Abstract
Murine cytomegalovirus (MCMV) interferes with the MHC class I pathway of antigen presentation. The type I transmembrane glycoprotein gp40, encoded by the gene m152, retains major histocompatibility complex (MHC) class I complexes in the endoplasmic reticulum (ER)–Golgi intermediate compartment (ERGIC)/cis–Golgi. These MHC class I complexes are stable, show an extended half-life and do not exchange β2-microglobulin, whereas gp40 reaches an endosomal/lysosomal compartment where it subsequently is degraded. The analysis of regions within the viral protein that are essential for MHC class I retention revealed that a secreted form of gp40, lacking the cytoplasmic tail and the transmembrane region, still has the capacity to retain MHC class I complexes. Continuous expression of gp40 is not required for MHC class I retention. Our data indicate that the retention of MHC class I complexes in the ERGIC/cis–Golgi is triggered by gp40 and does not require the further presence of the viral protein.
Keywords: antigen presentation/ERGIC/cis-Golgi retention/immune evasion/MHC class I pathway/murine cytomegalovirus
Introduction
Herpesviruses establish life-long infections in their hosts even in the face of an active immune system. During co-evolution with their hosts, a number of viruses have developed immune evasive strategies, which comprise, for example, the inhibition of an inflammatory response by affecting the complement cascade or by interfering with cytokines, chemokines and interferons (Smith, 1994). The inhibition of major histocompatibility complex (MHC) class I-mediated antigen presentation, resulting in the prevention of cytotoxic T-cell recognition of virally infected cells, is another strategy used by all herpesvirus families studied so far (Hengel et al., 1998; Johnson and Hill, 1998).
Peptides destined for antigen presentation are generated in the cytosol by the proteasome. The peptides are translocated from the cytosol into the lumen of the endoplasmic reticulum (ER) by specific transporters (TAP, transporter associated with antigen processing), where they associate with newly synthesized MHC class I heavy chain and β2-microglobulin (β2m). The assembly of this ternary MHC class I complex (peptide, heavy chain and β2m) involves a number of chaperones, such as calnexin, calreticulin and tapasin. Properly folded and assembled MHC class I complexes are transported via the constitutive secretory pathway to the cell surface for antigen presentation to CD8+ T cells (Pamer and Cresswell, 1998).
Viruses use several different strategies to affect the antigen presentation pathway, e.g. interference with MHC class I assembly, blockade of MHC class I transport along the secretory pathway or MHC class I degradation (Hengel and Koszinowski, 1997). Remarkably, no cellular homologs have been found for any of these viral proteins. Although in herpesviruses these proteins are members of gene families of related viral proteins (Chee et al., 1990; Rawlinson et al., 1996), family members employ different strategies to affect MHC class I function. Therefore, the mechanism by which these viral proteins act on the MHC class I pathway has to be elucidated individually for each gene product.
Viral proteins affecting MHC class I expression act by direct protein–protein interaction with either MHC class I or proteins involved in MHC class I maturation. US6 of human cytomegalovirus (HCMV) (Ahn et al., 1997; Hengel et al., 1997; Lehner et al., 1997) and ICP47 of herpes simplex virus (HSV) type 1 and type 2 (Früh et al., 1995; Hill et al., 1995; Ahn et al., 1996b) bind to the peptide transporter TAP to prevent peptide loading of MHC class I molecules. Other viral proteins form complexes directly with MHC class I molecules. The HCMV gene products of US2 and US11 mediate the rapid destruction of newly synthesized MHC class I molecules. They dislocate bound MHC class I molecules from the ER into the cytosol, where they become degraded by the proteasome (Wiertz et al., 1996a,b). The glycoproteins gp48 and gp34 of murine cytomegalovirus (MCMV), which belong to the same gene family, act at a later stage of MHC class I maturation: gp48 targets MHC class I complexes via a di-leucine motif (LL) in its cytoplasmic tail into an endosomal/lysosomal compartment for degradation (Reusch et al., 1999), whereas gp34 accompanies MHC complexes to the cell surface to exert an unknown function (Kleijnen et al., 1997). The paradigm for a viral glycoprotein interfering with antigen presentation, E3/19K of adenovirus, was detected through its binding to MHC class I molecules (Signäs et al., 1982; Pääbo et al., 1986). Its cytoplasmic tail contains a double lysine (KKXX) motif, which mediates the retrieval of E3/19K and associated MHC class I complexes back into the ER (Burgert and Kvist, 1985; Jackson et al., 1990; Burgert, 1996). Like E3/19K, the US3-encoded glycoprotein of HCMV binds directly to MHC class I molecules and arrests them in the ER (Ahn et al., 1996a; Jones et al., 1996).
We recently reported that the MCMV glycoprotein gp40 encoded by the gene m152 inhibits the transport of MHC class I molecules, resulting in their accumulation in the ER cis–Golgi intermediate compartment (ERGIC)/cis–Golgi (Thäle et al., 1995; Ziegler et al., 1997). m152/gp40 inhibits the transport of all murine MHC class I alleles tested so far (Kd, Kk, Kb, Dd, Lq and Ld; Thäle et al., 1995; Ziegler et al., 1997; A.Gutermann, personal communication). The retained MHC molecules consist of the complete trimolecular complex of heavy chain, β2m and peptide, as shown by quantitative peptide extraction (Del Val et al., 1992). In contrast to E3/19K, the amino acid sequence of gp40 does not contain any known retention or retrieval signal. Moreover, no direct evidence was provided that m152/gp40 stably associates with MHC class I molecules.
Here we report that a secreted m152/gp40 variant protein consisting of only the luminal part of gp40 still has MHC class I retention capacity. The m152/gp40 does not form a stable complex with the retained MHC class I molecules. Whereas MHC class I molecules are arrested in the ERGIC/cis–Golgi, gp40 molecules reach an endosomal/lysosomal compartment. The continuous MHC class I retention is only triggered by m152/gp40, and does not require its further presence.
Results
Expression of gp40 deletion mutants by recombinant vaccinia virus
Only for a few viral proteins interacting with MHC class I molecules have the functionally relevant domains been determined (Pääbo et al., 1986; Jackson et al., 1990; Reusch et al., 1999). These studies showed that the luminal part of the protein confers MHC class I complex binding, whereas the transmembrane region or the cytoplasmic tail determine the intracellular location.
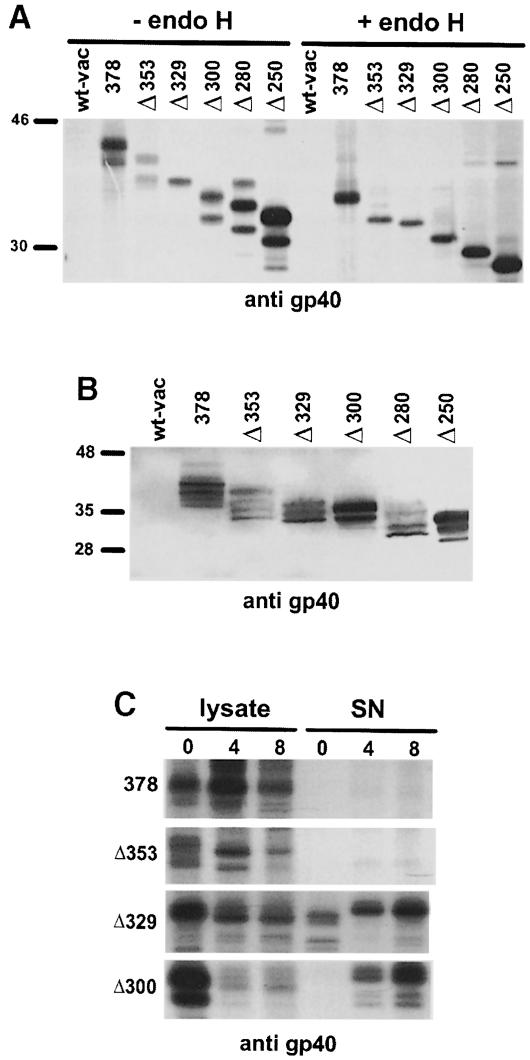
To identify the domains of gp40 essential for MHC class I retention, we constructed a series of C-terminal gp40 deletion mutants (Figure 1A) and expressed them by recombinant vaccinia viruses. Immunoprecipitation of gp40 results in the appearance of two characteristic protein species on SDS–PAGE (40 and 37 kDa), which represent differently glycosylated forms of the m152 gene product (Ziegler et al., 1997). Most of the gp40 mutants also showed two protein bands that are reduced to a single protein species upon deglycosylation by endoglycosidase H (endo H; Figure 2A). Endo H hydrolyzes N-glycans of the high mannose type, which are present in the ER and cis–Golgi. For unknown reasons, the mutant Δ329 exhibits a single band (–endo H); presumably one N-glycosylated species is generated predominantly. All mutants migrated at the expected molecular weight and were expressed in comparable quantities. Differences in the steady-state level, shown by Western blot analysis of total cellular lysates (Figure 2B), were in a range acceptable for further functional analysis.
Fig. 1. Schematic representation of gp40 deletion mutants and chimeric proteins. (A) Proportional representation of the m152 gene product gp40 (378) with signal peptide (sp), glycosylation sites (branched symbols), transmembrane region (tm) and cytoplasmic tail (ct). The bar indicates the binding region of the peptide antiserum to gp40 (p11). The number of the deletion mutants refers to the last amino acid expressed in the truncated protein. (B) The number in the chimeric proteins indicates the last amino acid of gp40 to which the cytoplasmic tail of gp48 (-gp48ct) or the transmembrane region of CD4 (-CD4tm) was fused. The bar indicates the binding region of the mAb to the cytoplasmic tail of gp48 (CROMA229). The numbers of the two intramolecular deletion mutants indicate the depleted region. (C) Properties of the mutant gp40 proteins described in this study: the release of the proteins into the culture supernatant, their transport to lysosomes and ability to block the transport of MHC class I molecules. nd, not determined.
Fig. 2. Expression of gp40 deletion mutants by recombinant vaccinia virus. (A) NIH 3T3 cells were infected with wild-type vaccinia virus (wt-vac), recombinant vaccinia virus expressing the complete m152 ORF (378) and the C-terminal deletion mutants (Δ353, Δ329, Δ300, Δ280 and Δ250). At 5 h post-infection, cells were pulse-labeled for 30 min with [35S]cysteine/methionine. The gp40 derivatives were precipitated with peptide antiserum to gp40 (p11). Half of the precipitates were either digested with endoglycosidase H (endo H) or mock treated before separation by 10% SDS–PAGE. (B) Western blot analysis of NIH 3T3 cells infected with wt-vac and the indicated recombinant vaccinia viruses, respectively. At 12 h post-infection, total cellular lysates were prepared and separated by 10% SDS–PAGE. After blotting, binding of the mAb against gp40 (gpM3D10) was visualized with alkaline phosphatase-conjugated secondary antibody and color substrate. (C) NIH 3T3 cells were infected with wt-vac and the indicated recombinant vaccinia viruses, respectively. At 5 h post-infection, cells were pulse-labeled for 1 h with [35S]cysteine/methionine and chased for the indicated times. Cell lysates were prepared and culture supernatants were collected. The gp40 derivatives were precipitated with peptide antiserum p11. Precipitated proteins were separated by 10% SDS–PAGE and visualized by autoradiography.
To test whether deletion mutants of gp40 lacking the transmembrane region are secreted into the culture medium, we precipitated gp40 (378) and the mutants Δ353, Δ329 and Δ300 from cellular lysates and culture medium (Figure 2C). As expected, the gp40 derivatives lacking the membrane anchor region (Δ329 and Δ300) appeared in the supernatant within 4 h of chase.
The secreted luminal part of gp40 still has the capacity to retain MHC class I molecules
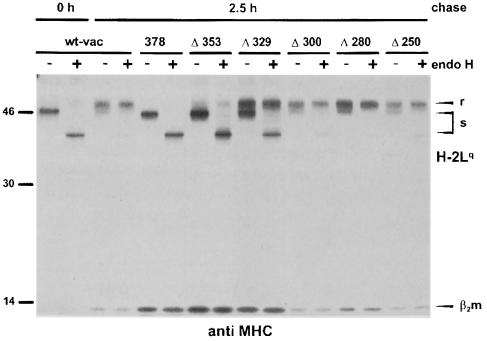
In wild-type vaccinia virus (wt-vac)-infected cells, MHC class I complexes (H-2Lq) acquired endo H resistance after 2.5 h of chase, indicating that they passed the Golgi to become presented at the cell surface (Figure 3). In contrast, the glycans of MHC class I complexes arrested in the ERGIC/cis–Golgi by gp40 (378) did not undergo the typical Golgi processing and remained endo H sensitive. In cells expressing the gp40 mutant lacking the cytoplasmic tail (Δ353), the MHC class I molecules also remained endo H-sensitive, consistent with earlier data (Ziegler et al., 1997). As judged by the relationship between endo H-resistant and -sensitive forms, the secreted mutant Δ329 retained ∼30% of the precipitated MHC class I molecules. The three deletion mutants Δ300, Δ280 and Δ250 did not show a significant retention of Lq molecules. The same results were obtained by the analysis of MHC class I allele Kd, expressed in B12 cells (data not shown). These data show that the luminal part of gp40 still has a residual capacity to retain MHC class I complexes.
Fig. 3. Retention of MHC class I complexes by gp40 deletion mutants. NIH 3T3 cells were infected with wt-vac and the indicated recombinant vaccinia viruses, respectively. At 5 h post-infection, cells were pulse-labeled for 30 min with [35S]cysteine/methionine. Cell lysates were either prepared directly after labeling (0 h) to isolate nascent MHC class I molecules or after a 2.5 h chase period. MHC class I molecules (H2-Lq) were precipitated with the mAb 28-14-8S. Half of the precipitated proteins were digested with endo H. The proteins were separated by SDS–PAGE and visualized by auto- radiography. r, endo H-resistant; s, endo H-sensitive MHC class molecules.
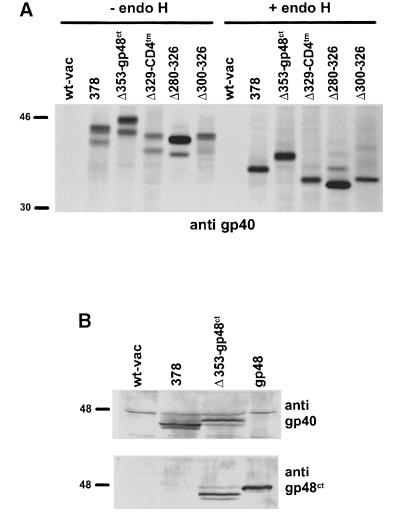
To examine whether the different activity of Δ353 and Δ329 reflects the lack of gp40 membrane anchoring or indicates a specific property of the gp40 transmembrane region, a chimeric protein was generated consisting of the luminal part of gp40 (Δ329) and the transmembrane region of CD4 (Δ329–CD4tm; Figures 1B and 4A). As the chimeric protein Δ329–CD4tm induced a strong transport block of the MHC molecules (Figure 5A), the fusion of the luminal domain of gp40 to the transmembrane region of CD4 completely restored the retention of MHC class I complexes. Thus, we conclude that the luminal part of gp40 contains the domain responsible for MHC class I retention.
Fig. 4. Expression of the chimeric proteins and the intramolecular gp40 deletion mutants by recombinant vaccinia virus. (A) NIH 3T3 cells were infected with wt-vac, recombinant vaccinia virus expressing the entire m152 ORF (378), the chimeric proteins Δ353–gp48ct and Δ329–CD4tm, and the intramolecular deletion mutants (Δ280–326 and Δ300–326). At 5 h post-infection, cells were pulse-labeled for 30 min with [35S]cysteine/methionine. gp40 derivatives were precipitated with peptide antiserum p11. Half of the precipitates were either digested with endo H or mock treated before separation by 10% SDS–PAGE. (B) Western blot analysis of NIH 3T3 cells infected with either wt-vac or the indicated recombinant vaccinia viruses, and NIH 3T3 cells stably expressing m06/gp48. Total cellular lysates were separated by 10% SDS–PAGE and subsequently blotted. Expression of the luminal part of gp40 was analyzed by using an mAb against gp40 (gpM3D10, upper panel). Expression of the cytoplasmic tail of gp48 was tested by an mAb against the cytoplasmic tail of gp48 (CROMA229, lower panel). The proteins were stained with an alkaline phosphatase-conjugated secondary antibody and color substrate.
