Abstract
Infertility is one of the most prevalent public health problems facing young adult males in today’s society. A clear, treatable cause of infertility cannot be determined in a large number of these patients, and a growing body of evidence suggests that infertility in many of these men may be due to genetic causes. Studies utilizing animal models, and most importantly, mouse knockout technology, have been integral not only for the study of normal spermatogenesis but also for identifying proteins essential for this process, which in turn are candidate genes for causing human male infertility. Successful spermatogenesis depends on a delicate balance of local signaling factors, and this review focuses specifically on the genes that encode these factors. Normal functioning of all testicular cell types is not only essential for normal fertility but, as recently hypothesized, may also be crucial to prevent germ cell oncogenesis. Analysis of these processes using mouse models in vivo has provided investigators with an invaluable tool to effectively translate basic science research to the research of human disease and infertility.
Keywords: male infertility, Sertoli cell, spermatogenesis, knockout mouse, testis signaling
Mouse Models of Spermatogenesis
Animal models have been indispensable to the study of a number of biological processes, and fertility research is no exception. Histological analysis in 1871 allowed Viktor von Ebner to first describe the cycle of spermatogenesis in the rat (Ref. 1, 2). In 1878, the studies of Enrico Sertoli provided detailed evidence of the origin of the cell types in the testis, correctly distinguishing the types of maturing germ cells – spermatogonia, spermatocytes, and spermatids – and also correctly identifying their support cell, which von Ebner later termed the “Sertoli cell” (Ref. 1, 2) (See Figure 1 for an overview of spermatogenesis (Ref. 3)). For many years thereafter, understanding of the mechanisms of spermatogenic regulation was limited, and research was heavily focused on proteins that were affected by a few naturally occurring genetic mutations. However, knowledge of the molecular basis of infertility exponentially increased with the advent of mouse gene knockout technology. Since up to 30% of male infertility cases are likely due to genetic causes, mouse models of infertility may be instrumental in directing treatment of these patients (Ref. 4). This review will focus on spermatogenesis with an emphasis on how the local signaling environment regulates germ cells and how mouse models of infertility have enriched our knowledge of testis development which may directly relate to the genetic causes of human male infertility.
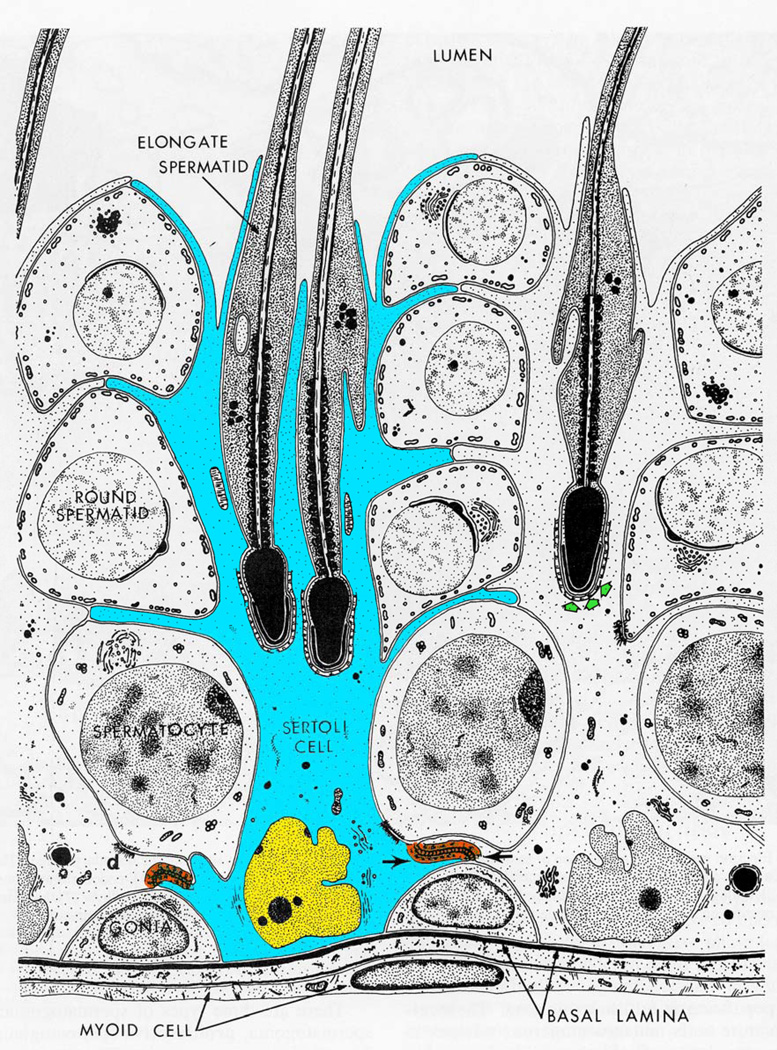
Figure 1.
An overview of spermatogenesis. The seminiferous epithelium is supported by the Sertoli cell (blue). As germ cells mature, they progress from the basal compartment near the basal lamina (basement membrane) of the seminiferous tubule to the adluminal compartment in the center of the tubule. Mitotic spermatogonia (gonia) differentiate into preleptotene spermatocytes and enter meiosis. It is at this time of maturation that the germ cells transit the blood-testis barrier (orange) and enter the adluminal compartment. After a prolonged period in meiotic prophase, spermatocytes (4N) undergo meiotic divisions to form round spermatids (1N). Round spermatids undergo extensive morphological remodeling to differentiate into elongate spermatids which are subsequently released into the tubular lumen. As germ cells progress from mitotic spermatogonia to post-meiotic mature elongating spermatids, they depend on continuous signaling and support from Sertoli cells. Figure adapted from (3).
Spermatogonial Regulation
Spermatogonia are the progenitor population of male germ cells. Unlike the ovary, which has a fixed pool of oocytes for the lifetime of an organism, testicular germ line stem cells continually proliferate. Similar to other stem cell types, the most important function of spermatogonial stem cells is to balance self-renewal with differentiation. If there is not adequate differentiation, sperm counts will suffer. On the other hand, if there is not adequate self-renewal, the pool of germ cells will progressively become exhausted. This balance is dependent on both extrinsic and intrinsic factors. The extrinsic factors are derived from the spermatogonial stem cell niche, an environment that is propagated by signaling factors from the vasculature (Ref. 5) and also those originating more locally in adjacent peritubular myoid cells, Leydig cells, and, most importantly, Sertoli cells (Ref. 6). A number of factors are important for the niche including glial cell line-derived neurotrophic factor (GDNF), kit ligand (KITL), activin A, and bone morphogenic protein 4 (BMP4), to name a few (Ref. 7). In vitro and in vivo studies have shown the impact of these signaling factors on intrinsic spermatogonial stem cell factors and spermatogonial self-renewal versus differentiation (Figure 2).
