Abstract
Major histocompatibility complex (MHC) class II molecules bind and present to CD4+ T cells peptides derived from endocytosed antigens. Class II molecules associate in the endoplasmic reticulum with invariant chain (Ii), which (i) mediates the delivery of the class II–Ii complexes into the endocytic compartments where the antigenic peptides are generated; and (ii) blocks the peptide-binding site of the class II molecules until they reach their destination. Once there, Ii must be removed to allow peptide binding. The bulk of Ii–class II complexes reach late endocytic compartments where Ii is eliminated in a reaction in which the cysteine protease cathepsin S and the accessory molecule H–2DM play an essential role. Here, we here show that Ii is also eliminated in early endosomal compartments without the intervention of cysteine proteases or H–2DM. The Ii-free class II molecules generated by this alternative mechanism first bind high molecular weight polypeptides and then mature into peptide-loaded complexes.
Keywords: endosomes/invariant chain/MHC class II
Introduction
CD4+ T cells recognize foreign antigens displayed by major histocompatibility complex (MHC) class II molecules on the surface of antigen-presenting cells (APCs) (Wolf and Ploegh, 1995). The antigenic determinants bound by class II molecules are peptides derived from proteins that are endocytosed by the APCs and degraded as they progress into the endocytic pathway (Watts, 1997).
Class II molecules consist of αβ heterodimers whose structure forms a peptide-binding site capable of accommodating a large variety of peptides that differ in both sequence and length (Rudensky et al., 1991; Chicz et al., 1993). Newly synthesized αβ dimers associate in the endoplasmic reticulum (ER) with invariant chain (Ii), a protein that serves as a chaperone for class II molecules and directs their delivery into the endocytic route (Cresswell, 1994). In forming the αβ–Ii complex, a specialized region of Ii, CLIP (class II invariant chain-derived peptide; Chicz et al., 1993), is inserted into the peptide-binding site of the αβ dimer in a manner similar to an antigenic peptide (Bijlmakers et al., 1994; Romagnoli and Germain, 1994; Ghosh et al., 1995). This interaction is necessary to stabilize the αβ dimer (Sadegh-Nasseri et al., 1994; Zhong et al., 1996) and to prevent the premature occupancy of its binding site by polypeptides that reside in the ER (Busch et al., 1996).
Upon entry into the endocytic route, destruction of Ii and displacement of CLIP are required to allow association of antigenic peptides with the binding site of αβ dimers (Wolf and Ploegh, 1995). Since the array of available antigenic determinants probably varies at different locations along the endocytic route, it is important to establish where the class II molecules will acquire their peptide-binding capacity. This is determined, first, by the point of entry of the class II molecules into the endocytic pathway, and secondly, by the intracellular distribution of the active proteases that degrade Ii. Subcellular fractionation and morphological analysis have defined different compartments along the endocytic route involved in generation of class II–peptide complexes in murine and human cell lines (Pierre and Mellman, 1998b). Whether and to what extent they co-exist in the same APC is not entirely clear (Geuze, 1998).
The activity of the vacuolar H+-ATPase, responsible for acidification of vacuolar compartments, is required not only for normal functioning of proteases with a low pH optimum, but also for the ordered progression of cargo along the endocytic pathway. Application of selective inhibitors of this enzyme, the macrolide antibiotics bafilomycin A1 and concanamycin B (ConB), raises the pH of lysosomal compartments. These drugs interrupt the early to late endosomal and/or late endosomal to lysosomal transition without blocking surface deposition of mature glycoproteins or secretion of soluble proteins (Yilla et al., 1993; Clague et al., 1994; Benaroch et al., 1995; van Weert et al., 1995). Here we combine biochemical analysis and subcellular fractionation studies of mouse APCs to show that ConB bisects the endocytic pathway into two components, both of which can generate Ii-free class II molecules, but that differ in their dependence on cysteine proteases and H–2DM.
When Ii-bound class II molecules progress normally along the endocytic route, the bulk of Ii is degraded in several stages (Chapman, 1998), in a process in which the cysteine protease cathepsin S (Cat S) plays an essential role (Riese et al., 1996, 1998; Villadangos et al., 1997; Nakagawa et al., 1999; Shi et al., 1999). The resulting product, an αβ–CLIP complex, is the substrate recognized by H–2DM (in humans, HLA–DM), an MHC class II-like molecule that catalyzes the exchange of CLIP for antigenic peptides (Vogt and Kropshofer, 1999). However, when endocytic acidification and traffic are disrupted by ConB, class II molecules are retained largely in early endocytic compartments where Ii is eliminated without the intervention of cysteine proteases or H–2DM. The Ii-free αβ dimers generated by this alternative mechanism bind high molecular weight polypeptides and form distinct complexes that can be resolved by SDS–PAGE. The class II molecules included in these complexes mature into regular peptide-loaded dimers after removal of ConB. Such complexes can also be observed transiently in control cells, showing that a fraction of the class II molecules expressed by normal APCs follows this distinct pathway of maturation during their transit through early endosomes. Thus, a population of class II molecules can bind antigenic epitopes that may exist only as part of longer polypeptide precursors in the early components of the endocytic route.
Results
The vacuolar H+-ATPase inhibitor ConB has two major effects: (i) the deficit in acidification inhibits the activity of low pH-dependent lysosomal proteases; and (ii) trafficking from early to late endosomes and/or from late endosomes to lysosomes is interrupted (Yilla et al., 1993; Clague et al., 1994; Benaroch et al., 1995; van Weert et al., 1995). Assessment of the effects of ConB on maturation of mouse MHC class II molecules should provide new insights into events that occur in compartments located upstream of the blockade imposed by the drug.
The Cat S-dependent mechanism of degradation of Ii
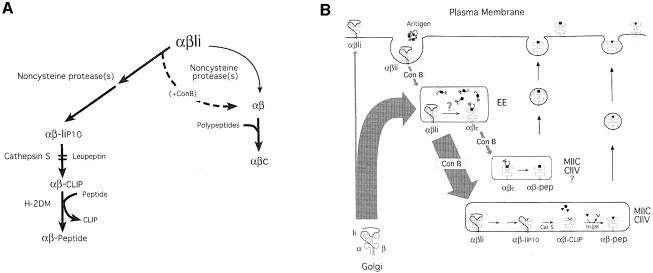
As a reference, we will first describe biochemically the process of conversion of αβ–Ii into αβ–peptide, which relies on the cysteine protease Cat S (Riese et al., 1996, 1998; Villadangos et al., 1997; Nakagawa et al., 1999; Shi et al., 1999). A schematic representation of this process is shown in Figure 6A.
Fig. 6. A model for the removal of Ii from class II molecules at two different locations of the endocytic route. (A) Schematic representation of the two mechanisms of Ii elimination and the intermediates that they generate. See text for details. (B) Compartmentalization of the Cat S/H-2DM-dependent and -independent mechanisms for removal of Ii. See Dicussion for details.
