Abstract
Background
Echinacea is widely used to treat common cold.
Objective
To assess potential benefits of echinacea as common cold treatment.
Design
Randomized controlled trial with four parallel groups: 1) no pills, 2) placebo pills (blinded), 3) echinacea pills (blinded), or 4) echinacea pills (open-label). (NCT00065715)
Setting
Community-based trial.
Participants
People aged 12 to 80 years with new onset common cold.
Interventions
Extracts of Echinacea purpurea and E. angustifolia root were used to make tablets standardized to alkamide content. Indistinguishable placebo tablets contained only inert ingredients.
Measurements
The primary outcome was area-under-the-curve global severity, with severity assessed twice daily by self report on the Wisconsin Upper Respiratory Symptom Survey (WURSS-21). Secondary outcomes included interleukin-8 and neutrophil count from nasal wash assessed at intake and two days later.
Results
Of 719 enrolled, 713 completed the protocol. Participants were 64% female and 88% white, with mean age 33.7 years. Mean global severity was 236 and 258 for blinded and unblinded echinacea, 264 for blinded placebo, and 286 for those without pills. Contrasting the two blinded groups yields a 28 point (95% CI = −69 to 13) trend toward benefit for echinacea (p=0.089). Mean illness duration for the blinded and unblinded echinacea groups was 6.34 and 6.76 days, respectively, compared to 6.87 days for blinded placebo and 7.03 for no pills. Contrasting blinded groups yields a 0.53 day (95% CI = −1.25 to 0.19) trend toward benefit (p = 0.075). Median change interleukin-8 (pg/mL) and neutrophil cell count were: no pills (30, 1), blinded placebo (39, 1), blinded echinacea (58, 2), and open-label echinacea (70, 1), also not statistically significant.
Limitations
Higher-than-expected variability limited power to detect small-but-potentially-important benefits.
Conclusions
The observed shorter illness duration and lower severity seen in the echinacea groups were not statistically significant. These results do not support the ability of this dose of this echinacea formulation to substantively change the course of the common cold.
Keywords: clinical trial, common cold, echinacea, upper respiratory infection
Introduction
Acute viral respiratory infection (common cold) is humanity’s most frequent illness. Etiologic agents include rhinovirus, coronavirus, influenza, parainfluenza, respiratory syncytial virus, adenovirus, enterovirus, and metapneumovirus.(1–3) While influenza-caused illness is the most serious and is often categorized separately, symptoms are usually indistinguishable from those produced by other viruses.(4–7) Excluding influenza, economic costs of acute respiratory infection are estimated around $40 billion, putting acute respiratory infection in the top 10 most expensive illnesses.(8) Most of this impact comes from the estimated 20 million doctor visits and 40 million school and work days lost each year. Available treatments are at best modestly effective at reducing symptoms.(9) None have been proven to shorten illness duration.
The botanical genus Echinacea is native to North America, where indigenous peoples used various echinacea preparations for many different illnesses.(10) Nevertheless, much of the foundational biomedical research on echinacea was done in Germany, where the plant was introduced in the 1920s, and used for a variety of illnesses, including respiratory infection.(11;12) Immunoactivity, including macrophage activation and cytokine expression, has been widely reported,(13–22) but specific pathways, pharmacokinetics, and mechanism of action of the various phytochemical constituents are incompletely understood.(23–29) Most commercially available echinacea products derive primarily from two species, Echinacea purpurea and E. angustifolia,(30;31) and can be divided into two general categories. The first consists of stabilized fresh juice of aerial parts of E. purpurea, and is rich in hydrophilic derivatives such as polysaccharides and glycoproteins. The second is an aqueous-ethanolic extract of root material (E.angustifolia and/or E. purpurea) and is richer in lipophilic consituents such as alkamides. Other potentially active constituents, such as echinacoside, cynarin, and caffeic, caftaric, chichoric and chlorogenic acids, are found in various concentrations among various formulations. When designing the study in 2002 we decided to use a root-based alkamide-rich preparation. Research published since that time has tended to support that decision.(32–38)
By the mid-1990s, when echinacea had become popular in the U.S., several hundred scientific studies on echinacea had already been published, including a dozen randomized trials testing echinacea for preventing or treating common cold.(39) Virtually all of these early trials reported either statistically significant benefit or trends toward benefit.(40) However, all were manufacturer-sponsored and all were of moderate-to-poor quality. In this context we found it necessary to conduct our own trial in 1999–2000, which turned out flatly negative.(41) Since then, there have been several new trials, a few positive,(42–44) and a few negative.(45–48) Systematic reviews and meta-analyses have varied in terms of inclusion criteria, review methods, results and interpretation.(49–53) With the question of echinacea’s effectiveness still unresolved, we designed and conducted the trial reported here.
Methods
Research question
This trial was sponsored by the National Center for Complementary and Alternative Medicine at the National Institutes of Health. We took the somewhat unconventional approach of asking three independent research questions: 1) Are there placebo effects associated with blinded versus open-label pills? 2) Do doctor-patient interactions influence cold outcomes? 3) Are there effects attributable specifically to echinacea, as assessed by blinded comparison? The current paper addresses this third specific aim. Papers addressing the first two aims will be available elsewhere.
Design overview
This trial employed a two-way factorial design, randomizing subjects in one direction to a 33.3% chance of no clinical interaction, standard clinical interaction, or enhanced clinical interaction. In the other direction, subjects were randomized to no pills, blinded placebo, blinded echinacea, or unblinded open-label echinacea, with a 25% chance of each condition. An article explaining rationale and methodology has been published.(54)
Setting and participants
The study was conducted at two sites within Dane County, Wisconsin, USA. Study promotion included newspaper advertising, posters, community talks, targeted mailings, emails, and word-of-mouth. Prospective participants called an advertised phone number and were screened for eligibility. Those eligible were met in person for informed consent, following procedures approved by the University of Wisconsin (U.W.) Institutional Review Board. After consent, participants rated themselves on several self-report questionnaires. An envelope was then opened to reveal allocation to no pills, blinded pills, or open-label echinacea. The first dose of pills was taken at the consent visit. Participants self-rated symptoms twice daily until their colds had resolved, up to a maximum of 14 days. Nasal wash collected at enrollment and two days later was analyzed for interleukin-8 (IL-8) and neutrophil count.(55–58) Participants were met for an exit interview after their illness had resolved.
Inclusion/exclusion
Prospective participants were required to answer “Yes” to either: “Do you think that you have a cold?” or “Do you think you are coming down with a cold?” Using Jackson’s criteria,(59) participants had to report at least one of four cold symptoms: A) nasal discharge; B) nasal obstruction; C) sneezing; and/or D) sore throat. Symptoms had to start within 36 hours before enrollment. Participants needed a Jackson score of 2 or higher, summing symptom scores, with 0=absent, 1=mild, 2=moderate, 3=severe. The eight Jackson symptoms include the four mentioned above, plus headache, malaise, chilliness, and cough. Participants had to be 18 years or older, or 12 to 17 with parental permission. Exclusions included use of antibiotics, antivirals, nasal steroids, decongestants, antihistamines, combination cold formulas, echinacea, zinc or vitamin C. To avoid confounding from allergy or asthma symptoms, we excluded anyone with a history of allergic rhinitis who reported sneezing or itching of the nose or eyes, and anyone with a history of asthma who reported current cough, wheezing or shortness of breath. People with autoimmune and immune deficiency disease were excluded by self-report, as were pregnant women.