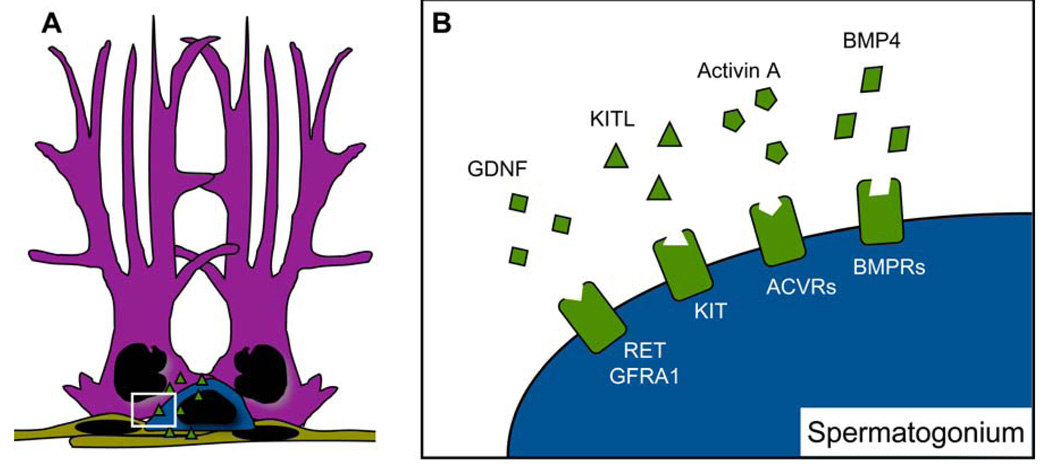
Figure 2.
The spermatogonial stem cell niche. Sertoli cells (purple), peritubular myoid cells (tan), and Leydig cells (not shown) all secrete signaling factors (green triangles) that form the spermatogonial stem cell environment, or niche. The basal lamina (not shown) is a shared product of Sertoli and peritubular myoid cells that regulates integrins and harbors growth factors to facilitate regulation of the stem cell niche (A). Important signaling proteins of the stem cell niche include glial cell line-derived neurotrophic factor (GDNF), kit ligand (KITL), activin A, and bone morphogenic protein 4 (BMP4). GDNF signals through RET and GDNF family receptor A1 (GFRA1) to promote self-renewal, and KITL, activin A, and BMP4 signal through KIT receptor (KIT), activin receptors (ACVRs), and BMP receptors (BMPRs), respectively, to promote differentiation (B). Reviewed in (6).
Glial Cell Line-Derived Neurotrophic Factor
GDNF, which was first discovered as a pro-survival factor for midbrain dopaminergic neurons, is a distant member of the transforming growth family β (TGFβ) superfamily that is expressed and secreted by Sertoli cells (Ref. 8, 9, 10). A subset of spermatogonia express the major GDNF receptors, RET (ret proto-oncogene) and GFRA1 (GDNF family receptor alpha 1) (Ref. 8, 10, 11), and this led to interest in investigating the roles of GDNF signaling in spermatogonial stem cells in vitro. It was found that not only did GDNF enhance stem cell maintenance (Ref. 12), but it was also found to be essential for the maintenance of stem cell activity in long term cultures (Ref. 13, 14). These results led to interest in GDNF receptors as markers of “stemness” in spermatogonial stem cell cultures. However, studies utilizing GFRA1 antibodies in magnetic activated cell sorting procedures to enrich for GFRA1+ cells found that although a subset of spermatogonial stem cells did express this GDNF receptor, the overall stem cell pool was heterogeneous with respect to receptor expression (Ref. 15, 16). In contrast to those findings, siRNA-mediated knockdown of Gfra1 in mouse type A spermatogonial cultures led to suppression of proliferation and induction of markers of spermatogonial differentiation (Ref. 10). In addition, phosphorylation of tyrosine 1062 of RET, a known binding site for many of its downstream signaling components, was reduced in the absence of GFRA1 (Ref. 10) suggesting that RET may also play a pivotal role in the prevention of differentiation and the maintenance of self-renewal of this cell type.
Transgenic mouse models were critical in the discovery of the functional importance of GDNF to the spermatogonial stem cell niche and the translation of the in vitro findings to a live organism. Gdnf-heterozygous mutant mice were fertile but contained a number of seminiferous tubules with progressive depletion of spermatogonial pools, and conversely, transgenic mice that overexpressed GDNF accumulated masses of undifferentiated spermatogonia postnatally (Ref. 8). To circumvent the neonatal lethality of Gdnf-, Gfra1-, and Ret knockout mice, testes from these mice were transplanted into the back of castrated nude mice (Ref. 11). Consistent with signaling of GDNF through a receptor complex that incudes RET and GFRA1, the null testes phenocopied each other and exhibited impaired spermatogonial stem cell proliferation, decreased markers of spermatogonia stem cells, and increased markers of differentiation in the spermatogonial population (Ref. 11). Knockin mice have also been utilized to replace tyrosine 1062 of RET with a phenylalanine codon, rendering it incapable of phosphorylation. The testes from these mice showed marked atrophy, and RET-expressing spermatogonia became almost undetectable by 3 weeks of age, revealing in vivo that Y1062 of RET is essential for the self-renewal of spermatogonial stem cells (Ref. 17).
Kit ligand
Spontaneous mutations in the mouse Steel (Sl) locus encoding kit ligand (official gene symbol kitl, also known as stem cell factor) were the first clues in the identification of the importance of KITL in spermatogenesis (Ref. 18, 19). KITL is a signaling factor synthesized by Sertoli cells in the postnatal testis (Ref. 20) that binds and activates the KIT receptor (official gene symbol kit, also known as c-kit) located on differentiating spermatogonia and early spermatocytes (Ref. 21). Similar to KITL, the importance of KIT in spermatogenesis was well known due to spontaneous mutations in the white spotting (W) locus that encodes it (Ref. 18, 19). Early work utilizing one such mutant (Wv) led to the discovery that Wv-heterozygous mice that were made cryptorchid to eliminate all differentiating germs cells and leave only undifferentiated spermatogonia were never able to progress to differentiating spermatogonia after surgical reversal (Ref. 22). Injection of the ACK2 blocking antibody that prevents KIT activation led to reduced mitosis of differentiating spermatogonia but had no effect on the mitosis of undifferentiated spermatogonia or the meiosis of spermatocytes (Ref. 21). The effect on undifferentiated spermatogonia was further corroborated by later studies revealing that SSCs do not express KIT mRNA or protein (Ref. 23, 24). When wild-type spermatogonial stem cells were transplanted into KITL mutant mice, undifferentiated spermatogonia were able to proliferate, but the cells were never able to differentiate into more mature spermatogonia (Ref. 25). However, these same cells when re-transplanted to KIT mutant testes were able to differentiate and reestablish complete spermatogenesis (Ref. 25). Further proof of the importance of Sertoli cell expression of KITL and germ cell expression of KIT came when KITL mutant germ cells were transplanted into KIT mutant testes and were shown to successfully rescue the infertility in those mice (Ref. 26).
Mouse models have also been critical in discovering the relationship between intrinsic stem cell factors and KIT/KITL signaling. One such intrinsic protein is zinc finger and BTB domain containing 16 (ZBTB16 also known as Plzf or Zfp145), a DNA sequence-specific transcriptional factor. ZBTB16 is expressed in undifferentiated spermatogonia, and mice with mutations in the Zbtb16 locus have progressive infertility due to depletion of the spermatogonial stem cell pool (Ref. 27, 28). ZBTB16 directly represses the transcription of Kit, and undifferentiated spermatogonia from Zbtb16-null mice have a relative enrichment of KIT-positive cells, leading to the hypothesis that ZBTB16-mediated repression of Kit is at least partially responsible for its function in maintaining the undifferentiated state in spermatogonia (Ref. 29).