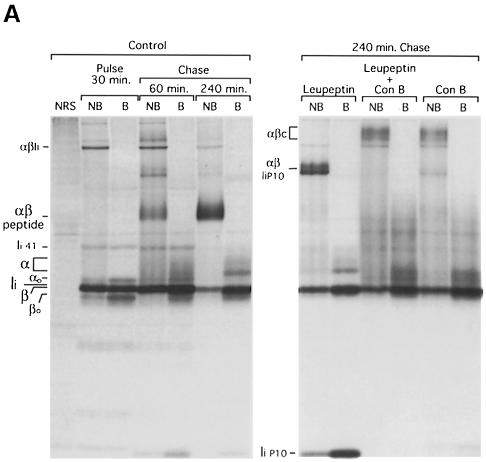
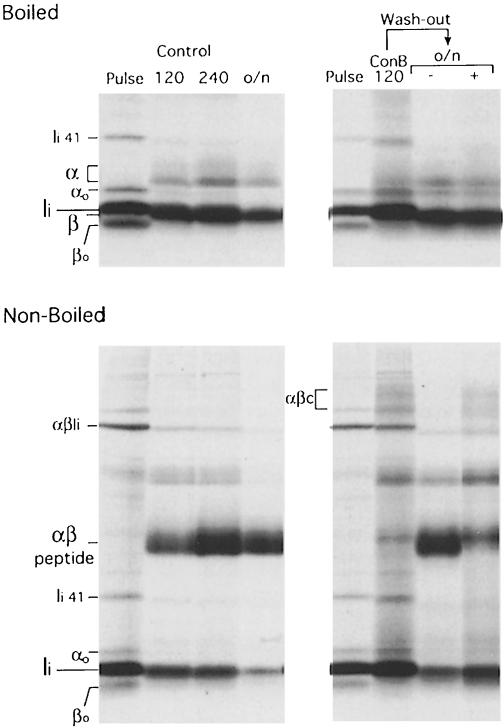
Control mouse splenocytes were pulse-labeled with [35S]methionine/cysteine for 30 min and chased for 1 and 4 h. At each time point, cells were lysed, and the I-Ab molecules immunoprecipitated with the monoclonal antibody (mAb) N22, which recognizes αβ dimers with or without Ii but none of the three subunits individually (Villadangos et al., 1997). Mature I-Ab αβ–peptide complexes are resistant to dissociation in SDS at room temperature. The N22 immunoprecipitates were thus divided into two and analyzed by SDS–PAGE with or without previous boiling to score for formation of αβ–peptide (Figure 1A, control). After the pulse, all labeled class II molecules were associated with Ii. Some αβ–Ii trimers resisted dissociation in SDS at room temperature and could be resolved by SDS–PAGE as a complex with distinct mobility (Figure 1A, pulse NB), but no mature αβ–peptide complexes (αβm) were observed. During the chase: (i) the mobility of both α and β was reduced due to N–linked carbohydrate modifications in the Golgi (the mature β chain thus migrates at the same position as Ii); (ii) Ii was destroyed; and (iii) αβ–peptide complexes (αβm) accumulated.
Fig. 1. Ii is eliminated in ConB-treated cells without the intervention of cysteine proteases. (A) Mouse splenocytes were pulse-labeled for 30 min and chased for 60 and 240 min in the absence (control) or the presence of 1 mM leupeptin, 20 nM ConB or both drugs combined. MHC class II molecules were immunoprecipitated with mAb N22. Each immunoprecipitate was analyzed by reducing 12.5% SDS–PAGE without (NB) or after (B) boiling. The positions of immature (αo and βo) and mature (α and β) I-Ab subunits, invariant chain (Ii), the p41 form of Ii (Ii 41) (Cresswell, 1994) and the Ii degradation intermediate IiP10 are indicated. SDS-stable αβ–Ii, αβ–peptide, αβ–IiP10 and (ConB-induced) αβc complexes are also indicated. The first lane (NRS) was loaded with an immunoprecipitate obtained with normal rabbit serum plus normal mouse serum from the lysate of control cells chased for 60 min. (B) N22 immunoprecipitates obtained from pulse-labeled cells, or cells chased for 240 min without (control) or with ConB or leupeptin as in (A), were denatured by boiling in SDS. One-tenth of the sample was set apart, and the remainder used for re-immunoprecipitation with anti-I-Aα and anti-I-Aβ rabbit sera, and sequentially with a rabbit serum against the N–terminal region of Ii. The N22 immunoprecipitate and each re-immunoprecipitated sample were loaded on a reducing 12.5% SDS–polyacrylamide gel. (C) Quantitation of the re-immunoprecipitations in (B). The amount of α, β and Ii in each of the pulse, control, leupeptin and ConB sets was quantitated in a phosphorimager. To correct for differences in the total amount of sample, the values in each set were normalized relative to β. The amount of each subunit relative to the amount present in the pulse-labeled sample was calculated.
The cysteine protease Cat S is necessary for complete destruction of Ii in splenocytes. Thus, if APCs are treated with the cysteine protease inhibitor leupeptin (Figure 1A, leupeptin), or do not express Cat S owing to disruption of the Cat S gene (Figure 2A), destruction of Ii is interrupted prior to formation of CLIP. I-Ab molecules bound to the Ii degradation intermediate IiP10 thus accumulate (Figure 6A) (Nakagawa et al., 1999; Shi et al., 1999). Like the αβ–peptide complex, the αβ–IiP10 complex can be resolved by SDS–PAGE when the immunoprecipitate is not boiled (Kasai et al., 1996; Brachet et al., 1997; Villadangos et al., 1997). Generation of αβ–IiP10 is a normal stage in the maturation of class II molecules, as indicated by the transient presence of this complex also in control cells (Figure 1A, control, 60 min chase).
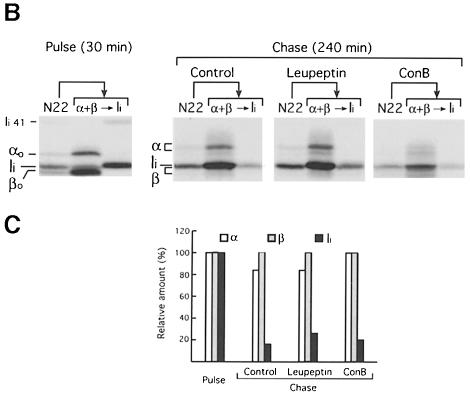
Fig. 2. Ii is eliminated in Cat S–/– and H-2DM–/– cells treated with ConB. Cat S–/– (A) or H-2DM–/– (B) splenocytes were pulse-labeled for 30 min and chased for 240 min in the absence or presence of 20 nM ConB, immunoprecipitated with mAb N22 and analyzed as in Figure 1. Labels are as in Figure 1. The positions of CLIP and I–Ab–CLIP that accumulate in H-2DM–/–-untreated cells are indicated (Fung-Leung et al., 1996; Martin et al., 1996; Miyazaki et al., 1996).