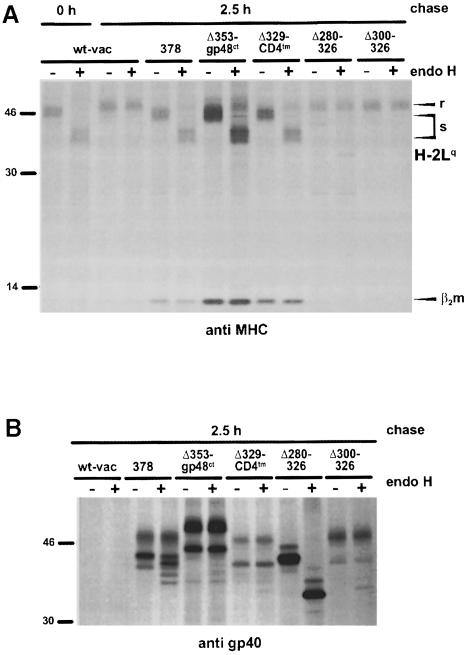
Fig. 5. MHC class I retention mediated by the gp40 chimeric proteins and intramolecular deletion mutants. NIH 3T3 cells were infected with wt-vac and the indicated recombinant vaccinia viruses, respectively. At 5 h post-infection, cells were pulse-labeled for 30 min with [35S]cysteine/methionine. Cell lysates were prepared after the indicated chase periods. MHC class I molecules (H2-Lq) and gp40 derivatives were precipitated with the mAb 28-14-8S (A) and the peptide antiserum against gp40 (B), respectively. Half of the precipitated proteins were digested with endo H. The proteins were separated by SDS–PAGE and visualized by autoradiography. Note that in addition to the endo H-resistant form of the two differently glycosylated protein species corresponding to gp40 and gp37, a slower migrating endo H-resistant protein form is visualized under chase conditions.
In addition, the transmembrane region and cytoplasmic tail of gp40 were fused to the gp40 luminal domains that were shortened by a further 29 (Δ300–326) and 49 (Δ280–326) amino acids, respectively (Figures 1B and 4A). In contrast to the full-length gp40 luminal domain fused to the CD4 transmembrane region (Δ329–CD4tm), these constructs did not regain functional activity (Figure 5A).
Intracellular separation of gp40 and MHC class I complexes
To rule out the possibility that the antibodies we used selectively bound to gp40 molecules that do not complex with MHC class I molecules, we generated a tagged gp40 protein. In this tagged chimera, the cytoplasmic tail of gp40 was exchanged by that of m06/gp48 (Δ353–gp48ct; Figures 1B and 4), which is recognized by the monoclonal antibody (mAb) CROMA229 (Reusch et al., 1999). As the cytoplasmic tail of gp48 contains a di-leucine (LL) motif, which is essential for targeting associated MHC class I molecules to lysosomes (Reusch et al., 1999), we also investigated whether MHC class I molecules would have a different fate in the presence of the chimeric protein Δ353–gp48ct.
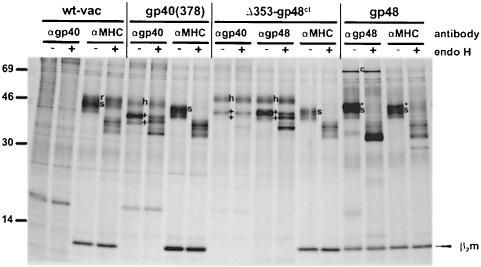
Independently of the antibodies used, gp40 and Δ353–gp48ct did not co-precipitate with MHC class I complexes (Figure 6), suggesting that a stable complex between both proteins does not exist. In contrast to the MHC class I molecules that remained endo H sensitive (Figure 5A), the proteins gp40 (378), Δ353–gp48ct and Δ329–CD4tm acquired resistance to endo H during a chase period of 2.5 h (Figure 5B), indicating that gp40 and MHC class I molecules were separated. Thus, in the presence of Δ353–gp48ct, the luminal part of gp40 defined the fate of MHC class I complexes.
Fig. 6. gp40 and MHC class I molecules do not co-precipitate. NIH 3T3 cells were infected for 5 h with wt-vac and the indicated recombinant vaccina viruses. As a control, NIH-3T3 cells stably expressing gp48 were used to show co-precipitation of MHC class I molecules with the complexed viral protein gp48. Cells were metabolically labeled for 3 h with [35S]methionine/cysteine to cover both newly synthesized and matured proteins. Cells were lysed with digitonin and proteins were precipitated with the indicated antibodies (αgp40, peptide antiserum p11; αgp48, mAb CROMA229; αMHC, mAB 28-14-8S). Half of the proteins were digested with endo H and subsequently separated on 11.5–13.5% SDS–PAGE. Endo H sensitivity of MHC class I complexes is indicated as s, and resistance as r. The two differently glycosylated protein species of the m152 gene product (gp40/37) are marked as + and the slower migrating, highly glycosylated form as h. gp48 is identified as * and c indicates calnexin.
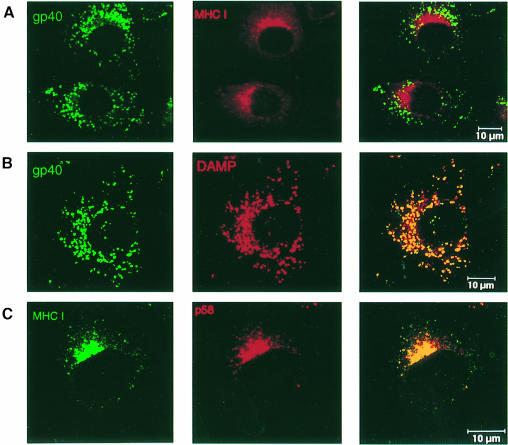
To examine the subcellular distribution of gp40 and MHC class I molecules under steady-state conditions, fibroblasts stably expressing gp40 were analyzed by confocal laser scanning microscopy. Double immunofluorescence staining showed that the bulk of gp40 molecules and MHC class I complexes do not co-localize (Figure 7). gp40 molecules co-localized with 3′-amino-N-methyl dipropylamine (DAMP) (Figure 7B) and Lamp-1 (data not shown), markers specific for the endosomal/lysosomal compartment (Hughes and August, 1981; Orci et al., 1994), whereas MHC class I complexes co-localized with the ERGIC marker p58 (Figure 7C) (Saraste and Svensson, 1991).
Fig. 7. Intracellular separation of gp40 and the arrested MHC class I complexes. B12 cells stably expressing gp40 were analyzed by confocal laser scanning microscopy. To inhibit lysosomal degradation, cells were incubated with 50 μg/ml leupeptin 12 h prior to double immunostaining. (A) The subcellular distribution of gp40 (green) was monitored with mAb gpM3D10, and MHC class I complexes (red) were detected with mAb SF1.1.1 (anti H2-Kd). (B) The staining pattern of gp40 (green) was compared with that of DAMP (red), a specific marker for acidic vesicles (endosomes/lysosomes). (C) Staining of MHC class I complexes (green) was compared with that of the ERGIC marker p58 (red).
The cytoplasmic domain of gp40 contains a tyrosine-based amino acid sequence (YRLV) similar to the general endosomal/lysosomal targeting motif YXXΦ (Y, tyrosine; X, any amino acid; Φ, hydrophobic amino acid) (Guarnieri et al., 1993). The deletion mutant lacking the cytoplasmic tail (Δ353) allowed us to examine the relevance of this motif. Confocal laser scanning microscopy showed that the truncated protein still co-localized with DAMP and Lamp-1 (data not shown), indicating that the YRLV motif is not essential for lysosomal targeting of gp40.