The previous studies emphasized the relationship between KIT/KITL signaling and spermatogonial differentiation. However, studies in Kit mutant mice indicated that KIT/KITL signaling is not necessary for the initial differentiation of juvenile undifferentiated spermatogonia into KIT-positive cells but is necessary for the maintenance and proliferation of those cells (Ref. 30). Adding to this finding is a recent study that discovered that KIT mutant testes contain spermatogonial stem cells that could be enriched in culture and reintroduced into recipient KIT mutant testes to successfully reestablish all stages of spermatogenesis (Ref. 31). This result casts doubt on the dogma that KIT/KITL signaling is an absolute requirement for spermatogonial differentiation. In another interesting study, it was shown that GDNF and fibroblast growth factor 2 can reprogram KIT-positive spermatogonia to dedifferentiate into spermatogonial stem cells, raising the possibility that “stemness” can be acquired by differentiating progenitor cells in the testis (Ref. 32). Although it may be quite complicated, the studies in the GDNF and KITL pathways support the hypothesis that there is a balance between self-renewal and differentiation of spermatogonial stem cells, and studies in humans support the hypothesis that alterations in this balance can lead to infertility and/or cancer. This topic will be discussed in greater length in a later section.
Meiotic Initiation
After undifferentiated spermatogonia commit to entering a differentiation pathway, these cells undergo a couple of mitotic divisions and then differentiate into primary spermatocytes that subsequently undergo meiotic division. Initiation of meiosis is dependent upon intrinsic as well as extrinsic signals, similar to what has been discussed about spermatogonial maintenance in the previous section. Also, similar to their role in maintaining the spermatogonial stem cell niche, Sertoli cells are critical in controlling the extrinsic signaling environment that influences meiotic initiation. Much of the work in this field has focused on the role of retinoic acid signaling and the regulation of the permissive meiotic environment (Ref. 33).
Extrinsic Retinoic Acid Signaling
Historically, it has been observed that vitamin A deficiency in rodents causes spermatogenic defects and complete loss of meiotic germ cells (Ref. 43). This was the first evidence that retinoids, the metabolic derivatives of vitamin A, are important to spermatogenesis. During embryogenesis, the mesonephros expresses a major enzyme of retinoic acid synthesis (ALDH1A2) and, consequently, is the main source of retinoic acid in the embryonic gonad (Ref. 37). Postnatally, Sertoli cell expression of ALDH1A1 may play a more prominent role in retinoic acid (RA) production as Aldh1a1-null testes have reduced expression of RA-sensitive genes at postnatal day 5 (Ref. 35, 44). Although there appears to be abundant levels of RA present throughout gonadal development, there are also prominent sex-specific differences in RA availability to the germ cells, the discovery of which helped to clarify the role of RA in meiotic initiation.
Meiosis begins embryonically in females and postnatally in males. A major retinoid-degrading enzyme – cytochrome P450, family 26, subfamily b, polypeptide 1 (CYP26B1) – is expressed sex-specifically in Sertoli cells of the male gonads (Ref. 37). Deletion of Cyp26b1 causes male germ cells to enter meiosis precociously at the same embryonic time point that female germ cells normally initiate meiosis (E13.5) (Ref. 37, 38), and CYP26B1-null embryonic testes have elevated RA levels (Ref. 38). Addition of a P450 antagonist to E12.5 gonadal cultures reduces the expression of spermatogonial stem cell markers and increases the expression of meiotic markers (Ref. 37), and meiosis is induced in gonadal cultures if they are treated with a synthetic retinoid that is resistant to CYP26B1-mediated metabolism (Ref. 38). Further cementing the importance of the role of RA in meiotic initiation is the observation that meiotic markers are suppressed in female gonadal cultures that have been treated with a synthetic antagonist of RA (Ref. 37).
Intrinsic Competency Factors
Stimulated by retinoic acid gene 8 (Stra8) is an RA-responsive gene that is expressed in premeiotic germ cells in both the male and female gonads (Ref. 41, 45, 46). RA treatment of cultured gonocytes, spermatogonia, or whole neonatal testes strongly induces Stra8 expression (Ref. 45). Stra8 knockout males show a block in spermatogenesis immediately preceding meiotic prophase and show no signs of meiotic recombination or chromosomal synapsis (Ref. 41). So, these results beg the question – since RA is quite abundant in many tissue types in development, why are germ cells the only cell type to respond to RA by initiating meiosis? Meiotic competence must not only depend on an extrinsic signal but also an intrinsically permissive environment.
Deleted in azoospermia-like (Dazl) is a germ cell-specific gene that is expressed in the embryonic male and female gonads (Ref. 47, 48). Dazl knockout female gonads have defective meiotic chromosome condensation and synapsis and severely suppressed endogenous Stra8 mRNA levels (Ref. 49). In contrast to fetal ovaries, wild-type fetal testes do not normally undergo meiotic initiation, but when cultured in the presence of RA, they respond by upregulating Stra8 mRNA (Ref. 49). However, culturing of Dazl-null male gonads with RA does not result in Stra8 mRNA upregulation (Ref. 49). Interestingly, germ cells in Stra8-null testes express an important protein for meiotic synapsis, synaptonemal complex protein 3 (SYCP3), but it is not loaded onto chromosomes as it normally is during proper meiosis (Ref. 41). DAZL directly binds to the 3’-untranslated region of Sycp3 mRNA and enhances its translation (Ref. 50), and Sycp3 is severely suppressed in Dazl-null germ cells (Ref. 49). Taken together, these results suggest that DAZL enables meiotic competence of germ cells by promoting SYCP3 expression and that RA-induced STRA8 promotes meiotic progression by enabling the proper localization of SYCP3.
Spermatozoan Maturation
As germ cells progress through the meiotic divisions of spermatogenesis, they migrate from the basal layer of the seminiferous tubule towards the adluminal compartment and are eventually released into the tubular lumen as mature spermatozoa. During this maturation process, Sertoli cells are responsible for a number of important functions including: 1) re-forming the blood-testis barrier as the germ cells transit from the basal to adluminal compartments, 2) providing proper signaling and nutrients, 3) secreting tubular fluid to maintain the seminiferous tubular lumen, and 4) remodeling the constantly changing junctions between Sertoli cells and maturing germ cells (Ref. 2, 3). Improper Sertoli cell functioning during spermatogenesis could cause a myriad of spermatozoan defects including misshaping of the sperm head, improper formation of the sperm tail leading to reduced fertility, or decreased sperm counts, just to name a few. Androgen signaling through the androgen receptor is a major signaling pathway that is crucial for proper Sertoli cell regulation and support of spermatogenesis (Ref. 51).
Androgen Receptor
In 1970, Lyon and Hawkes isolated the testicular feminization (Tfm) mouse mutant, an X-linked mutation that caused XY mice to develop external female genitalia, cryptorchidism, and a spermatogenic block at the spermatocyte to round spermatid transition (Ref. 52). Mapping of the Tfm mutation revealed a single base deletion in the amino-terminus of the gene encoding the androgen receptor (Ar) that results in a frameshift mutation and premature termination of AR at amino acid 412 (Ref. 53). Later studies showed that Tfm mutant mice had reduced Sertoli cell numbers at birth, but by puberty, Tfm mutant testes were no different from wild-type testes that were made cryptorchid surgically as shown by their similar histology and gene expression levels (Ref. 54). Knockout mice that were engineered to have a null allele of Ar phenocopy the Tfm mutant mice, but researchers were still left wondering if the spermatogenic defects in these mice were due to defects in androgen signaling or cryptorchidism (Ref. 51, 55).