ConB impairs MHC class II maturation but allows removal of Ii
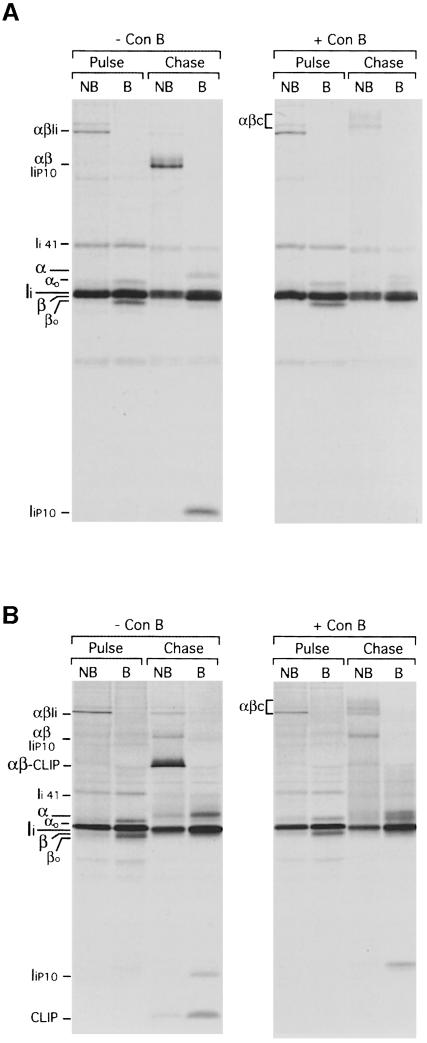
In splenocytes exposed to ConB, generation of αβ–peptide (αβm) was also impaired (Figure 1A, ConB). ConB did not interfere with formation of αβ–Ii complexes, as judged by the similar amounts of each of these polypeptides that were immunoprecipitated at the end of the pulse from both control and ConB-treated cells (Figures 2 and 4). The failure to generate αβ–peptide when ConB was present was not due to a blockade in Ii degradation because (i) the amount of full-length Ii remaining after the chase in ConB-treated cells was similar to that in control or in leupeptin-treated cells (Figure 1A, compare the boiled immunoprecipitates from the ‘control, 240 min’, ‘leupeptin’ and ‘ConB’ samples); and (ii) ConB-treated cells did not accumulate any significant amount of Ii degradation intermediates.
Fig. 4. Reversion of ConB treatment allows formation of αβ–peptide. The panels on the left show N22 immunoprecipitates run on a 12.5% SDS–polyacrylamide gel without (lower panel) or after (upper panel) boiling, obtained from splenocytes pulse-labeled for 30 min and chased for 120 min, 240 min or overnight (o/n). In the panels on the right, the N22 immunoprecipitates were obtained from cells that had been pulse-labeled for 30 min in 20 nM ConB (pulse), then chased for 120 min in 5 nM ConB (ConB 120), and then washed and incubated overnight (o/n) in the absence (–) or presence (+) of 5 nM ConB. The high molecular weight regions of the lanes containing the boiled immunoprecipitates (upper panels) were similar to those displayed in Figures 1–3.
A direct estimate of the amount of Ii remaining at the end of the chase (Figure 1A) was difficult because mature I–Abβ and Ii co-migrate. To overcome this problem, the α, β and Ii subunits were re-immunoprecipitated from N22 immunoprecipitates obtained from pulsed cells, and from cells chased without or with leupeptin or ConB (Figure 1B), and quantitated (Figure 1C). The ratio of α and β did not change among the four immunoprecipitates, but the amount of full-length Ii decreased dramatically, and to the same extent, in all the chased samples compared with their pulsed counterpart. We conclude that Ii is eliminated in both control and in ConB-treated mouse APCs. Similar results were observed using splenocytes from a Balb/c (H-2d) mouse (not shown).
The mobility of some of the I-Abα and β chains recovered after treatment with ConB was slightly different from that of the control or leupeptin-treated samples (Figure 1B), suggesting that N–linked glycan modifications of I–Ab were altered in ConB-treated cells. This was not due to prolonged retention of I–Ab in the ER of cells treated with ConB, because the mobility of most of β was unaffected. In fact, αβ–peptide complexes containing incompletely modified α chains could be generated when the effect of ConB was reversed (see below). Thus, despite the subtle difference in carbohydrate modifications shown by I–Ab in the presence of ConB, our results are consistent with prior observations that ConB does not affect transfer of molecules synthesized in the ER through the Golgi and into the endocytic route (Yilla et al., 1993; Clague et al., 1994; Benaroch et al., 1995; van Weert et al., 1995). Small alterations in N–linked glycans are also known to occur on glycoproteins secreted by ConB-treated HepG2 cells (Yilla et al., 1993).
Ii is eliminated in ConB-treated cells by a mechanism independent of cysteine proteases and H–2DM
Ii might be degraded in ConB-treated cells because the cysteine protease(s) that normally degrades Ii in mouse splenocytes might be less dependent on proper acidification, and thus insensitive to ConB treatment. If so, such enzymes should still be inhibited by leupeptin. To test this, we pulse–chased cells in the presence of both leupeptin and ConB (Figure 1A). This treatment resulted in elimination of full-length Ii and Ii fragments, indicating that when mouse splenocytes are treated with ConB, Ii is eliminated in a manner independent of leupeptin-sensitive cysteine proteases.
We confirmed this by testing the effect of ConB on cells that do not express Cat S. Degradation of Ii in cells from Cat S–/– mice is blocked at the IiP10 stage (Figure 2A, –ConB) (Nakagawa et al., 1999; Shi et al., 1999). Incubation of Cat S–/– APCs with ConB had no effect on the formation of αβ–Ii complexes (compare the immunoprecipitates from control and ConB-treated cells after the pulse). At the end of the chase, not only full-length Ii, but also IiP10 had been eliminated in ConB-treated Cat S–/– APCs (Figure 2A, +ConB). Elimination of Ii also occurred in ConB-treated splenocytes from mice deficient in Cat D (an aspartate protease), Cat B and Cat L (two cysteine proteases) (not shown). These enzymes are dispensable for Ii degradation in untreated splenocytes (Villadangos et al., 1997; Deussing et al., 1998; Nakagawa et al., 1998).
In the presence of ConB, a leupeptin-insensitive protease could perhaps substitute for Cat S, and still cleave IiP10 to generate CLIP. We therefore analyzed by pulse–chase the effect of ConB on H–2DM–/– APCs, in which I–Ab–CLIP complexes accumulate (Fung-Leung et al., 1996; Martin et al., 1996; Miyazaki et al., 1996). At the end of the chase, the amount of full-length Ii or of Ii fragments that remained in control and ConB-treated H–2DM–/– cells was comparable (Figure 2B). However, CLIP was absent from the ConB-treated sample. In conclusion, treatment of mouse splenocytes with ConB allows class II molecules to bypass the requirement for Cat S and for H–2DM for removal of Ii.
An alternative mechanism for removal of Ii in early endosomes
If Ii is destroyed, what then prevents the formation of αβ–peptide complexes in ConB-treated mouse APCs? The class II molecules devoid of Ii that are generated in the presence of ConB might accumulate in a state that required further processing. In fact, class II molecules immunoprecipitated from cells incubated with ConB contained a complex stable in SDS in the 150–200 kDa region which we refer to as αβc (Figures 1A and 2). The composition of αβc will be addressed below.