Randomization, allocation & blinding
SAS software was used to generate a single block of 804 unique identification numbers so that each of 12 cells (3 clinician groups by 4 pill groups) was represented equally. Using these codes, the U.W. Hospitals Pharmaceutical Research Center Investigational Drug Service prepared consecutively numbered sealed envelopes directing allocation. An envelope-within-envelope strategy was employed, so that opening of the larger outer envelope by the research assistant directly following consent revealed whether the participant was to receive no pill, blinded pill, or open label pill. Allocation concealment for the two blinded pill groups was accomplished using identical-appearing coated tablets in identical-appearing plastic pill bottles. For the two thirds of the sample who would see a clinician, a second smaller envelope directing allocation to standard or enhanced visit was opened by the study clinician prior to entering the exam room. The randomized allocation key was not shared with investigators until after all data were collected, entered, cleaned, and analysis strategies determined. Blinding was tested at exit interview by asking participants to which group they thought they had been assigned.
Echinacea & placebo
Echinacea and identical placebo tablets were manufactured by MediHerb, Warwick, Australia. Echinacea tablets contained the equivalent of 675 mg E. purpurea root standardized to 2.1mg alkamides and 600 mg E.angustifolia root standardized to 2.1mg alkamides. Tableting excipients included calcium acid phosphate, cellulose, silica, sodium starch glycollate, hypromellose and magnesium stearate. Placebo and echinacea tablets contained the same proportions of inert ingredients, and were covered with identical digestible coatings.
Two tablets were ingested at enrollment, followed by two-tablet doses three more times within 24 hours of enrollment. Dosing then went to one tablet four times daily for the next four days. Thus, each participant ingested the equivalent of 10.2g of dried echinacea root during the first 24 hours and the equivalent of 5.1g during each of the next four days.
Outcomes and follow-up
The primary outcome was prospectively defined as area-under-the-curve global severity, with duration and severity assessed by twice daily self report. Duration began at enrollment and continued through the last time the participant answered “Yes” to “Do you think you still have a cold?” The date and time of filling out questionnaires was recorded, allowing duration to be quantified as a continuous measure. To confirm that the illness had ended, this last “Yes” had to be followed by “No” for two days in a row. We chose to limit monitoring to a maximum of 14 days to reduce potential bias from extended length illnesses.
Illness severity was assessed twice daily on the Wisconsin Upper Respiratory Symptom Survey (WURSS-21), a validated illness-specific quality-of-life outcome instrument.(60;61) WURSS-21 items assesses symptom severity and functional impairment with 1=very mild, 3=mild, 5=moderate and 7=severe. The first item assesses overall illness severity, and the last item assesses change-since-yesterday. Summing scores on the intervening 19 items provides a global measure of illness severity. Summing across time points yields area-under-the-curve global severity, which we calculated using trapezoidal approximation.
Secondary outcomes included self-report on psychosocial questionnaires and biomarkers of immune response and inflammation. Self-report measures included general health-related quality of life, perceived stress, interpersonal support, optimism, and mood states. General health was assessed daily using the Short Form (SF-8) scale,(62) a 24-hour recall version of the highly validated SF-36. The SF-8 yields separate physical and mental health scores using an item-weighted algorithm.(62) Perceived stress was assessed at baseline, day 3, and exit using Cohen’s Perceived Stress Scale (PSS-4),(63–65) and daily using a 100mm visual analogue scale that we developed for this study. Interpersonal support and optimism were measured at baseline, day 3, and exit using the Ryff Personal Relationships (PR-9) scale (66) and the Life Orientation Test (LOT-6).(67)
Adverse effects & safety monitoring
While allergic reactions to echinacea have been reported, there are no known major or dose-dependent risks of adverse effects.(50) We assessed possible side effects by asking participants at the exit interview whether they had experienced any of the following at any time during their illness: bad taste, diarrhea, headache, nausea, rash, or stomach upset. In addition, we used open-ended questions to ask about possible side effects at the day 3 follow-up visit and during telephone contact. A data safety and monitoring committee met once yearly to review enrollment and side effect data.
Data collection, entry & cleaning
Questionnaire booklets filled out by participants were scanned into electronic files by the U.W. Educational Testing Service. Data collected during telephone monitoring were recorded on paper, then hand-entered twice, with resolution of discrepancies by comparison to paper.
Statistical analysis
The trial was designed to have 80% power to detect a 20% between-group difference in area-under-the-curve global severity. A priori power calculations were based on data collected with a predecessor instrument of the WURSS-21. Assuming α=0.05, β=0.20, one-sided testing, and proportionally stable standard deviations, the protocol planned enrollment of N=800 participants to achieve N=720 protocol completers. Intervention groups were kept blinded throughout data cleaning, assessment of missingness and response, and initial descriptive analyses. To arrive at area-under-the-curve global severity, we first averaged morning and evening scores for each item of the WURSS-21. If either morning or evening data were missing, existing data was used. We used Little’s Missing Completely at Random (MCAR) test to assess possible patterns of missingness for WURSS-21 items.(68) Where appropriate, we used a multiple imputation strategy using the expectancy maximization algorithm, as outlined by Schafer.(69) For data with skewed distribution, Box Cox transformation was considered. Primary efficacy analysis was done by comparing results in the blinded echinacea and blinded placebo groups. Contrasts of group means and medians were done with T-test and Mann-Whitney U test, respectively. Potential treatment effects were assessed with a general linear model (GLM) (70) using the NCSS statistical software program.(71) Covariates designated as potential confounders and controlled for in the GLM model included: duration of symptomatic illness prior to enrollment, illness severity at enrollment, age, gender, ethnicity, education, income, smoking status, mental and physical general health, and allocation to clinician-related visits. To test blinding we used Fisher’s exact test of proportional difference. To avoid hazards associated with multiple testing, we chose to limit statistical testing to primary outcomes in primary comparison groups, and to compare secondary outcomes in terms of confidence intervals rather than p-values.
Funding
The trial was sponsored by the National Center for Complementary and Alternative Medicine at the National Institutes of Health (grant # R01AT001428). MediHerb (Queensland, Australia) provided the echinacea and placebo tablets, and conducted phytochemical content analysis, but did not contribute financially.