Conditional knockout mice were developed using Cre-loxP technology to knock out the androgen receptor in specific cells or tissues. A number of different laboratories made Sertoli cell-specific knockouts of Ar (Ref. 56, 57, 58). None of these conditional knockout mice had cryptorchidism but did vary in the severity of the spermatogenic block, with some experiencing a block between the spermatocyte and spermatid stages (Ref. 56, 57) and another showing a block between the round spermatid and elongating spermatid stages (Ref. 58). Despite these differences, all of these Sertoli cell-specific knockouts of Ar were shown to have defects in both the permissive and instructive environment driving the later stages of spermatogenesis. The permissive environment of late spermatogenesis refers to the adluminal compartment that is protected by the blood-testis barrier. The integrity of the blood-testis barrier is essential to shield the developing germ cells from being influenced by signaling from any source besides Sertoli cells or other germ cells and also functions as an immunological barrier. Transcript levels of a variety of genes involved in tubular restructuring and blood-testis barrier maintenance, including proteases and protease inhibitors, cell adhesion molecules, and tight junction components, were affected in all of the aforementioned Sertoli cell-specific Ar knockout mouse models (Ref. 59, 60, 61). One functional assay indeed showed that Sertoli cell-specific Ar knockout mice experienced disruption of blood-testis barrier integrity (Ref. 62). The instructive environment was also affected as mRNAs for proteins involved in transport and metabolism were misregulated (Ref. 59, 61). Interestingly, one of the genes that was upregulated in the knockout was alcohol dehydrogenase 1, the rate limiting enzyme of retinol to retinoic acid conversion, which raised the possibility of cross talk between AR and RA pathways (Ref. 59).
Clinical Implications/Applications
Research using mouse models has produced a wealth of knowledge about the signaling pathways and intrinsic factors that are essential to spermatogenesis. Applying this knowledge to human disease is vitally important, and some steps have been taken in that direction (reviewed in (Ref. 4)). There are a number of chromosomal abnormalities and gene mutations, including Klinefelter’s syndrome and cystic fibrosis transmembrane conductance regulator mutations, respectively, that have been identified as related to male infertility, but the following section will focus instead on the pathways that we have discussed in previous sections. We will also discuss the relationship of these molecular pathways in the pathogenesis of testicular cancer and the relationship between male infertility and cancer.
Genetics of Male Infertility
It is estimated that 15% of male infertility patients suffer from chromosomal alterations and single gene mutations that may be the cause of disease (reviewed in (Ref. 64, 65)). One common chromosomal aberration found in patients with severe infertility is microdeletions in the long arm of the Y chromosome, which is estimated to cause 10–15% of azoospermia (absence of sperm in the ejaculate) and 5–10% of severe oligozoospermia (low sperm counts) (Ref. 64). This commonly deleted portion of the Y chromosome has been divided into 3 “azoospermia factor” regions: AZFa, AZFb, and AZFc. Deletions of AZFc are frequently found in patients with severe disease (3–7%), and this locus contains DAZ1–4, the Y chromosome paralogs of the autosomal gene, DAZL (Ref. 64). In one study that covered a decade of treating infertility, 71.4% of patients with an AZFc deletion had sperm in their testes that could be retrieved, and two-thirds of those cases resulted in pregnancy via assisted reproductive techniques (Ref. 66). In a new twist, new AZFc haplotypes termed “gr/gr” or b2/b3, which both result in loss of DAZ3 and DAZ4, have been identified as linked to infertility but have also been found in normozoospermic males as well (Ref. 64, 65). As suggested from knockout of Dazl in mice, which causes loss of germ cells and complete absence of gamete production (Ref. 39), DAZL in humans has been examined as a candidate for male infertility. One study identified 4 novel missense mutations in DAZL with one homozygous DAZL-null male patient presenting as infertile (Ref. 67). This group hypothesized that DAZL mutations may account for the variations in infertility found in patients with partial deletions in AZFc; however, they were not able to discern a link between autosomal DAZL function and severity of male infertility in the population they examined (Ref. 68).
Idiopathic infertility that is not related to chromosomal alterations may be due to single gene mutations or polymorphisms. One of the most widely appreciated and best-characterized genes found mutated in infertile men is the androgen receptor, estimated to be associated with infertility in 2–3% of all cases of azoospermia/oligozoospermia (Ref. 64). The AR gene can be mutated in a number of ways resulting in mild to complete androgen insensitivity. The cases of complete androgen insensitivity phenocopy the Tfm mice, in which the 46,XY patients present as phenotypic females with undeveloped gonads, whereas the mild forms present as phenotypic males with low sperm counts. In the phenotypic males with low sperm counts, studies have found that there is a significant relationship between AR mutations and infertility; however, no one has been able to match a specific AR polymorphism to disease severity (Ref. 69, 70). Polymorphisms that have been examined in the AR gene relate to two sites in exon 1 that exhibit variable stretches of CAG or GGC repeats (Ref. 64). In a mouse model of Kennedy disease (a neurodegenerative disorders caused by CAG expansion in the AR gene), 113 glutamine codons were knocked into exon 1 of Ar (Ref. 71). These mice experienced progressive infertility not as a result of loss of AR function but instead due to insoluble AR fractions created in Sertoli cell nuclei that led to cytoskeletal abnormalities and decreased support of germ cells (Ref. 71). When examining CAG polymorphisms in groups of infertile patients, different groups had different results, but overall, many studies found a relationship between CAG expansion and infertility (Ref. 72). Unlike in the mouse, it is unclear whether this low level of CAG expansion affects AR transactivation.
Germ Cell Tumors and Testicular Dysgenesis Syndrome
Type II testicular germ cell tumors (TGCTs), including seminomas and non-seminomas, arise from malignant primordial germ cells or gonocytes. They are the most prevalent type of male germ cell tumor and account for 1% of all cancers in male Caucasians and up to 60% of malignancies in those between 20 and 40 years-old (Ref. 73). Multiple studies have linked TGCT occurrence to subfertility, cryptorchidism, and genitourinary tract malformations (Ref. 73). This knowledge has led to the testicular dysgenesis syndrome (TDS) hypothesis (Ref. 74). According to the TDS hypothesis, TGCTs form embryonically when a localized disturbance in niche environment impairs the differentiation of fetal gonocytes. These gonocytes do not mature and remain in the testis as carcinoma in situ. Carcinoma in situ cells proliferate during the peripubertal period and, with the help of abnormal signaling, acquire genetic changes that progress to TGCT in the adult. Environmental factors and genetic mutations and polymorphisms in the pathways that were discussed in previous sections are all hypothesized to be vital to the development of TGCTs as they all affect the gonocyte niche.