Transient interaction between gp40 and MHC class I molecules
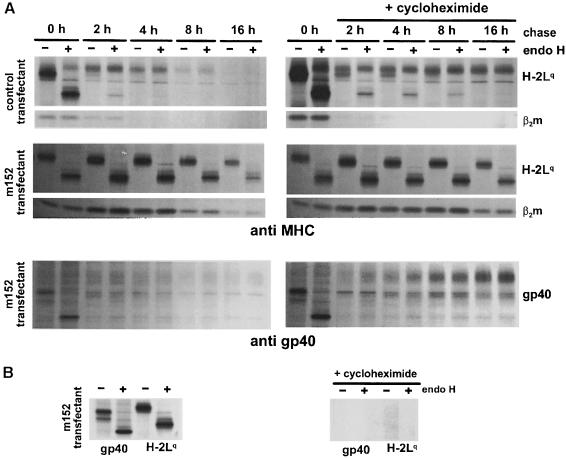
To gain more information about the retention mechanism, transfectants stably expressing gp40 were used to analyze the half-life and stability of the viral protein and the retained MHC class I complexes under pulse–chase conditions. A fraction of the precipitated proteins was digested with endo H to examine the transport through the medial–Golgi (Figure 8A, left panels). In control transfectants, the precipitated Lq molecules become endo H resistant after 2 h of chase and have a half-life of ∼4 h. In contrast, MHC class I complexes precipitated from cells expressing gp40 remain endo H sensitive for the whole chase period of 16 h and have a half-life of ∼8 h. Thus, retention of MHC class I complexes results in an increased half-life. β2m co-precipitates with the heavy chain. After a 4 h chase period, the 35S-labeled β2m disappears in control transfectants, which is probably due to the exchange of labeled β2m with cold β2m present in cells or the culture medium. In contrast, in m152 transfectants, β2m co-precipitated stoichiometrically with the heavy chain: quantitative analysis of phosphoimager blots showed an average 1:1.3 ratio of heavy chain and β2m throughout the whole chase period. Thus, MHC class I retention is associated with a decreased exchange of labeled β2m. In the cell line stably expressing gp40, the half-life of gp40 was <2 h (Figure 8) and the bulk of gp40 became endo H resistant during this time (data not shown). In summary, gp40 and retained MHC class I complexes differ with respect to their half-life and intracellular distribution.
Fig. 8. Continuous expression of gp40 is not necessary for the retention of MHC class I complexes. (A) NIH 3T3 cells stably expressing the m152 gene product gp40 and control transfectants were pulse-labeled for 45 min with [35S]cysteine/methionine. Cells were chased for the indicated time, with (right panels) or without (left panels) 50 μg/ml cycloheximide. MHC class I molecules (H2-Lq) and gp40 were precipitated with the mAbs 28-14-8S and gpM3D10, respectively. Half of the precipitated proteins were digested with endo H. The proteins were separated by SDS–PAGE and visualized by autoradiography. In the presence of cycloheximide, gp40 acquired the slow migrating endo H-resistant phenotype, comparable with gp40 expression shown in Figures 5B and 6, indicative of the transport through the Golgi. In addition, the half-life of MHC class I molecules and gp40 was increased, presumably caused by a side effect of the drug, the suppression of endogenous protein degradation by an action upon the lysosomal pathway (Seglen, 1983). (B) To control for the activity of cycloheximide, m152 transfectants were treated for 2 h with (right panel) or without (left panel) 50 μg/ml cycloheximide and subsequently pulsed with [35S]cysteine/methionine for 45 min. Cell lysates were prepared and treated as described in (A).
Although gp40 and the MHC class I molecules have a different fate, the possibility remains that a continuous exchange of newly synthesized gp40 molecules at the MHC class I complexes ensures retention. To address this question, continuous synthesis of gp40 was blocked by addition of the protein synthesis inhibitor cycloheximide to the chase medium. In the presence of cycloheximide, MHC class I Lq molecules (Figure 8, right panels) and Kd molecules (data not shown) still remained endo H sensitive over the whole 16 h chase period, indicating that the retention of MHC class I complexes in the ERGIC/cis–Golgi does not require a continuous synthesis of gp40.
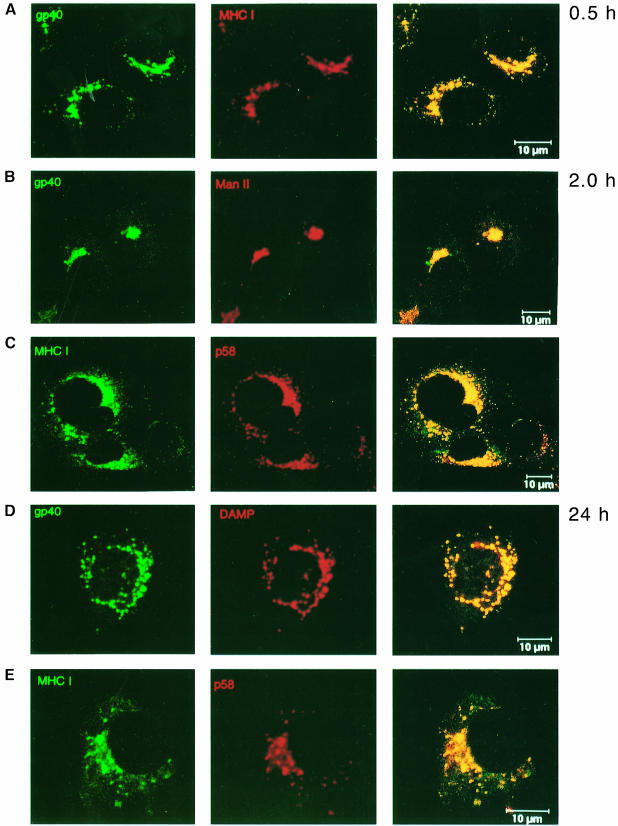
To visualize the transport dynamics of newly synthesized gp40 and MHC class I molecules, m152 transfectants were analyzed by confocal laser scanning microscopy (Figure 9). In order to observe distinct populations of gp40 and MHC class I molecules, cells were treated with cycloheximide and actinomycin D. Double immunofluorescence staining showed that after 30 min of protein synthesis gp40 and MHC class I molecules co-localized, probably in the ER/cis–Golgi. After 2 h of protein synthesis, most of the gp40 co-localized with mannosidase II (ManII), a marker of the medial–Golgi, whereas MHC class I complexes co-localized with the ERGIC marker p58. After 24 h, gp40 co-localized with the endosomal/lysosomal compartment marker DAMP, whereas retained MHC class I complexes still co-localized with the ERGIC marker p58, confirming the biochemical evidence that the retention of MHC class I molecules does not require the continuous synthesis and presence of gp40.
Fig. 9. Transport dynamics of newly synthesized gp40 and MHC class I molecules. B12 cells stably expressing gp40 were treated for 10 h with 20 μg/ml cycloheximide to accumulate mRNA by inhibiting translation. After washing, cycloheximide was replaced by 5 μg/ml actinomycin D to inhibit further transcription and to allow translation of the accumulated mRNA. To avoid lysosomal degradation, 50 μg/ml leupeptin was added. Cells were fixed and permeabilized at the indicated times after addition of actinomycin D. (A) At 0.5 h after protein release, gp40 (green, mAb gpM3D10) co-localized with the MHC class I Kd molecules (red, mAb SF1.1.1). (B) Two hours later, gp40 (green) co-localized with the medial– Golgi marker ManII (red). (D) After 24 h, gp40 co-localized with DAMP, which stains acidic vesicles (endosomes/lysosomes). (C and E) During this time course, the retained MHC class I molecules (green) co-localized with the ERGIC marker p58 (red). Compared with the half-life of gp40, the time required for the transport of gp40 to the endosomes/lysosomes was increased by cycloheximide and actinomycin D treatment. This is probably due to delayed transport kinetics of the involved cellular compartments after protein release.