Results
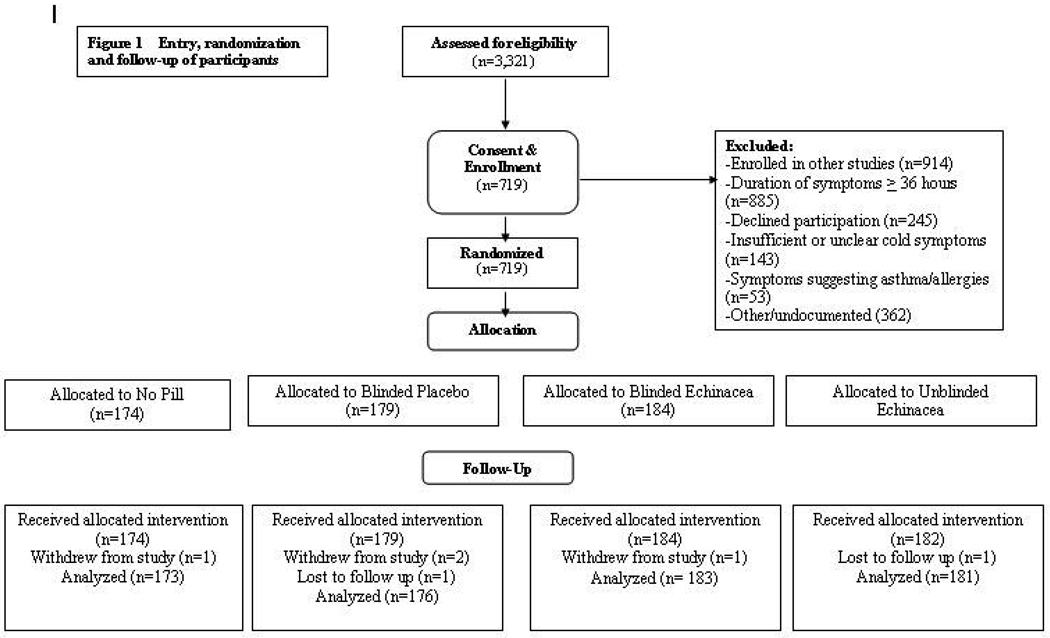
Enrollment opened in January 2004 and ended in August 2008. Of the 3,321 screened, 719 were enrolled and randomized (Figure 1). Retention was high. Two people were lost to follow-up, and four withdrew before primary outcome data could be gathered. Reasons for withdrawal were: too sick or too busy to fill out questionnaires, and/or desire to take non-protocol medications. Approximately 98% of intended data were collected. The largest data gap was with nasal wash, where 33 people either refused the second nasal wash or failed to return within time limits (24 to 72 hours after first wash). Following Little’s MCAR test, there were no discernible patterns of missingness in the 0.27% of missing WURSS items. Imputation of WURSS-21 items and calculation of global severity and duration values were done prior to unblinding, using methods outlined above.
Figure 1.
Participants were 64% female and 88% white, with 84% reporting at least some college education. Age ranged from 12 to 80 years, with mean 33.7 and standard deviation 14.4. Some 12.8% were current smokers. Baseline measures appeared similar across the four groups (Table 1). There were 522 enrolled in Madison and 197 in Verona. Comparing mean age (33.3 vs 34.9; p=0.20), gender (63.4% vs 66.0% female; p=0.52), and those with at least some college education (84.6% vs 82.3%; p=0.47), no significant between-site differences were found.
Table 1.
Baseline characteristics of participants
| All n=719 |
No pill n=174 |
Unblinded Echinacea n=182 |
Blinded Placebo n=179 |
Blinded Echinacea n=184 |
|
|---|---|---|---|---|---|
| Age: mean (SD) | 33.7 (14.4) | 32.3 (14.2) | 33.9 (14.5) | 33.2 (13.5) | 35.4 (15.3) |
| Gender: % female | 64.1 | 60.9 | 65.9 | 63.7 | 65.8 |
| Race/ethnicity: % non-white | 12.1 | 13.8 | 8.2 | 12.3 | 14.1 |
| Tobacco: % current smoker | 12.8 | 14.4 | 11.6 | 11.2 | 14.1 |
| Lower income: % reporting household income ≤ $25,000 | 35.9 | 40.4 | 32.6 | 35.7 | 35.1 |
| Higher education: % with at least some college | 84.0 | 84.0 | 86.4 | 85.6 | 80.0 |
| Hours of symptoms prior to enrollment: mean (SD) | 22.8 (8.6) | 23.6 (8.0) | 22.3 (9.2) | 23.3 (8.5) | 22.0 (8.5) |
| WURSS-21 at enrollment: mean (SD) | 85.4 (51.4) | 84.3 (50.0) | 82.9 (46.6) | 89.8 (54.4) | 84.7 (54.3) |
| SF-8 physical health: mean (SD) | 48.5 (6.1) | 48.7 (6.2) | 48.7 (5.5) | 48.2 (6.1) | 48.2 (6.6) |
| SF-8 mental health: mean (SD) | 43.5 (9.7) | 42.7 (9.8) | 43.7 (10.1) | 43.4 (9.1) | 44.3 (9.6) |
SD = Standard deviation
Primary outcomes
Average area-under-the-curve global severity and illness duration were lower in the blinded and open-label echinacea groups than in either the blinded placebo or no pill groups (Table 2). Mean global severity was 236 and 258 for blinded and unblinded echinacea, respectively, 264 for blinded placebo, and 286 for those without pills. Primary efficacy analysis contrasting global severity in the blinded echinacea and placebo groups yields a mean difference of 28 points (95% CI = −69 to 13). Statistical testing yields T=1.34 and p = 0.089. Because of skewness, the Mann-Whitney U test contrasting median severities of 206 for blinded placebo to 193 for blinded echinacea may be more appropriate, and yields z = 0.97 and p= 0.17. Mean illness duration for the blinded and unblinded echinacea groups was 6.34 and 6.76 days, respectively, compared to 6.87 days for blinded placebo and 7.03 for no pills. Efficacy analysis contrasting illness duration for blinded echinacea vs. blinded placebo yields a mean difference of 0.53 days (95% CI = −1.25 to 0.19), a T-value of 1.97, and p = 0.075. Contrasting the two blinded groups using a general linear model to control for potential confounders also failed to find statistically significant differences (p=0.42 for area-under-the-curve severity; p=0.74 for duration.) Distribution of both global severity was skewed, hence Box Cox transformation was used for that model. Reported p-values are based on one-sided testing and do not adjust for multiple testing.
Table 2.