Hormonal Disruption
A rat model of the early pathogenesis of TDS gave clues towards defining pathways that may be affected in human disease (Ref. 75). Dibutyl phthalate administration to gravid mothers induced cryptorchidism, hypospadias, infertility, and testis abnormalities in their male offspring, similar to those seen in human TDS (Ref. 75). Cryptorchid testes often showed areas of focal dysgenesis containing partially formed testicular cords with mislocalized Sertoli cells, Leydig cells, and gonocytes, and this focal dysgenesis might have been caused by suppressed testosterone in utero affecting peritubular myoid cell function (Ref. 75). Dysgenic and “Sertoli cell only” tubules contained Sertoli cells expressing markers of immaturity (Ref. 75). Since this model of early pathogenesis was very similar to human disease (Ref. 76), gene expression analysis in this system could lead to the discovery of important pathways in human disease. One such study found that phthalate esters, including dibutyl phthalate, affected a number of systems including genes necessary for proper steroid hormone synthesis (i.e., testosterone) and genes important for germ cell-Sertoli cell junctions and signaling (including KIT) (Ref. 77). In humans, there has not been a definitive study linking phthalate ester exposure to TDS, but one study found that high exposure to prenatal phthalates corresponded to decreased anogenital distance, penile width, and testicular descent in male infants – all signs of low prenatal testosterone (Ref. 78).
Non-Hormonal Factors
One major finding from the study assessing gene expression changes in the rat model of TDS was the decreased expression of KIT receptor in the dysgenic testes (Ref. 77). Conversely, Kitl-heterozygous mice have increased incidence of TGCTs (Ref. 79). Is it possible that KIT activating mutations are selected for in the Kitl-heterozygous mice and that the downregulation of KIT receptor may be the major reason why rat models of TDS using phthalate esters do not recapitulate the TGCTs seen in human disease? Activating mutations of KIT are often seen in TGCTs (Ref. 80, 81) and have been associated with bilateral tumor development (Ref. 82, 83). The use of KITL immunostaining has also been proposed as novel diagnostic marker for early malignant germ cells (Ref. 84). In a mouse model, mutation of the tyrosine codon of KIT that is necessary for phosphatidylinositol (PI) 3’-kinase activation led to infertility (Ref. 85). This was in direct contrast to one human study that found a KIT mutation that was associated with TGCT pathogenesis that also constitutively activated PI3 kinase in vitro (Ref. 81). Another in vitro study indicated that KIT activation of PI3 kinase upregulates cyclin D3 and promotes cell cycle progression, which would explain its dichotomous role in the aforementioned studies (Ref. 86).
One cohort study and one retrospective literature review found that even when excluding cryptorchidism as a confounding factor, there is still a significant positive association between subfertility and increased risk of testicular cancer (Ref. 87, 88). All of these studies that have determined genetic link for infertility and cancer raise the concern that better screening for genetic causes of infertility may be vital so that these patients can be referred for genetic counseling and possibly preimplantation genetic screening if they are attempting to conceive using assisted reproductive technologies (ART).
Research in Progress and Outstanding Research Questions
Although there has been a lot of outstanding research in both the clinical and basic science fields, more needs to be done to translate “wet lab” benchwork into bedside patient therapies. Testicular dysgenesis syndrome and embryonic stem (ES) cell technology are two fields that have both benefited from extensive basic science research.
Testicular Cancer
We previously outlined a couple of areas that have seen major progress with regards to translational research, but we also would like to highlight some areas that may need more attention. The first genetic study trying to link AR mutations/trinucleotide repeat expansions to testicular cancer saw an increased incidence of null mutations of AR and certain trinucleotide repeat polymorphisms in TGCT patients (Ref. 89), but not much has been done on this topic since that study. Another important deletion in infertility, the gr/gr haplotype (DAZ3/DAZ4), has been associated with an increased risk of developing TGCTs in one report (Ref. 90). Studying the functions of AR and DAZ orthologs in humans will be vital to determining the causes of TDS.
Although we have compelling mouse models for the role of KIT/KITL in infertility and compelling human data for the role of this signaling system in TGCTs, very little is known about KIT or KITL mutations in human infertility. We were only able to find two studies that looked at KIT in infertility (Ref. 86, 91), with only one of them finding a positive association (Ref. 86). A mouse model in which GDNF was overexpressed demonstrated an early phenotype of infertility but at later time points developed seminomatous tumors (Ref. 92). Although this model does not accurately recapitulate human disease, it is unclear if the GDNF pathway is important to human TDS, as we were not able to find any human studies examining this ligand or its receptors. Again, better understanding of the genetics of human male infertility with regards to the KITL and GDNF pathways may lead to better understanding of TDS. Research in this field is moving in the right direction with the establishment of the International Testicular Cancer Linkage Consortium (Ref. 93).
Promise of ES cell technology/SSC culturing
New studies using DAZ and its homologs are revolutionizing the field of ES cell technology and highlighting some of the differences between mouse and human ES cells. Overexpression of DAZL in mouse ES cells caused cells to differentiate into phenotypic male and female germ cells without any extrinsic signaling (Ref. 94). In a human ES cell study, on the other hand, researchers had to overexpress all DAZ homologs (DAZ1–4, DAZL, and BOULE) and supplemented the differentiation medium with multiple BMP ligands (BMP4, 7, and 8b) to make the human cells meiotically competent (Ref. 95). Interestingly, both culture systems seemed to also result in the appearance of Sertoli-like cells which raises the possibility that germ cells may also be influencing the development of Sertoli cells and not just the other way around or, alternatively, that the crosstalk between both cell types is necessary for their coordinated development. The surprisingly different requirements of mouse and human ES cells to achieve meiotic competence may just be a reflection of the innate differences between mouse and human ES cells, as mouse ES cells have many strong germ cell markers and may represent a germ cell-like lineage, unlike human ES cells that may be more like cells from the epiblast (Ref. 96). These studies also support the theories that DAZ homologs are the causative factor in infertility in patients with Y-chromosome microdeletions and that DAZ homologs are intrinsic meiotic competence factors that control germ cell identity.
Future Directions
Of the approximately 15% of all U.S. couples that experience infertility, one to two-thirds of these cases are due, at least in part, to male factor infertility (Ref. 97, 98). Scientific breakthroughs in ART have revolutionized the field of reproductive endocrinology and allowed many of these couples to conceive biological offspring. However, there are still many uncertainties regarding the long-term consequences of ART, especially in light of the genetic issues that we have outlined throughout. Treatments of severe male factor infertility are limited (Ref. 99, 100), and the need for more treatments is enormous especially for couples experiencing infertility due to male factor alone. Although the idea of gene therapy for the treatment of male infertility seems far-fetched (Ref. 101) and genetic treatment of male factor infertility may or may not reduce the monetary cost of ART, treatments of male infertility would mitigate the medical cost placed on the female patient who must endure hormonal injections ridden with side effects and improve the health of the fetus, since many of the long term consequences of reproductive technologies are still unknown (Ref. 102, 103, 104, 105).
Table 1.
