractionates with Bos1p (solid line), an ER marker. (B) Top panel: the first peak of Bet1p (broken line) co-fractionates with Anp1p (solid line), an early Golgi marker. Bottom panel: the second peak of Bet1p (broken line) co-fractionates with Bos1p (solid line). (C) Top panel: TRAPP subunits Bet3p (♦) and p33 (▪) co-fractionate with Och1-HA (□), another early Golgi marker. Bottom panel: the peaks of Och1p (□) and Bos1p (♦) are distinct on this gradient, showing that the Golgi and ER are well resolved. (D) In contrast to TRAPP subunits, Uso1p (□) is largely soluble and is not detected on membranes.
All SNAREs are homologous to three different neuronal proteins: synaptobrevin (v-SNARE), syntaxin and SNAP-25 (t-SNAREs) (for a review see Ferro-Novick and Jahn, 1994). In yeast ER to Golgi transport, Sed5p is the syntaxin-like homolog (Hardwick and Pelham, 1992), Bos1p and Sec22p are homologous to synaptobrevin (Dascher et al., 1991; Shim et al., 1991; Sacher et al., 1997) and Bet1p contains a domain that is related to SNAP-25 (Stone et al., 1997). Although Bos1p fractionates as a single peak with Sec61p (Figure 1A; data not shown), previous studies have shown that it also resides on vesicles (Newman et al., 1992; Lian and Ferro-Novick, 1993). In contrast, Bet1p fractionates with Anp1p, an early Golgi marker, as well as Bos1p (Figure 1B). The finding that a significant amount of Bet1p resides on Golgi membranes is consistent with the observation that Bet1p is related to SNAP-25, the neuronal t-SNARE (Söllner et al., 1993). We also examined the localization of Sec22p and found that, like Bet1p, it fractionates with the Golgi and ER compartments (Figure 4C). Thus, each of the SNAREs examined in this study can be found on at least two compartments.
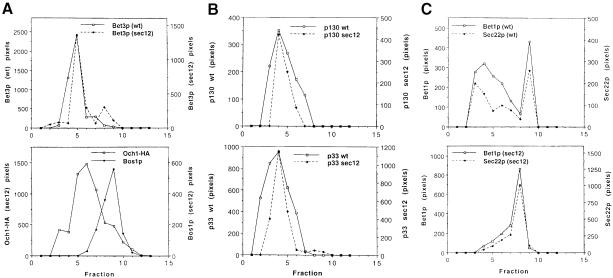
Fig. 4. Fractionation of Bet3p in wild-type and sec12 at 37°C. Wild-type and sec12 cells were shifted to 37°C for 1 h, followed by lysis and loading on sucrose gradients. Data in (A), (B) and (C) are from separate gradients. (A) Top panel: Bet3p fractionates similarly in wild-type (solid line) and sec12 (broken line) at 37°C. We consider the minor peak of Bet3p in fraction 8 to be insignificant as it was not reproduced in other gradients. Bottom panel: the peaks of Och1p (□) and Bos1p (♦) in lysates from sec12 are distinct, showing that Golgi and ER membranes are resolved in the mutant. (B) TRAPP subunits p130 (top panel) and p33 (bottom panel) also fractionate similarly in wild-type (solid line) and sec12 (broken line). (C) Top panel: Bet1p (solid line) and Sec22p (broken line) co-fractionate in Golgi and ER peaks in lysates from wild-type. Bottom panel: in lysates from sec12, Bet1p (solid line) and Sec22p (broken line) redistribute to the ER in sec12 at 37°C and are no longer detected in Golgi fractions.
Since the SNAREs reside on multiple compartments, we investigated the localization of Bet3p with respect to another Golgi marker. We chose Och1p for our analysis, as previous studies implied that its localization would be more static than that of the SNAREs (Schröder et al., 1995). Och1p is the first known Golgi-modifying enzyme that proteins encounter as they enter the cis-Golgi compartment. It modifies N-linked glycans by adding the initiating α-1,6-mannose to the core oligosaccharide backbone (Nakayama et al., 1992). For this study, we followed the localization of a hemagglutinin (HA)-tagged version of Och1p, which has been described previously (Harris and Waters, 1996). Examination of Och1-HA and Bet3p on gradients revealed that they co-fractionate (Figure 1C). In addition to Bet3p, TRAPP contains nine other subunits (Bet5p, p20, p23, p31, p33, p65, p85, p120 and p130). The subunits p130, p33, p20, p23 and Bet5p also co-fractionate with Och1-HA (Figure 1C; data not shown). Thus, Bet3p and other TRAPP subunits co-localize with the Golgi marker Och1p, and not with ER membranes.
The localization of the tethering factors Uso1p and Sec35p was also examined on these gradients. Uso1p was detected in the first two fractions of these gradients where soluble proteins fractionate and was not detected on Golgi membranes (Figure 1D). This finding is consistent with the fact that Uso1p was purified as a soluble factor required for vesicle tethering in vitro (Barlowe, 1997). We also examined the fractionation pattern of Sec35p on these gradients. As previously reported, we found the membrane association of Sec35p to be variable (VanRheenen et al., 1998). In several gradients, a soluble as well as a membrane-associated fraction of Sec35p, that co-fractionated with Golgi markers, was observed (data not shown).