Retained MHC class I molecules are not transported to the cell surface after BFA release
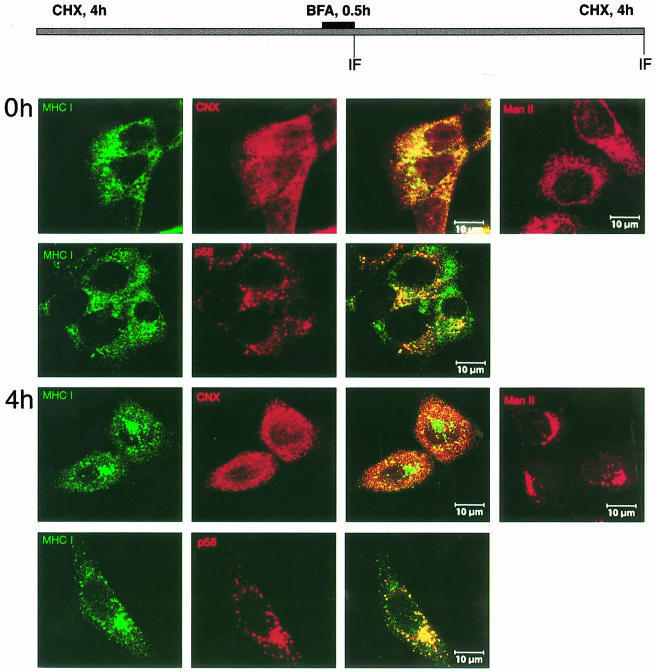
The retention of MHC class I molecules in the ERGIC/cis–Golgi could be explained either by a signal within gp40 or by a gp40-induced modification of the MHC molecules, which then defines their subcellular location. If gp40 directly guides MHC class I molecules to the ERGIC/cis–Golgi, relocation of the MHC class I molecules to the ER in the absence of gp40 should enable their physiological export to the cell surface. To this end, cells devoid of gp40 by cycloheximide treatment were incubated with brefeldin A (BFA) (Figure 10). BFA relocates cis- and medial–Golgi structures, but not the ERGIC/p58 compartment, into the ER (Scheel et al., 1997). As a control, relocation of the medial–Golgi marker ManII was followed, which showed a reticular ER staining after BFA treatment (0 h) and a typical Golgi staining after the release of BFA (4 h). Immediately after BFA release (0 h), the retained MHC class I molecules are localized in the ERGIC (p58+), but also in a reticular compartment which is stained by the ER marker calnexin (CNX). Remarkably, after release of BFA (4 h), the MHC class I molecules do not reach the plasma membrane, but are relocated to the compartment defined by p58. Therefore, we conclude that gp40 induces a biochemical modification of MHC molecules that prevents, even in the absence of gp40, the physiological export of the ER-relocated MHC class I molecules.
Fig. 10. Effect of BFA on the distribution of retained Kd molecules in the absence of gp40. m152 transfectants were incubated with cycloheximide for 4 h, then BFA was added for 0.5 h. Subsequently, cells were washed three times in the presence of cycloheximide (CHX) before incubation was continued for another 4 h. Double staining was performed at 0 and 4 h after BFA release with mAb against Kd (SF1.1.1., green), polyclonal antibodies against the ER marker calnexin (CNX, red) and the ERGIC marker p58 (red), respectively. BFA-induced changes of the Golgi were followed with polyclonal antibody against ManII (right panels).
Discussion
gp40 retains mouse MHC class I molecules in murine cells, but not human MHC molecules in human cells. Furthermore, gp40 does not retain human MHC class I molecules in murine cells, but retains mouse MHC class I molecules expressed in human cells (Ziegler et al., 1997). Thus, we presume that the interaction between gp40 and MHC class I molecules is direct. In this study, we have mapped the domain of gp40 required for ERGIC/cis–Golgi retention of MHC class I molecules to the luminal part. By constructing serial deletions of gp40, we have shown that neither the cytolasmic tail nor the transmembrane part is absolutely essential for gp40 function. A secreted version of gp40 retains significant functional activity, which is fully restored by anchoring it to the membrane via the transmembrane segment of CD4. Two conclusions are drawn: (i) the luminal domain of gp40 (Δ329) contains the activity responsible for MHC retention; and (ii) full activity requires membrane attachment, which probably provides the appropriate orientation and perhaps supports correct folding of the domain. Membrane attachment of Δ300 and Δ280 (constructs Δ300–326 and Δ280–326) did not restore retention of MHC class I complexes (Figures 1 and 5). Thus, the sequence between amino acids 300 and 329 is crucial for gp40 function. Whether or not these amino acids are required directly for the effect on MHC molecules or whether they are important for correct folding of the luminal domain remains to be investigated. The mutant Δ280–326, for example, is not transported through the Golgi (Figure 5B), as is the case for full-length gp40 and other gp40 derivatives.
Retention of MHC molecules does not depend on a known retention motif within the viral protein. First, gp40 does not exhibit a prolonged residence within the ERGIC, where MHC molecules are retained, but rather is transported to endosomes/lysosomes. Secondly, consistent with these properties, we cannot identify any amino acid motif within the viral protein that conforms to a known ER retention/retrieval signal such as KKXX or the polarity of the transmembrane segment (Jackson et al., 1990; Rayner and Pelham, 1997). Accordingly, deletion of the cytoplasmic tail does not eliminate the MHC retention function of gp40. Interestingly, this mutant form (Δ353) still reaches a lysosomal compartment, while the soluble Δ329 mutant does not. Therefore, we conclude that (i) the YXXΦ motif in the cytoplasmic tail of gp40 is not required for lysosomal sorting and (ii) the transmembrane segment of gp40, perhaps in conjunction with the luminal domain, contains the information for lysosomal targeting. This idea is supported by the fact that only a minor fraction of the mutant gp40 molecule in which the transmembrane segment of gp40 is replaced by that of CD4 (Δ329–CD4tm) reaches the lysosomes.
Most remarkably, we find a differential fate of MHC molecules and gp40. While gp40 has a very short half-life (t1/2 ∼2 h), retained MHC molecules are very stable (t1/2 ∼8 h). Consistent with this behavior, the MHC molecules remain endo H sensitive over the whole observation period of up to 16 h, whereas gp40 and most mutant derivatives become endo H resistant (Figures 5 and 8). This indicates that gp40 is transported through the medial–Golgi, while MHC molecules are not. This conclusion is confirmed by confocal immunofluorescence microscopy, which shows that the steady-state distribution of gp40 is in an endosomal/lysosomal compartment while that of MHC molecules is in the ERGIC/cis–Golgi (Figure 7). The retention of MHC molecules in the ERGIC could be explained by continuous exchange of newly synthesized gp40 at the MHC molecules. Pulse–chase experiments in the presence of cycloheximide, which prevents new protein synthesis, demonstrated that this is not the case. Again gp40 molecules, now migrating with a slower mobility, become endo H resistant, whereas MHC molecules are fixed in an endo H-sensitive state (Figure 8). Using a similar pulse–chase technique in combination with confocal immunofluorescence microscopy (Figure 9), we show that newly synthesized MHC molecules and gp40 initially are located in the same compartment (ER/ERGIC), but 2 h later they reach distinct compartments: gp40 is located in the medial–Golgi whereas MHC molecules reside in structures co-localizing with the ERGIC marker p58 (Saraste and Svensson, 1991). Moreover, we are unable to demonstrate a complex between gp40 and MHC class I molecules by co-immunoprecipitation, even under mild solubilization conditions. As our gp40-specific antibodies react with the luminal part, the possibility exists that they do not recognize an MHC–gp40 complex. However, no complex was detected with the chimeric protein Δ353–gp48ct, representing an epitope-tagged gp40 variant, which allowed us to use antibodies against the cytoplasmic tail of gp48. The same antibodies readily co-precipitate MHC molecules with wild-type gp48 (Figure 6; Reusch et al., 1999). Thus, the putative gp40–MHC class I molecule complex did not escape our attention due to the lack of appropriate antibodies. In addition, we were unable to identify reproducibly a complex between gp40 and MHC class I molecules in cross-linking experiments, under conditions where we clearly conjugated both MHC class I with the transiently acting chaperone calnexin, and gp40 with calnexin (data not shown).
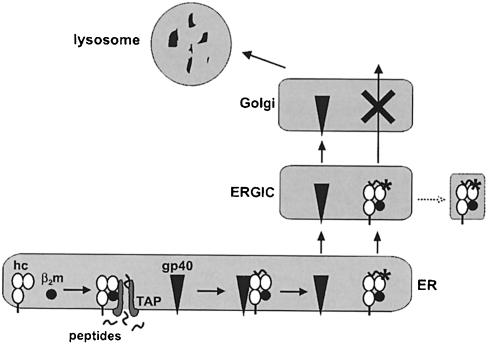
Taken together, our experimental evidence suggests that the retention of MHC molecules in the ERGIC/cis–Golgi mediated by the MCMV glycoprotein gp40 does not require a stable physical interaction. Rather, the two proteins seem to interact transiently. During this time period, gp40 seems to induce a modification within the MHC molecule, which inhibits their further transport. The viral protein subsequently reaches an endosomal/lysosomal compartment (Figure 11). This property of gp40 contrasts with the functional activity of all other known viral proteins that interfere with the MHC class I antigen presentation pathway (Burgert, 1996; Hengel et al., 1998). Thus, the gp40-mediated retention of MHC molecules appears to be based on a novel mechanism.
Fig. 11. Schematic representation of the mechanism proposed for the gp40-mediated MHC class I retention. After assembly of the trimolecular MHC class I complex, consisting of heavy chain (hc), β2-microglobulin (β2m) and peptide (provided by the transporter associated with antigen processing, TAP), interaction of gp40 and MHC class I takes place. gp40 triggers a reaction that results in the alteration of MHC class I complexes (*) and their retention. gp40 dissociates from MHC class I complexes, passes the Golgi and reaches an endosomal/lysosomal compartment for degradation. MHC class I complexes exit the endoplasmic reticulum and enter the ER–Golgi intermediate compartment (ERGIC), where they are retained either by their as yet unknown modification (*) and/or by interfering with a cellular protein. Alternatively, the retained complexes might be delivered to dead-end vesicles (dotted line).
We propose that gp40 triggers or catalyzes a reaction that results in the arrest of the MHC class I molecules in the ERGIC/cis–Golgi compartment. Therefore, the question arises of what marks the MHC class I complexes for retention. We have no evidence that the retained MHC class I molecules are misfolded or incompletely assembled because such molecules would be degraded rapidly in the cytosol by the proteasome (Hughes et al., 1997). Originally this route of MHC class I dislocation and degradation was described by the action of US2 and US11 of HCMV (Wiertz et al., 1996a), which is now known to be a general fate of proteins that fail to pass the ER quality control (Hammond and Helenius, 1995; Wiertz et al., 1996b). In contrast, gp40-retained mouse MHC class I molecules consist of the complete trimolecular complex of heavy chain, β2m and peptide (Del Val et al., 1992), are very stable, with an increased half-life (Figure 8), and they are not degraded by proteasome-dependent proteolysis in the cytosol (data not shown).
We hypothesize that gp40 induces an alteration of the MHC class I complexes, which could be any post-translational modification, e.g. phosphorylation of amino acids or carbohydrates and the lack of β2m exchange (see, for example, Figures 3, 5 and 8) could indicate a conformational change of the retained MHC class I molecules. A modification that defines the subcellular localization of the retained MHC class I molecules is supported by the relocation of MHC molecules in the ERGIC after BFA release in the absence of gp40 (Figure 10). The modification might inhibit binding of MHC class I molecules to an obligate transport receptor, acting as a positive selecting device, such as cargo receptors of the p24 family of proteins (Fiedler et al., 1996), thereby excluding them from further transport. Alternatively, the modification might serve as an ERGIC retention signal which connects proteins to the retrograde transport machinery for permanent retrieval (Rothman and Wieland, 1996). Currently, the compartment of MHC class I residence is not clearly defined. Although the turnover of the arrested MHC class I molecules seems to be decreased further by treatment with BFA and leupeptin, we never found endo H-resistant MHC class I complexes. Since the structures in which the retained MHC class I complexes accumulate do not swell upon chloroquine treatment (data not shown), they probably do not belong to the endosome/lysosome system (Dunn, 1990). Thus, the retained complexes might be sequestered in a compartment similar to Russell bodies (Valetti et al., 1991), which turns over very slowly (Figure 11).
Materials and methods
Cloning procedures and expression of gp40, deletion mutants and chimeric proteins
To obtain cell transfectants, the m152 open reading frame (ORF) was cloned by SalI–XhoI from m152/pBK-CMV (Ziegler et al., 1997) into the SalI restriction site of B45-Neo, which was kindly provided by Dr E.R.Podack (Ohe et al., 1995). The plasmids were transfected into embryonic mouse NIH 3T3 fibroblasts (ATCC CRL 1658) by electroporation and selected with 500 μg/ml G418.
For the generation of recombinant vaccinia viruses, the m152 gene sequence (DDBJ/EMBL/GenBank accession No. U53330) was cloned from the EcoRI O fragment of the MCMV EcoRI plasmid library, kindly provided by Dr D.Spector (University of California, San Diego, CA). The m152 ORF was cloned into the vector pCDM7 (Aruffo and Seed, 1987) by three-fragment ligation: the NheI–XhoI fragment of the EcoRI O plasmid was ligated with the XhoI–BamHI and XbaI–BamHI fragments of the vector pCDM7. The m152 3′ deletion mutants were generated by PCR using the EcoRI O fragment as template. The forward primer (5′gtc tcc gcc gta ctc tcc cga cgt3′, PCR1for), binding at the BssHII site within the m152 ORF, and the following reverse primers were used: 5′-cgcgggctcgagtcattatcgtccaccagaatacat-3′ (Δ250), 5′-cgcgggctcgagtcattaagcgtaccatcccgtcgg-3′ (Δ280), 5′-cgcgggctcgagtcattaatcgatcgacgacaacag-3′ (Δ300), 5′-cgcgggctcgagtcattagatggagtactccgcgga-3′ (Δ329) and 5′-cgcgggctcgagtcattacttcaccagatacatgaa-3′ (Δ353). The PCR products were cloned using BssHII and XhoI restriction sites into the plasmid m152-pCDM7. The m152 deletion constructs were subcloned into the vector pBREP, a modified B45-Neo plasmid, by HindIII–XhoI and then transferred as AvrII–BamHI fragments into the NheI–BamHI restriction sites of p7.5K131ad. The plasmid p7.5K131ad was generated by insertion of an adaptor (forward: 5′-gatctcctaggactagtgatatcggatccg-3′; reverse: 5′-aattcggatccgatatcactagtcctagga-3′) into the plasmid p7.5K131 (Cranage et al., 1986) as a new multiple cloning site. The chimeric protein Δ329–CD4tm was cloned by PCR with the above-mentioned BssHII forward primer and a 138 bp reverse primer (5′-cgcgggctcgagttatcaccggca– cctgacacagaagaagatgcctagcccaatgaaaagcaggaggccggcgacgccccccagcacaa– tcagggccatcccgccgtcgacgatggagtactccgcggagacgggagg-3′). The reverse primer codes for nine amino acids of the membrane-proximal luminal part of gp40, a four amino acid spacer (VDGG), 24 amino acids of the transmembrane region of CD4 (DDBJ/EMBL/GenBank accession No. M35160) and three downstream amino acids (RCR) of the cytoplasmic tail of CD4. The PCR product was cloned by BssHII–XhoI into the plasmid m152/p7.5K131ad. For the generation of the chimeric protein Δ353–gp48ct, the cytoplasmic tail of gp48 (MCMV gene m06 ORF 5327:6339) was fused to amino acid residue 353 of gp40 by overlapping PCR. For the first PCR, the BssHII forward primer (PCR1for) and the reverse primer 5′-cttcaccagatacatgaagacg-3′ (PCR1rev) were used to amplify the m152 sequence from the EcoRI O fragment. To amplify the m06 cytoplasmic tail sequence and to generate a connection to the m152 sequence, the second PCR was performed on MCMV genomic DNA, using the forward primer 5′-cgtcttcatgtatctggtgaagggccgcgagccgctagctagactgc-3′ (PCR2for) and the reverse primer 5′-cgcgggctcgagtttattatttggtaagcaagggggaagtg-3′ (PCR2rev). To fuse both sequences, a subsequent PCR was performed on both PCR products using the forward primer PCR1for and the reverse primer PCR2rev. In similar fashion, the internal deletion mutants Δ300–326 and Δ280–326 were generated using the BssHII primer (PCR1for) and the reverse primers 5′-gaagaagatggagtactcatcgatcgacgacaacag-3′ (Δ300–326) and 5′-gaagaagatggagtactcag– cgtaccatcccgtcgg-3′ (Δ280–326), respectively. The same template was used for the second PCR using the forward primer 5′-gagtactccatcttc– ttc-3′ and the m152-reverse primer 5′-cgcgggctcgagttatcaccacacgcggcag– ttg-3′. The third PCR was performed on the first and the second PCR products using the PCR1for primer and the m152-reverse primer. The resulting PCR products were cloned by BssHII–XhoI into the plasmid m152/p7.5K131ad, 3′ to the 7.5K promotor. The constructs were then used to generate recombinant vaccinia viruses after homologous recombination with the vaccinia strain Copenhagen. Vaccinia recombinants were selected by infection of HU TK– 143 cells in the presence of 100 μg/ml bromodeoxyuridine (Sigma, Munich, Germany).
Cells
NIH 3T3 (ATCC CRL 1658) cells were cultured in Dulbecco‘s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) newborn calf serum (NCS). NIH 3T3 fibroblasts transfected with the B45-Neo vector were cultured in the same medium supplemented with 500 μg/ml G418 (Gibco-BRL). The B45-Neo-transfected subclone B12 of an immortalized cell line of BALB/c fibroblasts (Del Val et al., 1991) was cultured as above, except that medium was supplemented with 10% (v/v) fetal calf serum instead of NCS.
Generation of monoclonal antibodies
Monoclonal antibodies against gp40 (gpM3D10 and gpM6D4) were generated using a GST–m152 fusion protein. The m152 ORF was subcloned into pGEX-4T-1 (Pharmacia Biotech, Uppsala, Sweden) using BamHI and XhoI restriction sites. The resulting GST–m152 fusion protein was expressed in Escherichia coli BL-21 and purified using glutathione–Sepharose (Pharmacia) as described in the manufacturer's protocol (Smith and Johnson, 1988). Monoclonal antibodies were generated as described earlier (Kremmer et al., 1997).
Reagents
The protease inhibitor leupeptin (Boehringer, Mannheim) was used at a final concentration of 200 μM. BFA (Sigma) was dissolved in ethanol at a concentration of 5 mg/ml and used in a 1:1000 dilution. The proteasome inhibitor lactacystin (Sigma) was used at a final concentration of 20 μM. The protein synthesis inhibitor cycloheximide (Sigma) was prepared as a 2 mg/ml stock solution and used at a final concentration of 50 μg/ml.
Immunofluorescence
Immunofluorescence was performed as described previously (Ziegler et al., 1997). For double immunofluorescence staining of gp40 and MHC class I molecules, the rat mAb gpM3D10 (anti-gp40) and the mouse mAb SF1.1.1 (anti H2-Kd, ATCC HB159) were used. Fluorescein isothiocyanate (FITC)-conjugated goat anti-rat immunoglobulin G (IgG) F(ab)2 fragments and lissamine rhodamine (LRSC)-conjugated goat anti-mouse IgG (Dianova, Hamburg) served as secondary antibodies. For staining of endosomes/lysosomes, cells were incubated with DAMP (Molecular Probes, Leiden, The Netherlands) for 30 min and chased for 45 min (Orci et al., 1994). DAMP was detected with Texas red-X-conjugated rabbit anti-dinitrophenyl-keyhole limpet hemocyanin (Molecular Probes). Specific staining of medial–Golgi structures was performed by using a rabbit polyclonal antibody directed to ManII (Moremen et al., 1991). As a specific marker for the ERGIC, a rabbit polyclonal antibody against p58 was used (Saraste and Svensson, 1991). The rabbit polyclonal antibodies were identified with Texas red-X-conjugated goat anti-rabbit IgG (Dianova, Hamburg). The immunofluorescence staining was analyzed by confocal laser scanning microscopy (Leica, Heerbrugg, Switzerland; microscope DMIRB; scanner TCSNT).
Metabolic labeling, immunoprecipitation and endo H treatment
Immunoprecipitation was performed as described previously (Del Val et al., 1992). In brief, cells were labeled with [35S]cysteine/methionine (Amersham, Braunschweig, Germany) at a concentration of 500 μCi/ml at 37°C for 30 min and chased in the presence of 10 mM non-labeled methionine. Cells were lysed in buffer [140 mM NaCl, 5 mM MgCl2, 20 mM Tris pH 7.6 and 1 mM phenylmethylsulfonyl fluoride (PMSF)] containing 1% (w/v) digitonin (Calbiochem, Bad Soden, Germany) or 1% (v/v) NP-40 (Sigma). Cell lysates were pre-cleared by adding anti-rabbit and anti-mouse IgG, respectively. Immune complexes were retrieved with protein A–Sepharose. Quantitative precipitations of MHC molecules were performed with mAb 28-14-8S (ATCC HB27) specific for Lq. To ensure quantitative retrieval of immune complexes, the lysates were incubated twice with protein A–Sepharose. gp40 was precipitated with peptide antiserum (Ziegler et al., 1997) or mAbs gpM3D10 and gpM6D4, respectively. For endo H (Boehringer, Mannheim, Germany) digestion, the immune complexes were eluted from protein A–Sepharose by boiling in 0.1% SDS/0.1 M β-mercaptoethanol. The samples were mock treated or incubated with 2 mU of endo H at 37°C overnight. Digestion was stopped by addition of sample buffer. Subsequently, the precipitates were analyzed by SDS–PAGE.
Acknowledgments
Acknowledgements
We are grateful to Dr R.Podack for the B45-Neo plasmid, Dr W.Brune for transfectants and Dr J.Haas for reading the manuscript. This study was supported by grants of the Deutsche Forschungsgemeinschaft and the Bundesministerium für Bildung und Forschung.