Mouse knockouts of select meiotic genes
| Gene Symbol |
Gene Name | Reproductive Phenotype | Fertility Status |
Age of phenotype |
Ref. |
|---|---|---|---|---|---|
| Aldh1a1 | aldehyde dehydrogenase family 1, subfamily A1 | Testes have reduced expression of retinoic acid-sensitive genes (such as Stra8) | Fertile | P5 | (Ref. 34, 35) |
| Aldh1a2 | aldehyde dehydrogenase family 1, subfamily A2 | Knockout mice show severe morphological defects by embryonic day 9.5 | Lethal | N/A | (Ref. 36) |
| Cyp26b1 | cytochrome P450, family 26, subfamily b, polypeptide 1 | Precocious meiotic entry due to increased embryonic levels of gonadal retinoic acid; subsequent pachytene arrest and apoptosis | Infertile | E13.5 | (Ref. 37, 38) |
| Dazl | deleted in azoospermia-like | Reduced embryonic development and survival of XY germ cells, reduced expression of germ cell markers, increased germ cell apoptosis | Infertile | E14.5-15.5 | (Ref. 39, 40) |
| Stra8 | stimulated by retinoic acid gene 8 | Failure of meiotic prophase with loss of meiotic chromosome cohesion, synapsis, and recombination | Infertile | P10 | (Ref. 41) |
| Sycp3 | synaptonemal complex protein 3 | Defects in chromosome synapsis during meiosis, increased germ cell apoptosis at the zygotene stage | Infertile | P12 | (Ref. 42) |
N/A, not applicable; E, embryonic day; P, postnatal day
Table 2.
Select mouse mutants with defects in androgen receptor expression and/or function
| Mutation | Cre Promoter | Cell type affected |
Reproductive Phenotype | Fertility Status |
Ref. |
|---|---|---|---|---|---|
| Spontaneous (Tfm) | Not applicable | All | Feminized external genitalia, hypogonadal, cryptorchidism with a spermatogenesis block | Infertile | (Ref. 52, 53) |
| Flox (deleted exon 2) | β-Actin | All | Female-like appearance, hypogonadal, low serum testosterone, spermatogenic arrest at pachytene stage | Infertile | (Ref. 55) |
| Flox (deleted exon 2) | Anti-Müllerian Hormone (Guillou) | Sertoli cells | Normal testis descent and male urogenital tract, hypogonadal, block in meiotic progression with severely decreased spermatids | Infertile | (Ref. 56) |
| Flox (deleted exon 2) | Anti-Müllerian Hormone (Guillou) | Sertoli cells | Normal testis descent and male urogenital tract, spermatogenic arrest at the diplotene premeiotic stage, low serum testosterone | Infertile | (Ref. 57) |
| Flox (inverted exon 1) | Anti-Müllerian Hormone (Braun) | Sertoli Cells | Normal testis descent and male urogenital tract, unaffected meiotic progression, spermatogenic block from the late-round to elongating spermatid stage | Infertile | (Ref. 58) |
| Flox (deleted exon 3) | Anti-Müllerian Hormone (Guillou) | Sertoli cells | Normal testis descent and male urogenital tract, block in meiotic progression with severely decreased spermatids, normal serum testosterone | Infertile | (Ref. 63) |
Inverted exon 1, loss of start codon and deletion of entire protein; deleted exon 2, frameshift deletion of first zinc finger of the AR DNA binding domain and premature termination of the protein; deleted exon 3, in frame deletion of second zinc finger domain of the AR DNA binding domain
Acknowledgments
Acknowledgements and funding
Research in the Matzuk laboratory on male reproduction and testicular cancer has been supported by National Institutes of Health (U01HD060496, R01HD057880, P01HD036289, and R01CA60651). R.L.N. is a student in the Medical Scientist Training Program at Baylor College of Medicine and has been supported in part by the Edward J. and Josephine G. Hudson Scholar Fund. We would also like to thank our peer reviewers for their help with improving this review.
Footnotes
Further reading, resources, and contacts
American Society for Reproductive Medicine http://www.asrm.org/
Society for Assisted Reproductive Technology http://www.sart.org/
European Society of Human Reproduction and Embryology http://www.eshre.com/page.aspx/3
OMIM: Testicular tumors http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=273300/
International Male Contraception Coalition http://www.malecontraceptives.org/
Mammalian Reproductive Genetics http://mrg.genetics.washington.edu/
Papers with testicular expression profiling:
Schultz, N., Hamra, F.K. and Garbers, D.L. (2003) A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci U S A 100, 12201–12206
Shima, J.E. et al. (2004) The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol Reprod 71, 319–330
Chalmel, F. et al. (2007) The conserved transcriptome in human and rodent male gametogenesis. Proc Natl Acad Sci U S A 104, 8346–8351
References
- 1.Russell LD, Griswold MD. The Sertoli cell. 1st, eds. Clearwater, FL: Cache River Press; 1993. [Google Scholar]
- 2.Skinner MK, Griswold MD. Sertoli cell biology. Boston: Elsevier Academic Press, Amsterdam; 2005. [Google Scholar]
- 3.Russell LD. Histological and histopathological evaluation of the testis. 1st, eds. Clearwater, Fl: Cache River Press; 1990. [Google Scholar]
- 4.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 6.Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263–286. doi: 10.1146/annurev.cellbio.24.110707.175355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Rooij DG. The spermatogonial stem cell niche. Microsc Res Tech. 2009;72:580–585. doi: 10.1002/jemt.20699. [DOI] [PubMed] [Google Scholar]
- 8.Meng X, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 9.Yue F, et al. Induction of midbrain dopaminergic neurons from primate embryonic stem cells by coculture with sertoli cells. Stem Cells. 2006;24:1695–1706. doi: 10.1634/stemcells.2005-0409. [DOI] [PubMed] [Google Scholar]
- 10.He Z, et al. Gfra1 silencing in mouse spermatogonial stem cells results in their differentiation via the inactivation of RET tyrosine kinase. Biol Reprod. 2007;77:723–733. doi: 10.1095/biolreprod.107.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naughton CK, et al. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- 12.Nagano M, et al. Maintenance of mouse male germ line stem cells in vitro. Biol Reprod. 2003;68:2207–2214. doi: 10.1095/biolreprod.102.014050. [DOI] [PubMed] [Google Scholar]
- 13.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanatsu-Shinohara M, et al. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 15.Buageaw A, et al. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod. 2005;73:1011–1016. doi: 10.1095/biolreprod.105.043810. [DOI] [PubMed] [Google Scholar]
- 16.Ebata KT, Zhang X, Nagano MC. Expression patterns of cell-surface molecules on male germ line stem cells during postnatal mouse development. Mol Reprod Dev. 2005;72:171–181. doi: 10.1002/mrd.20324. [DOI] [PubMed] [Google Scholar]
- 17.Jijiwa M, et al. GDNF-mediated signaling via RET tyrosine 1062 is essential for maintenance of spermatogonial stem cells. Genes Cells. 2008;13:365–374. doi: 10.1111/j.1365-2443.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- 18.Mauduit C, Hamamah S, Benahmed M. Stem cell factor/c-kit system in spermatogenesis. Hum Reprod Update. 1999;5:535–545. doi: 10.1093/humupd/5.5.535. [DOI] [PubMed] [Google Scholar]
- 19.Bedell MA, Mahakali Zama A. Genetic analysis of Kit ligand functions during mouse spermatogenesis. J Androl. 2004;25:188–199. doi: 10.1002/j.1939-4640.2004.tb02779.x. [DOI] [PubMed] [Google Scholar]