Unlike the SNAREs, Bet3p does not cycle between the Golgi and ER
The emerging view of the Golgi complex as a dynamic structure has been strengthened by findings that many Golgi proteins are not localized statically to their respective compartments. In particular, work in mammalian cells has shown that many Golgi proteins cycle through the ER compartment during their lifetime (Cole et al., 1998). Proteins involved in vesicle transport or protein retrieval would be expected to cycle between these compartments (ER–Golgi SNAREs, ER–Golgi retrieval machinery). The localization patterns of these proteins are a result of their steady-state dynamics, rather than a static localization. For proteins in flux between the Golgi and ER, a recycling assay has been used to demonstrate that certain proteins localized in the Golgi at steady state can become trapped in the ER when budding from the ER is blocked (Schröder et al., 1995). This assay makes use of the sec12 mutant. Sec12p is the guanine nucleotide exchange factor for Sar1p, a component of the COPII coat complex (Barlowe and Schekman, 1993). At the restrictive temperature, the budding of transport vesicles from the ER is blocked in this mutant. Retrograde transport continues, mediated by either COPI vesicles or a COPI-independent transport event (Girod et al., 1999; White et al., 1999). Because recycling from the Golgi to the ER continues in the absence of forward transport, proteins whose Golgi localization at steady state is dependent on cycling undergo a redistribution to the ER where they become trapped. The Golgi protein Emp47p relocates from the Golgi to the ER using this assay (Schröder et al., 1995), and Sed5p behaves similarly, recycling to the ER in sec12 within 15 min of a shift to the restrictive temperature (Wooding and Pelham, 1998).
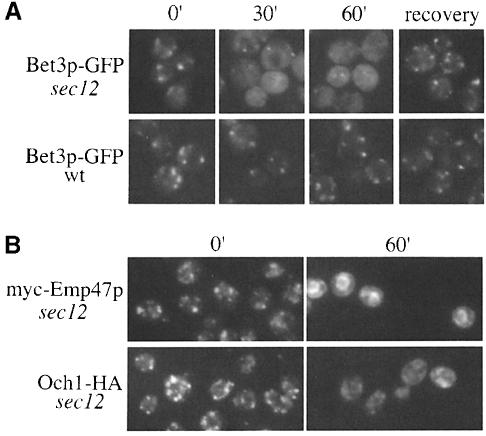
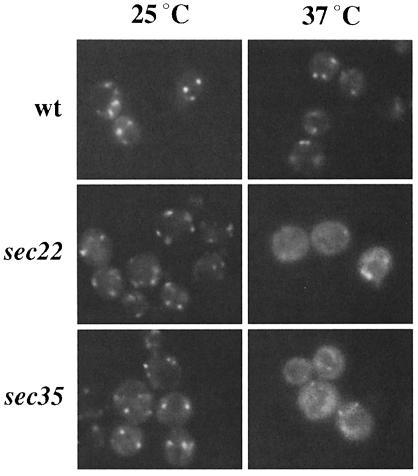
To examine the localization of Bet3p in the recycling assay, we constructed a sec12-4 strain that expressed Bet3p fused to the green fluorescent protein (Bet3p–GFP) as the sole copy of Bet3p. Previous studies have shown that Bet3p–GFP resides on elongated punctate structures (Sacher et al., 1998). While the Golgi pattern of Bet3p–GFP was not affected by incubation of wild-type cells at 37°C, Bet3p–GFP appeared to become finely punctate in sec12 (Figure 2A). This apparent dispersal of the Golgi signal became apparent by 30 min, when the elongated puncta became visibly smaller. This change was accompanied by an increased haziness of the cytoplasm. Importantly, shifting to the restrictive temperature did not cause Bet3p–GFP to redistribute to the ER, as no ER signal was apparent even after 60 min. After 1 h, Golgi dots could often still be seen in the cells, but they were less numerous and were obscured by the fine punctate appearance of the cytoplasm. Elongated Golgi reappeared after shifting the cells back to the permissive temperature in the presence of cycloheximide, indicating that this effect was reversible (Figure 2A). This phenomenon was also seen in another mutant (sec23-1) that blocks budding from the ER (data not shown), as well as in sec22-3 and sec35-1, a fusion and a tethering mutant, respectively (Figure 3). These results are similar to those of Wooding and Pelham (1998), who recently reported that early and late Golgi compartments disperse when forward transport between the ER and Golgi is blocked. We followed myc-Emp47p in sec12 (RH3048) as a positive control for our studies and found that, as previously reported (Schröder et al., 1995), it recycled efficiently to the ER (Figure 2B). We also monitored the localization of the Golgi marker Och1-HA in the sec12 recycling assay. As expected, Och1-HA also dispersed (Figure 2B). Thus, Bet3p–GFP does not recycle via retrograde Golgi vesicles and become trapped on the ER. Consistent with these findings, we failed to detect Bet3p on purified ER-derived transport vesicles (data not shown).
Fig. 2. Bet3p–GFP and Och1-HA become finely punctate in sec12 at 37°C. Wild-type and sec12 cells, containing Bet3p–GFP, Och1-HA or myc-Emp47p, were shifted to 37°C for the indicated times followed by appropriate preparation for imaging under a Zeiss fluorescence light microscope. (A) Bet3p–GFP was imaged in live cells at the indicated time points ranging from 0 (cells at 25°C prior to shift) to 60 min. Following the 1 h incubation at 37°C, cells subsequently were shifted to 25°C and incubated in the presence of 20 μg/ml cycloheximide for 1 h to allow for recovery. The punctate signal from Bet3p–GFP becomes hazy after 30 min at 37°C, and returns upon a shift back to permissive temperature. (B) As controls, the localization of myc-Emp47p, a recycling Golgi protein, and Och1-HA was followed in sec12. The tagged proteins were detected using indirect immuno- fluorescence as described in Materials and methods. Och1-HA is detected on elongated punctate structures at the 0 min time point, which disperse after 60 min at 37°C. In contrast and as previously reported (Schröder et al., 1995), myc-Emp47p recycles to the ER under these conditions.
Fig. 3. Bet3p–GFP disperses in a fusion and tethering mutant. Wild-type, sec22 and sec35 strains containing Bet3p–GFP were shifted to 37°C for 1 h.
Since Bet3p is a member of a peripheral membrane protein complex, it remained formally possible that Bet3p–GFP becomes more soluble in sec12 and the fusion and tethering mutants, and that an increase in the soluble pool accounted for the haziness of the cytoplasm rather than the dispersal of the Golgi. In order to address this possibility, we followed the localization of Bet3p and Och1-HA on sucrose gradients in wild type, sec12, sec22 and sec35. The small amount of soluble Bet3p present in wild-type lysates was not detectable by the lysis method we employed. If Bet3p becomes more soluble after a shift to 37°C, then an increase in the soluble pool (fraction 1) would be apparent on the sucrose velocity gradient described above. This analysis revealed that the fractionation pattern of Bet3p (Figure 4A), p130, p33, p23, p20 and Bet5p (Figure 4B; data not shown) remained the same in wild-type and sec12 lysates. Importantly, Bet3p and the other subunits of TRAPP did not become more soluble and did not fractionate with the ER in sec12. We speculate that the entire TRAPP complex remains bound to Golgi membranes under these conditions, as in vivo labeling studies revealed that the complex remains assembled in the sec12 mutant subsequent to a 1 h shift at the restrictive temperature (data not shown). In contrast, the SNAREs Bet1p, Sec22p (Figure 4C) and Sed5p (data not shown) all redistributed to ER fractions under these conditions. Bet3p, as well as TRAPP subunits p130 and p33, also remained associated with the Golgi and was not found in the soluble or ER fractions in lysates from sec22 and sec35 (data not shown). Thus, these findings demonstrate that TRAPP remains bound to the Golgi when forward transport to the Golgi is blocked.
The binding of transport vesicles to the Golgi requires Bet3p
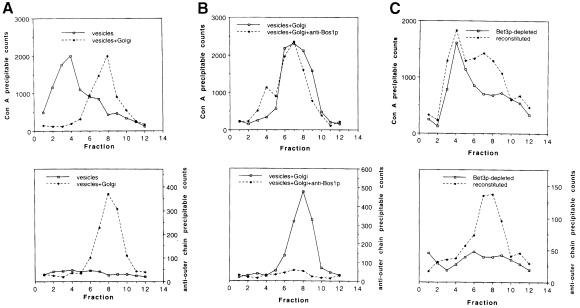
Using an in vitro assay that reconstitutes transport from the ER to the Golgi complex (Ruohola et al., 1988; Groesch et al., 1990), we demonstrated previously that Bet3p-depleted Golgi fail to support the docking and/or fusion of transport vesicles to the Golgi apparatus (Sacher et al., 1998). Transport was reconstituted by the addition of cytosol that contains Bet3p. To determine whether Bet3p facilitates the binding of vesicles to the Golgi, or their fusion, we modified the transport assay to distinguish between these events. Briefly, vesicles containing radiolabeled pro-α-factor were incubated with and without Golgi membranes in the presence of cytosol and an ATP–regenerating system. At the end of the reaction, the products were analyzed on a sucrose velocity gradient, modified to resolve transport vesicles from Golgi membranes, as described in Materials and methods. Pro-α-factor transport was monitored by precipitating the reaction product with the solid phase lectin absorbent concanavalin A (Con A)–Sepharose, and membrane fusion was detected with an antiserum that recognizes Golgi-specific outer chain carbohydrate modifications. Transport vesicles were found at the top of the gradient (Figure 5A, top), while Golgi membranes (Figure 5A, top) and vesicles bound to the Golgi (Figure 5B, top) fractionated further into the gradient where Golgi markers previously have been shown to migrate. Vesicle fusion was confirmed by the presence of outer chain carbohydrate on pro-α-factor (Figure 5A, bottom).
Fig. 5. Fractions depleted of Bet3p in vivo fail to support the binding of ER-derived vesicles to Golgi membranes in vitro. Bet3p-depleted fractions were prepared and assayed as described previously (Groesch et al., 1992; Sacher et al., 1998). The reaction product was analyzed on a sucrose velocity gradient as described in Materials and methods. The slight changes made in the velocity gradient are sufficient to allow separation of ER–Golgi transport vesicles from Golgi membranes. (A) Top panel: free vesicles (solid line) and vesicles that have fused with the Golgi (broken line) migrate to distinct locations. Bottom panel: pro-α-factor is ubject to outer chain modification when vesicles are incubated in the presence of Golgi membranes (broken line), indicating that fusion with the Golgi has occurred. (B) Top panel: in the presence of anti-Bos1p antibodies (broken line), vesicles bind to Golgi membranes. Bottom panel: in the presence of anti-Bos1p antibodies, the vesicles that migrate with the Golgi have not fused (broken line), as indicated by the lack of outer chain modification. (C) Top panel: the depletion of Bet3p prevents vesicles from binding to Golgi membranes (solid line). The addition of cytosol containing Bet3p partially reconstitutes vesicle docking (broken line). Bottom panel: vesicle fusion is reconstituted by the addition of cytosol containing Bet3p, as indicated by the presence of outer chain modifications (broken line).
We previously demonstrated that an antibody raised against the v-SNARE Bos1p specifically inhibits the late stages of ER to Golgi transport in vitro, but not budding from the ER (Lian and Ferro-Novick, 1993). When this antibody was added to the reaction, the peak of Con A-precipitable pro-α-factor co-migrated with Golgi (Figure 5B, top), but lacked outer chain carbohydrate (Figure 5B, bottom), consistent with the notion that carrier vesicles had docked but not fused. The shift we observed was not a consequence of antibody-induced cross-linking of membranes, as vesicles incubated with antibody in the absence of Golgi did not migrate further into the gradient (data not shown). The small peak of Con A-precipitable counts in fraction 4 probably represents vesicles that have not docked. Our findings are consistent with a previous report showing that a temperature-sensitive defect in bos1 prevents the release of COPII vesicles from semi-intact cells. The pro-α-factor retained in the cells was not outer chain modified, suggesting that vesicles had tethered, but not fused, to the Golgi (Cao et al., 1998).
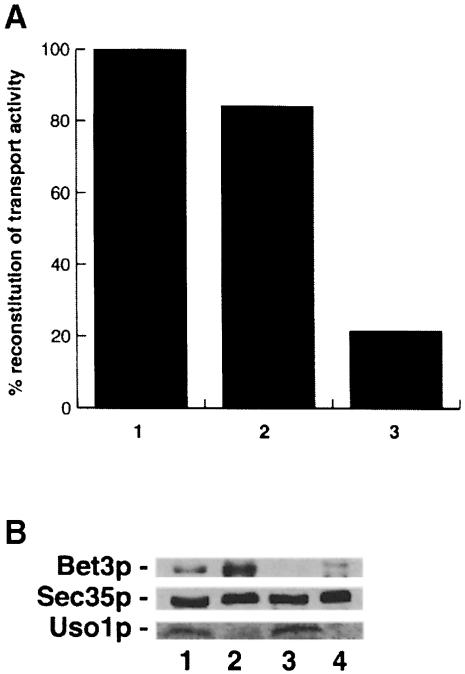
When Bet3p-depleted Golgi was incubated with vesicles in the presence of Bet3p-depleted cytosol, the vesicles that formed failed to bind to Golgi (Figure 5C, top). The depletion of Bet3p did not affect the amounts of the tethering factors Uso1p or Sec35p (see Figure 6B), indicating that these factors are not sufficient for docking vesicles in the absence of Bet3p. In the presence of cytosol that contains Bet3p, a fraction of the Con A-precipitable material shifted into the gradient where the Golgi migrates (Figure 5C, top). The Con A-precipitable counts in this fraction, but not in the vesicle fraction, could be precipitated with outer chain antibody, indicating that the vesicles that bound also fused (Figure 5C, bottom). In order to show that the reconstitution of transport activity was dependent on Bet3p, we employed a strain (SFNY904) in which the sole copy of Bet3p was fused at the C-terminus to protein A (Bet3p–PrA). Bet3p–PrA is fully functional in vivo. Incubation of cytosol prepared from this strain with IgG–Sepharose substantially depleted Bet3p–PrA (data not shown). The Bet3p–PrA-depleted cytosol, but not mock-treated (for preparation, see Materials and methods) or untreated cytosol, showed a significant reduction in the reconstitution of transport activity (Figure 6A). Thus, the reconstitution of activity by the addition of wild-type cytosol is a direct consequence of adding Bet3p to the assay. These data demonstrate that the binding of ER to Golgi carrier vesicles to their acceptor compartment requires Bet3p.
Fig. 6. Bet3p is required for the reconstitution of transport activity in vitro. (A) Reconstitution of ER to Golgi transport in vitro was measured with anti-outer chain antiserum. The data shown are representative. Untreated (column 1) or mock-treated cytosol (column 2) prepared from a strain (SFNY 904) containing Bet3p fused to protein A restored transport activity in vitro to the Bet3p-depleted Golgi. Treatment of cytosol from the tagged strain (SFNY904) with IgG–Sepharose reduced its ability to reconstitute activity (column 3). The activity of Bet3p-depleted cytosol assayed in the presence of Bet3p-depleted membranes was 15% that of wild-type fractions. A 1.7-fold increase in activity was observed in the reconstituted sample. (B) Wild-type and depleted fractions were blotted for the presence of Bet3p, Sec35p and Uso1p. Shown here are cytosolic (lanes 1 and 3) and membrane (lanes 2 and 4) fractions prepared from wild-type (lanes 1 and 2) and the Bet3p-depleted strain (lanes 3 and 4). The ability to detect Bet3p is significantly reduced in fractions from the Bet3p-depleted strain (compare lanes 1 and 2 with lanes 3 and 4), while the levels of Sec35p and Uso1p are unaffected. Lanes 1–4 contain equal amounts of protein (250 μg).
Discussion
Previous studies have demonstrated that Bet3p resides and functions on the Golgi where it acts prior to SNARE complex formation (Rossi et al., 1995; Sacher et al., 1998). Here we show that unlike the v-SNARE Bos1p, which is needed for membrane fusion, Bet3p is required for an earlier event in vesicle consumption. Bet3p and other TRAPP subunits bind to the Golgi in a stable fashion, remaining bound under conditions which disrupt membrane traffic and cause the Golgi apparatus to disperse. Considering the persistent association of TRAPP subunits with Golgi membranes, and the requirement for Bet3p in the vesicle docking event, TRAPP may serve as a landmark on the Golgi for incoming vesicle traffic.
Like TRAPP, Uso1p and Sec35p act upstream of the SNAREs and play a role in docking vesicles to the Golgi (Barlowe, 1997; VanRheenen et al., 1999). In contrast to TRAPP, the proposed vesicle-tethering components Sec35p and Uso1p (Barlowe, 1997; VanRheenen et al., 1998) are more soluble components of the secretory pathway whose membrane association is variable. Uso1p was completely soluble and the Sec34p–Sec35p complex was released from membranes more readily than TRAPP under the experimental conditions used in this study. It is interesting to note in this regard that TRAPP subunits are resistant to extraction with Triton X-100, suggesting that TRAPP may bind to a matrix (Sacher et al., 2000). It may be this characteristic of TRAPP which ensures that it remains stably bound to Golgi membranes.
Interestingly, deletions of Uso1p, Sec35p and Ypt1p can be bypassed by gene products (Bos1p, Bet1p, Sec22p, Sly1-20p, Uso1p and Ypt1p) acting in the late stages of ER to Golgi transport (Dascher et al., 1991; Lian et al., 1994; Sapperstein et al., 1996; Stone et al., 1997; VanRheenen et al., 1998). In contrast, we find that a deletion of BET5 (bet5Δ::HIS3) cannot be bypassed by the overexpression of YPT1, USO1, SEC35, BOS1, BET1 or SEC22, or combinations of BOS1 with BET1, BOS1 with SEC22 and the expression of SLY1-20 (a dominant mutation in SLY1, t-SNARE-associated protein) from a CEN vector (data not shown). This result supports the idea that TRAPP plays a critical role in the targeting and docking of vesicles to the Golgi.
Here we have established a role for Bet3p, a component of TRAPP, in the docking of vesicles to Golgi membranes. The function of Bet3p and TRAPP in ER–Golgi transport may be analogous to that of the exocyst in post-Golgi secretion. The exocyst defines the site for polarized secretion in yeast during the budding of a daughter cell from its mother (Terbush and Novick, 1995; Finger et al., 1998), while Bet3p and the other TRAPP subunits may be a landmark on the Golgi. In this regard, Bet3p fulfills three important criteria. First, it does not recycle between the Golgi and the ER compartments. Secondly, it does not disassociate from the Golgi in the absence of anterograde membrane traffic. As a marker for Golgi, Bet3p and possibly other TRAPP subunits should mark docking sites at all times and not be subject to recruitment in response to incoming vesicle traffic. Finally, Bet3p acts prior to membrane fusion and is required for the binding of transport vesicles to the Golgi in vitro. Despite their similar roles, the known subunits of TRAPP do not bear any significant homology to components of the exocyst (Sacher et al., 1998), which may be indicative of the specialized stage-specific roles of the two complexes. It is worth noting that the conditions used to deplete Bet3p do not affect the levels of the tethering factors Sec35p and Uso1p (Figure 6B). Therefore, it appears that Sec35p and Uso1p are unable to facilitate vesicle docking in the absence of Bet3p, suggesting that TRAPP may function with, or prior to, these tethering factors. While it is clear that Uso1p, the Sec34p–Sec35p complex and TRAPP all play important roles in docking vesicles to the Golgi, further experiments are required to determine how these components cooperate to perform this task.
An intriguing question that remains to be addressed is how TRAPP maintains its localization on early Golgi compartments. As a peripheral membrane complex, the localization of TRAPP to Golgi membranes probably involves binding to a receptor protein(s). A membrane-bound receptor would most probably arrive at the Golgi through the secretory pathway. In order to account for the exclusive localization of TRAPP to one membrane, the receptor must be transported to the Golgi in an inactive state, or non-binding conformation. Once it arrives at the Golgi, the receptor may become modified, enabling TRAPP to bind to it. Alternatively, or in addition, the receptor may be a component of a matrix. This would be consistent with the observation that TRAPP subunits are resistant to extraction with Triton X-100 (Sacher et al., 2000). With these possibilities in mind, the question of how this stable association with the Golgi is maintained in the midst of constant membrane traffic remains to be answered. Ongoing studies of Bet3p and the other subunits of TRAPP should help to elucidate the answer to this question.
Materials and methods
Antibodies and Western blotting
The standard protocol for Western blotting with ECL reagents was performed (Amersham Life Science). Rabbit polyclonal antibodies against Anp1p were obtained from S.Munro and used at 1:8000. Rabbit polyclonal antibodies against Sed5p were affinity purified and used at a concentration of 1 μg/ml. For immunofluorescence procedures, mouse monoclonal antibodies generated against the HA epitope were obtained from BAbCO, Richmond, CA (MMS-101R), and used at a dilution of 1:2000. Secondary CY3-conjugated antibodies specific for mouse immunoglobulins were purchased from Jackson ImmunoResearch Laboratories, Inc. and used at a dilution of 1:500.
Subcellular fractionation
Velocity sedimentation was performed using sucrose gradients as previously described (Antebi and Fink, 1992) with minor modifications. Cells were grown overnight in minimal media containing leucine to early log phase (OD599 = 0.5–1.0). For temperature shift experiments, cells were harvested, resuspended in pre-warmed media (minimal plus leucine) and incubated at 37°C for 1 h. Approximately 400 OD599 units of cells were harvested at 4°C and washed once in cold 10 mM sodium azide. Cells were converted to spheroplasts during a 1 h incubation in buffer (1.4 M sorbitol, 50 mM potassium phosphate pH 7.5, 10 mM sodium azide, 0.4% 2-mercaptoethanol, 1 mg/ml zymolyase-T) at 37°C with gentle shaking. The spheroplasts were pelleted through an 8 ml sorbitol cushion (1.7 M sorbitol, 50 mM potassium phosphate pH 7.5) at 600 g for 12 min and lysed in 6 ml of cold lysis buffer (20 mM triethanolamine, 12.5% sucrose, 1 mM EDTA, 1× protease inhibitor cocktail) by 7–10 passages through a 25 gauge needle as previously described (Schröder et al., 1995). Unbroken cells were pelleted by spinning the lysate at 500 g. After confirming by microscopy that the lysate was clear of unbroken cells, 1 ml of lysate was loaded onto the top of a sucrose gradient consisting of eleven 1 ml steps (18, 22, 26, 30, 34, 38, 42, 46, 50, 54 and 60% sucrose w/w in 10 mM HEPES pH 7.5, 1 mM MgCl2). Gradients were centrifuged at 38 000 r.p.m. in an SW40.1 rotor (Beckman Instruments) at 4°C for 2 h 20 min. One milliliter fractions were collected from the top of the gradient. Fractions were boiled in SDS sample buffer, and 80 μl aliquots of each fraction were analyzed by SDS–PAGE and immunodetection. In some experiments, fractions were first subjected to trichloroacetic acid (TCA) precipitation as described by Hardwick and Pelham (1992) before analysis by SDS–PAGE and immunoblotting. Antibodies were detected using the ECL method (Amersham Life Science) and the bands were quantified using Bio Image® software. The data plotted represent a quantification of the number of pixels in a band, a measurement that is proportional to band intensity.
Fluorescence microscopy and indirect immunofluorescence
For the temperature shift experiments, wild-type and mutant strains were grown to early log phase, harvested and resuspended in pre-warmed media. Incubations were performed at 37°C for the indicated times. Cells subsequently were harvested at 4°C, resuspended in 0.5 ml of ice-cold YPD medium and viewed directly on a Zeiss fluorescence light microscope. Localization of Och1-HA and myc-Emp47p was performed using an immunofluorescence protocol that incorporates modifications from Bonangelino et al. (1997) and Berkower et al. (1994). Images were acquired using a charged-coupled device camera (Photometrics), and adjusted using the Adobe Photoshop program.
In vitro transport assay
Standard in vitro transport assays and components for the assay were described previously (Ruohola et al., 1988; Groesch et al., 1992; Lian and Ferro-Novick, 1993) except that the cytosolic fraction was prepared by centrifugation of an S1000 fraction for 4 h at 200 000 g. Bet3p-depleted fractions were prepared as described previously (Sacher et al., 1998). Typically five reactions were performed in one tube for each gradient. When anti-Bos1p antibody was used, the assay was performed in the presence of 10 μg (per five reactions) of affinity-purified IgG. At the end of the assay, donor membranes were sedimented during a 1 min centrifugation in an Eppendorf centrifuge. The supernatant (which contains vesicles and Golgi) was removed and the volume adjusted to 1 ml with transport buffer (115 mM potassium acetate, 2.5 mM magnesium acetate, 25 mM HEPES pH 7.2). The sample was then loaded on the top of a sucrose step gradient (Antebi and Fink, 1992) with the following modifications (sucrose solutions are w/w in transport buffer): 0.5 ml of 50% sucrose, 0.5 ml of 46% sucrose, 1 ml of 42% sucrose, 1.5 ml of 38% sucrose, 1.5 ml of 34% sucrose, 1.5 ml of 30% sucrose, 1.5 ml of 26% sucrose, 1.5 ml of 22% sucrose and 1.5 ml of 18% sucrose. The gradient was centrifuged for 2.5 h at 38 000 r.p.m. in a Beckman SW41 rotor. Fractions (1 ml) were collected from the top of the gradient and 200 μl of each fraction were treated with Con A– Sepharose as described before (Groesch et al., 1992). Half the sample was used for liquid scintillation counting and the remainder was immunoprecipitated with an anti-outer chain antiserum (Lian and Ferro–Novick, 1993).
For the experiment shown in Figure 6, the transport assay was performed as described previously (Sacher et al., 1998). At the end of the reaction, samples were treated with Con A–Sepharose and then anti-outer chain serum. For the depletion of Bet3p–PrA from cytosol prepared from SFNY904, samples (two reactions) were incubated with an ∼20 μl bed volume of IgG–Sepharose for 4 h at 4°C. Mock-treated cytosol prepared from SFNY904 was treated under identical conditions except that Sepharose CL-4B, instead of IgG–Sepharose, was used (Figure 6). Cytosol prepared from SFNY26-3A was mock treated with IgG–Sepharose and no loss of transport activity was observed (data not shown). The construction of SFNY904 will be described elsewhere (Sacher et al., 2000).
Acknowledgments
Acknowledgements
We thank Dr Hans Dieter Schmitt and Dr Gerry Waters for plasmids, Dr Sean Munro for antibodies directed against Anp1p, and Monica Andreoli for technical assistance. M.S. was supported as an Associate of the Howard Hughes Medical Institute.
References
- Antebi A. and Fink, R.G. (1992) The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a Golgi-like distribution. Mol. Biol. Cell, 3, 633–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. (1997) Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J. Cell Biol., 139, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. and Schekman, R. (1993) SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature, 365, 347–349. [DOI] [PubMed] [Google Scholar]
- Berkower C., Loayza, D. and Michaelis, S. (1994) Metabolic instability and constitutive endocytosis of STE6, the α-factor transporter of Saccharomyces cerevisiae. Mol. Biol. Cell, 5, 1185–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonangelino C.J., Catlett, N.L. and Weisman, L.S. (1997) Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol. Biol. Cell, 17, 6847–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P., Kearns, B., Champion, K., Keranen, S., Bankaitis, V. and Novick, P. (1994) Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell, 79, 245–258. [DOI] [PubMed] [Google Scholar]
- Cao X., Ballew, N. and Barlowe, C. (1998) Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J., 17, 2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole N.B., Ellenberg, J., Song, J., DiEuliis, D. and Lippincott-Schwartz, J. (1998) Retrograde transport of Golgi-localized proteins to the ER. J. Cell Biol., 140, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C., Ossig, R., Gallwitz, D. and Schmitt, H.D. (1991) Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol. Cell. Biol., 11, 872–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S. and Jahn, R. (1994) Vesicle fusion from yeast to man. Nature, 370, 191–193. [DOI] [PubMed] [Google Scholar]
- Finger F.P., Hughes, T.E. and Novick, P. (1998) Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell, 92, 559–571. [DOI] [PubMed] [Google Scholar]
- Garcia E.P., McPherson, P.S., Chilcote, T.J., Takei, K. and DeCamilli, P. (1995) rbSec1A and B co-localize with syntaxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J. Cell Biol., 129, 105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod A., Storrie, B., Simpson, J.C., Johannes, L., Goud, B., Roberts, L.M., Lord, J.M., Nilsson, T. and Pepperkok, R. (1999) Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nature Cell Biol., 1, 423–430. [DOI] [PubMed] [Google Scholar]
- Groesch M.E., Ruohola, H., Bacon, R., Rossi, G. and Ferro-Novick, S. (1990) Isolation of a functional vesicular transport intermediate that mediates ER to Golgi transport in yeast. J. Cell Biol., 111, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groesch M.E., Rossi, G. and Ferro-Novick, S. (1992) Reconstitution of endoplasmic reticulum to Golgi transport in yeast: in vitro assay to characterize secretory mutants and functional transport vesicles. Methods Enzymol., 219, 137–152. [DOI] [PubMed] [Google Scholar]
- Hardwick K.G. and Pelham, H.R.B. (1992) SED5 encodes a 39-kd integral membrane protein required for vesicle transport between the ER and the Golgi complex. J. Cell Biol., 119, 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S.L. and Waters, M.G. (1996) Localization of a yeast early Golgi mannosyltransferase, Och1p, involves retrograde transport. J. Cell Biol., 132, 985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthius J.C.M., Nichols, B.J., Dhruvakumar, S. and Pelham, H.R.B. (1998) Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J., 17, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui N., Nakamura, N., Sonnichsen, B., Shima, D.T., Nilsson, T. and Warren, G. (1997) An isoform of the Golgi t-SNARE, syntaxin 5, with an endoplasmic reticulum retrieval signal. Mol. Biol. Cell, 8, 1777–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Scarpa, A., Zhang, L., Stone, S., Feliciano, E. and Ferro-Novick, S. (1998) A high copy suppressor screen reveals genetic interactions between BET3 and a new gene: evidence for a novel complex in ER to Golgi transport. Genetics, 149, 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-W., Sacher, M., Scarpa, A., Quinn, A.M. and Ferro-Novick, S. (1999) High-copy suppressor analysis reveals a physical interaction between Sec34p and Sec35p, a protein implicated in vesicle docking. Mol. Biol. Cell, 10, 3317–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J.P. and Ferro-Novick, S. (1993) Bos1p, an integral membrane protein of the endoplasmic reticulum to Golgi transport vesicles, is required for their fusion competence. Cell, 73, 734–745. [DOI] [PubMed] [Google Scholar]
- Lian J.P., Stone, S., Jiang, Y., Lyons, P. and Ferro-Novick, S. (1994) Ypt1p implicated in v-SNARE activation. Nature, 372, 698–701. [DOI] [PubMed] [Google Scholar]
- Mayer A., Wickner, W. and Haas, A. (1996) Sec18 (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell, 85, 83–94. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Nagasu, T., Shimma, Y., Kuromitsu, J. and Jigami, Y. (1992) OCH1 encodes a novel membrane-bound mannosyltransferase: outer chain elongation of asparagine-linked oligosaccharides. EMBO J., 11, 2511–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A.P., Groesch, M.E. and Ferro-Novick, S. (1992) Bos1p, a membrane protein required for ER to Golgi transport in yeast, co-purifies with the carrier vesicles and with Bet1p and the ER membrane. EMBO J., 11, 3609–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G., Kolstad, K., Stone, S., Palluault, F. and Ferro-Novick, S. (1995) BET3 encodes a novel hydrophilic protein that acts in conjunction with yeast SNAREs. Mol. Biol. Cell, 6, 1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.E. (1994) Mechanisms of intracellular transport. Nature, 372, 55–63. [DOI] [PubMed] [Google Scholar]
- Ruohola H., Kabcenell, A.K. and Ferro-Novick, S. (1988) Reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex in yeast: the acceptor Golgi compartment is defective in the sec23 mutant. J. Cell Biol., 107, 1465–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M., Stone, S. and Ferro-Novick, S. (1997) The synaptobrevin-related domains of Bos1p and Sec22p bind to the syntaxin-like region of Sed5p. J. Biol. Chem., 272, 17134–17138. [DOI] [PubMed] [Google Scholar]
- Sacher M., et al. (1998) TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J., 17, 2494–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M., Barrowman,J., Schieltz,D., Yates,J.R.,III and Ferro-Novick,S. (2000) Identification and characterization of five new subunits of TRAPP. Eur. J. Cell Biol., in press. [DOI] [PubMed] [Google Scholar]
- Sapperstein S.K., Walter, D.M., Grosvenor, A.R., Heuser, J.E. and Waters, M.G. (1995) p115 is a general vesicular transport factor related to the yeast endoplasmic reticulum to Golgi transport factor Uso1p. Proc. Natl Acad. Sci. USA, 17, 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapperstein S.K., Lupashin, V.V., Schmitt, H.D. and Waters, M.G. (1996) Assembly of the ER to Golgi SNARE complex requires Uso1p. J. Cell Biol., 132, 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder S., Schimmöller, F., Singer-Krüger, B. and Riezman, H. (1995) The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1-1 mutation in α-COP. J. Cell Biol., 131, 895–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J., Newman, A.P. and Ferro-Novick, S. (1991) The BOS1 gene encodes an essential 27-kd membrane protein that is required for vesicluar transport from the ER to the Golgi complex in yeast. J. Cell Biol., 113, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Whiteheart, S.W., Brunner, M., Erdjument-Bromage, H., Geromanos, S., Tempst, P. and Rothman, J.E. (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature, 362, 318–324. [DOI] [PubMed] [Google Scholar]
- Stone S., Sacher, M., Mao, Y., Carr, C., Lyons, P., Quinn, A.M. and Ferro-Novick, S. (1997) Bet1p activates the v-SNARE, Bos1p. Mol. Biol. Cell, 8, 1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush D.R. and Novick, P. (1995) Sec6, Sec8 and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J. Cell Biol., 130, 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Sato, K. and Wickner, W. (1998) Defining the functions of trans-SNARE pairs. Nature, 396, 543–548. [DOI] [PubMed] [Google Scholar]
- VanRheenen S.M., Cao, X., Lupashin, V.V., Barlowe, C. and Waters, M.G. (1998) Sec35p, a novel peripheral membrane protein, is required for ER to Golgi vesicle docking. J. Cell Biol., 141, 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen S.M., Cao, X., Sapperstein, S.K., Chiang, E.C., Lupashin, V.V., Barlowe, C. and Waters, M.G. (1999) Sec34p, a protein required for vesicle tethering to the yeast Golgi apparatus, is in a complex with Sec35p. J. Cell Biol., 147, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman, B.V., McNew, J.A., Westermann, B., Gmachl, M., Parlati, F., Söllner, T. and Rothman, J.E. (1998) SNAREpins: minimal machinery for membrane fusion. Cell, 92, 759–772. [DOI] [PubMed] [Google Scholar]
- White J., et al. (1999) Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol., 147, 743–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding S. and Pelham, H.R.B. (1998) The dynamics of Golgi protein traffic visualized in living yeast cells. Mol. Biol. Cell, 9, 2667–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]