Primary outcomes: Global severity and duration of illness
| Group assignment | No pill |
Unblinded Echinacea |
Blinded Placebo |
Blinded Echinacea |
Between Blinded Group Differences |
|---|---|---|---|---|---|
| # providing main outcome data | n=173 | n=181 | n=176 | n=183 | |
| Median global severity (95% CI) | 220 (189–238) | 195 (169–213) | 206 (177–256) | 193 (163–218) | −13 (−37.8, 38.4) |
| Mean global severity (SD) | 286 (246) | 258 (214) | 264 (212) | 236 (182) | −28 (−69.0, 13.0) |
| *Adjusted global severity (95% CI) | 10.3 (9.9–10.7) | 10.1 (9.7–10.5) | 10.0 (9.7–10.4) | 10.1 (9.7–10.4) | 0.10 (−0.60, 0.40) |
| Median duration (95% CI) | 6.42 (6.13–7.21) | 6.16 (5.31–6.60) | 6.47 (5.82–7.12) | 6.04 (5.30–6.53) | −0.43 (−1.01, 0.95) |
| Mean duration (SD) | 7.03 (3.49) | 6.76 (3.48) | 6.87 (3.62) | 6.34 (3.31) | −0.53 (−1.25, 0.19) |
| Subset of those enrolled within 24 hours of first symptom | n=80 | n=97 | n=79 | n=95 | |
| Median global severity (95% CI) | 221 (177–277) | 177 (140–213) | 199 (162–259) | 196 (160–250) | −3.0 (−51.5, 49.2) |
| Mean global severity (SD) | 281 (225) | 250 (218) | 257 (207) | 246 (186) | −11.0 (−69.8, 47.8) |
| *Adjusted global severity (95% CI) | 10.6 (9.7–11.6) | 10.1 (9.3–10.8) | 9.7 (8.6–10.7) | 10.1 (9.1–11.1) | 0.41 (−1.83, 1.03) |
| Median duration (95% CI) | 6.66 (6.13–7.30) | 6.15 (5.06–7.00) | 6.38 (4.78–7.37) | 6.07 (4.98–6.68) | −0.31 (−1.13, 1.10) |
| Mean duration (SD) | 6.83 (3.23) | 6.62 (3.47) | 6.67 (3.52) | 6.47 (3.31) | −0.20 (−1.22, 0.82) |
SD = Standard deviation CI = Confidence interval Duration = days of illness
Global severity = area under the time severity curve, with severity assessed by the WURSS-21
Adjusted results from general linear model controlling for: duration of symptoms prior to enrollment, symptom severity at enrollment, age, gender, ethnicity, education, income, smoking status, physical health, mental health, and factorial allocation to clinician-related visits. Distribution of global severity was skewed, hence Box Cox transformation was used to better satisfy statistical assumptions.
Because echinacea is thought to work through immune stimulation with early dosing important, we did a subgroup analysis on the 351 people who were enrolled within 24 hours of their first symptom (Table 2). Compared to either the no pill or blinded placebo groups, illness duration and global severity were lower for both echinacea groups. Nevertheless, none of these between group comparisons in this secondary analysis were statistically significant. Results and conclusions did not change significantly after applying a general linear model, controlling for covariates mentioned above.
Secondary outcomes
Analysis of secondary outcomes did not demonstrate effects clearly attributable to echinacea (Table 3). Nasal neutrophil count and IL-8 in nasal wash tended to rise faster in the two echinacea groups than in either control group, but these differences were not statistically significant. Self-reported health measures including physical and mental health (SF-8), stress (PSS-4), optimism (LOT), and social support (Ryff PR) did not appear to be influenced by random assignment to echinacea.
Table 3.
Secondary outcomes (day 3 assessments)
| No Pill | Unblinded Echinacea |
Blinded Placebo |
Blinded Echinacea |
Between Blinded Group Differences |
|
|---|---|---|---|---|---|
| Biomarker Data | n=164 | n=171 | n=168 | n=170 | |
| Median change IL-8 (95% CI) | 30 (2–89) | 70 (18–134) | 39 (12–106) | 58 (18–105) | 19.0 (−75.2, 72.0) |
| Median change neutrophils (95%CI) | 1 (−1–4) | 1 (0–4) | 1 (−1–4) | 2 (0–5) | 1.0 (−4.0, 3.0) |
| Self-report data (mean) | n=174 | n=182 | n=179 | n=184 | |
| Physical health SF-8 (95%CI) | 48.0 (47.1–49.0) | 47.7 (46.8–48.6) | 46.9 (45.9–48.0) | 47.3 (46.2–48.4) | 0.40 (−1.13, 1.93) |
| Mental health SF-8 (95%CI) | 43.8 (42.3–45.3) | 43.7 (42.2–45.2) | 42.5 (41.0–43.9) | 44.4 (43.1–45.7) | 1.90 (−0.06, 3.86) |
| Feeling thermometer (95%CI) | 60.3 (57.9–62.9) | 62.5 (59.7–65.3) | 62.5 (59.5–65.5) | 63.6 (60.8–66.4) | 1.10 (−2.88, 5.08) |
| Stress (PSS-4) (95%CI) | 4.3 (3.9–4.8) | 4.5 (4.1–4.9) | 4.6 (4.1–5.0) | 4.5 (4.0–5.0) | 0.10 (−0.76, 0.56) |
| Stress (VAS) (95%CI) | 38.3 (34.3–42.3) | 40.0 (36.2–43.8) | 38.0 (34.5–41.5) | 36.6 (32.9–40.3) | 1.40 (−6.33, 3.53) |
| Optimism (LOT-6) (95%CI) | 22.7 (22.1–23.4) | 22.9 (22.4–23.6) | 22.1 (21.5–22.7) | 23.1 (22.5–23.7) | 1.00 (0.16, 1.84) |
| Social support (Ryff PR) (95%CI) | 45.1 (44.1–46.3) | 45.6 (44.6–46.8) | 44.5 (43.1–45.6) | 45.4 (44.2–46.4) | 0.90 (−0.65, 2.45) |
Values for IL-8 and neutrophil count are median change from Day 1 intake to Day 3
Neutrophil counts are number of cells per high power field
IL-8 units are pg/mL
Side effects
Frequency of potential adverse effects was similar (statistically indistinguishable) in the four groups (Table 4). The only possible exception was headache, where 62% of those in the no pill group reported having had a headache at some time during their illness, compared to less than 50% in the three pill groups. Responses to open-ended questions asking about possible side effects during monitoring did not show any patterns of side effects attributable to echinacea.
Table 4.
Potential adverse effects
| No pill n=174 |
Unblinded Echinacea n=182 |
Blinded Placebo n=179 |
Blinded Placebo n=179 |
Between Blinded Group Differences |
|
|---|---|---|---|---|---|
| Bad taste (95%CI) | NA (NA) | 8.9 (4.7–13.1) | 9.1 (7.2–16.8) | 12.4 (7.6–17.3) | 3.3 (−3.34, 10.1) |
| Diarrhea (95%CI) | 5.4 (2.0–8.9) | 9.4 (5.2–13.7) | 12.0 (7.2–16.8) | 9.6 (5.3–13.9) | −2.4 (−8.70, 4.90) |
| Headache (95%CI) | 62.1 (54.7–69.4) | 47.8 (40.5–55.1) | 49.1 (41.7–56.5) | 46.3 (39.0–53.7) | −2.8 (−12.7, 8.18) |
| Nausea (95%CI) | 10.2 (5.6–14.9) | 6.7 (3.0–10.3) | 12.6 (7.7–17.5) | 15.8 (10.4–21.2) | 3.2 (−4.17, 10.8) |
| Rash (95%CI) | 1.8 (0.0–3.8) | 1.7 (0.0–3.5) | 1.1 (0.0–2.7) | 1.1 (0.0–2.7) | 0.0 (−3.08, 3.01) |
| Stomach upset (95%CI) | 16.3 (10.7–21.9) | 13.3 (8.4–18.3) | 12.0 (7.2–16.8) | 14.7 (9.5–19.9) | 2.7 (−4.04, 10.7) |
Values displayed are the per cent of subjects who at exit interview indicated that they had this symptom at some time during their illness.