- 20.Munsie M, et al. Expression of stem cell factor in the postnatal rat testis. Mol Reprod Dev. 1997;47:19–25. doi: 10.1002/(SICI)1098-2795(199705)47:1<19::AID-MRD3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 21.Yoshinaga K, et al. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991;113:689–699. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- 22.Koshimizu U, et al. White-spotting mutations affect the regenerative differentiation of testicular germ cells: demonstration by experimental cryptorchidism and its surgical reversal. Biol Reprod. 1991;45:642–648. doi: 10.1095/biolreprod45.4.642. [DOI] [PubMed] [Google Scholar]
- 23.Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prabhu SM, et al. Expression of c-Kit receptor mRNA and protein in the developing, adult and irradiated rodent testis. Reproduction. 2006;131:489–499. doi: 10.1530/rep.1.00968. [DOI] [PubMed] [Google Scholar]
- 25.Ohta H, et al. Regulation of proliferation and differentiation in spermatogonial stem cells: the role of c-kit and its ligand SCF. Development. 2000;127:2125–2131. doi: 10.1242/dev.127.10.2125. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa T, et al. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costoya JA, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- 28.Buaas FW, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 29.Filipponi D, et al. Repression of kit expression by Plzf in germ cells. Mol Cell Biol. 2007;27:6770–6781. doi: 10.1128/MCB.00479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohta H, Tohda A, Nishimune Y. Proliferation and differentiation of spermatogonial stem cells in the w/wv mutant mouse testis. Biol Reprod. 2003;69:1815–1821. doi: 10.1095/biolreprod.103.019323. [DOI] [PubMed] [Google Scholar]
- 31.Kubota H, et al. Spermatogonial stem cells derived from infertile Wv/Wv mice self-renew in vitro and generate progeny following transplantation. Biol Reprod. 2009;81:293–301. doi: 10.1095/biolreprod.109.075960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barroca V, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–196. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 33.Edson MA, Nagaraja AK, Matzuk MM. The Mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan X, et al. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol Cell Biol. 2003;23:4637–4648. doi: 10.1128/MCB.23.13.4637-4648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vernet N, et al. Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. EMBO J. 2006;25:5816–5825. doi: 10.1038/sj.emboj.7601447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niederreither K, et al. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 37.Bowles J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 38.MacLean G, et al. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology. 2007;148:4560–4567. doi: 10.1210/en.2007-0492. [DOI] [PubMed] [Google Scholar]
- 39.Ruggiu M, et al. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389:73–77. doi: 10.1038/37987. [DOI] [PubMed] [Google Scholar]
- 40.Lin Y, Page DC. Dazl deficiency leads to embryonic arrest of germ cell development in XY C57BL/6 mice. Dev Biol. 2005;288:309–316. doi: 10.1016/j.ydbio.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 41.Anderson EL, et al. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A. 2008;105:14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan L, et al. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell. 2000;5:73–83. doi: 10.1016/s1097-2765(00)80404-9. [DOI] [PubMed] [Google Scholar]
- 43.Griswold MD, et al. Function of vitamin A in normal and synchronized seminiferous tubules. Ann N Y Acad Sci. 1989;564:154–172. doi: 10.1111/j.1749-6632.1989.tb25895.x. [DOI] [PubMed] [Google Scholar]
- 44.Bowles J, et al. Male-specific expression of Aldh1a1 in mouse and chicken fetal testes: Implications for retinoid balance in gonad development. Dev Dyn. 2009;238:2073–2080. doi: 10.1002/dvdy.22024. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Q, et al. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod. 2008;79:35–42. doi: 10.1095/biolreprod.107.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- 47.Seligman J, Page DC. The Dazh gene is expressed in male and female embryonic gonads before germ cell sex differentiation. Biochem Biophys Res Commun. 1998;245:878–882. doi: 10.1006/bbrc.1998.8530. [DOI] [PubMed] [Google Scholar]
- 48.Cooke HJ, et al. A murine homologue of the human DAZ gene is autosomal and expressed only in male and female gonads. Hum Mol Genet. 1996;5:513–516. doi: 10.1093/hmg/5.4.513. [DOI] [PubMed] [Google Scholar]
- 49.Lin Y, et al. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science. 2008;322:1685–1687. doi: 10.1126/science.1166340. [DOI] [PubMed] [Google Scholar]
- 50.Reynolds N, et al. Translation of the synaptonemal complex component Sycp3 is enhanced in vivo by the germ cell specific regulator Dazl. RNA. 2007;13:974–981. doi: 10.1261/rna.465507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kerkhofs S, et al. Androgen receptor knockout and knock-in mouse models. J Mol Endocrinol. 2009;42:11–17. doi: 10.1677/JME-08-0122. [DOI] [PubMed] [Google Scholar]
- 52.Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- 53.He WW, Kumar MV, Tindall DJ. A frame-shift mutation in the androgen receptor gene causes complete androgen insensitivity in the testicular-feminized mouse. Nucleic Acids Res. 1991;19:2373–2378. doi: 10.1093/nar/19.9.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnston H, et al. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology. 2004;145:318–329. doi: 10.1210/en.2003-1055. [DOI] [PubMed] [Google Scholar]
- 55.Yeh S, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Gendt K, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang C, et al. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 59.Eacker SM, et al. Transcriptional profiling of androgen receptor (AR) mutants suggests instructive and permissive roles of AR signaling in germ cell development. Mol Endocrinol. 2007;21:895–907. doi: 10.1210/me.2006-0113. [DOI] [PubMed] [Google Scholar]
- 60.Denolet E, et al. The effect of a sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol Endocrinol. 2006;20:321–334. doi: 10.1210/me.2005-0113. [DOI] [PubMed] [Google Scholar]
- 61.Wang RS, et al. Androgen receptor in sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology. 2006;147:5624–5633. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- 62.Meng J, et al. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim P, et al. Sertoli cell androgen receptor DNA binding domain is essential for the completion of spermatogenesis. Endocrinology. 2009;150:4755–4765. doi: 10.1210/en.2009-0416. [DOI] [PubMed] [Google Scholar]
- 64.Ferlin A, et al. Male infertility: role of genetic background. Reprod Biomed Online. 2007;14:734–745. doi: 10.1016/s1472-6483(10)60677-3. [DOI] [PubMed] [Google Scholar]
- 65.Krausz C, Giachini C. Genetic risk factors in male infertility. Arch Androl. 2007;53:125–133. doi: 10.1080/01485010701271786. [DOI] [PubMed] [Google Scholar]
- 66.Stahl PJ, et al. A decade of experience emphasizes that testing for Y microdeletions is essential in American men with azoospermia and severe oligozoospermia. Fertil Steril. 2009 doi: 10.1016/j.fertnstert.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Tung JY, et al. Novel missense mutations of the Deleted-in-AZoospermia-Like (DAZL) gene in infertile women and men. Reprod Biol Endocrinol. 2006;4:40. doi: 10.1186/1477-7827-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen P, et al. Phenotypic Expression of Partial AZFc Deletions is Independent of the Variations in DAZL and BOULE in a Han Population. J Androl. 2009 doi: 10.2164/jandrol.108.007187. [DOI] [PubMed] [Google Scholar]