Like the αβ–IiP10 complex, αβc occurs transiently in control cells (Figures 1A, 60 min chase, and 5B; see also supplementary Figure 2; the supplementary data are available in The EMBO Journal Online), suggesting that αβc might also represent a normal intermediate in the maturation of class II molecules. We did not detect αβc at the cell surface by iodination followed by immunoprecipitation with N22 (data not shown), showing that αβc resides intracellularly. The αβc complex cannot be a precursor of αβ–IiP10, because αβc lacks Ii. Nor can αβ–IiP10 precede formation of αβ, because, in that case, the combined effect of leupeptin and ConB would elicit accumulation of αβ–IiP10, not αβc (Figure 1A). Its increased presence in ConB-treated cells implies involvement of an acidic compartment. Our hypothesis was that two proteolytic pathways for Ii removal may operate simultaneously in normal APCs: one, predominant in normal APCs, and dependent on Cat S is characterized by the formation of the αβ–IiP10 intermediate; the other, independent of cysteine proteases, would proceed through formation of the αβc complex and predominate when ConB is present (Figure 6A). What was causing the accumulation of αβc in ConB-treated cells? Is αβc indeed a precursor of αβ–peptide complexes?
ConB causes the retention of class II molecules in early endocytic compartments of mouse APCs
ConB could be causing the retention of αβ dimers in compartments devoid of short peptides, or whose physicochemical conditions were hostile to peptide binding. We addressed this possibility by subcellular fractionation.
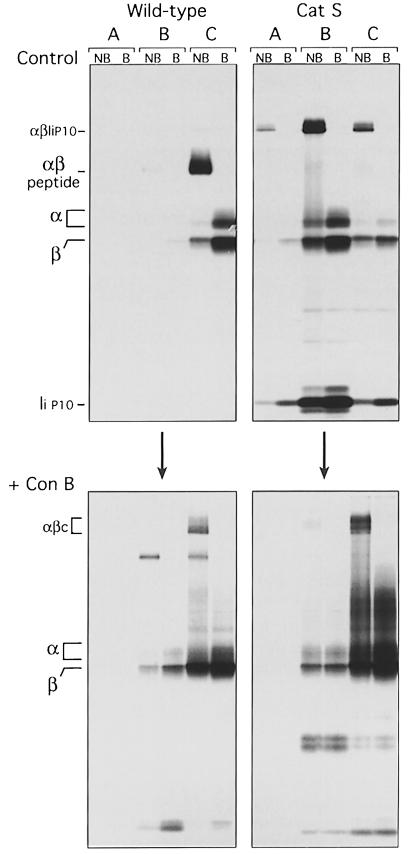
Control or Cat S–/– splenocytes were pulse–chased in the absence or the presence of ConB, homogenized, and the post-nuclear supernatant fractionated in a 27% Percoll gradient. The bottom of this gradient, enriched with lysosomes, constitutes what we refer to here as fraction A. The top of the gradient was then applied to a 10% Percoll gradient that yielded fractions B, enriched in late endosomes, and C, that contained ER, Golgi, plasma membranes and early endosomes (Castellino and Germain, 1995; Driessen et al., 1999). Class II molecules were then immunoprecipitated from the A, B and C fractions of each cell type/treatment and analyzed by SDS–PAGE as above (Figure 3).
Fig. 3. ConB provokes the retention of I–Ab in early endosomes. Wild-type or Cat S–/– splenocytes were pulse-labeled for 60 min and chased for 180 min in the absence (upper panels) or presence (lower panels) of ConB. Cells were homogenized, and the post-nuclear supernatant subjected to subcellular fractionation in a two-step Percoll gradient (Castellino and Germain, 1995; Driessen et al., 1999). I–Ab was immunoprecipitated from fractions enriched in lysosomes (fraction A), late endosomes (fraction B), and early endosomes, ER, Golgi and plasma membranes (fraction C). The immunoprecipitates were analyzed by SDS–PAGE as in Figures 1 and 2.
The majority of newly synthesized MHC class II molecules intersect the endocytic route at or near the early endosomal–late endosomal boundary. The αβ–I complexes then proceed to later compartments where Ii is degraded. The resulting αβ dimers then bind antigenic peptides and are transported to the cell surface (Geuze, 1998; Driessen et al., 1999). Consistent with this scheme, the large majority of labeled class II molecules obtained from control wild-type mice after 3 h of chase had already matured into peptide-bound complexes and resided at the plasma membrane (contained in fraction C; Figure 3, wild-type, control), as confirmed by surface biotinylation (data not shown). In the absence of Cat S, αβ–IiP10 complexes accumulated in late endocytic compartments (fraction B, Cat S control) (Amigorena et al., 1995; Brachet et al., 1997; Pierre and Mellman, 1998a; Driessen et al., 1999).
Addition of ConB to the control or the Cat S–/– splenocytes during the pulse–chase experiment caused the accumulation of class II molecules in fraction C (Figure 3, +ConB). The shift in the intracellular distribution of class II in ConB-treated Cat S–/– cells was particularly informative regarding the effect of this drug: the progression from the early endosomes into later compartments is severely compromised in ConB-treated cells (Yilla et al., 1993; Clague et al., 1994; Benaroch et al., 1995; van Weert et al., 1995). Our fractionation experiments cannot distinguish between the trans-Golgi network (TGN) and early endosomes, both of which are mildly acidic compartments. Given the increased representation of active lysosomal proteases in early endosomes, as compared with the TGN, we favor the involvement of early endosomes as the key compartment targeted by ConB. Class II molecules retained in the early endosomes (fraction C) lose Ii via a cysteine protease-independent mechanism, and accumulate in the form of αβc complexes.
When ConB is removed, αβc complexes are converted into αβ–peptide
What is the fate of the αβc complexes? Are they degraded or, like αβ–IiP10, are they converted into αβ–peptide? We investigated the fate of αβc complexes that accumulated in ConB-treated cells by washing out the drug (Figure 4). Reversion of the effect of ConB could be achieved only if, after a pulse in the presence of 20 nM ConB, the chase was limited to 2 h in 5 nM ConB. Under these conditions, the effect of ConB was readily observed, with inhibition of formation of αβm and accumulation of αβc (compare ‘Control 120’ and ‘ConB 120’ in Figure 4). Following the ConB wash-out, return of the cells to 5 nM ConB and incubation overnight allowed formation of only a minor amount of peptide-loaded αβm complexes, with much class II still in the form of αβc, or not forming any SDS-stable complex at all (Figure 4, o/n+). In contrast, removal of ConB following the 2 h treatment resulted in conversion of most I-Ab molecules into αβm dimers and disappearance of αβc (Figure 4, o/n–). While we cannot argue that this conversion is quantitative, the results nonetheless indicate that a sizable fraction of αβc can be converted to αβm. We conclude that αβc, once formed, can be converted into conventional αβ–peptide complexes.
When the αβm dimers immunoprecipitated from cells subjected to wash-out of ConB were destroyed by boiling, both the completely and the partially modified forms of the α chain were released from the αβ–peptide complexes (Figure 4, upper panel). Thus, the presence of αβ dimers with incompletely modified carbohydrates in ConB-treated cells was not an indication of retention of class II in pre-Golgi compartments, but of partially inhibited N–linked glycan modifications, as observed for other glycoproteins in ConB-treated cells (Yilla et al., 1993).
A novel intermediate in the maturation of I-Ab
The results above show that newly synthesized αβ–Ii trimers can undergo Ii elimination via a cysteine protease/H–2DM-independent mechanism in early endosomes. The Ii-free αβ dimers generated in this manner form a complex stable in SDS, αβc, which can mature into αβ–peptide. The absence of any substantial amount of Ii in this complex was confirmed by Western blotting (supplementary Figure 1).