References
- Ahn K., Angulo, A., Ghazal, P., Peterson, P.A., Yang, Y. and Früh, K. (1996a) Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc. Natl Acad. Sci. USA, 93, 10990–10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K., Meyer, T.H., Uebel, S., Sempe, P., Djaballah, H., Yang, Y., Peterson, P.A., Früh, K. and Tampe, R. (1996b) Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J., 15, 3247–3255. [PMC free article] [PubMed] [Google Scholar]
- Ahn K., Gruhler, A., Galocha, B., Jones, T.R., Wiertz, E.J., Ploegh, H.L., Peterson, P.A., Yang, Y. and Früh, K. (1997) The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity, 6, 613–621. [DOI] [PubMed] [Google Scholar]
- Aruffo A. and Seed, B. (1987) Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc. Natl Acad. Sci. USA, 84, 8573–8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H.-G. (1996) Subversion of the MHC class I antigen-presentation pathway by adenoviruses and herpes simplex viruses. Trends Microbiol., 4, 107–112. [DOI] [PubMed] [Google Scholar]
- Burgert H.-G. and Kvist, S. (1985) An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell, 41, 987–997. [DOI] [PubMed] [Google Scholar]
- Chee M.S., et al. (1990) Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol., 154, 125–169. [DOI] [PubMed] [Google Scholar]
- Cranage M.P., et al. (1986) Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J., 5, 3057–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Val M., Schlicht, H.J., Ruppert, T., Reddehase, M.J. and Koszinowski, U.H. (1991) Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell, 66, 1145–1153. [DOI] [PubMed] [Google Scholar]
- Del Val M., Hengel, H., Hacker, H., Hartlaub, U., Ruppert, T., Lucin, P. and Koszinowski, U.H. (1992) Cytomegalovirus prevents antigen presentation by blocking the transport of peptide-loaded major histocompatibility complex class I molecules into the medial–Golgi compartment. J. Exp. Med., 176, 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. (1990) Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J. Cell Biol., 110, 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K., Veit, M., Stamnes, M.A. and Rothman, J.E. (1996) Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science, 273, 1396–1399. [DOI] [PubMed] [Google Scholar]
- Früh K., Ahn, K., Djaballah, H., Sempe, P., van Endert, P., Tampe, R., Peterson, P.A. and Yang, Y. (1995) A viral inhibitor of peptide transporters for antigen presentation. Nature, 375, 415–418. [DOI] [PubMed] [Google Scholar]
- Guarnieri F.G., Arterburn, L.M., Penno, M.B., Cha, Y. and August, J.T. (1993) The motif Tyr-X-X-hydrophobic residue mediates lysosomal membrane targeting of lysosome-associated membrane protein 1. J. Biol. Chem., 268, 1941–1946. [PubMed] [Google Scholar]
- Hammond C. and Helenius, A. (1995) Quality control in the secretory pathway. Curr. Opin. Cell Biol., 7, 523–529. [DOI] [PubMed] [Google Scholar]
- Hengel H. and Koszinowski, U.H. (1997) Interference with antigen processing by viruses [see comments]. Curr. Opin. Immunol., 9, 470–476. [DOI] [PubMed] [Google Scholar]
- Hengel H., Koopmann, J.O., Flohr, T., Muranyi, W., Goulmy, E., Hammerling, G.J., Koszinowski, U.H. and Momburg, F. (1997) A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity, 6, 623–632. [DOI] [PubMed] [Google Scholar]
- Hengel H., Brune, W. and Koszinowski, U.H. (1998) Immune evasion by cytomegalovirus—survival strategies of a highly adapted opportunist. Trends Microbiol., 6, 190–197. [DOI] [PubMed] [Google Scholar]
- Hill A., Jugovic, P., York, I., Russ, G., Bennink, J., Yewdell, J., Ploegh, H. and Johnson, D. (1995) Herpes simplex virus turns off the TAP to evade host immunity. Nature, 375, 411–415. [DOI] [PubMed] [Google Scholar]
- Hughes E.A., Hammond, C. and Cresswell, P. (1997) Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome. Proc. Natl Acad. Sci. USA, 94, 1896–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes E.N. and August, J.T. (1981) Characterization of plasma membrane proteins identified by monoclonal antibodies. J. Biol. Chem., 256, 664–671. [PubMed] [Google Scholar]
- Jackson M.R., Nilsson, T. and Peterson, P.A. (1990) Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J., 9, 3153–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.C. and Hill, A.B. (1998) Herpesvirus evasion of the immune system. Curr. Top. Microbiol. Immunol., 232, 149–177. [DOI] [PubMed] [Google Scholar]
- Jones T.R., Wiertz, E.J., Sun, L., Fish, K.N., Nelson, J.A. and Ploegh, H.L. (1996) Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc. Natl Acad. Sci. USA, 93, 11327–11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijnen M.F., Huppa, J.B., Lucin, P., Mukherjee, S., Farrell, H., Campbell, A.E., Koszinowski, U.H., Hill, A.B. and Ploegh, H.L. (1997) A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. EMBO J., 16, 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremmer E., Ohst, K., Kiefer, J., Brewis, N. and Walter, G. (1997) Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: abundant expression of both forms in cells. Mol. Cell. Biol., 17, 1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner P.J., Karttunen, J.T., Wilkinson, G.W. and Cresswell, P. (1997) The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc. Natl Acad. Sci. USA, 94, 6904–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen K.W., Touster, O. and Robbins, P.W. (1991) Novel purification of the catalytic domain of Golgi α-mannosidase II. Characterization and comparison with the intact enzyme. J. Biol. Chem., 266, 16876–16885. [PubMed] [Google Scholar]
- Ohe Y., Zhao, D., Saijo, N. and Podack, E.R. (1995) Construction of a novel bovine papillomavirus vector without detectable transforming activity suitable for gene transfer. Hum. Gene Ther., 6, 325–333. [DOI] [PubMed] [Google Scholar]
- Orci L., Halban, P., Perrelet, A., Amherdt, M., Ravazzola, M. and Anderson, R.G. (1994) pH-independent and -dependent cleavage of proinsulin in the same secretory vesicle. J. Cell Biol., 126, 1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pääbo S., Weber, F., Nilsson, T., Schaffner, W. and Peterson, P.A. (1986) Structural and functional dissection of an MHC class I antigen-binding adenovirus glycoprotein. EMBO J., 5, 1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer E. and Cresswell, P. (1998) Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol., 16, 323–358. [DOI] [PubMed] [Google Scholar]
- Rawlinson W.D., Farrell, H.E. and Barrell, B.G. (1996) Analysis of the complete DNA sequence of murine cytomegalovirus. J. Virol., 70, 8833–8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner J.C. and Pelham, H.R. (1997) Transmembrane domain-dependent sorting of proteins to the ER and plasma membrane in yeast. EMBO J., 16, 1832–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch U., Muranyi, W., Lucin, P., Burgert, H.G., Hengel, H. and Koszinowski, U.H. (1999) A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J., 18, 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.E. and Wieland, F.T. (1996) Protein sorting by transport vesicles. Science, 272, 227–234. [DOI] [PubMed] [Google Scholar]
- Saraste J. and Svensson, K. (1991) Distribution of the intermediate elements operating in ER to Golgi transport. J. Cell Sci., 100, 415–430. [DOI] [PubMed] [Google Scholar]
- Scheel J., Pepperkok, R., Lowe, M., Griffiths, G. and Kreis, T.E. (1997) Dissociation of coatomer from membranes is required for brefeldin A-induced transfer of Golgi enzymes to the endoplasmatic reticulum. J. Cell Biol., 137, 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P.O. (1983) Inhibitors of lysosomal function. Methods Enzymol., 96, 737–764. [DOI] [PubMed] [Google Scholar]
- Signäs C., Katze, M.G., Persson, H. and Philipson, L. (1982) An adenovirus glycoprotein binds heavy chains of class I transplantation antigens from man and mouse. Nature, 299, 175–178. [DOI] [PubMed] [Google Scholar]
- Smith D.B. and Johnson, K.S. (1988) Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene, 67, 31–40. [DOI] [PubMed] [Google Scholar]
- Smith G.L. (1994) Virus strategies for evasion of the host response to infection. Trends Microbiol., 2, 81–88. [DOI] [PubMed] [Google Scholar]
- Thäle R., Szepan, U., Hengel, H., Geginat, G., Lucin, P. and Koszinowski, U.H. (1995) Identification of the mouse cytomegalovirus genomic region affecting major histocompatibility complex class I molecule transport. J. Virol., 69, 6098–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valetti C., Grossi, C.E., Milstein, C. and Sitia, R. (1991) Russell bodies: a general response of secretory cells to synthesis of a mutant immunoglobulin which can neither exit from, nor be degraded in, the endoplasmic reticulum. J. Cell Biol., 115, 983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiertz E.J., Jones, T.R., Sun, L., Bogyo, M., Geuze, H.J. and Ploegh, H.L. (1996a) The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell, 84, 769–779. [DOI] [PubMed] [Google Scholar]
- Wiertz E.J., Tortorella, D., Bogyo, M., Yu, J., Mothes, W., Jones, T.R., Rapoport, T.A. and Ploegh, H.L. (1996b) Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction [see comments]. Nature, 384, 432–438. [DOI] [PubMed] [Google Scholar]
- Ziegler H., Thäle, R., Lucin, P., Muranyi, W., Flohr, T., Hengel, H., Farrell, H., Rawlinson, W. and Koszinowski, U.H. (1997) A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis–Golgi compartments. Immunity, 6, 57–66. [DOI] [PubMed] [Google Scholar]