NA = Not applicable
Adherence
Adherence to dosing regimen was assessed by asking participants “Did you take all your pills as directed?”, and by counting pills in returned pill bottles. Of the 545 people allocated pills, 518 (95%) reported taking pills as directed. Of the 524 bottles returned, 486 (93%) were empty, 27 (5%) had 4 or fewer pills, and 11 (2%) had 5 or more pills left in the bottles There was no indication that those receiving echinacea took their pills differently than those receiving placebo. See Table 5.
Table 5.
Adherence to pill regimen
| Adherence to pill regimen | Blinded Placebo |
Blinded Echinacea |
Unblinded Echinacea |
|---|---|---|---|
| Total number receiving pills in bottles | 179 | 184 | 182 |
| Reported taking all pills as directed | 169 | 173 | 176 |
| Reported not taking all pills as directed | 8 | 8 | 4 |
| Lost, withdrawn or missing data | 2 | 3 | 2 |
| Empty pill bottles returned | 161 | 162 | 163 |
| Pill bottles returned with pills left | 10 | 15 | 13 |
| Bottles not returned or missing data | 8 | 7 | 6 |
| Testing of blinding | Do you believe that you were given echinacea or placebo? | ||
| Echinacea* | 56 | 69 | 107 |
| Placebo* | 72 | 54 | 3 |
| Don’t know / Won’t guess | 49 | 58 | 3 |
| Missing data, not answered | 2 | 3 | 69 |
Fisher’s exact test of proportional difference tested whether the trend toward guessing assignment correctly was statistically significant. This yielded p-value = 0.053 and 95% confidence interval for proportional difference (−0.002, 0.246)
Test of blinding
Blinding appeared to be intact. Of the 363 receiving blinded pills, 141 (39%) guessed their assignment correctly, 110 (30%) guessed incorrectly, and 107 (29%) refused to guess (Table 5). Of 179 assigned blinded placebo, 72 (40%) correctly guessed their assignment, compared to 69 (38%) receiving blinded echinacea. Including only those who were willing to guess pill assignment, a Fisher’s exact test of proportional difference yielded p-value 0.053 (95% CI −0.002, 0.246). While this does not allow us to reject the null and conclude blind-breaking, it does leave open the possibility that a few people were able to correctly ascertain to which group they had been assigned.
Phytochemical analysis
Laboratories of the manufacturer (MediHerb, Australia) and the natural products analysis company Chromadex (Clearwater, FL) conducted independent phytochemical assays at successive time points from 2004 to 2007. Both companies used high performance liquid chromatography with reference standards of known purified ingredients. Table 6 shows the lowest and highest results from MediHerb’s four lab assays and Chromadex’s three assays. Phytochemical concentrations appeared stable over time, with no trends towards lower concentration in later years (data not shown).
Table 6.
Phytochemical composition of echinacea tablets
| MediHerb low | MediHerb high | Chromadex low | Chromadex high |
|
|---|---|---|---|---|
| Caftaric acid | 1.85 | 2.43 | 1.32 | 2.14 |
| Chlorogenic acid | NA | NA | 0.07 | 0.38 |
| Cynarin | NA | NA | 0.35 | 0.83 |
| Cichoric acid | 7.63 | 10.04 | 5.13 | 6.84 |
| Echinacoside | 4.09 | 5.30 | 3.80 | 3.87 |
| Total phenolics = CAs | 12.98 | 16.87 | 9.80 | 13.30 |
| DDYIA | NA | NA | 0.52 | 2.05 |
| DDIA | NA | NA | 0.15 | 0.16 |
| DZTIA | NA | NA | 1.05 | 10.2 |
| Total 2-enes | 0.54 | 0.89 | NA | NA |
| Total 2,4 dienes | 2.48 | 3.57 | NA | NA |
| Total alkamides | 3.06 | 4.46 | 1.73 | 12.4 |
Phytochemical content analyzed independently by MediHerb (4 assays) and Chromadex (3 assays) during years 2004 to 2007. No time trends were seen.
All results are in mg/tablet
Dosing regimen was: 2 tabs 4 times per day for 1st day, then 1 tab 4 times per day for next 4 days
NA = not analyzed
CAs = cichoric acid derivatives
Specific alkamides measured by Chromadex were:
DDYIA = dodec-2-ene-8,10-diynoic acid isobutylamide
DDIA = dodeca-2(E),4(E)-dienoic acid isobutylamide
DZTIA = dodeca-2(E),4(E),8(Z),10(Z)-tetraenoic acid isobutylamide
Conclusions & Discussion
This dose regimen of this echinacea formulation did not make a large impact on the course of the common cold, compared either to blinded placebo or to no pills. Trends, however, were in the direction of benefit, amounting to an average half day reduction in the duration of a week-long cold, or an approximate 10% reduction in overall severity. Our own previous research suggests that a minority of people – no more than 1 in 4 – would judge this level of benefit worthwhile, given the cost, inconvenience, and possible side effects.(72–74) Nevertheless, while these results do not allow us to reject the null hypothesis and confidently claim evidence-of-benefit, data are also insufficient to exclude the possibility of a clinically significant effect. Confidence intervals of between-group differences allow for the possibility of a 24-hour reduction in duration and a 20% reduction in overall severity attributable to echinacea, both of which might be accepted as clinically significant by many or most cold-sufferers.(72–74)
This trial has a number of limitations. Participants in our study had community-acquired self-reported colds in Dane County, Wisconsin, USA. Etiological agents and psychosocial factors influencing colds may be different in other populations or geographic areas. We made no attempt to base inclusion on viral etiology, hence some of the illnesses represented here may be caused by influenza as well as other viruses. While age range was wide and both sexes were well-represented, racial and ethnic diversity was limited. Perhaps more importantly, this trial may have been underpowered. The power estimates for this trial used data existing at that time, showing a ratio of standard deviation to mean of 0.70. Equivalent data from the current trial provide a ratio of 0.80. Looking at data gathered from 1999 to 2008, we now conclude that a conventional randomized controlled trial would need slightly more than 200 people in each of two arms to have 80% power to detect a 20% difference in global severity, using the WURSS-21.(61) A trial using illness duration or pre-specified day-to-day change as primary outcome could be smaller, but results would be less meaningful. We should also note that these results reflect only one of many possible types of echinacea formulation. While the dosing and array of phytochemical constituents shown in Table 6 are reasonably representative of currently available echinacea preparations, it is quite possible that a substantively different formulation would give substantially different results. Finally, it is worth remembering that randomized trials provide results in terms of group averages, which may obscure benefits (or harms) for individuals or subgroups.
In conclusion, our own interpretation is that there is likely a small beneficial effect attributable to echinacea’s pharmacological activity. This interpretation comes not only from the trends observed in this trial, but from a reasonably substantial body of scientific evidence, including several positively-reported trials and a few cautiously optimistic meta-analyses.(50–53) Nevertheless, if there is indeed underlying benefit, it is not large, and is not clearly demonstrated by this trial’s results. Unfortunately, echinacea is not the long sought cure for the common cold. Individual choices about whether to use echinacea to treat common cold should be guided by personal health values and preferences, as well as by the limited evidence available.
Acknowledgements
The National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health provided primary support for this research project (1-R01-AT-1428). NCCAM had also provided Dr. Barrett with a Patient-Oriented Career Development Grant (K23 AT00051) which supported development of the R01 grant proposal. The Robert Wood Johnson Foundation Generalist Physician Faculty Scholars Program provided career development support to Dr. Barrett during the design and data collection phase of this project. MediHerb provided the placebo and echinacea tablets used in this trial, and conducted phytochemical assays, both free of charge. When NIH funds ran out before data collection had been completed, Deans Robert Golden and Paul DeLuca of the University of Wisconsin School of Medicine and Public Health facilitated financial support to allow the project to reach enrollment goals. The authors would like to thank St. Marys Hospital for allowing us to use the Employee Health clinic room for doctor visits and nasal wash collection, the U.W. Department of Family Medicine for providing an institutional base and collegial support, and especially to Mary Beth Plane PhD and Terry Little for assistance with editing and formatting. Finally, we would like to extend our greatest appreciation to the many research participants who generously contributed with their time and energy during a period of illness.
Grant Support
The trial was sponsored by the National Center for Complementary and Alternative Medicine at the National Institutes of Health (grant #R01AT001428).
Footnotes
Authors’ Contributions and Affiliations
Bruce Barrett MD PhD designed the research protocol, supervised data collection and analysis, and wrote the manuscript. bruce.barrett@fammed.wisc.edu Principal Investigator University of Wisconsin, Madison
Roger Brown PhD (Co-Investigator) contributed to the design, conducted statistical analysis, and contributed to the manuscript. rlbrown3@wisc.edu University of Wisconsin, Madison
David P Rakel MD (Co-Investigator) contributed to the design, participated in implementing the study and contributed to the manuscript drakel@uwhealth.org University of Wisconsin, Madison
Marlon Mundt PhD (Co-Investigator) contributed to the design, conducted statistical analysis, and contributed to the manuscript. marlon.mundt@fammed.wisc.edu Statistician, Scientist, University of Wisconsin, Madison
Kerry Bone Dip Phyto (Co-Investigator) directed manufacture of the echinacea and placebo pills, and contributed to the manuscript. kerry.bone@integria.com MediHerb, Warwick, Australia and School of Health, University of New England, Armidale, Australia
Shari Barlow BA (Research specialist) enrolled and monitored subjects, managed data, and contributed to the manuscript. shari.barlow@fammed.wisc.edu University of Wisconsin, Madison
Tola Ewers MS (Graduate student) assisted with statistical analysis and contributed to the manuscript. lmewers@wisc.edu University of Wisconsin, Madison
Reference List
- 1.Gwaltney JM. Virology and immunology of the common cold. Rhinology. 1985;23:265–271. [PubMed] [Google Scholar]
- 2.Monto AS. Viral respiratory infections in the community: Epidemiology, agents, and interventions. American Journal of Medicine. 1995;99:24S–27S. doi: 10.1016/S0002-9343(99)80307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. New England Journal of Medicine. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrat F, Vergu E, Ferguson NM, Lemaitre M, Cauchemez S, Leach S, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167:775–785. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 5.Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA. 2005;293:987–997. doi: 10.1001/jama.293.8.987. [DOI] [PubMed] [Google Scholar]
- 6.Eccles R. Understanding the symptoms of the common cold and influenza. The Lancet Infectious Diseases. 2005;5:718–725. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jefferson T. Influenza. Clin Evid (Online) 2009;2009:1–36. [Google Scholar]
- 8.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Archives of Internal Medicine. 2003;163:487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 9.Turner RB. New considerations in the treatment and prevention of rhinovirus infections. Pediatric Annals. 2005;34:53–57. doi: 10.3928/0090-4481-20050101-12. [DOI] [PubMed] [Google Scholar]
- 10.Flannery MA. From Rudbeckia to Echinacea: The emergence of the purple cone flower in modern therapeutics. Pharmacy in History. 1999;41:52–59. [PubMed] [Google Scholar]
- 11.Bauer R, Wagner H. Echinacea species as potential immunostimulatory drugs. In: Wagner H, Farnsworth NR, editors. Economic and Medicinal Plant Research. New York: Academic Press Limited; 1991. pp. 253–318. [Google Scholar]
- 12.Melchart D, Linde K, Worku F, Bauer R, Wagner H. Immunomodulation with Echinacea - a systematic review of controlled trials. Phytomedicine. 1994;1:245–254. doi: 10.1016/S0944-7113(11)80072-3. [DOI] [PubMed] [Google Scholar]
- 13.Barrett B. Medicinal properties of Echinacea: A critical review. Phytomedicine. 2003;10:66–86. doi: 10.1078/094471103321648692. [DOI] [PubMed] [Google Scholar]
- 14.Barrett B. Echinacea and ginseng for immunomodulation and protection from respiratory infection. PharmacologyOnLine. 2006;3:392–407. [Google Scholar]
- 15.Bauer R. Chemistry, analysis and immunological investigations of Echinacea phytopharmaceuticals. In: Wagner H, editor. Immunomodulatory Agents from Plants. Basel, Boston, Berlin: Birkhauser Verlag; 1999. pp. 41–88. [Google Scholar]
- 16.Block KI, Mead MN. Immune system effects of echinacea, ginseng, and astragalus: a review. Integrative Cancer Therapies. 2003;2:247–267. doi: 10.1177/1534735403256419. [DOI] [PubMed] [Google Scholar]
- 17.Bukovsky M, Vaverkova S, Kostalova D. Immunomodulating activity of Echinacea gloriosa L., Echinacea angustifolia DC., and Rudbeckia speciosa Wenderoth ethanol-water extracts. Pol J Pharmacol. 1995;47:175–177. [PubMed] [Google Scholar]
- 18.Goel V, Chang C, Slama JV, Barton R, Bauer R, Gahler R, et al. Alkylamides of Echinacea purpurea stimulate alveolar macrophage function in normal rats. International Immunopharmacology. 2002;2:381–387. doi: 10.1016/s1567-5769(01)00163-1. [DOI] [PubMed] [Google Scholar]
- 19.Guiotto P, Woelkart K, Grabnar I, Voinovich D, Perissutti B, Invernizzi S, et al. Pharmacokinetics and immunomodulatory effects of phytotherapeutic lozenges (bonbons) with Echinacea purpurea extract. Phytomedicine. 2008;15:547–554. doi: 10.1016/j.phymed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Wagner H, Stuppner H, Schafer W, Zenk M. Immunologically active polysaccharides of Echinacea purpurea cell cultures. Phytochemistry. 1988;27:119–126. [Google Scholar]
- 21.Woelkart K, Bauer R. The role of alkamides as an active principle of echinacea. Planta Med. 2007;73:615–623. doi: 10.1055/s-2007-981531. [DOI] [PubMed] [Google Scholar]
- 22.Zhai Z, Haney D, Wu L, Solco A, Murphy PA, Wurtele ES, et al. Alcohol extracts of Echinacea inhibit production of nitric oxide and tumor necrosis factor-alpha by macrophages in vitro. Food Agric Immunol. 2007;18:221–236. doi: 10.1080/09540100701797363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernath J, Percival SS. The oxidative burst of HL-60 cells is modified differently by individual echinacea species and plant parts. FASEB. 2001;15:A1228. [Google Scholar]
- 24.Gorski JC, Huang SM, Pinto A, Hamman MA, Hilligoss JK, Zaheer NA, et al. The effect of echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clinical Pharmacology & Therapeutics. 2004;75:89–100. doi: 10.1016/j.clpt.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Jager H, Meinel L, Dietz B, Lapke C, Bauer R, Merkle HP, et al. Transport of alkamides from Echinacea species through Caco-2 monolayers. Planta Medica. 2002;68:469–471. doi: 10.1055/s-2002-32076. [DOI] [PubMed] [Google Scholar]
- 26.Matthias A, Gillam EM, Penman KG, Matovic NJ, Bone KM, De Voss JJ, et al. Cytochrome P450 enzyme-mediated degradation of Echinacea alkylamides in human liver microsomes. Chemico-Biological Interactions. 2005;155:62–70. doi: 10.1016/j.cbi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Woelkart K, Xu W, Pei Y, Makriyannis A, Picone RP, Bauer R. The endocannabinoid system as a target for alkamides from Echinacea angustifolia roots. Planta Medica. 2005;71:701–705. doi: 10.1055/s-2005-871290. [DOI] [PubMed] [Google Scholar]
- 28.Woelkart K, Marth E, Suter A, Schoop R, Raggam RB, Koidl C, et al. Bioavailability and pharmacokinetics of Echinacea purpurea preparations and their interaction with the immune system. Int J Clin Pharmacol Ther. 2006;44:401–408. doi: 10.5414/cpp44401. [DOI] [PubMed] [Google Scholar]
- 29.Senchina DS, McCann DA, Asp JM, Johnson JA, Cunnick JE, Kaiser MS, et al. Changes in immunomodulatory properties of Echinacea spp. root infusions and tinctures stored at 4 degrees C for four days. Clinica Chimica Acta. 2005;355:67–82. doi: 10.1016/j.cccn.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 30.American Herbal Pharmacopoeia. Echinacea purpurea Root: Standards of analysis, quality control, and therapeutics. Santa Cruz, CA: American Herbal Pharmacopoeia; 2004. [Google Scholar]
- 31.American Herbal Pharmacopoeia. Echinacea angustifolia Root: Standards of analysis, quality control, and therapeutics. Scotts Valley (CA): American Herbal Pharmacopoeia and Therapeutic Compendium; 2010. [Google Scholar]
- 32.Gertsch J, Schoop R, Kuenzle U, Suter A. Echinacea alkylamides modulate TNF-alpha gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Letters. 2004;577:563–569. doi: 10.1016/j.febslet.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 33.Hinz B, Woelkart K, Bauer R. Alkamides from Echinacea inhibit cyclooxygenase-2 activity in human neuroglioma cells. Biochem Biophys Res Commun. 2007;360:441–446. doi: 10.1016/j.bbrc.2007.06.073. [DOI] [PubMed] [Google Scholar]
- 34.Lalone CA, Hammer KD, Wu L, Bae J, Leyva N, Liu Y, et al. Echinacea species and alkamides inhibit prostaglandin E(2) production in RAW264.7 mouse macrophage cells. J Agric Food Chem. 2007;55:7314–7322. doi: 10.1021/jf063711a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthias A, Addison RS, Penman KG, Dickinson RG, Bone KM, Lehmann RP. Echinacea alkamide disposition and pharmacokinetics in humans after tablet ingestion. Life Sciences. 2005;77:2018–2029. doi: 10.1016/j.lfs.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Woelkart K, Dittrich P, Beubler E, Pinl F, Schoop R, Suter A, et al. Pharmacokinetics of the main alkamides after administration of three different Echinacea purpurea preparations in humans. Planta Med. 2008;74:651–656. doi: 10.1055/s-2008-1034284. [DOI] [PubMed] [Google Scholar]
- 37.Woelkart K, Bauer R. The role of alkamides as an active principle of echinacea. Planta Med. 2007;73:615–623. doi: 10.1055/s-2007-981531. [DOI] [PubMed] [Google Scholar]
- 38.Zhai Z, Haney D, Wu L, Solco A, Murphy PA, Wurtele ES, et al. Alcohol extracts of Echinacea inhibit production of nitric oxide and tumor necrosis factor-alpha by macrophages in vitro. Food Agric Immunol. 2007;18:221–236. doi: 10.1080/09540100701797363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrett B, Vohmann M, Calabrese C. Echinacea for upper respiratory infection: Evidence-based clinical review. Journal of Family Practice. 1999;48:628–635. [PubMed] [Google Scholar]
- 40.Giles JT, Palat CT, Chien SH, Chang ZG, Kennedy DT. Evaluation of Echinacea for treatment of the common cold. Pharmacotherapy. 2000;20:690–697. doi: 10.1592/phco.20.7.690.35173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett BP, Brown RL, Locken K, Maberry R, Bobula JA, D'Alessio D. Treatment of the common cold with unrefined echinacea: A randomized, double-blind, placebo-controlled trial. Annals of Internal Medicine. 2002;137:939–946. doi: 10.7326/0003-4819-137-12-200212170-00006. [DOI] [PubMed] [Google Scholar]
- 42.Cohen HA, Varsano I, Kahan E, Sarrell EM, Uziel Y. Effectiveness of an herbal preparation containing Echinacea, Propolis, and Vitamin C in preventing respiratory tract infections in children: A randomized, double-blind, placebo-controlled, multicenter study. Archives of Pediatrics & Adolescent Medicine. 2004;158:217–221. doi: 10.1001/archpedi.158.3.217. [DOI] [PubMed] [Google Scholar]
- 43.Goel V, Lovlin R, Barton R, Lyon MR, Bauer R, Lee TD, et al. Efficacy of a standardized echinacea preparation (Echinilin) for the treatment of the common cold: a randomized, double-blind, placebo-controlled trial. Journal of Clinical Pharmacy & Therapeutics. 2004;29:75–83. doi: 10.1111/j.1365-2710.2003.00542.x. [DOI] [PubMed] [Google Scholar]
- 44.Sperber SJ, Shah LP, Gilbert RD, Ritchey TW, Monto AS. Echinacea purpurea for prevention of experimental rhinovirus colds. Clinical Infectious Diseases. 2004;38:1367–1371. doi: 10.1086/386324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor JA, Weber W, Standish L, Quinn H, Goesling J, McGann M, et al. Efficacy and safety of echinacea in treating upper respiratory tract infections in children: a randomized controlled trial. JAMA. 2003;290:2824–2830. doi: 10.1001/jama.290.21.2824. [DOI] [PubMed] [Google Scholar]
- 46.Turner RB, Riker DK, Gangemi JD. Ineffectiveness of Echinacea for prevention of experimental rhinovirus colds. Antimicrobial agents and chemotherapy. 2000;44:1708–1709. doi: 10.1128/aac.44.6.1708-1709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi JD . An evaluation of Echinacea angustifolia in experimental rhinovirus infections. New England Journal of Medicine. 2005;353:341–348. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 48.Yale SH, Liu K. Echinacea purpurea therapy for the treatment of the common cold: a randomized, double-blind, placebo-controlled clinical trial. Archives of Internal Medicine. 2004;164:1237–1241. doi: 10.1001/archinte.164.11.1237. [DOI] [PubMed] [Google Scholar]
- 49.Caruso TJ, Gwaltney JM., Jr Treatment of the common cold with echinacea: a structured review. Clinical Infectious Diseases. 2005;40:807–810. doi: 10.1086/428061. [DOI] [PubMed] [Google Scholar]
- 50.Huntley AL, Thompson CJ, Ernst E. The safety of herbal medicinal products derived from echinacea species: a systematic review. Drug Safety. 2005;28:387–400. doi: 10.2165/00002018-200528050-00003. [DOI] [PubMed] [Google Scholar]
- 51.Linde K, Barrett B, Wolkart K, Bauer R, Melchart D. Echinacea for preventing and treating the common cold. Cochrane Database of Systematic Reviews. 2006 doi: 10.1002/14651858.CD000530.pub2. CD000530. [DOI] [PubMed] [Google Scholar]
- 52.Schoop R, Klein P, Suter A, Johnston SL. Echinacea in the prevention of induced Rhinovirus colds. Clinical Therapeutics. 2006;28:1–10. doi: 10.1016/j.clinthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Shah SA, Sander S, White CM, Rinaldi M, Coleman CI. Evaluation of echinacea for the prevention and treatment of the common cold: a meta-analysis. Lancet Infect Dis. 2007;7:473–480. doi: 10.1016/S1473-3099(07)70160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barrett B, Rakel D, Chewning B, Marchand L, Rabago D, Brown R, et al. Rationale and methods for a trial assessing placebo, echinacea, and doctor-patient interaction in the common cold. Explore (NY) 2007;3:561–572. doi: 10.1016/j.explore.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Becker S, Koren HS, Henke DC. Interleukin-8 expression in normal nasal epithelium and its modulation by infection with respiratory syncytial virus and cytokines tumor necrosis factor, Interleukin-1, and Interleukin-6. Am J Respir Cell Mol Biol. 1993;8:20–27. doi: 10.1165/ajrcmb/8.1.20. [DOI] [PubMed] [Google Scholar]
- 56.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. American Journal of Respiratory & Critical Care Medicine. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 57.Noah TL, Henderson FW, Wortman IA, Devlin RB, Handy J, Koren HS, et al. Nasal cytokine production in viral acute upper respiratory infection of childhood. Journal of Infectious Disease. 1995;171:584–592. doi: 10.1093/infdis/171.3.584. [DOI] [PubMed] [Google Scholar]
- 58.Teran LM, Johnston SL, Schroder JM, Church MK, Holgate ST. Role of nasal interleukin-8 in neutrophil recruitment and activation in children with virus induced asthma. Am J Respir Cri Care Me. 1997;155:1362–1366. doi: 10.1164/ajrccm.155.4.9105080. [DOI] [PubMed] [Google Scholar]
- 59.Jackson GG, Dowling HF, Muldoon RL. Present concepts of the common cold. Am J Public Health. 1962;52:940–945. doi: 10.2105/ajph.52.6.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barrett B, Brown R, Mundt M, Safdar N, Dye L, Maberry R, et al. The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. Journal of Clinical Epidemiology. 2005;58:609–617. doi: 10.1016/j.jclinepi.2004.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barrett B, Brown RE, Mundt MP, Thomas GR, Barlow SK, Highstrom AD, et al. Validation of a short form Wisconsin Upper Respiratory Symptom Survey (WURSS-21) Health and Quality of Life Outcomes. 2009;7 doi: 10.1186/1477-7525-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ware JE, Kosinski M, Dewey JE, Gandek B. How to score and interpret single-item health status measures: A manual for users of the SF-8 health survey. Lincoln RI: QualityMetric; 2001. [Google Scholar]
- 63.Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychology. 1998;17:214–223. doi: 10.1037//0278-6133.17.3.214. [DOI] [PubMed] [Google Scholar]
- 64.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. The Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 65.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapam S, Oskamp S, editors. The social psychology of health: Claremont Symposium on applied social psychology. Sage Publications; Newbury Park, CA: 1988. [Google Scholar]
- 66.Ryff CD, Singer B. Psychological well-being: Meaning, measurement, and implications for psychotherapy research. Psychother Psychosom. 1996;65:14–23. doi: 10.1159/000289026. [DOI] [PubMed] [Google Scholar]
- 67.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A re-evaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;1078 doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 68.Little RJA. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–1202. [Google Scholar]
- 69.Schafer JL. Analysis of Incomplete Multivariate Data. London: Chapman & Hall; 1997. [Google Scholar]
- 70.McCullagh P, Nelder JA. Generalized Linear Models. London: Chapman & Hall; 1989. [Google Scholar]
- 71.Hintze J. NCSS Statistical Package. Kaysville, Utah: NCSS, LLC; 2007. www.ncss.com. [Google Scholar]
- 72.Barrett B, Brown R, Mundt M, Dye L, Alt J, Safdar N, et al. Using benefit harm tradeoffs to estimate sufficiently important difference: the case of the common cold. Medical Decision Making. 2005;25:47–55. doi: 10.1177/0272989X04273147. [DOI] [PubMed] [Google Scholar]
- 73.Barrett B, Harahan B, Brown D, Zhang Z, Brown R. Sufficiently important difference for common cold: severity reduction. Ann Fam Med. 2007;5:216–223. doi: 10.1370/afm.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barrett B, Brown R, Mundt M. Comparison of anchor-based and distributional approaches in estimating important difference in common cold. Quality of Life Research. 2008;17:75–85. doi: 10.1007/s11136-007-9277-2. [DOI] [PubMed] [Google Scholar]