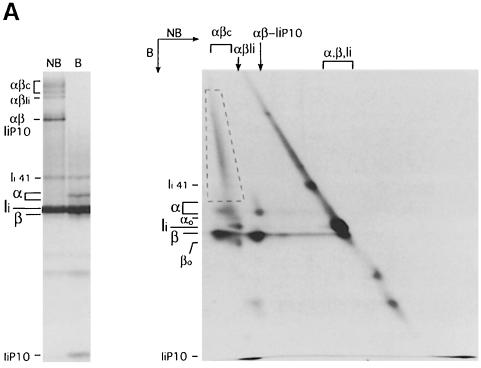
Boiling of N22 immunoprecipitates rich in αβc released α and β chains along with heterodisperse material, which suggested that a complex mixture of polypeptides was included in this complex (Figures 1–4). To better address the composition of αβc, we subjected an immunoprecipitate obtained from cells pulse–chased in the presence of ConB to two-dimensional SDS–PAGE analysis (Figure 5). This immunoprecipitate contained αβ–Ii, αβ–IiP10 and αβc complexes, providing a control for the composition of the ER-resident precursor (αβ–Ii) and of the αβ–IiP10 intermediate generated in late endocytic compartments. The αβ–Ii and αβ–IiP10 complexes were resolved in the second dimension into their respective components: immature α and β chains plus Ii in the former case, mature α and β chains plus IiP10 in the latter. The αβc complex was composed mostly of mature α and β chains (with the same mobility as their counterparts in the αβ–IiP10 complex). Associated with these αβ heterodimers, a heterogeneous mixture of polypeptides was visible as a diagonal streak in the second dimension, and spanning the region that encompassed αβc in the first dimension (dashed box). We estimate the size range of these polypeptides to be 35–200 kDa. Consistent with the observation that Ii is eliminated in cells treated with ConB, neither full-length Ii nor a fragment of Ii were detected in the αβc complex.
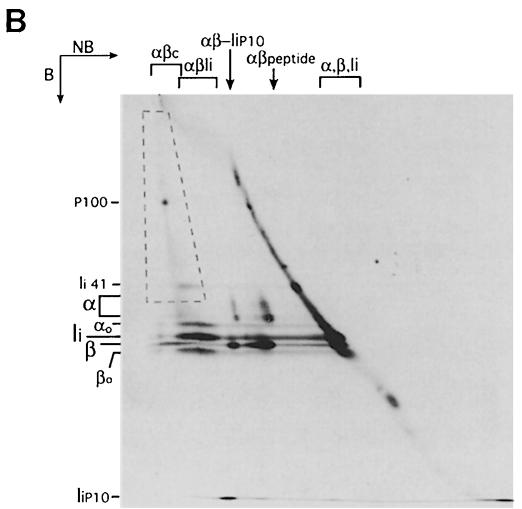
Fig. 5. The αβc complex consists of αβ dimers associated with high molecular weight polypeptides. (A) Class II molecules were immunoprecipitated from splenocytes pulsed for 30 min and chased for 240 min in the presence of ConB. A fraction of the immunoprecipitate was run on a 12.5% SDS–polyacrylamide gel without (NB) or after (B) boiling as a reference (left panel). The remainder was run first on a tubular 12.5% SDS–polyacrylamide gel without boiling. The gel was then boiled, put on top of a 10% SDS–polyacrylamide gel and run in the second dimension (right). The position of the different SDS-stable complexes and free α, β and Ii in the first dimension is indicated on top of the two-dimensional gel. The positions of immature (αo and βo) and mature (α and β) I-Ab subunits, Ii, the p41 form of Ii (Ii 41) and the Ii degradation intermediate IiP10 are indicated on the left of each gel. The dashed box encloses the area occupied by αβc-associated high molecular weight polypeptides. The percentage of polyacrylamide used in the second dimension of the two-dimensional gel (10%) is lower than in the single dimension PAGE (12.5%), causing the difference in the relative distance among polypeptides. (B) Class II molecules were immunoprecipitated from splenocytes pulse-labeled for 30 min and chased for 60 min (no drugs added). The immunoprecipitate was analyzed by two-dimensional SDS–PAGE as in (A). The position of a predominant polypeptide included in αβc is indicated (P100).
Formation of αβc is not an artifact of ConB treatment. This complex is observed in immunoprecipitates obtained from control APCs during a pulse–chase experiment at the 60 min time point (Figure 1A). The composition of the αβc complex generated in normal cells is similar to that described for the complex accumulated in ConB-treated cells, as is evident from analysis of such an immunoprecipitate by two-dimensional SDS–PAGE (Figure 5B, see supplementary Figure 2 for a complete two-dimensional SDS–PAGE analysis of a pulse–chase experiment). In this case, a single polypeptide of ∼100 kDa stood out among the species contained in αβc. We have not investigated the identity of this polypeptide further. The αβc complex could be resolved by size exclusion chromatography of cell lysates, indicating that it was not generated as an artifact of the immunoprecipitation (supplementary Figure 3).
Discussion
We show here that normal splenic APCs possess two distinct mechanisms for the removal of Ii from newly synthesized αβ–Ii complexes. The predominant pathway involves late endocytic compartments and relies on the thiol protease Cat S (Amigorena et al., 1995; Riese et al., 1996, 1998; Brachet et al., 1997; Villadangos et al., 1997; Driessen et al., 1999; Nakagawa et al., 1999; Shi et al., 1999). An alternative mechanism was revealed by applying a block in the endocytic pathway with the vacuolar H+-ATPase inhibitor ConB. In the absence of such pharmacological manipulation, endocytosis proceeds too rapidly to follow accurately the events that occur in early endosomes. ConB in effect bisects the endocytic route, and permits analysis of the reactions as they occur upstream of the ConB block, presumably early in the endocytic pathway.
Two mechanisms for elimination of Ii in distinct endocytic compartments
We propose a model in which Ii can be eliminated at two different locations in the endocytic pathway (Figure 6). If αβ–Ii trafficking proceeds normally, the bulk of Ii is degraded in late endocytic compartments to generate the αβ–IiP10 complex (Amigorena et al., 1995; Brachet et al., 1997; Driessen et al., 1999). The IiP10 fragment is then cleaved by Cat S to produce αβ–CLIP (Riese et al., 1996, 1998; Villadangos et al., 1997; Nakagawa et al., 1999; Shi et al., 1999), which is converted to αβ–peptide in a reaction catalyzed by H–2DM (Vogt and Kropshofer, 1999). The second mechanism occurs in early endosomes, is therefore enhanced in cells in which delivery to later endosomal compartments has been interrupted by application of ConB, and leads to formation of αβc (Figure 6). The lack of Ii in the αβc complex was demonstrated by re-immunoprecipitation (Figure 1B), two-dimensional SDS–PAGE analysis (Figure 5), immunoblotting (supplementary Figure 1) and SDS–PAGE of αβc complexes separated by size exclusion chromatography (supplementary Figure 3). Elimination of Ii in ConB-treated cells does not require cysteine proteases because (i) formation of αβc is not inhibited by leupeptin and (ii) αβc occurs in control and in ConB-treated cells deficient in Cat S (Figure 2A), B or L (not shown).
How is Ii eliminated in the presence of ConB? There are at least two possible mechanisms. First, the enzymes that convert Ii to the P10 stage are not inhibited by leupeptin, and the same protease(s) might substitute for Cat S in ConB-treated cells. However, this would lead to formation of αβ–CLIP complexes that should accumulate in H–2DM-deficient cells treated with ConB, whereas in fact the opposite was observed (Figure 2B). Alternatively, in the early endosomes where class II molecules are retained in the presence of ConB, both the IiP10 fragment and CLIP may be dislodged from αβ dimers independently of H–2DM. The efficiency of removal of CLIP-containing fragments of Ii from class II molecules depends on the structure of the fragments themselves (Kropshofer et al., 1995; Sanderson et al., 1996), the physicochemical conditions of the reaction (Avva and Cresswell, 1994; Denzin et al., 1996), the MHC class II allelic product considered (Avva and Cresswell, 1994; Stebbins et al., 1995; Villadangos et al., 1997) and the intervention of other accessory molecules such as H–2DO (Denzin et al., 1997; van Ham et al., 1997; Kropshofer et al., 1998). It is conceivable that in the compartments in which class II molecules are retained in ConB-treated cells, both CLIP and IiP10 can be eliminated even when Cat S or H–2DM are inactive.
Subcellular fractionation of human and mouse B-cell lines has shown that class II molecules can acquire peptide at various locations along the endocytic route (Castellino and Germain, 1995; Driessen et al., 1999). Therefore, degradation of Ii and loading of antigenic determinants may occur synchronously in several endocytic compartments but involving distinct intermediates. Lindner and Unanue (1996) have demonstrated binding of exogenously added hen egg lysozyme (HEL) to I–Ak. Formation of I–Ak–HEL occurred intracellularly, was leupeptin insensitive and did not require H–2DM, suggesting that the mechanisms of loading of I–Ak with full-length HEL and HEL-derived peptides were different.
Association of class II molecules with precursors of antigenic peptides
The removal of Ii that occurs in ConB-treated cells results in formation of a high molecular weight complex stable in SDS: αβc. The αβc complex occurs transiently in cells not exposed to any drug (Figures 1A and 5B, and supplementary Figure 2), and thus may play a role in class II maturation in normal APCs. This complex is not an artifact of immunoprecipitation, because it can be resolved by chromatography prior to immunoprecipitation (supplementary Figure 3). It is also unlikely that it forms post-lysis because we consistently observe a similar amount of αβc at each time point in the course of different pulse–chase experiments.
Analysis of the composition of αβc by two-dimensional SDS–PAGE shows the presence of a complex mixture of biosynthetically labeled polypeptides along with αβ dimers (Figure 5). These polypeptides are associated with I–Ab in a manner similar to Ii or antigenic peptides, in that they survive treatment in SDS at room temperature. Busch et al. have reported the formation of complexes of ER-resident proteins with class II molecules synthesized in the absence of Ii (Busch et al., 1996). However, the αβc complex that we describe here is generated in a post-Golgi compartment, because most of the I–Abα and β chains present in the complex had undergone complex N–linked sugar modifications (Figure 5, and supplementary Figures 2 and 3) and because the complex did not become apparent until after the pulse (Figures 1A and 2, and supplementary Figure 2). Previous studies have shown that class II molecules can associate intracellularly with intact HEL (mol. wt 14 kDa) (Lindner and Unanue, 1996; Castellino et al., 1998). Our results here show that antigenic capture can occur in the endocytic route with much longer and heterogeneous polypeptides.
What is the role of αβc? By eliminating Ii from a fraction of αβ–Ii complexes in early endosomes, some αβ heterodimers might have access to antigenic determinants that exist only as larger polypeptides in these compartments, but would otherwise be destroyed in the more proteolytically active, later endosomal compartments downstream of the ConB block (Figure 6). Formation of αβc does not represent a ‘dead-end’, because the αβ dimers that accumulate as αβc in the presence of ConB matured to αβ–peptide complexes after removal of the drug (Figure 4). This could involve trimming of the long antigenic precursors or, alternatively, simple loss of the larger polypeptides might allow replacement with other peptides, with or without the assistance of H–2DM, upon reaching a location where such peptides are abundant. The ability to generate class II molecules competent for peptide binding in different intracellular locations would provide the APCs with a capacity to scan for pathogen- derived epitopes in a wider range of compartments.
It is difficult to estimate with precision the relative amount of class II molecules that mature following the αβc intermediate mechanism or the αβ–IiP10 intermediate mechanism. How much of each intermediate is present at any time point during the course of a pulse–chase experiment? Such a comparison can be made reliably only by resolving the complexes by two-dimensional SDS–PAGE: in single dimension gels, the presence of the methionine-rich IiP10 fragment in αβ–IiP10 makes this complex much more apparent in autoradiography than a similar amount αβ–polypeptide (αβc) complexes. Figure 5B and supplementary Figure 2 clearly show that the αβ–IiP10 complex is predominant over αβc at any time point. This comparison is hampered because whereas most of αβ–IiP10 is SDS-stable at room temperature, many αβ–polypeptide complexes may be unstable and not appear as αβc in SDS–PAGE, just as most of αβ–Ii is not resolved as a separate SDS-stable species. Thus, the amount of class II molecules that mature following the alternative mechanism that we describe here is probably larger than is apparent from the amount of αβc complexes that can be resolved during a pulse–chase experiment. In addition, the proportion of class II molecules that lose Ii in early endocytic compartments may vary among MHC allelic products, as does the requirement for Ii for folding in the ER, or the dependency on Cat S and/or H–2DM to mature fully into peptide-bound complexes (Avva and Cresswell, 1994; Bikoff et al., 1995; Stebbins et al., 1995; Wolf et al., 1998). Human B cells treated with ConB do not degrade Ii, and accumulate αβ–Ii trimers in late endocytic/MIIC compartments (Benaroch et al., 1995). What could account for this difference with mouse splenocytes? It is possible that the complement of proteases in the endocytic pathway, and/or their activities, differ for human B lymphoblastoid cells and mouse splenocytes, as they do among different APCs of the same species (Nakagawa et al., 1998, 1999; Shi et al., 1999). Alternatively, in mouse splenocytes, ConB could have more profound effects on transfer from early to late endosomes, whereas in human B cells most αβ–Ii might still be transferred to later compartments. Indeed, the published reports are consistent with cell type-dependent differences in the actual transitions in the endocytic pathway that are targeted by ConB (Yilla et al., 1993; Clague et al., 1994; Benaroch et al., 1995; van Weert et al., 1995). Because the activity of proteases that reside in late endosomes is likely to be more dependent on proper acidification than that of their counterparts in earlier compartments, human B cells would accumulate αβ–Ii trimers more readily. A third possibility might be that human class II molecules enter the endocytic route at later compartments than their mouse counterparts (Benaroch et al., 1995). Still, HLA class II molecules from ConB-treated human B cells co-precipitate with heterodisperse material, which also suggests the formation of some αβc-type complexes, an issue that we have not yet explored. Hence, the model of two pathways for degradation of Ii, as discussed here for mouse splenocytes, may also apply to human B cells.
In conclusion, our results show that APCs are equipped with distinct mechanisms to provide antigen-receptive class II molecules at different stations of the endocytic route. The simultaneous presence of these mechanisms increases the possibilities of displaying foreign epitopes that will be recognized by CD4+ T cells.
Materials and methods
Drugs
Leupeptin was from Boehringer Mannheim (Indianapolis, IN) and ConB was obtained from Ajinimoto Co. (Kanagawa, Japan).
Antibodies
N22 is a hamster mAb that recognizes mouse MHC class II molecules (Metlay et al., 1990; Villadangos et al., 1997), and was a gift of Dr R.M.Steinman (Rockefeller University, New York). The rabbit serum raised against the N–terminal region of Ii (anti-Nt-Ii) (Barois et al., 1997) was the gift of Dr Davoust (Centre d'Immunologie INSERM-CNRS, Marseille, France). The anti-I-Aα and anti-I-Aβ rabbit sera were the gift of Dr R.N.Germain (National Institutes of Health, Bethesda, MD). Similar reagents were generated in our laboratory as described elsewhere (Sant et al., 1991).
Mice
C57bl/6 mice were from the Jackson Laboratory (Bar Harbor, ME). Cat S–/– mice, generated in a 129 (H-2b) background, have been described elsewhere (Shi et al., 1999). H–2DM–/– mice were a kind gift of Dr L.van Kaer (Vanderbilt University, Nashville, TN). Animals used in this study were maintained at the animal facilities of the Massachusetts Institute of Technology and Harvard Medical School in compliance with institutional guidelines.
Metabolic labeling and immunoprecipitation
Metabolic labeling and immunoprecipitation were carried out exactly as described (Villadangos et al., 1997).
Reversion of ConB treatment
Splenocytes were pulsed for 30 min in 20 nM ConB as described above, spun down and resuspended in chase medium containing 5 nM ConB. After 120 min of chase, cells were washed twice in chase medium and incubated overnight in the same medium with or without 5 nM ConB.
Subcellular fractionation
Splenocytes, pulse–chased as above, were subjected to homogenization and subcellular fractionation in two-step Percoll gradients as described previously (Castellino and Germain, 1995; Driessen et al., 1999).
Supplementary data
Supplementary data to this paper (supplementary Figures 1, 2 and 3) are available in The Embo Journal Online.
Acknowledgments
Acknowledgements
We wish to thank Drs Jean Davoust, Ron Germain, Jim Miller and Ralph Steinman for providing antibodies, and Dr Luc Van Kaer for H–2M–/– mice. We also wish to thank our colleagues M.F.Sernee and Dr E.Wang for their help with the FPLC, Dr J.Huppa for help with the 2D SDS–PAGE analyses, and L.Pilapil for excellent technical assistance. This research was supported by National Institutes of Health Grants 5–RO1-AI34893 and 2-P30-CA14051 (H.L.P.). J.A.V. was the recipient of a fellowship from the Lady Tata Memorial Trust (UK). C.D. was supported by the Deutsche Forschungsgemeinschaft.
References
- Amigorena S., Webster, P., Drake, J., Newcomb, J., Cresswell, P. and Mellman, I. (1995) Invariant chain cleavage and peptide loading in major histocompatibility complex class II vesicles. J. Exp. Med., 181, 1729–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avva R.R. and Cresswell, P. (1994) In vivo and in vitro formation and dissociation of HLA–DR complexes with invariant chain-derived peptides. Immunity, 1, 763–774. [DOI] [PubMed] [Google Scholar]
- Barois N., Forquet, F. and Davoust, J. (1997) Selective modulation of the major histocompatibility complex class II antigen presentation pathway following B cell receptor ligation and protein kinase C activation. J. Biol. Chem., 272, 3641–3647. [DOI] [PubMed] [Google Scholar]
- Benaroch P., Yilla, M., Raposo, G., Ito, K., Miwa, K., Geuze, H.J. and Ploegh, H.L. (1995) How MHC class II molecules reach the endocytic pathway. EMBO J., 14, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlmakers M.J., Benaroch, P. and Ploegh, H.L. (1994) Assembly of HLA DR1 molecules translated in vitro: binding of peptide in the endoplasmic reticulum precludes association with invariant chain. EMBO J., 13, 2699–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikoff E.K., Germain, R.N. and Robertson, E.J. (1995) Allelic differences affecting invariant chain dependency of MHC class II subunit assembly. Immunity, 2, 301–310. [DOI] [PubMed] [Google Scholar]
- Brachet V., Raposo, G., Amigorena, S. and Mellman, I. (1997) Ii chain controls the transport of major histocompatibility complex class II molecules to and from lysosomes. J. Cell Biol., 137, 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch R., Cloutier, I., Sekaly, R.P. and Hammerling, G.J. (1996) Invariant chain protects class II histocompatibility antigens from binding intact polypeptides in the endoplasmic reticulum. EMBO J., 15, 418–428. [PMC free article] [PubMed] [Google Scholar]
- Castellino F. and Germain, R.N. (1995) Extensive trafficking of MHC class II–invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity, 2, 73–88. [DOI] [PubMed] [Google Scholar]
- Castellino F., Zappacosta, F., Coligan, J.E. and Germain, R.N. (1998) Large protein fragments as substrates for endocytic antigen capture by MHC class II molecules. J. Immunol., 161, 4048–4057. [PubMed] [Google Scholar]
- Chapman H.A. (1998) Endosomal proteolysis and MHC class II function. Curr. Opin. Immunol., 10, 93–102. [DOI] [PubMed] [Google Scholar]
- Chicz R.M., Urban, R.G., Gorga, J.C., Vignali, D.A., Lane, W.S. and Strominger, J.L. (1993) Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J. Exp. Med., 178, 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague M.J., Urbe, S., Aniento, F. and Gruenberg, J. (1994) Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem., 269, 21–24. [PubMed] [Google Scholar]
- Cresswell P. (1994) Assembly, transport and function of MHC class II molecules. Annu. Rev. Immunol., 12, 259–293. [DOI] [PubMed] [Google Scholar]
- Denzin L.K., Hammond, C. and Cresswell, P. (1996) HLA-DM interactions with intermediates in HLA-DR maturation and a role for HLA-DM in stabilizing empty HLA-DR molecules. J. Exp. Med., 184, 2153–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzin L.K., Sant'Angelo, D.B., Hammond, C., Surman, M.J. and Cresswell, P. (1997) Negative regulation by HLA-DO of MHC class II-restricted antigen processing. Science, 278, 106–109. [DOI] [PubMed] [Google Scholar]
- Deussing J., Roth, W., Saftig, P., Peters, C., Ploegh, H.L. and Villadangos, J.A. (1998) Cathepsins B and D are dispensable for major histocompatibility complex class II-mediated antigen presentation. Proc. Natl Acad. Sci. USA, 95, 4516–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen C., Bryant, R.A., Lennon-Dumenil, A.M., Villadangos, J.A., Bryant, P.W., Shi, G.P., Chapman, H.A. and Ploegh, H.L. (1999) Cathepsin S controls the trafficking and maturation of MHC class II molecules in dendritic cells. J. Cell Biol., 147, 775–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Leung W.P., Surh, C.D., Liljedahl, M., Pang, J., Leturcq, D., Peterson, P.A., Webb, S.R. and Karlsson, L. (1996) Antigen presentation and T cell development in H2-M-deficient mice. Science, 271, 1278–1281. [DOI] [PubMed] [Google Scholar]
- Geuze H.J. (1998) The role of endosomes and lysosomes in MHC class II functioning. Immunol. Today, 19, 282–287. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Amaya, M., Mellins, E. and Wiley, D.C. (1995) The structure of an intermediate in class II MHC maturation: CLIP bound to HLA–DR3. Nature, 378, 457–462. [DOI] [PubMed] [Google Scholar]
- Kasai M., Hirokawa, K., Kajino, K., Ogasawara, K., Tatsumi, M., Hermel, E., Monaco, J.J. and Mizuochi, T. (1996) Difference in antigen presentation pathways between cortical and medullary thymic epithelial cells. Eur. J. Immunol., 26, 2101–2107. [DOI] [PubMed] [Google Scholar]
- Kropshofer H., Vogt, A.B., Stern, L.J. and Hammerling, G.J. (1995) Self-release of CLIP in peptide loading of HLA-DR molecules. Science, 270, 1357–1359. [DOI] [PubMed] [Google Scholar]
- Kropshofer H., Vogt, A.B., Thery, C., Armandola, E.A., Li, B.C., Moldenhauer, G., Amigorena, S. and Hammerling, G.J. (1998) A role for HLA-DO as a co-chaperone of HLA-DM in peptide loading of MHC class II molecules. EMBO J., 17, 2971–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner R. and Unanue, E.R. (1996) Distinct antigen MHC class II complexes generated by separate processing pathways. EMBO J., 15, 6910–6920. [PMC free article] [PubMed] [Google Scholar]
- Martin W.D., Hicks, G.G., Mendiratta, S.K., Leva, H.I., Ruley, H.E. and Van Kaer, L. (1996) H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation and T cell repertoire selection. Cell, 84, 543–550. [DOI] [PubMed] [Google Scholar]
- Metlay J.P., Witmer-Pack, M.D., Agger, R., Crowley, M.T., Lawless, D. and Steinman, R.M. (1990) The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J. Exp. Med., 171, 1753–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T., Wolf, P., Tourne, S., Waltzinger, C., Dierich, A., Barois, N., Ploegh, H., Benoist, C. and Mathis, D. (1996) Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell, 84, 531–541. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., et al. (1998) Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science, 280, 450–453. [DOI] [PubMed] [Google Scholar]
- Nakagawa T.Y., et al. (1999) Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity, 10, 207–217. [DOI] [PubMed] [Google Scholar]
- Pierre P. and Mellman, I. (1998a) Developmental regulation of invariant chain proteolysis controls MHC class II trafficking in mouse dendritic cells. Cell, 93, 1135–1145. [DOI] [PubMed] [Google Scholar]
- Pierre P. and Mellman, I. (1998b) Exploring the mechanisms of antigen processing by cell fractionation. Curr. Opin. Immunol., 10, 145–153. [DOI] [PubMed] [Google Scholar]
- Riese R.J., Wolf, P.R., Bromme, D., Natkin, L.R., Villadangos, J.A., Ploegh, H.L. and Chapman, H.A. (1996) Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity, 4, 357–366. [DOI] [PubMed] [Google Scholar]
- Riese R.J., Mitchell, R.N., Villadangos, J.A., Shi, G.P., Palmer, J.T., Karp, E.R., De Sanctis, G.T., Ploegh, H.L. and Chapman, H.A. (1998) Cathepsin S activity regulates antigen presentation and immunity. J. Clin. Invest., 101, 2351–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli P. and Germain, R.N. (1994) The CLIP region of invariant chain plays a critical role in regulating major histocompatibility complex class II folding, transport and peptide occupancy. J. Exp. Med., 180, 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky A., Rath, S., Preston-Hurlburt, P., Murphy, D.B. and Janeway, C.A.,Jr (1991) On the complexity of self. Nature, 353, 660–662. [DOI] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., Stern, L.J., Wiley, D.C. and Germain, R.N. (1994) MHC class II function preserved by low-affinity peptide interactions preceding stable binding. Nature, 370, 647–650. [DOI] [PubMed] [Google Scholar]
- Sanderson F., Thomas, C., Neefjes, J. and Trowsdale, J. (1996) Association between HLA-DM and HLA-DR in vivo. Immunity, 4, 87–96. [DOI] [PubMed] [Google Scholar]
- Sant A.J., Hendrix, L.R., Coligan, J.E., Maloy, W.L. and Germain, R.N. (1991) Defective intracellular transport as a common mechanism limiting expression of inappropriately paired class II major histocompatibility complex α/β chains. J. Exp. Med., 174, 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G.P., Villadangos, J.A., Dranoff, G., Small, C., Gu, L., Haley, K.J., Riese, R., Ploegh, H.L. and Chapman, H.A. (1999) Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity, 10, 197–206. [DOI] [PubMed] [Google Scholar]
- Stebbins C.C., Loss, G.E., Jr, Elias, C.G., Chervonsky, A. and Sant, A.J. (1995) The requirement for DM in class II-restricted antigen presentation and SDS-stable dimer formation is allele and species dependent. J. Exp. Med., 181, 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ham S.M., et al. (1997) HLA-DO is a negative modulator of HLA-DM-mediated MHC class II peptide loading. Curr. Biol., 7, 950–957. [DOI] [PubMed] [Google Scholar]
- van Weert A.W., Dunn, K.W., Gueze, H.J., Maxfield, F.R. and Stoorvogel, W. (1995) Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J. Cell Biol., 130, 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadangos J.A., Riese, R.J., Peters, C., Chapman, H.A. and Ploegh, H.L. (1997) Degradation of mouse invariant chain: roles of cathepsins S and D and the influence of major histocompatibility complex polymorphism. J. Exp. Med., 186, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt A.B. and Kropshofer, H. (1999) HLA-DM—an endosomal and lysosomal chaperone for the immune system. Trends Biochem. Sci., 24, 150–154. [DOI] [PubMed] [Google Scholar]
- Watts C. (1997) Capture and processing of exogenous antigens for presentation on MHC molecules. Annu. Rev. Immunol., 15, 821–850. [DOI] [PubMed] [Google Scholar]
- Wolf P.R. and Ploegh, H.L. (1995) How MHC class II molecules acquire peptide cargo: biosynthesis and trafficking through the endocytic pathway. Annu. Rev. Cell Dev. Biol., 11, 267–306. [DOI] [PubMed] [Google Scholar]
- Yilla M., Tan, A., Ito, K., Miwa, K. and Ploegh, H.L. (1993) Involvement of the vacuolar H (+)-ATPases in the secretory pathway of HepG2 cells. J. Biol. Chem., 268, 19092–19100. [PubMed] [Google Scholar]
- Zhong G., Castellino, F., Romagnoli, P. and Germain, R.N. (1996) Evidence that binding site occupancy is necessary and sufficient for effective major histocompatibility complex (MHC) class II transport through the secretory pathway redefines the primary function of class II-associated invariant chain peptides (CLIP). J. Exp. Med., 184, 2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]