- 69.Gottlieb B, et al. The androgen receptor gene mutations database (ARDB): 2004 update. Hum Mutat. 2004;23:527–533. doi: 10.1002/humu.20044. [DOI] [PubMed] [Google Scholar]
- 70.Ferlin A, et al. Male infertility and androgen receptor gene mutations: clinical features and identification of seven novel mutations. Clin Endocrinol (Oxf) 2006;65:606–610. doi: 10.1111/j.1365-2265.2006.02635.x. [DOI] [PubMed] [Google Scholar]
- 71.Yu Z, et al. Abnormalities of germ cell maturation and sertoli cell cytoskeleton in androgen receptor 113 CAG knock-in mice reveal toxic effects of the mutant protein. Am J Pathol. 2006;168:195–204. doi: 10.2353/ajpath.2006.050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rajender S, Singh L, Thangaraj K. Phenotypic heterogeneity of mutations in androgen receptor gene. Asian J Androl. 2007;9:147–179. doi: 10.1111/j.1745-7262.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 73.Looijenga LH. Human testicular (non)seminomatous germ cell tumours: the clinical implications of recent pathobiological insights. J Pathol. 2009;218:146–162. doi: 10.1002/path.2522. [DOI] [PubMed] [Google Scholar]
- 74.Sonne SB, et al. Testicular dysgenesis syndrome and the origin of carcinoma in situ testis. Int J Androl. 2008;31:275–287. doi: 10.1111/j.1365-2605.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 75.Fisher JS, et al. Human 'testicular dysgenesis syndrome': a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod. 2003;18:1383–1394. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- 76.Sharpe RM. Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract Res Clin Endocrinol Metab. 2006;20:91–110. doi: 10.1016/j.beem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Liu K, et al. Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol Reprod. 2005;73:180–192. doi: 10.1095/biolreprod.104.039404. [DOI] [PubMed] [Google Scholar]
- 78.Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heaney JD, et al. Loss of the transmembrane but not the soluble kit ligand isoform increases testicular germ cell tumor susceptibility in mice. Cancer Res. 2008;68:5193–5197. doi: 10.1158/0008-5472.CAN-08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tian Q, et al. Activating c-kit gene mutations in human germ cell tumors. Am J Pathol. 1999;154:1643–1647. doi: 10.1016/S0002-9440(10)65419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kemmer K, et al. KIT mutations are common in testicular seminomas. Am J Pathol. 2004;164:305–313. doi: 10.1016/S0002-9440(10)63120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Looijenga LH, et al. Stem cell factor receptor (c-KIT) codon 816 mutations predict development of bilateral testicular germ-cell tumors. Cancer Res. 2003;63:7674–7678. [PubMed] [Google Scholar]
- 83.McIntyre A, et al. Amplification and overexpression of the KIT gene is associated with progression in the seminoma subtype of testicular germ cell tumors of adolescents and adults. Cancer Res. 2005;65:8085–8089. doi: 10.1158/0008-5472.CAN-05-0471. [DOI] [PubMed] [Google Scholar]
- 84.Stoop H, et al. Stem cell factor as a novel diagnostic marker for early malignant germ cells. J Pathol. 2008;216:43–54. doi: 10.1002/path.2378. [DOI] [PubMed] [Google Scholar]
- 85.Blume-Jensen P, et al. Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3'-kinase is essential for male fertility. Nat Genet. 2000;24:157–162. doi: 10.1038/72814. [DOI] [PubMed] [Google Scholar]
- 86.Feng LX, Ravindranath N, Dym M. Stem cell factor/c-kit up-regulates cyclin D3 and promotes cell cycle progression via the phosphoinositide 3-kinase/p70 S6 kinase pathway in spermatogonia. J Biol Chem. 2000;275:25572–25576. doi: 10.1074/jbc.M002218200. [DOI] [PubMed] [Google Scholar]
- 87.Jacobsen R, et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ. 2000;321:789–792. doi: 10.1136/bmj.321.7264.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peng X, et al. The association risk of male subfertility and testicular cancer: a systematic review. PLoS One. 2009;4:e5591. doi: 10.1371/journal.pone.0005591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garolla A, et al. Molecular analysis of the androgen receptor gene in testicular cancer. Endocr Relat Cancer. 2005;12:645–655. doi: 10.1677/erc.1.00954. [DOI] [PubMed] [Google Scholar]
- 90.Nathanson KL, et al. The Y deletion gr/gr and susceptibility to testicular germ cell tumor. Am J Hum Genet. 2005;77:1034–1043. doi: 10.1086/498455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grimaldi P, et al. Molecular genetics of male infertility: stem cell factor/c-kit system. Am J Reprod Immunol. 2002;48:27–33. doi: 10.1034/j.1600-0897.2002.01095.x. [DOI] [PubMed] [Google Scholar]
- 92.Meng X, et al. Promotion of seminomatous tumors by targeted overexpression of glial cell line-derived neurotrophic factor in mouse testis. Cancer Res. 2001;61:3267–3271. [PubMed] [Google Scholar]
- 93.Mai PL, et al. The International Testicular Cancer Linkage Consortium: A clinicopathologic descriptive analysis of 461 familial malignant testicular germ cell tumor kindred. Urol Oncol. 2009 doi: 10.1016/j.urolonc.2008.10.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu Z, et al. Dazl Promotes Germ Cell Differentiation from Embryonic Stem Cells. J Mol Cell Biol. 2009;1:93–103. doi: 10.1093/jmcb/mjp026. [DOI] [PubMed] [Google Scholar]
- 95.Kee K, et al. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tesar PJ, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 97.Rebar RW, DeCherney AH. Assisted reproductive technology in the United States. N Engl J Med. 2004;350:1603–1604. doi: 10.1056/NEJMp048046. [DOI] [PubMed] [Google Scholar]
- 98.McLachlan RI, et al. Genetic disorders and spermatogenesis. Reprod Fertil Dev. 1998;10:97–104. doi: 10.1071/r98029. [DOI] [PubMed] [Google Scholar]
- 99.Donoso P, Tournaye H, Devroey P. Which is the best sperm retrieval technique for non-obstructive azoospermia? A systematic review. Hum Reprod Update. 2007;13:539–549. doi: 10.1093/humupd/dmm029. [DOI] [PubMed] [Google Scholar]
- 100.[No authors listed] Round spermatid nucleus injection (ROSNI) Fertil Steril. 2008;90:S199–S201. doi: 10.1016/j.fertnstert.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 101.Boekelheide K, Sigman M. Is gene therapy for the treatment of male infertility feasible? Nat Clin Pract Urol. 2008;5:590–593. doi: 10.1038/ncpuro1234. [DOI] [PubMed] [Google Scholar]
- 102.Lamb DJ. Debate: is ICSI a genetic time bomb? Yes. J Androl. 1999;20:23–33. [PubMed] [Google Scholar]
- 103.Neri QV, Takeuchi T, Palermo GD. An update of assisted reproductive technologies results in the United States. Ann N Y Acad Sci. 2008;1127:41–48. doi: 10.1196/annals.1434.017. [DOI] [PubMed] [Google Scholar]
- 104.Alukal JP, Lipshultz LI. Safety of assisted reproduction, assessed by risk of abnormalities in children born after use of in vitro fertilization techniques. Nat Clin Pract Urol. 2008;5:140–150. doi: 10.1038/ncpuro1045. [DOI] [PubMed] [Google Scholar]
- 105.Alukal JP, Lamb DJ. Intracytoplasmic sperm injection (ICSI)--what are the risks? Urol Clin North Am. 2008;35:277–288. doi: 10.1016/j.ucl.2008.01.004. ix-x. [DOI] [PMC free article] [PubMed] [Google Scholar]