Abstract
The tight interplay between endoplasmic-reticulum-(ER-) and mitochondria-mediated Ca2+ signaling is a key determinant of cellular health and cellular fate through the control of apoptosis and autophagy. Proteins that prevent or promote apoptosis and autophagy can affect intracellular Ca2+ dynamics and homeostasis through binding and modulation of the intracellular Ca2+-release and Ca2+-uptake mechanisms. During aging, oxidative stress becomes an additional factor that affects ER and mitochondrial function and thus their role in Ca2+ signaling. Importantly, mitochondrial dysfunction and sustained mitochondrial damage are likely to underlie part of the aging process. In this paper, we will discuss the different mechanisms that control intracellular Ca2+ signaling with respect to apoptosis and autophagy and review how these processes are affected during aging through accumulation of reactive oxygen species.
1. Intracellular Ca2+ Signaling
Intracellular Ca2+ signaling is important in the regulation of multiple cellular processes, including development, proliferation, secretion, gene activation, and cell death. The formation of these Ca2+ signals is dependent on many cellular Ca2+-binding and Ca2+-transporting proteins, present in the various cell compartments of which the endoplasmic reticulum (ER) forms the main intracellular Ca2+ store [1]. The resting cytosolic [Ca2+] remains very low (∼100 nM), through active extrusion of Ca2+ by pumps in the plasma membrane or in intracellular organelles, like the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) pump in the ER. Due to SERCA activity and intraluminal Ca2+-binding proteins, the ER can accumulate Ca2+ in more than thousandfold excess compared to the cytosol [1, 2]. In the ER lumen, Ca2+ functions as an important cofactor for ER chaperones, thereby aiding in the proper folding of newly synthesized proteins [3]. Reciprocally, the Ca2+-binding chaperones affect the Ca2+ capacity of the ER by buffering Ca2+ [2]. In addition, two tetrameric ER Ca2+-release channels exist that, upon stimulation, release Ca2+ into the cytosol, thereby provoking Ca2+ signaling: the inositol 1,4,5-trisphosphate (IP3) receptor (IP3R) and the ryanodine receptor (RyR). They are similar in function and structure but differ in regulation, conductance, and expression profile [4, 5]. The rise in cytosolic [Ca2+] following its release from the ER results in various Ca2+-dependent intracellular events. The exact cellular outcome depends on the spatiotemporal characteristics of the generated Ca2+ signal [6]. Since close contact sites between the ER and the mitochondria, involving direct molecular links with the IP3R, exist (Figure 1), it is clear that ER-originating Ca2+ signals critically affect the mitochondrial function.
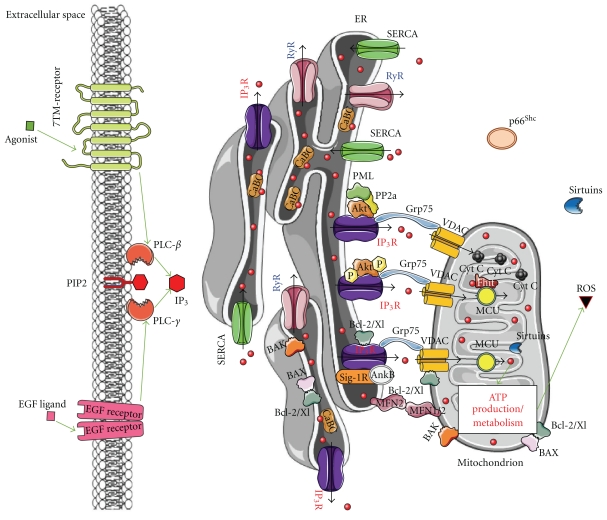
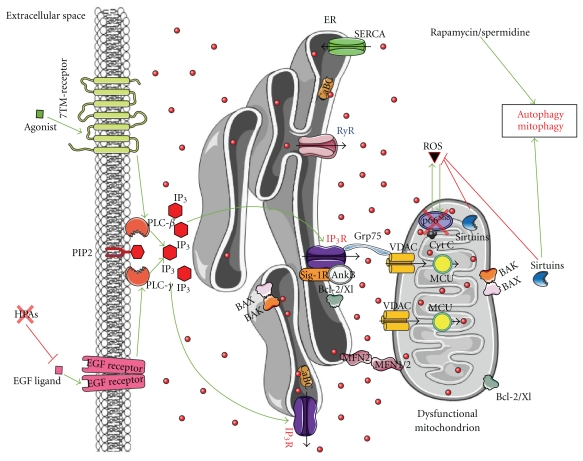
Figure 1.
In a healthy cell, ER Ca2+-handling components tightly regulate mitochondrial function and bioenergetics, representing the different key players involved in intracellular Ca2+ signalling with particular emphasis on the ER-mitochondria connections. The ER-Ca2+ content is regulated by channels and pumps (IP3Rs, RyRs, SERCAs) and by Ca2+-binding chaperones (CaBCs). IP3 stimulates ER Ca2+ release and consequently the transfer of Ca2+ (red dots) from ER to mitochondria. Mitochondrial Ca2+, transported via VDAC, is directly or indirectly involved in cellular energy metabolism and in the secondary production of reactive oxygen species (ROS). It is clear that IP3R-mediated Ca2+ release ought to be tightly regulated to sustain mitochondrial activity and function. As a consequence, Ca2+-flux properties of IP3Rs are tightly and dynamically regulated by accessory proteins involved in cell death and survival, like Bcl-2, Bcl-Xl, PKB/Akt, Sigma-1 receptor (Sig-1R)/Ankyrin B (AnkB), and the recently identified PML. It is important to note that different regulatory mechanisms occur at the IP3R, which may help cell survival (like Bcl-2, Bcl-Xl, PKB/Akt) or help to promote cell death (like PML). The latter is essential to prevent the survival of altered, damaged, or oncogenic cells. Thus, a tight balance between both outcomes is a requisite for cellular health and homeostasis, and a dynamic switch from prosurvival to prodeath is likely essential. In this paradigm, the production of ROS might contribute to the survival of cells by efficient detection of damaged/altered mitochondria and their removal by autophagy, while preventing excessive apoptosis. In addition, controlled apoptosis is likely to be important to eliminate cells, in which the removal of altered mitocondria by autophagy is not sufficient, thereby avoiding tumor genesis. In this process, the recently identified tumor suppressor PML may play a crucial role as it promotes IP3R-mediated Ca2+ transfer from the ER into the mitochondria by dephosphorylating and suppressing PKB/Akt activity through PP2A. While PKB/Akt is known to suppress IP3R-channel activity by phosphorylation of the IP3R, the recruitment of PP2A via PML at the interorganellar ER/mitochondrial complex dephosphorylates and inactivates PKB/Akt. This suppresses PKB-dependent phosphorylation of IP3R and thus promotes Ca2+ release through this channel and Ca2+ transfer into the mitochondria. At the mitochondrial level, the tumor suppressor Fhit has been shown to increase the affinity for the mitochondrial Ca2+ uniporter (MCU), thereby enhancing the uptake of mitochondrial Ca2+ at low and physiologically relevant levels of agonist-induced Ca2+ signals. Green arrows: stimulation; red lines: inhibition; black arrows: Ca2+ flux.
During aging, ER Ca2+ homeostasis alters and becomes dysregulated [7]. Most observations support a decline in ER [Ca2+] and in ER Ca2+ release (due to lower activity of SERCA, IP3R, and RyR), but contradictory findings have been published, possibly related to the cell type under investigation (Figure 2). In addition, ER Ca2+ release and subsequent Ca2+ uptake by mitochondria regulate reactive oxygen species (ROS) production, autophagy, and cell death, processes implicated in aging.
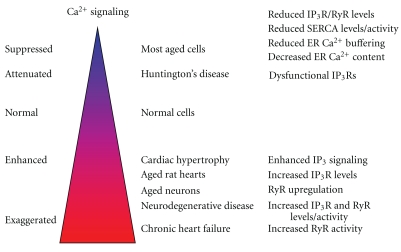
Figure 2.
Altered Ca2+ signaling during aging and in age-related diseases. The Ca2+ dyshomeostasis during age is dependent on the cell type and the context. Most aged cells display decreased ER Ca2+ content and release, due to declined IP3R or RyR levels, reduced SERCA activity, and decreased Ca2+ buffering by intraluminal Ca2+-binding chaperones. However, in neurons and rat hearts, an enhanced Ca2+ signaling is found, caused by increasing IP3R or RyR activity. Age-related diseases (neurodegeneration, cardiac hypertrophy, and chronic heart failure) are also characterized by enhanced Ca2+ signaling. However, this property may be disease dependent, since a mouse model for Huntington's disease displayed attenuated IP3R1 activity due to impaired binding of Grp78 to IP3R1. Hence, caution should be taken with general claims.
In a previous review [8], we have focused on mechanisms regulating the Ca2+ content in the ER and its relevance for the development of physiological versus pathophysiological Ca2+ signalling. In the present review, we will focus on the subsequent step which is the mechanisms responsible for controlling Ca2+ transfer from the ER to the mitochondria. The Ca2+ level in the mitochondrial matrix plays an important role in the progression of apoptosis and autophagy [9, 10]. Here, we will especially analyze how the Ca2+ transfer to the mitochondria as well as apoptosis and autophagy are affected by the aging process in general and by reactive oxygen species in particular.
2. Mitochondrial Ca2+ Handling
In contrast with the role of the ER, the role of the mitochondria in physiological Ca2+ handling was underestimated or even ignored for a long time, but due to the seminal work of Rizzuto and his colleagues [11], this role is now generally accepted.
The electrochemical gradient (Δψm = −180 mV) between the inside and outside of energized mitochondria forms the driving force for the Ca2+ uptake in the mitochondrial matrix, which implies the transfer of Ca2+ ions over both the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM).
The Ca2+ ions taken up into the mitochondrial matrix stimulate the mitochondrial ATP production by regulating the activities of isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and pyruvate dehydrogenase, three dehydrogenases of the Krebs cycle [12, 13]. Also other mitochondrial processes as fatty acid oxidation, amino acid catabolism, aspartate and glutamate carriers, the adenine-nucleotide translocase, Mn-superoxide dismutase, and F1-ATPase activity, are regulated by mitochondrial Ca2+ [12, 14, 15].
The ATP produced by the mitochondria is subsequently transferred to the cytoplasm; it will so especially regulate the activity of ATP-sensitive proteins localized in the close vicinity of the mitochondria. Two major proteins involved in Ca2+ transport, the SERCA, responsible for loading the ER, and the IP3Rs, responsible for Ca2+ release from the ER, are stimulated by ATP. The bidirectional relation between Ca2+ release and ATP production allows for a positive feedback regulation between ER and mitochondria during increased energetic demand [16].
The uptake of Ca2+ in the mitochondria will also affect Ca2+ signaling. The local Ca2+ concentration near the mitochondria will depend on both the amount of Ca2+ released by the IP3R and that taken up by the mitochondria. This will in turn depend on the efficiency of the coupling between both. Since both the SERCA pumps and the IP3Rs are also regulated by Ca2+, the local Ca2+ concentration in the vicinity of the mitochondria will determine the refilling of the ER and eventually the spatiotemporal characteristics of the subsequent Ca2+ signals. The way in which the Ca2+ signals are affected depends on the exact subcellular localization of the mitochondria, the production of ROS, the local Ca2+ concentration, the IP3R isoform expressed, and may as well involve stimulation as inhibition of the signals [16–19]. Furthermore, the connection between mitochondria and the ER can be highly dynamic as the local Ca2+ concentration can also affect mitochondrial motility and ER-mitochondria associations in various ways [20].
3. Transport Proteins Involved in the Transfer of Ca2+ between ER and Mitochondria
3.1. IP3Rs
The first key player is the IP3R, the main Ca2+-release channel in the ER of most cell types. The IP3R consists of 4 subunits of about 310 kDa each (i.e., about 2700 a.a.). In mammals, three different IP3R isoforms are expressed (IP3R1, IP3R2, and IP3R3) while diversity is increased by splicing and the formation of both homo- and heteromeric channels [4, 21, 22]. All IP3R isoforms are activated by IP3, though with varying affinity [23]. Low Ca2+ concentrations stimulate but high Ca2+ concentrations inhibit the IP3Rs [24–27]. Further modulation of the IP3Rs is performed by ATP, phosphorylation, and protein-protein interactions [4, 28–30].
For efficient Ca2+ transfer between ER and mitochondria, it is important that IP3Rs are localized very close to the mitochondrial Ca2+-uptake sites. As different IP3R isoforms exist, an important point is whether interaction with the mitochondria is isoform specific [31]. In CHO cells, IP3R3 is the least expressed isoform, but it demonstrated the highest degree of colocalization with the mitochondria and consequently its silencing had the most profound effects on mitochondrial Ca2+ signals [32]. However, this does not represent a general rule as, for example, in astrocytes IP3R2 was found to preferentially colocalize within the mitochondria [33]. These differences in intracellular localization of the IP3R isoforms may be due to differences in relative expression levels of the various IP3R isoforms and in subcellular localization among different cell types [34]. Moreover, the physiological setting [35] and the differentiation status [36] determine the subcellular localization of the various IP3R isoforms in a given cell type.
3.2. Voltage-Dependent Anion Channels: The Main Ca2+-Transport System across the OMM
The Ca2+ fluxes through the OMM are mainly determined by voltage-dependent anion channels (VDAC). Of the 3 existing VDAC isoforms, VDAC1 is the most abundant in most cell types [37]. It was demonstrated that the transient overexpression of VDAC in various cell types led to an increased Ca2+ concentration in the mitochondria, leading to a higher susceptibility for ceramide-induced cell death [38].
VDAC, however, allows also the transport of other ions and metabolites, including ATP. It has therefore multiple functions in the cell and is a central player in the crosstalk between the cytoplasm and mitochondria. In this manner, VDAC is also implicated in the induction of apoptosis by various stimuli [15].
The permeabilization of the OMM is a crucial step in apoptosis, but how this is exactly performed is not yet clear. Proteins belonging to the B-cell CLL/lymphoma-2 (Bcl-2)-protein family appear anyway to be necessary [39, 40]. Several Bcl-2-family members can affect the permeability of the OMM, for example, by binding to VDAC and regulating its properties or by forming multimeric channel complexes. Independently of the mechanism by which the increase in permeability of the OMM is achieved, it allows the release of the apoptogenic factors present in the intermembrane space to the cytoplasm and the progression of apoptosis [15, 40–42].
3.3. Ca2+-Transport Systems across the IMM
In contrast to the Ca2+-transport system across the OMM, that of the IMM is not yet well characterized. For a long time, the main IMM Ca2+-transport system was named the mitochondrial Ca2+ uniporter. Additionally, a so-called rapid mode of mitochondrial Ca2+ uptake was described, but the nature of neither was known [43].
Three different highly Ca2+-selective channels that may contribute to this process were meanwhile characterized, that is, MiCa [44], mCa1, and mCa2 [45]. Two of these channels, MiCa and mCa1, have properties compatible with the former uniporter and may represent species- and/or cell-type-dependent variability [43]. At the molecular level, the mitochondrial Ca2+-uptake channels are not yet identified, but evidence for a role of a number of proteins has been presented [46, 47]. Recently, a Ca2+-binding protein, named MICU1, which appears essential for mitochondrial Ca2+ uptake, was described [48]. It is, however, not known whether it actually forms (part of) a Ca2+ channel or functions as Ca2+ buffer or Ca2+ sensor. Interestingly, the tumor suppressor protein Fhit (fragile histidine triad) seems to promote mitochondrial Ca2+ uptake by increasing the affinity of the mitochondrial Ca2+ uniporter at the ER/mitochondrial microdomain [49].
Finally, the permeabilization transition pore (PTP) is another channel of still unknown nature [50]. It is voltage and Ca2+ dependent and is sensitive to cyclosporine A. It is not selective for Ca2+ as the open conformation of the PTP has a high conductance for all ions, including Ca2+, and for molecules up to 1500 Da [51]. Its long-time activation leads to the demise of the cell, either by apoptosis or else by necrosis, depending on whether PTP opening occurs in only a small part of the mitochondria or in all of them, respectively [51, 52].
In addition, Ca2+/Na+ and Ca2+/H+ exchangers are also present in the IMM. Their main function is probably to export Ca2+ from the matrix, but they may also contribute to Ca2+ uptake under certain conditions [43].
4. Structural and Regulatory Proteins Involved in the Control of Ca2+ Transfer between ER and Mitochondria
Mitochondria-associated ER membranes (MAMs) were originally described as sites for lipid synthesis and lipid transfer between ER and mitochondria [53]. These MAMs are, however, also ideally suited for Ca2+ exchange [14]. Several proteins may participate in the stabilization of those MAMs and, through this stabilization, affect Ca2+ transfer between ER and mitochondria. Other proteins may be directly involved in regulating the Ca2+-transport proteins described above.
4.1. Glucose-Regulated Protein 75
Glucose-regulated protein 75 (Grp75) belongs to the Hsp70 family of chaperones but is not inducible by heat shock [54, 55]. Importantly, it can couple the IP3R to VDAC1 and allows for a better transfer of the Ca2+ ions from the ER to the mitochondrial matrix [56]. The increased Ca2+ signals in the mitochondria were not due to an increased ER-mitochondria contact area. These results indicate that Grp75 is probably not the main determinant for the ER-mitochondrial linkage but regulates the Ca2+ flux between ER and mitochondria by controlling the interaction between the IP3R and VDAC1.
4.2. Sigma-1 Receptor
The ER chaperone proteins known as sigma receptors are targets for certain neurosteroids. Based on their biochemical and pharmacological properties, two subclasses, sigma-1 and sigma-2 receptors, are distinguished but only the sigma-1 receptor was cloned and properly characterized [57, 58]. The sigma-1 receptor is involved in many physiological functions as well as in several pathological conditions [58].
Sigma-1 receptors are especially enriched at the MAMs [59]. A specific interaction between the Ca2+-binding chaperone BiP and the sigma-1 receptor was described [59]. This interaction depends on the ER Ca2+ concentration: a decrease in ER Ca2+ concentration leads to their dissociation, whereby both proteins become active chaperones.
The sigma-1 receptor regulates several ion channels, including the IP3Rs [58]. Agonists of sigma-1 receptors could so potentiate agonist-induced Ca2+ release in NG108 cells [60]. Hereby, an interaction between the sigma-1 receptor, cytoskeletal ankyrin B, and IP3R3 was demonstrated [61]. In CHO cells, the sigma-1 receptor also interacted with IP3R3, but here ankyrin was not observed in the complex. Finally, a specific role was found for the sigma-1 receptor stabilizing the IP3R3 present at the MAMs, and so regulating Ca2+ transfer between ER and mitochondria [59].
4.3. Mitofusins
Mitofusin 1 and 2 are two dynamin-related GTPases acting on mitochondria. Mitofusin 2 is enriched at MAMs. The absence of mitofusin 2 not only affected ER and mitochondrial morphology but also reduced the number of contact points between ER and mitochondria by about 40% [62]. Mitofusin 2 on the ER appeared necessary for connecting the two organelles by directly interacting with either mitofusin 1 or mitofusin 2 on the OMM. Moreover, the diminished interaction observed in the absence of mitofusin 2 affected Ca2+ transfer between the ER and the mitochondria. A too strong ER-mitochondria interaction may also be detrimental as overexpression of mitofusin 2 led to apoptosis [63].
4.4. Bcl-2-Family Members
Bcl-2 is the prototype of a large family containing both anti- and proapoptotic proteins. The antiapoptotic members of this family, including Bcl-2 itself, are characterized by the presence of 4 Bcl-2-homology (BH) domains (BH1 to 4). The proapoptotic members either have 3 BH domains (BH1, BH2, and BH3) as, for example, Bax and Bak, or only a single BH3 domain, as for example, Bim, Bid, and Bad (the so-called BH3-only proteins) [39].
The BH1, BH2, and BH3 domains of the antiapoptotic proteins, as Bcl-2 and Bcl-Xl, form together a hydrophobic cleft that can bind the amphipathic α-helical BH3 domain of proapoptotic proteins. In this manner, the antiapoptotic Bcl-2 family members antagonize apoptosis at the level of the mitochondria by binding and neutralizing proapoptotic Bax and Bak [39, 64]. In addition to this mitochondrial function, antiapoptotic Bcl-2 family members also act on the ER Ca2+ homeostasis [65, 66]. The exact mechanism is, however, not yet clarified, and effects on several Ca2+-binding or Ca2+-transporting proteins were described, including on the IP3R [67–69].
Although there is an agreement that the antiapoptotic proteins as Bcl-2 bind to the IP3R, there is among the various studies a discrepancy with respect to the exact binding site and to the functional consequences. The results obtained are summarized here below.
Firstly, cells lacking Bax/Bak displayed a decreased ER Ca2+-store content, which was associated with an increased (i) amount of Bcl-2 bound to the IP3R, (ii) protein-kinase-A-(PKA-) dependent phosphorylation of the IP3R, and (iii) Ca2+ leak rate from the ER. Hence, increasing the ratio of antiapoptotic over proapoptotic Bcl-2-family members seemed to decrease the ER Ca2+-store content by promoting the Ca2+ leak via hyperphosphorylation and hyperactivation of the IP3R [70].
Secondly, IP3Rs were described to be activated by Bcl-Xl. Bcl-Xl bound to all three IP3R isoforms, thereby sensitizing them to low IP3 concentrations [71, 72]. The interaction site was demonstrated to be the C-terminal part of IP3R1 [71]. The binding of Bcl-Xl to the IP3Rs is important for the protection of cells against apoptotic stimuli, since the overexpression of Bcl-Xl in IP3R triple-knockout (TKO) cells did not provoke resistance against apoptotic stimuli. By ectopically overexpressing the different IP3R isoforms in the TKO cells, it was found that all IP3R isoforms were sensitized by Bcl-Xl and so conferred resistance against apoptotic stimuli. However, a decline in steady-state ER Ca2+ levels was only found in TKO cells ectopically expressing IP3R3 [72], suggesting that decreased ER Ca2+ levels are not a requisite for cellular protection against apoptosis. The antiapoptotic action may therefore be due to the enhanced Ca2+-spiking activity resulting from the sensitization of the IP3Rs, and be mediated either by increased mitochondrial bioenergetics or by modulation of transcriptional activity and gene expression [71, 72]. A similar mechanism was recently proposed for Bcl-2 and Mcl-1 [73].
Thirdly, an inhibition of the IP3-induced Ca2+ release by Bcl-2 was also demonstrated [74]. In contrast to the work discussed above, the interaction site was mapped to the regulatory domain of IP3R1; moreover, the interaction was mediated through the BH4 domain of Bcl-2, a domain which is not involved in the interaction with the C-terminus of the IP3R [73, 75]. A peptide corresponding to the Bcl-2-binding site on IP3R1 specifically disrupted this interaction and in this way counteracted the functional effects of Bcl-2 on the IP3R [75, 76].
4.5. PKB/Akt and Promyelocytic Leukemia Protein
Another regulatory mechanism of the Ca2+-flux properties of the IP3R is its phosphorylation via PKB/Akt [29, 77, 78]. Upon prosurvival stimulation of cells, the prosurvival kinase PKB/Akt binds and phosphorylates the IP3R, thereby reducing its Ca2+-release activity. This mechanism underpins the increased resistance of cells towards apoptotic stimuli by inhibiting the Ca2+ flux into the mitochondria and may be perused by tumor cells, yielding a survival advantage. The latter has been shown to occur in glioblastoma cells that display hyperactive PKB/Akt, leading to IP3R hyperphosphorylation and suppression of IP3R-channel activity [77].
Very recently, extranuclear promyelocytic leukemia protein (PML) has been shown to be present at the ER and mitochondrial-associated membranes, thereby promoting ER Ca2+ release. At these microdomains, PML controls the Ca2+-flux properties of the IP3R by recruiting PP2A, which dephosphorylates PKB/Akt. The latter suppresses its kinase activity and thus the PKB/Akt-mediated phosphorylation of the IP3R, resulting in increased IP3R-mediated Ca2+ transfer into the mitochondria and thus OMM permeabilization [79, 80]. This mechanism supplements the other known functions of PML in the nucleus of higher eukaryotes. PML nuclear bodies seem to contribute to its tumor suppressive action by inhibiting cell cycle progression and promoting cell death [81].
5. The Transfer of Ca2+ between the IP3R and Mitochondria in Apoptosis and Autophagy
From the previous it is clear that Ca2+ transfer from the ER to the mitochondrial matrix is crucial for regulating mitochondrial functions, including bioenergetics. The mitochondrial Ca2+ signal can, however, also control the choice between cell survival and cell death, as it can participate in the induction and progression of apoptosis and autophagy [9, 10].
5.1. IP3Rs and Mitochondrial Ca2+ in Apoptosis and Necrosis
Different studies have placed the IP3R as central player in the transfer of Ca2+ into the mitochondria. Many cell types display the propagation of agonist-induced Ca2+ signals into the interior of the mitochondria [11, 82].
Ca2+ uptake in the mitochondria is crucial for multiple important cellular functions, but the risk of mitochondrial Ca2+ overload exists, which may result in the induction of cell death. At a high concentration, mitochondrial Ca2+ supports opening of the PTP in the IMM [51, 83]. This opening leads to the release of ions (including Ca2+) and molecules (including ATP), mitochondrial depolarization, ROS production, cessation of oxidative phosphorylation followed by ATP hydrolysis, matrix swelling by osmotic forces, remodeling of the IMM, and eventually rupture of the OMM [52]. Subsequently various apoptogenic factors, including cytochrome C (CytC), apoptosis-inducing factor, Smac/Diablo, HtrA2/Omi, and endonuclease G, are released from the mitochondria [40]. These apoptogenic factors will activate effector caspases, as caspase-3 and caspase-7, and lead the cell into the execution phase of apoptosis. Permeabilization of the OMM is therefore considered as the decisive event in the development of cell death [84]. Given the proximity of IP3Rs to the mitochondrial Ca2+-entry sites, IP3-induced Ca2+ spikes appear ideally suited for the stimulation of apoptosis [85], while the knockdown of the IP3R by siRNA led to the suppression of the Ca2+ transfer to the mitochondria.
In addition to this canonical pathway, the group of Mikoshiba recently showed that not only excessive IP3R-mediated Ca2+ release and the concomitant mitochondrial Ca2+ overload but also the loss of IP3R function may lead to apoptosis by lowering the mitochondrial membrane potential [86]. In this study, it was shown that ER stress in neuronal cell leads to attenuation of IP3R function by impairing the positive regulation of IP3R1 by the ER chaperone Grp78, which acts as a major regulator of the unfolded protein response and thus prevents ER stress. The loss of Grp78 binding to the luminal domain of the IP3R1 leads to impaired subunit assembly and thus dysfunctional channels. This property seems selective for IP3R1, since Grp78 knockdown attenuated IP3R1-mediated Ca2+ release but did not affect IP3R2- or IP3R3-mediated Ca2+ release. Hence, it is interesting to note that Ca2+ transfer from the ER to mitochondria requires a fine-tuned regulation, in which both suppressed and excessive Ca2+ transfer leads to apoptosis.
While a severe impairment of IP3R1 function and attenuated Ca2+ release lead to mitochondrial apoptosis, low-level Ca2+ signaling from ER to mitochondria or enhancing ER-originating Ca2+ oscillations elicits a prosurvival action by stimulating the mitochondrial energy production or by inducing transcription of specific genes [9, 31, 67, 69, 87]. In this paradigm, Bcl-Xl has been proposed to promote cell survival through its direct action on the IP3R by enhancing prosurvival Ca2+ signaling, increasing mitochondrial bio-energetics and activation of signaling via nuclear factor of activated T cells [71, 72].
Mitochondrial Ca2+ is a central factor in several neurodegenerative diseases as Alzheimer's disease, Parkinson's disease, and Huntington's disease [88]. The inhibition of cell death by preventing mitochondrial Ca2+ overload or by preventing the collapse of the mitochondrial membrane potential is likely therapeutically relevant for the treatment of these diseases. In contrast, enhancement of mitochondrial Ca2+ overload can lead to inhibition of tumor cell growth. Stimulation of the Ca2+ transfer between ER and mitochondria could lead to increased apoptosis and in this way inhibit uncontrolled cellular proliferation [89]. In this concept, it is not surprising that many tumor suppressor proteins emerge as regulators of the transfer of Ca2+ from the ER to the mitochondria, like Fhit and PML. Fhit acts at the mitochondrial level by increasing the affinity of the mitochondrial Ca2+ uniporter, thereby promoting mitochondrial Ca2+ elevations at low levels of agonist-induced Ca2+ signaling [49]. PML acts at the level of the ER, where it is recruited by the IP3R via a phosphorylation-dependent process involving Akt and PP2A, thereby promoting Ca2+ transfer between the ER and the mitochondria and inducing cell death [79, 80]. Mutations or ablation of proteins, like Fhit and PML, which may involve attenuated ER/mitochondrial Ca2+ transfers, has been associated with the development of tumors.
5.2. IP3Rs and Mitochondrial Ca2+ in Autophagy
Autophagy is a delivery pathway used for the lysosomal degradation of long-lived proteins, protein aggregates, damaged organelles, and foreign pathogens. In stress situations (e.g., nutrient starvation), this process offers the cell a fresh pool of building blocks and has thus a prosurvival function [90]. Cells in those conditions have to make the decision between survival (autophagy) and death (apoptosis). Important crosstalks exist between these two pathways [91, 92]. Interestingly, Ca2+ and IP3Rs have been implicated in both apoptosis and autophagy, although the role of Ca2+ in autophagy only recently emerged [9, 10, 93]. Nonetheless, Ca2+/IP3Rs may represent key players in the apoptosis-autophagy decision.
The first results on Ca2+ in autophagy even appeared contradictory. On the one hand, autophagy was activated by an increase of the cytosolic Ca2+ concentration [94–96]. On the other hand, autophagy was also activated by conditions that all would lead to a decrease of the IP3R activity and/or cytosolic Ca2+ concentration and therefore potentially of the mitochondrial Ca2+ concentration [97–100]. In a recent report, it was shown that IP3R activity is necessary to provide for a basal Ca2+ signal to the mitochondria, in order to control mitochondrial bioenergetics. IP3R knockdown or inhibition will blunt these Ca2+ signals, thereby compromising mitochondrial ATP production. The resulting increase in AMP/ATP ratio will subsequently activate autophagy via AMP-activated protein kinase (AMPK) [87].
Other results indicate that IP3Rs could inhibit autophagy through a scaffold function, via binding of both Bcl-2 and Beclin-1 (an essential autophagy protein), thereby promoting the anti-autophagic interaction between these two proteins. Treatment of HeLa cells with the IP3R inhibitor xestospongin B promoted the release of Beclin-1 from the IP3R-Bcl-2 complex, leading to autophagy activation [101].
So far, the data on Ca2+-stimulated autophagy concern the Ca2+ in the cytosol [94–96] or ER [102, 103]. It is not yet clear whether the IP3R is hereby involved, although treatment with an IP3R inhibitor did blunt cadmium-induced autophagy stimulation [95]. The exact mechanism by which Ca2+ promotes autophagy is also still under debate. AMPK-dependent [94], AMPK-independent [96], or ERK-dependent pathways [95] are all possible.
Taken together, these data indicate that a specific, low-intensity Ca2+ transfer from ER to mitochondria is necessary to inhibit autophagy, while an increase of the cytosolic Ca2+ concentration would activate autophagy.
6. