Abstract
Mechanisms by which the brain monitors and modulates performance are an important focus of recent research. The conflict-monitoring hypothesis posits that the ACC detects conflict between competing response pathways which, in turn, signals for enhanced control. The N2, an ERP component that has been localized to ACC, has been observed after high conflict stimuli. As a candidate index of the conflict signal, the N2 would be expected to be sensitive to the degree of response conflict present, a factor that depends on both the features of external stimuli and the internal control state. In the present study, we sought to explore the relationship between N2 amplitude and these variables through use of a modified Eriksen flankers task in which target–distracter compatibility was parametrically varied. We hypothesized that greater target–distracter incompatibility would result in higher levels of response conflict, as indexed by both behavior and the N2 component. Consistent with this prediction, there were parametric degradations in behavioral performance and increases in N2 amplitudes with increasing incompatibility. Further, increasingly incompatible stimuli led to the predicted parametric increases in control on subsequent incompatible trials as evidenced by enhanced performance and reduced N2 amplitudes. These findings suggest that the N2 component and associated behavioral performance are finely sensitive to the degree of response conflict present and to the control adjustments that result from modulations in conflict.
INTRODUCTION
Cognitive control describes the set of processes responsible for task-appropriate perceptual selection and response biasing, especially in face of distracting stimulus information or task-inappropriate responses tendencies (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Miller & Cohen, 2001). Evaluative mechanisms that monitor performance are thought to provide critical feedback in the modulation of control and have become a focus of great interest in recent years. The conflict hypothesis posits one such mechanism, by which there is monitoring for conflict between competing response pathways, signaling a need for additional control resources when high levels of conflict are detected (Botvinick et al., 2001, 2004). Numerous studies have provided empirical evidence consistent with such conflict-mediated control adjustments (Cho, Orr, Cohen, & Carter, 2009; Kunde & Wühr, 2006; Egner & Hirsch, 2005b; Ullsperger, Bylsma, & Botvinick, 2005; Kerns et al., 2004; Stürmer, Leuthold, Soetens, Schroter, & Sommer, 2002; Gratton, Coles, & Donchin, 1992), and the viability of conflict detection as a means of signaling for control has been established through computational modeling (Botvinick et al., 2001).
After the occurrence of a high-conflict processing event, behavioral adjustments appear to reflect a compensatory, augmented control state (Kerns et al., 2004; Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Gratton et al., 1992). For example, when instructed to respond to the central letter of an Eriksen flanker stimulus, an incongruent trial (e.g., the array, “HHSHH”) elicits conflict between competing “H” and “S” response pathways. The resulting, heightened control state gives rise to faster RTs and lower error rates (ERs) on subsequent incongruent trials through facilitation of task-relevant processing and inhibition of task-irrelevant input. Somewhat counterintuitively, the same heightened control state can lead to impaired performance on a subsequent congruent trial. Task-irrelevant inputs may facilitate processing of congruent stimuli (e.g., the array “SSSSS”) because both task-relevant and task-irrelevant (but in this case, facilitating the correct response) inputs map to the desired response outcome. As such, relatively impaired performance is expected for congruent trials after incongruent trials because the resultant-increased control reduces input from the irrelevant (but in this case helpful) stimulus dimension. These patterns of performance adjustment have been reliably observed and are consistent with the dynamic, bottom–up regulated control system proposed by the conflict hypothesis. Although alternative accounts explain these sequential effects through priming (Mayr, Awh, & Laurey, 2003) or feature integration (Hommel, Proctor, & Vu, 2004), several studies that have controlled for these factors have demonstrated reliable control adjustments (Verbruggen, Notebaert, Liefooghe, & Vandierendonck, 2006; Egner & Hirsch, 2005a; Ullsperger et al., 2005; Wühr, 2005; Botvinick et al., 2004).
The conflict hypothesis is further bolstered by evidence from EEG and fMRI studies probing the neural correlates of conflict detection and control adaptation. Neuroimaging studies have reported findings consistent with the proposal that the ACC indexes conflict (Carter et al., 1998), that this role can be dissociated from that of the dorsolateral pre-frontal cortex (DLPFC) in the strategic adjustment of control (MacDonald, Cohen, Stenger, & Carter, 2000) and that ACC activation is predictive of subsequent DLPFC activation and behavioral performance adjustments (Kerns et al., 2004). In EEG studies, the stimulus-locked ERP component known as the N2 has been consistently observed after the presentation of conflict stimuli in a number of different task paradigms (Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003; van Veen & Carter, 2002b; Liotti, Woldorff, Perez, & Mayberg, 2000; Kopp, Mattler, Goertz, & Rist, 1996). The N2 has a scalp distribution over the frontal midline and, with mathematical limitations of inverse solutions notwithstanding, source localization of the N2 consistently situates the signal in ACC (Ladouceur, Dahl, & Carter, 2007; Yeung et al., 2004; van Veen & Carter, 2002a, 2002b), consistent with fMRI findings using similar conflict paradigms (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004). Although the frontal N2 has been noted to be elicited by a diversity of processes such as mismatch or novelty, in the context of conflict tasks, the frontal N2 component is thought to index signals related to cognitive control processes (Folstein & Van Petten, 2008). As an index of conflict, the N2 is thought to correspond to the degree of conflict generated by competition between task-relevant and task-irrelevant inputs (Yeung & Cohen, 2006). This general assumption holds for experimental paradigms in which the N2 has been studied and has been explicitly addressed in recent work by Danielmeier, Wessel, Steinhauser, and Ullsperger (2009) in which N2 amplitude was found to increase with the proximity of distracting information. In the context of the flanker task (used in the current study), the N2 is thought to specifically index conflict at the response level. van Veen and Carter (2002a, 2002b) found that response but not stimulus conflict modulated the N2, whereas Freitas, Banai, and Clark (2009) also found that the N2 was likely indexing conflict after the initiation of responses, after completion of stimulus evaluation that was indexed by stimulus-locked lateralized readiness potentials (LRPs). Although these findings support the view that N2 reflects a conflict-monitoring signal, the sensitivity of the N2 to more detailed manipulations of conflict and control has not been fully tested.
In the present study, we sought to test three predictions that follow from the conflict hypothesis: (a) that behavioral performance and N2 amplitudes would reflect parametric variations in response conflict resulting from increasing degrees of incongruency, (b) that behavioral performance and N2 amplitudes could index the effects of control modulations because of parametric variations in previous trial incongruency, and (c) that behavioral performance adjustments relate to N2 amplitude in a manner consistent with expected modulations in control. The conflict hypothesis makes strong predictions regarding the influence of both current and recent processing demands on ACC activation and associated behavior. If these demands are reliably registered by the N2 as an index of ACC-based conflict signal, N2 amplitudes should track the expected degree of conflict and correlate with associated task performance.
The basic finding that ACC is responsive to conflict and predictive of subsequent control adjustment has been well established through a number of studies (Kerns, 2006; Liston, Matalon, Hare, Davidson, & Casey, 2006; Egner & Hirsch, 2005b; Weissman, Gopalakrishnan, Hazlett, & Woldorff, 2005; Badre & Wagner, 2004; Kerns et al., 2004; Fan, Flombaum, McCandliss, Thomas, & Posner, 2003). However, relatively few studies have sought to examine conflict detection in the context of more detailed, systematic manipulations of control states. In an fMRI study, Durston et al. (2003) parametrically manipulated control state through trial-type ordering. It was found that during a current incongruent trial, the activations of ACC, DLPFC, and superior parietal cortex increased as a function of the number of preceding compatible trials but decreased with an increasing number of preceding incompatible trials. It was reasoned that incremental increases in control would follow conflict detection by ACC on each successive incompatible trial that, in turn, could reduce the conflict signal on subsequent trials. Conversely, each successive compatible trial should reduce control, resulting in greater processing of irrelevant input and thus an increase in response conflict (Durston et al., 2003). Behavioral data supported this interpretation, revealing performance enhancement as a function of the number of preceding incongruent trials and performance impairment as a function of the number of congruent trials. The temporal progression of regional activations was consistent with the conflict-monitoring hypothesis, suggesting signaling of DLPFC by ACC and subsequent activation of superior parietal cortex, perhaps as a result of DLPFC-mediated regulation of selective attention. A study by Jones, Cho, Nystrom, Cohen, and Braver (2002) similarly found that ACC activation varied predictably with parametric manipulations in the degree of conflict elicited by stimulus history dependent priming effects. EEG studies by Danielmeier et al. (2009) and Iwaki, Miyatani, and Toshima (2003) examined N2 modulation by parametric variations in conflict as elicited by varying the degree of incongruency in the task stimuli. However, neither study addressed dependencies of N2 and related behavioral indices on variations in the control state. Wendt, Heldmann, Munte, and Kluwe (2007) studied sequential effects with a flanker paradigm, but without a parametric manipulation. They failed to find evidence of sequential modulation of performance or N2 amplitude by previous conflict. However, although the authors note that their N2 amplitude modulations were sufficiently large to adequately test for such sequential effects, they used a standard stimulus array size (five letters) in contrast with the larger array size (seven letters) in the current study that may provide an improved dynamic range to detect such effects. Further, as the authors note, there were a number of task design differences from the van Veen and Carter (2002a, 2002b) task version (lack of flanker-target onset asynchrony, the arrangement of flankers, increased proportion of incongruent trials, etc.) that may have led to decreased conflict-related effects.
In the present study, we sought to complement and extend previous neuroimaging studies by demonstrating that the N2 component is similarly responsive to parametric manipulations in the degree of conflict and that this component and associated behavioral performance indices reflect the control adjustments that result from such systematic manipulations. To this end, we developed a variant of the Eriksen flanker task (Eriksen & Eriksen, 1974) in which conflict levels were parametrically varied across stimuli. Each of four flanker stimulus conditions contained a different ratio of task-relevant to task-irrelevant input. We expected that greater contributions from task irrelevant input would result in increasing levels of response conflict and associated control adjustments and that these effects could be detected in both behavioral and ERP measures. On the basis of these assumptions, we made the following predictions: (a) During the current trial, performance impairment and N2 amplitude will increase with the level of response conflict elicited by the current stimulus. (b) Performance and N2 amplitudes will also depend on the level of response conflict elicited by the previous stimulus. Because control modulations are expected to be proportional to the level of conflict detected, we expected that higher levels of conflict on the previous trial would result in greater reductions in N2 amplitude on the present trial. And (c) because the N2 can be reliably characterized on a single subject basis, correlations between N2 amplitude and behavioral measures of performance adjustment should be observed. We expected to find that individuals who exhibit a more pronounced reduction in N2 amplitude, after conflict on the previous trial, will also exhibit greater performance adjustments. Both measures should reflect the degree to which the control state has been modulated and should thus be found to correlate across subjects.
Through the testing of these predictions, we sought to demonstrate that the N2 component and associated behavioral performance modulate predictably with the degree of task-relevant versus task-irrelevant content on present and previous trials and that the degree of modulation in the N2 would correlate with the degree of associated performance adjustments. Validation of these predictions would lend strong support to the claim that the N2 is a reliable index of conflict and that the neural signal indexed by this component is critical in the recruitment of control.
METHODS
Participants
Forty-nine undergraduate students from the University of Pittsburgh participated in the experiment (25 men, mean age = 18.4 years, SD = 2.83 years). All participants were right-handed and reported corrected-to-normal vision. Each individual provided written consent in accordance with the institutional review board of the University of Pittsburgh. All students earned partial course credit in an introductory level psychology class in return for their participation.
Procedure
E-Prime computer software (Psychological Software Tools, Pittsburgh, PA) was used for presentation of stimuli and recording of behavioral measures of performance. Modified Eriksen flanker stimuli were presented in white, 32-point, Courier font on a black background, and the subject was seated 77 cm from the presentation monitor. Each stimulus was composed of “H” and/or “S” letter components and arranged such that a central target letter was flanked by symmetric arrays of three distracter letters. The proportion of distracting flanker letters, compatible with the central target, was varied to create four different stimulus conditions. Flankers were 100% compatible with the central target for the congruent (CON) condition (e.g., SSSSSSS), 67% compatible for the incongruent-low (INCLO) condition (e.g., HSSSSSH), 33% compatible for the incongruent-intermediate (INCMED) condition (e.g., HHSSSHH), and 0% compatible for the incongruent-high (INCHI) condition (e.g., HHHSHHH). CON stimuli were presented on 70% of trials with each incongruent (INC) condition occurring equally often on the remaining 30% of trials. Participants were instructed to make button-press responses to the central letter in each stimulus using one of two buttons on a response pad, with a left index finger response required for “H” and a right index finger response required for “S.” Each participant completed a short practice with a minimum of 85% accurate responses before beginning the task. It has been reported that conflict effects are more robust under conditions of speed pressure (van Veen, 2006), so an RT deadline was determined on the basis of practice performance for each individual to encourage faster responding. For the first 14 participants, the mean RT of correct responses during the practice was used as the deadline for responses during the task. A more stringent deadline, set to the median of correct practice trial RTs, was subsequently adopted.
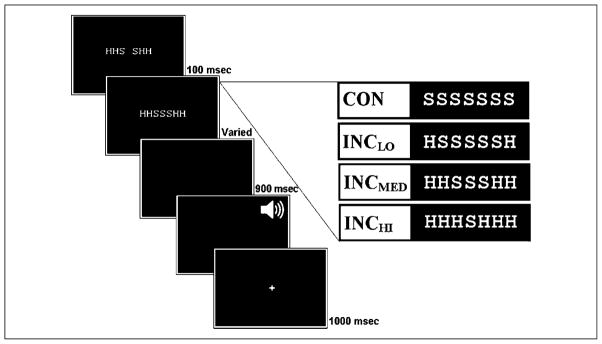
Flanker letters were presented 100 msec before target onset and remained visible for the duration of the target presentation, a period lasting until the individually determined RT deadline. Responses occurring after this deadline were considered late but were still recorded up to 1500 msec after target onset. Upon response production, there was a fixation period of 900 msec, followed by auditory feedback of brief duration (~2 msec) identifying correct, erroneous, and late responses. An intertrial interval of 1000 msec followed. A schematic of the trial procedure is presented in Figure 1. The experimental session consisted of six blocks of 98 trials, within which stimuli were presented in a pseudorandomized order.
Figure 1.
Schematic of trial procedure for the modified Eriksen flanker task. The example trial shown is for HHSSSHH. The stimuli to the right are examples for each of the congruency levels.
Electrophysiological Recordings
The EEG was recorded using a 128-channel Geodesic Sensor Net and NetStation software (Electrical Geodesics Inc., Eugene, OR) at a sampling rate of 250 Hz. Impedance measures for all channels were maintained below 40 kΩ during the recording session. All channels were referenced to a common vertex reference (Cz) during data collection and later rereferenced off-line to an average reference measure. Average ERPs were derived for each condition of interest after preprocessing of EEG data. The EEG was segmented into stimulus-locked epochs beginning 200 msec before target onset and lasting for 800 msec. Baseline correction was conducted with reference to the first 100 msec of each epoch. Within each epoch, channels in which differential amplitude exceeded 200 microvolts (μV) or was equivalent to zero and channels in which consecutive samples differed by more than 60 μV were identified as bad. Epochs containing 20 or more bad channels were visually inspected for exclusion from each data set. The EEGlab RUNICA tool was used to implement ICA-based ocular artifact correction (Delorme, Sejnowski, & Makeig, 2007), and then a 1- to 30-Hz band-pass infinite impulse response filter was applied. Any bad channel that persisted in the data after filtering was interpolated and reassessed using the artifact detection criteria noted above. Remaining epochs with 20 or more bad channels were visually inspected for exclusion a second time before a final baseline correction with reference to the first 100 msec of the epoch.
Statistical Analysis
One- and two-way repeated measures ANOVA tests were used to assess the degree to which experimental conditions predicted variability in behavioral and electrophysiological measures. A Greenhouse–Geisser correction was applied when a significant violation of sphericity was indicated by Mauchly’s test of sphericity. In addition, polynomial trend analysis was used to test for the predicted monotonic trends with parametric variation of congruency levels. Pearson correlation coefficients were also calculated to assess the relationship between behavioral and ERP indices of control adjustment across subjects. Before statistical analysis, ER values were arc-sine transformed to better approximate a normal distribution, and RT data were limited to correct trials preceded by correct trials to limit the effects of error-related changes in RT (Rabbitt, 1966). Responses made after the individually determined RT deadline were also excluded from both RT and ER analyses. Data from six participants were excluded altogether because these individuals failed to meet the RT deadline on over 40% of trials. Data from three additional participants were omitted from analysis of N2 by stimulus condition because there were insufficient epochs representing the INCHI condition for these individuals.
The N2 was defined as the fronto-central negativity around 310 msec after target presentation. Average N2 latency was calculated separately for each participant and used to define a window within which the N2 was identified for each condition of interest using an adaptive mean approach. The most negative peak within a 40-msec time window centered around the individual’s average latency was identified, and the mean voltage of the 24 msec around this peak was calculated for a fronto-central montage including electrodes Fz, Fcz, Cz, and Pz under the 10-10 system (ftp://ftp.egi.com/pub/documentation/technotes/200_ElectrodePositions.pdf). Averaging across all three INC conditions, N2 amplitude was found to be most prominent at channels Cz, Fz, and FCz. Although Fz showed the largest N2 amplitudes, Cz and FCz were chosen as the focus of subsequent analyses because they also showed substantial differentiation between conditions and were thus the best channels within which to explore possible parametric effects.
RESULTS
Within-trial Conflict Effects
Behavioral Results
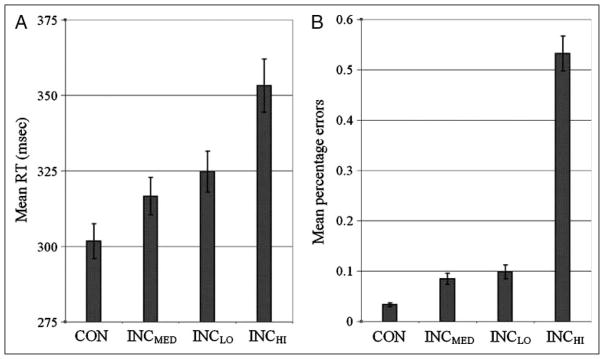
One-way repeated measures ANOVA tests with the factor Congruity (current trial, regardless of previous trial congruity) confirmed that behavioral measures varied significantly in accordance with stimulus congruity for both RT, F(3, 126) = 104.02, p < .001, and ER, F(3, 126) = 204.25, p < .001, measures. A monotonic increase in RT and ER was observed with increasing stimulus incongruity (see Table 1). Polynomial trend analyses revealed that increasing incongruity was associated with linear increases in RTs, F(1, 171) = 27.14, p < .001 (Figure 2A), and linear, F(1, 171) = 305.36, p < .001, and quadratic, F(1, 171) = 97.55, p < .001, increases in ERs (Figure 2B).
Table 1.
Mean and Standard Deviation of Behavioral Measures by Stimulus Congruity Condition
| Congruity | RT (msec) |
ER |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| CON | 302 | 38 | 0.033 | 0.024 |
| INCLO | 317 | 40 | 0.085 | 0.071 |
| INCMED | 325 | 44 | 0.099 | 0.092 |
| INCHI | 353 | 58 | 0.533 | 0.224 |
Figure 2.
Mean RTs (A) and ERs (B) by stimulus congruity condition. Mean RT was found to increase with stimulus incongruity (CON < INCLO < INCMED < INCHI; p < .001 for all comparisons). Mean ER also increased with stimulus incongruity (CON < INCLO < INCMED < INCHI) with CON versus INCLO and INCMED versus INCHI comparisons reaching significance (p < .001) and INCLO versus INCMED approaching significance at p = .113.
ERP Results
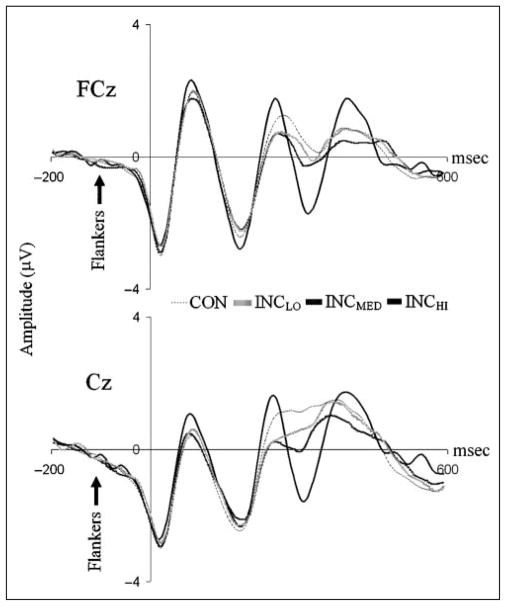
One-way ANOVA confirmed that N2 amplitude varied significantly across stimulus congruity conditions at both Cz, F(3, 117) = 32.69, p < .001, and FCz, F(3, 117) = 17.71, p < .001 (Figure 3). In line with patterns observed in behavioral measures, a monotonic increase in N2 amplitude with increasing stimulus incongruity was present at both channel locations (see Table 2). Polynomial trend analysis confirmed a significant linear increase in amplitude with increasing incongruity at both Cz, F(1, 159) = 47.78, p < .001, and FCz, F(1, 159) = 28.11, p < .001. A one-way ANOVA with N2 latency as the dependent variable revealed no significant variance in latency across congruity conditions.
Figure 3.
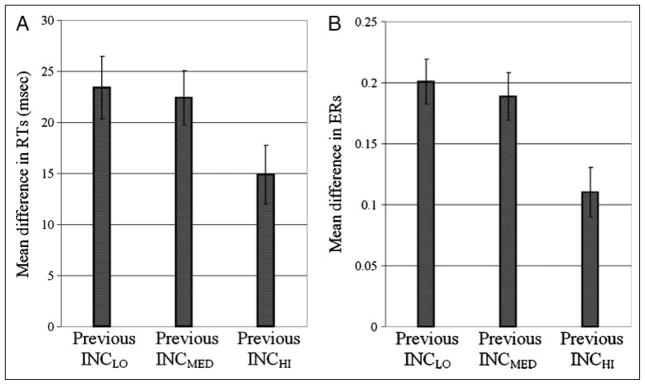
Behavioral adaptation as indexed by the mean difference between incongruent and congruent trials, given the incongruity of the previous stimulus for RT (A) and ER (B). After the INCHI condition behavioral adjustment, effects were greater than those after both the INCLO condition (for both RT, p = .004 and ER, p < .001) and the INCMED condition (for both RT, p = .021 and ER, p = .002). These findings are in line with our hypothesis that control recruitment, after conflict, depends on the level of conflict previously detected.
Table 2.
Mean and Standard Deviation of N2 Amplitude (μV) by Stimulus Congruity Condition and Channel
| Congruity | Cz |
FCz |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| CON | 0.75 | 1.26 | 0.18 | 1.19 |
| INCLO | 0.04 | 1.55 | −0.19 | 1.26 |
| INCMED | −0.38 | 1.62 | −0.51 | 1.76 |
| INCHI | −1.89 | 2.24 | −1.72 | 2.06 |
Conflict Adaptation Effects
Behavioral Results
To determine whether different levels of response conflict influence performance on subsequent trials, additional behavioral analyses were conducted. Control modulation by conflict is expected to result in impaired performance on low-conflict trials and enhanced performance on high conflict trials (see Introduction). To quantify performance benefits on high conflict trials, mean RTs and ERs were determined for INC trials (collapsing across INCLO, INCMED, and INCHI conditions1) after each particular INC trial type: INCLO, INCMED, and INCHI. Corresponding mean RT and ERs on CON trials after INCLO, INCMED, and INCHI stimuli were similarly determined. A 4 (Previous Congruity: CON, INCLO, INCMED, INCHI) × 2 (Present Congruity: CON, INC) repeated measures ANOVA identified a significant main effect of both previous, F(3, 126) = 9.95, p < .001, and present, F(1, 126) = 130.33, p < .001, congruity on RT. As expected, the interaction between previous and present congruity was also found to be significant, F(3, 126) = 6.02, p = .001. An analogous ANOVA for ERs similarly revealed significant main effects of previous, F(3, 126) = 6.73, p = .001, and present, F(1, 126) = 146.67, p < .001, congruity, as well as a significant interaction between these factors, F(3, 126) = 9.57, p < .001. Mean performance measures for CON trials after each type of INC stimulus were then subtracted from the analogous INC trial means such that a smaller difference reflected greater behavioral adaptation (for mean and difference scores by condition, see Table 3). For example, if a particular INC condition reduced RTs on subsequent INC trials and increased RTs on subsequent CON trials more than the other conditions, subtracting mean RT for the latter from that of the former would result in a lower value than that found for the other conditions. Summary indices of behavioral adaptation for RT and ER were then tested for polynomial trends. As defined above, behavioral adaptation after INCLO, INCMED, and INCHI conditions revealed significant linear trends for both RT, F(1, 128) = 4.43, p = .037, and ER, F(1, 128) = 10.81, p = .001. These findings remained consistent when exact stimulus-response repetitions were omitted to control for priming effects (the remaining transitions would involve switches between the target and the distractor letters, with any resulting negative priming and facilitation effects acting in opposite directions, although possibly with different magnitudes; Mayr & Awh, 2008). Mean values for RT and ER indices of behavioral adaptation are presented in Figure 4.
Table 3.
Mean and Difference Measures of Behavioral Adaptation by Previous Stimulus Congruity
| Previous Congruity | RT (msec) |
ER |
||||
|---|---|---|---|---|---|---|
| incCON | incINC | Difference | incCON | incINC | Difference | |
| INCLO | 301 | 324 | 23 | 0.040 | 0.241 | 0.201 |
| INCMED | 304 | 326 | 22 | 0.032 | 0.221 | 0.189 |
| INCHI | 314 | 329 | 15 | 0.044 | 0.154 | 0.110 |
Figure 4.
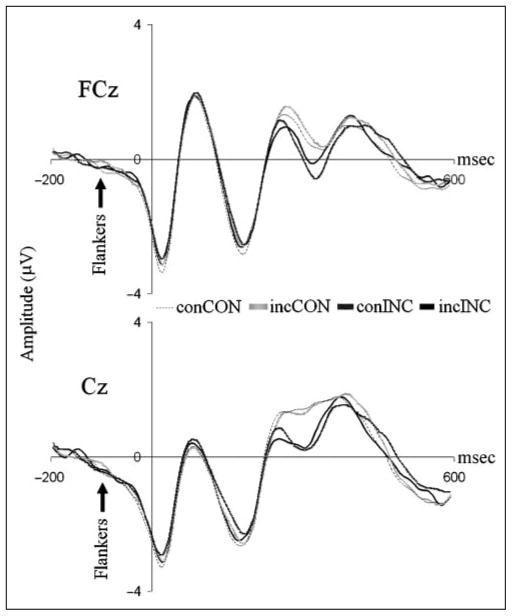
Stimulus-locked ERPs at FCz and Cz by stimulus congruity condition. N2 amplitude was found to increase with stimulus incongruity (CON < INCLO < INCMED < INCHI) at both FCz and Cz. Although there was no significant difference between N2 amplitudes after INCLO and INCMED stimuli at channel FCz, all other relevant comparisons were significant. The differentiation in N2 observed across stimulus conditions suggests that the amplitude of this component reflects the level of response conflict present.
ERP Results
A 4 (Previous Congruity: CON, INCLO, INCMED, INCHI) × 2 (Present Congruity: CON, INC) repeated measures ANOVA was conducted for each channel to determine whether N2 amplitudes were modulated by previous and present congruity, as predicted. A significant interaction between previous and present trial congruity was not observed for Cz or FCz, and the main effect of previous trial congruity was also not significant at both channels. The main effect of present trial congruity was significant at Cz, F(1, 126) = 27.55, p < .001, and FCz, F(1, 126) = 11.52, p = .002, as would be expected, given the results already reported above. Because N2 amplitudes were thought to reflect the degree of current trial conflict, the propensity for the N2 to be modulated by previous congruency was expected only for current INC trials but not for current CON trials that are not expected to engender conflict (cf. Freitas et al., 2009). Thus, we conducted an analysis focused on current INC trials to assess modulation by previous congruency. The mean amplitude for INC trials after INC trials was subtracted from the mean waveform for INC trials after CON trials. A one-sample t test against zero was significant for both Cz (M = −0.40, SD = 1.48 μV), t(42) =−1.78, p = .041, and FCz (M = −0.45 SD = 1.61 μV), t(42) = −1.83, p = .037, demonstrating that there was a significant modulation of current trial N2 amplitude by previous congruency. As confirmation, there were no such findings for analogous analyses for current CON trials (see Figure 5).
Figure 5.
Stimulus-locked ERPs at FCz and Cz by congruency (congruent vs. incongruent) of the previous and present trial. The main effect of previous stimulus congruity was nonsignificant at both FCz and Cz, and there was no significant previous–present congruency interaction. However, the mean voltage of conINC –incINC difference waves was significantly less than zero at the latency of the N2 for both FCz and Cz, suggesting that the congruity of the previous stimulus does affect subsequent N2 amplitude.
To further probe the effects of previous and present trial congruity on N2 amplitude, we determined mean N2 amplitudes on the basis of single trial data according to the following eight conditions of interest: CON followed by CON (conCON), INCLO followed by CON (incloCON), INCMED followed by CON (incmedCON), INCHI followed by CON (inchiCON), CON followed by any INC (conINC), INCLO followed by any INC (incloINC), INCMED followed by any INC (incmedINC), and INCHI followed by any INC (inchiINC). This single trial determination was performed with the expectation that detection of any fine-grained parametric variations in conflict may require averages derived from such precise determinations of single trial data. Pairs that were interrupted by block transitions or data exclusion were omitted, and only those pairs for which responses were correct on both the previous and the present trial were included in the analysis. N2 amplitude was determined for the second trial in each eligible pair. Single trial N2 amplitude was defined as the average voltage in a 40-msec window, centered at the average N2 latency for that participant. Single trial N2 amplitudes were subsequently averaged together for each of the eight conditions described above. Because not all subjects exhibited the expected effects of previous congruency, a subset of subjects (n = 22) that had larger N2 amplitudes for the conINC condition than for the inchiINC condition were selected for the group-level analysis (this selection criterion would be a necessary but not sufficient condition for the pattern of parametric variation we sought to test). Using this criterion to define a between-subjects factor (Conflict adaptation present vs. absent), together with within-subjects factors previous congruency and current congruency, a significant three-way interaction emerged for both Cz, F(3, 39) = 5.03, p < .005, and FCz, F(3, 39) = 10.59, p < .001. Given such a differentiation between groups distinguished on the present versus absence of the conflict adaptation effect, only those subjects who showed the basic effect were further assessed to test explicitly for parametric dependency on previous congruency. A 4 (Previous Congruity: CON, INCLO, INCMED, INCHI) × 2 (Present Congruity: CON, INC) repeated measures ANOVA was conducted for each channel to determine whether previous trial congruity affected present trial N2 amplitudes. A main effect of present trial congruity was not observed, but the main effect of previous trial congruity was significant at both channels, F(3, 63) = 6.66, p = .001, and F(3, 63) = 2.89, p = .042, for Cz and FCz, respectively. As predicted, the interaction between previous and present congruity was significant at both Cz, F(3, 63) = 2.88, p = .043, and FCz, F(3, 63) = 9.60, p < .001, with polynomial trend analyses revealing a significant linear dependence of current INC trial amplitudes on previous trial congruity at both Cz, F(1, 87) = 7.59, p = .007, and FCz, F(1, 87) = 10.42, p = .002. Thus, greater incongruity of the previous trial was associated with lower N2 amplitude on the current INC trial in keeping with predicted control adaptation effects. As expected, no significant linear or quadratic trends were observed for current CON trials for which conflict levels were expected to be too low to be sensitive to control-related modulations (see Figure 6).
Figure 6.
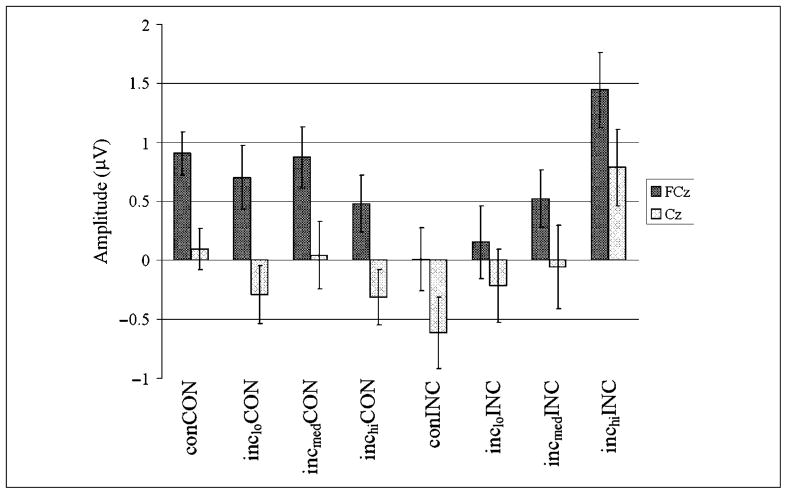
Mean N2 amplitude by stimulus congruity condition (CON, INCLO, INCMED, INCHI) of the previous trial and congruency (CON vs. INC) of the present trial for a subset of 22 subjects. No significant difference in N2 amplitude by previous stimulus congruity was found for current congruent trials, as predicted. For current incongruent trials, however, N2 amplitude was found to decrease with previous stimulus incongruity at both FCz and Cz. N2 amplitudes were significantly less negative after INCHI stimuli than any other condition at both FCz and Cz were significantly less negative after INCMED than con stimuli at FCz. These findings suggest that the level of conflict signaled on the previous trial predicts control recruitment that, in turn, determines subsequent N2 amplitude.
Correlation Analysis
We have suggested that modulation of control in response to conflict detection explains the effects of previous trial congruity on subsequent performance and N2 amplitudes. On this account, the magnitude of performance adjustments and control-related changes in N2 amplitude should be correlated across subjects. For each participant, mean RT for inchiINC, incmedINC, and incloINC was subtracted from the mean RT for the conINC condition. Differences in N2 amplitude between the inchiINC, incmedINC, and incloINC conditions and the conINC condition were like-wise calculated for both Cz and FCz. For each level of previous trial congruity, across-subject correlations were then determined for these difference measures. Control modulation is expected to result in more negative conINC N2 values, more positive incINC N2 values, slower conINC RTs, and faster incINC RTs. As such, a larger shift in control should result in a more negative conINC – incINC difference for N2 and a more positive conINC – incINC difference for RT, thus resulting in a negative correlation. Correlations observed for differences between conINC and incmedINC and between conINC and incloINC were all negative but did not reach significance at either channel (see Figure 7). Negative correlations were observed between differences in RT and N2 amplitude derived from the conINC and inchiINC conditions at both Cz, r(43) = −0.310, p = .043, and FCz, r(43) = −0.323, p = .035. Although both correlations were significant at the α = .05 level, they failed to meet the Bonferroni-corrected threshold (α = .017).
Figure 7.
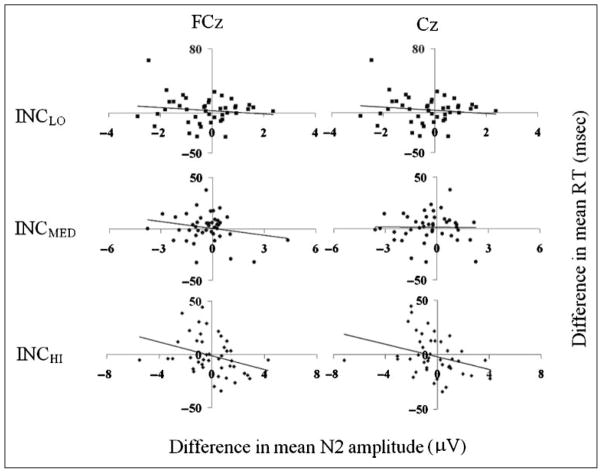
Across-subject correlations between the difference in RT observed for incongruent trials preceded by incongruent trials and those preceded by congruent trials (incINC – conINC) and corresponding differences in N2 amplitude for each incongruity condition. Control recruitment should result in decreased incINC RT, increased conINC RT, increased (less negative) incINC N2 amplitude, and decreased (more negative) conINC N2 amplitudes, so a negative correlation was expected. Negative correlations were observed for each level of previous stimulus incongruity and were significant for measures after the INCHI condition.
DISCUSSION
The results of the present study provide evidence consistent with the idea that the N2 indexes the conflict signal and that this signal guides subsequent modulation of control. Behavioral findings of increased RTs and ERs with greater target–distracter incompatibility suggested that our manipulation of response conflict worked as predicted. Also as predicted, N2 amplitudes followed the same pattern, increasing in negativity with increases in stimulus incongruity. Consistent with the proposed role of conflict detection in the recruitment of control resources, performance adjustments were found to increase, and incongruent N2 amplitudes were found to decrease with increasing incongruity of the previous trial’s stimulus, findings that provide additional support for such control modulations and indicate such adjustments are sensitive to relatively fine variations in the degree of conflict. Correlation analysis results were suggestive of associations between N2 modulation and behavioral adjustments but require further testing and replication. Together, these results are consistent with the idea that parametric variations in the degree of conflict can be indexed by both performance adjustments and associated covariations in N2 amplitude and that these measures track monotonically with parametric modulations in control processes.
Through the use of a parametric task design, the results of this study have demonstrated the sensitivity of conflict detection and control adjustment mechanisms within the conflict-control feedback loop to fine manipulations of the level of conflict. Although a similar study by Iwaki et al. (2003) also reported parametric manipulation of the N2 in response to flanker stimuli of varying target–distracter compatibility, the present investigation is the first EEG study to use this approach to examine sequential adjustment effects with regard to this manipulation, thus enabling a detailed examination of the dynamic modulation of cognitive control as indexed by behavioral performance and the N2 component. Two fMRI studies have examined sequential effects through parametric manipulations of either stimulus priming (Jones et al., 2002) or congruency (Durston et al., 2003) in recent trial history. In the current study, variations in the level of conflict were elicited more directly through manipulating the degree of incongruity in the stimulus itself. Even so, the sensitivity of the N2 and the associated behavioral measures to such subtle manipulations were analogous to the modulations in ACC-based signal in the fMRI studies, consistent with the idea that both the N2 and the fMRI ACC response are indexing a common neural signal that tracks conflict and that this signal is critical for the recruitment of top–down control.
Although the current study was motivated by the predictions of the conflict hypothesis, with the findings being consistent with this interpretive framework, there are several alternative accounts of ACC function that must also be considered. One such account is that of selection for action or response selection. This view contends that ACC coordinates the selection of perceptual information for goal-directed behavior (Turken, 1999; Posner & Dehaene, 1994; Pardo, Fox, & Raichle, 1991; Posner, Petersen, Fox, & Raichle, 1988). Selection for action is more difficult in the presence of task-irrelevant information, so demands associated with this process will generally increase with increasing levels of response conflict. At first glance, adjudicating between selection-for-action and conflict-monitoring processes may be challenging as both would be expected to increase with increasing stimulus incongruity. One approach to this question is outlined by Botvinick et al. (1999), in which the authors suggest that modulations in control after conflict detection are expected to reduce response conflict on subsequent incongruent trials while maintaining high selection-for-action demands, consistent with their findings in a fMRI study of the flanker task. Other studies also provide evidence that may be more consistent with a conflict-monitoring account for ACC, with the DLPFC being associated with control processes. MacDonald et al. (2000) used a version of the Stroop task in which a cue on each trial indicated the task Read Word (low control) versus Name Color (high control). The DLPFC (but not ACC) activation was predictive of subsequent decreases in congruency effects, and ACC (but not DLPFC) was sensitive to conflict during responses to the Stroop stimuli, consistent with the DLPFC being the locus of control and ACC playing an evaluative role. Also consistent with the DLPFC locus for control is a study reporting the effects of previous trial congruency on the Simon effect being eliminated by administering TMS to the DLPFC 500 to 300 msec before the next stimulus (Stürmer & Leuthold, 2003). Thus, the findings of these studies are consistent with the idea that the DLPFC is responsible for control and that ACC tracks conflict rather than mediating selection for action.
Another critical prediction of the selection-for-action view is that the N2 latency would also be modulated by selection-for-action demands (Gajewski, Stoerig, & Falkenstein, 2008). Indeed, if the N2 reflects a response selection process, its latency would be expected to be predictive of the timing of subsequent motor output. In contrast, a study by Yeung, Cohen, and Botvinick (2004) found that N2 latencies were relatively stable in the RT range examined in the current study (average RTs across conditions ranged from 300 to 350 msec; Yeung et al., 2004, found only a 1-msec difference in N2 latency between RT bins 300–350 and 400–450 msec), with the interval between the N2 peak and the RT tracking the degree of conflict (Yeung et al., 2004). Although N2 amplitude was clearly modulated by conflict in the present study, no appreciable latency differences were observed across task conditions, weighing against a selection-for-action account of the results. The lack of any systematic variations in N2 latency seems to be more consistent with a conflict-monitoring process that is not tied directly to response production. The response mapping in the current study did not allow the measurement of LRPs, which are scalp potentials that can index the degree of commitment of response in paradigms that use left- versus right-handed responses (Beste, Saft, Andrich, Gold, & Falkenstein, 2008), thus precluding a closer examination of the relationship between parametric variations in conflict and RTs. However, a recent study by Freitas et al. (2009) did examine N2 and LRP amplitudes during a flanker paradigm, measuring stimulus-locked LRPs as a measure of the time to complete stimulus evaluation before response generation. Consistent with our findings, N2 amplitude on incongruent trials was modulated by previous congruency. However, LRPs were not similarly modulated with the interpretation that the N2 conflict adaptation effects reflected control processes operating at stages later than stimulus evaluation, suggesting that the N2 may be more sensitive to conflict at the response rather than stimulus level in this paradigm. However, given that the LRP is a relative measure of bias toward the correct versus incorrect response, further clarity regarding stimulus versus response processing and how variations in control state contribute to N2 modulations may benefit methods that can evaluate each response activation independently. Interestingly, Larson, Kaufman, and Perlstein (2009) also found in an ERP study of the Stroop study a dissociation between the earlier N450 that showed modulation by current trial congruency but not conflict adaptation effects, whereas the later conflict slow potential showed both effects. There were a number of possibilities raised concerning why the N450 failed to show the conflict adaptation effect, but one was that the N450 might reflect ACC activity arising early after stimulus presentation whereas the conflict slow potential reflected a signaling for or implementation of controlled processing. Thus, one possibility is that earlier components may index stimulus-related processing conflict, whereas later components may be sensitive to response-related conflict that in turn is subject modulation by trial context.
Another account of ACC function, the error likelihood hypothesis, proposes that ACC is sensitive to adverse outcomes and stimuli, signaling their likely occurrence (Brown & Braver, 2005). As with selection for action, testing the error likelihood hypothesis against the conflict-monitoring hypothesis is often complicated by the positive relationship between error likelihood and response conflict. Indeed, as ERs were found to rise with increasing stimulus incongruity, error likelihood and response conflict might be expected to correlate within the context of the present task. Although the present study was not designed to explicitly adjudicate between the conflict hypothesis and the error likelihood hypothesis, one observation may be worth noting. Because flankers preceded target presentation by 100 msec, flanker information could potentially be used in the formation of error likelihood estimates. If this were the case, given that CON trials were presented 70% of the time and INCHI 10% of the time and that both CON and INCHI flankers would appear identical, such a homogeneous flanker array would be predictive of low error likelihood and, more importantly, predictive of equivalent error likelihoods for both the CON and the INCHI conditions, a prediction that would be at odds with our findings. Naturally, this line of reasoning is hardly conclusive given that a temporal window of 100 msec may be inadequate for accumulating sufficient information to produce an error likelihood estimate. Future studies may manipulate flanker-target onset asynchrony and the relative frequency of stimulus conditions to more thoroughly test these predictions.
It has also been proposed that ACC is more broadly involved in reward-based decision making (Holroyd & Coles, 2002). Expanding upon the error likelihood hypothesis, this account suggests that ACC accumulates information regarding past behavioral outcomes and uses this information to guide subsequent behavior on the basis of cost–benefit analysis (Brown & Braver, 2007). Recent work by Botvinick (2007) has sought to reconcile this account with conflict-monitoring by suggesting that conflict may also register with ACC as an aversive outcome. Botvinick suggests that ACC drives a type of avoidance learning that directs future behavior away from tasks and strategies that result in conflict and other adverse events, resulting in optimization of processing efficiency and reward acquisition. Although this new view unites a disparate body of findings into a parsimonious account of ACC function, many questions still remain. Foremost among them, upon consideration of the present results, is whether conflict also results in modulation of control, as originally proposed by the conflict hypothesis. The results of the present study appear to suggest that this is the case. Although ACC-mediated avoidance learning may, indeed, contribute to shifts in strategy (presumably between “attend flankers” and “ignore flankers”) during the course of the task, it seems unlikely that such shifts could explain the pattern of parametrically varied control recruitment we report here. To fully understand the role of ACC in performance monitoring and decision making, it will be important to determine whether unique control consequences arise from conflict detection and/or other adverse events that result in ACC activation.
In addition to alternative accounts of ACC function, there are also a number of considerations that determine the extent of cognitive control mechanisms on trial-to-trial effects or provide alternative accounts of such sequential effects. For instance, control modulation of conflict can diminish with time (Mayr & Awh, 2008), and target-to-distractor repetitions (Mayr & Awh, 2008) or stimulus priming (Mayr et al., 2003) can masquerade as conflict adaptation effects. Conversely, distractor-to-target repetitions can cause negative priming and thus obscure conflict adaptation, although in practice these potential effects appear inconsequential (Larson et al., 2009; Mayr & Awh, 2008). When stimuli have multiple, competing dimensions or features (e.g., Stroop task with color and word dimensions), additional mechanisms can also lead to trial-to-trial effects including task-set level priming (Allport, Styles, & Hsieh, 1994) and backward inhibition (Schuch & Koch, 2003; Mayr & Keele, 2000). Given the diversity of mechanisms that can potentially give rise to trial-to-trial effects, any claims regarding unique contributions of control-mediated effects must clearly be distinguished from a number of possible alternative accounts (Mayr et al., 2003; Mayr & Keele, 2000).
The present study suggests several avenues for future research that would clarify and extend our findings as well as address some of the limitations of our study. First, although the behavioral trial-to-trial effects showed clear parametric effects, the parametric nature of the ERP findings was much less robust, showing only in about half the subjects. Similarly, the correlations between the N2 and the associated modulation of subsequent RT were apparent only at the highest conflict level, likely because the N2 amplitudes and associated variance were sufficiently high to be sensitive such associations (these correlations did not survive Bonferroni correction due to similar comparisons for the lower conflict levels, suggesting future analyses could focus a priori on conditions likely to have sufficient variance to detect such relationships). Future studies could explore ERP indices that may be more sensitive to conflict (e.g., principle or independent components analysis), perhaps leading to more robust parametric variation of N2-like components by conflict or correlations that evidence relationships between N2 amplitudes and subsequent RT modulations. Another avenue for further study could investigate a claim of the conflict hypothesis that contends that the error-related negativity (ERN), another ERP that has been consistently source localized to ACC, is functionally equivalent to the N2, reflecting the same conflict detection signal (Yeung et al., 2004; van Veen & Carter, 2002a). If this is true, the ERN should also be sensitive to the parametric manipulation of conflict instituted in the present study. According to the conflict hypothesis, most errors occur when a response is prematurely initiated before the external stimulus has been sufficiently processed. Upon error production, continued processing of the stimulus results in activation of the correct response. This stimulus-driven activation of the correct response conflicts with the residual activation of the error response, leading to response conflict that is proportional to the strength of correct response activation. In effect, conflict is expected to increase with the proportion of task-relevant input present in the stimulus, a prediction directly opposite that put forth with regard to preresponse conflict registered by the N2. Scheffers and Coles (2000) did report such findings, with congruent errors showing greater ERN amplitudes versus those for incongruent errors. Within the context of the present study, we would thus predict that ERN amplitudes would be most negative for the CON condition followed by INCLO, INCMED, and INCHI, respectively (cf. Danielmeier et al., 2009). Unfortunately, ERs were insufficient for condition-specific ERN analysis within the current data set so this issue remains as a question for further study. An fMRI replication of the present study would also be valuable given our inability to directly measure DLPFC activation as an index of control recruitment. Although a number of previous studies have delineated the relationship between ACC and DLPFC activations in the context of performance adjustments (Kerns, 2006; Kerns et al., 2004), a direct test of this relationship using a parametric task design would further strengthen the case for the existence and fine sensitivity of such dynamic interactions in the service of regulating cognitive control mechanisms.
Acknowledgments
This work was supported by NIMH grants MH073955 awarded to R. Y. C. and MH047073 awarded to J. D. C. and a NARSAD Young Investigator Award to R. Y. C. Portions of these data were presented at the Society for Psychophysiological Research 2008 Annual Meeting, Austin, TX.
Footnotes
There were both practical and theoretical grounds for collapsing across the current INC types. First, because the proportions of trials for each INC type were purposefully set to be small (10%) to augment the effects of incongruency on trial-to-trial adjustments, the grouping of INC types for the current trial was necessary to have sufficient numbers of trials to analyze. Such grouping was thought to be justified because, by hypothesis, for any given current trial INC type, the degree of performance enhancement induced by a previous INC trial would be proportional to the degree of its incongruency, and any such parametric variations by previous INC should be preserved when averaging across the current INC trial types.
References
- Allport DA, Styles E, Hsieh S. Switching intentional set: Exploring the dynamic control of tasks. In: Umilta C, Moscovitch M, editors. Attention and Performance XV: Conscious and nonconscious information processing. Cambridge, MA: MIT Press; 1994. pp. 421–452. [Google Scholar]
- Badre D, Wagner AD. Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–487. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- Beste C, Saft C, Andrich J, Gold R, Falkenstein M. Stimulus-response compatibility in Huntington’s disease: A cognitive-neurophysiological analysis. Journal of Neurophysiology. 2008;99:1213–1223. doi: 10.1152/jn.01152.2007. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: Reconciling two perspectives on anterior cingulate function. Cognitive, Affective & Behavioral Neuroscience. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver TS, Yeung N, Ullsperger M, Carter CS, Cohen JD. The conflict monitoring hypothesis: Computational and empirical investigations. In: Posner MI, editor. Cognitive neuroscience of attention. New York: Guilford Publications; 2004. pp. 91–102. [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brown J, Braver T. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Brown J, Braver T. Risk prediction and aversion by anterior cingulate cortex. Cognitive, Affective & Behavioral Neuroscience. 2007;7:266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Cho RY, Orr JM, Cohen JD, Carter CS. Generalized signaling for control. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:1161–1177. doi: 10.1037/a0014491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, Wessel JR, Steinhauser M, Ullsperger M. Modulation of the error-related negativity by response conflict. Psychophysiology. 2009;46:1288–1298. doi: 10.1111/j.1469-8986.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Improved rejection of artifacts from EEG data using high-order statistics and independent component analysis. Neuroimage. 2007;34:1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, et al. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. Neuroimage. 2003;20:2135–2141. doi: 10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 2005a;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage. 2005b;24:539–547. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas AL, Banai R, Clark SL. When cognitive control is calibrated: Event-related potential correlates of adapting to information-processing conflict despite erroneous response preparation. Psychophysiology. 2009;46:1–8. doi: 10.1111/j.1469-8986.2009.00864.x. [DOI] [PubMed] [Google Scholar]
- Gajewski P, Stoerig P, Falkenstein M. ERP-correlates of response selection in a response conflict paradigm. Brain Research. 2008;1189:127–134. doi: 10.1016/j.brainres.2007.10.076. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. Optimizing the use of information: Strategic control of activation and responses. Journal of Experimental Psychology: General. 1992;4:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002 October;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Hommel B, Proctor RW, Vu KPL. A feature-integration account of sequential effects in the Simon task. Psychological Research. 2004;68:1–17. doi: 10.1007/s00426-003-0132-y. [DOI] [PubMed] [Google Scholar]
- Iwaki N, Miyatani M, Toshima T. A psychophysiological study on the function of the response-stop in the Eriksen task. Japanese Psychological Research. 2003;45:100–108. [Google Scholar]
- Jones AD, Cho RY, Nystrom LE, Cohen JD, Braver TS. A computational model of anterior cingulate function in speeded response tasks: Effects of frequency, sequence, and conflict. Cognitive, Affective & Behavioral Neuroscience. 2002;2:300–317. doi: 10.3758/cabn.2.4.300. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Anterior cingulate and prefrontal cortex activity in an FMRI study of trial-to-trial adjustments on the Simon task. Neuroimage. 2006;33:399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kopp B, Mattler U, Goertz R, Rist F. N2, P3 and the lateralized readiness potential in a nogo task involving selective response priming. Electroencephalography and Clinical Neurophysiology. 1996;99:19–27. doi: 10.1016/0921-884x(96)95617-9. [DOI] [PubMed] [Google Scholar]
- Kunde W, Wühr P. Sequential modulations of correspondence effects across spatial dimensions and tasks. Memory & Cognition. 2006;34:356–367. doi: 10.3758/bf03193413. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kaufman DA, Perlstein WM. Neural time course of conflict adaptation effects on the Stroop task. Neuropsychologia. 2009;47:663–670. doi: 10.1016/j.neuropsychologia.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Liotti M, Woldorff MG, Perez R, III, Mayberg HS. An ERP study of the temporal course of the Stroop color–word interference effect. Neuropsychologia. 2000;38:701–711. doi: 10.1016/s0028-3932(99)00106-2. [DOI] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare T, Davidson M, Casey B. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50:643–653. doi: 10.1016/j.neuron.2006.04.015. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E. The elusive link between conflict and conflict adaptation. Psychological Research. 2008;73:794–802. doi: 10.1007/s00426-008-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nature Neuroscience. 2003;6:450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- Mayr U, Keele SW. Changing internal constraints on action: The role of backward inhibition. Journal of Experimental Psychology: General. 2000;129:4–26. doi: 10.1037//0096-3445.129.1.4. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox P, Raichle ME. Localization of a system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends in Neurosciences. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, Fox PT, Raichle ME. Localization of operations in the human brain. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. Errors and error correction in choice reaction time tasks. Journal of Experimental Psychology. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG. Performance monitoring in a confusing world: Error-related brain activity, judgments of response accuracy, and types of errors. Journal of Experimental Psychology: Human Perception & Performance. 2000;26:141–151. doi: 10.1037//0096-1523.26.1.141. [DOI] [PubMed] [Google Scholar]
- Schuch S, Koch I. The role of response selection for inhibition of task sets in task shifting. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:92–105. doi: 10.1037//0096-1523.29.1.92. [DOI] [PubMed] [Google Scholar]
- Stürmer B, Leuthold H. Control over response priming in visuomotor processing: A lateralized event-related potential study. Experimental Brain Research. 2003;153:35–44. doi: 10.1007/s00221-003-1579-1. [DOI] [PubMed] [Google Scholar]
- Stürmer B, Leuthold H, Soetens E, Schroter H, Sommer W. Control over location-based response activation in the Simon task: Behavioral and electrophysiological evidence. Journal of Experimental Psychology: Human Perception & Performance. 2002;28:1345–1363. doi: 10.1037//0096-1523.28.6.1345. [DOI] [PubMed] [Google Scholar]
- Turken ASD. Response selection in the human anterior cingulate cortex. Nature Neuroscience. 1999;2:920–924. doi: 10.1038/13224. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Bylsma LM, Botvinick MM. The conflict adaptation effect: It’s not just priming. Cognitive, Affective & Behavioral Neuroscience. 2005;5:467–472. doi: 10.3758/cabn.5.4.467. [DOI] [PubMed] [Google Scholar]
- van Veen V. Unpublished doctoral dissertation. University of Pittsburgh; Pittsburgh: 2006. A neuroimaging approach to the relationship between attention and speed-accuracy tradeoff. [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior. 2002a;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002b;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Notebaert W, Liefooghe B, Vandierendonck A. Stimulus- and response-conflict-induced cognitive control in the flanker task. Psychonomic Bulletin & Review. 2006;13:328–333. doi: 10.3758/bf03193852. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cerebral Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Wendt M, Heldmann M, Munte TF, Kluwe RH. Disentangling sequential effects of stimulus- and response-related conflict and stimulus-response repetition using brain potentials. Journal of Cognitive Neuroscience. 2007;19:1104–1112. doi: 10.1162/jocn.2007.19.7.1104. [DOI] [PubMed] [Google Scholar]
- Wühr P. Evidence for gating of direct response activation in the Simon task. Psychonomic Bulletin & Review. 2005;12:282–288. doi: 10.3758/bf03196373. [DOI] [PubMed] [Google Scholar]
- Yeung N, Cohen JD. The impact of cognitive deficits on conflict monitoring. Predictable dissociations between the error-related negativity and N2. Psychological Science. 2006;17:164–171. doi: 10.1111/j.1467-9280.2006.01680.x. [DOI] [PubMed] [Google Scholar]
- Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]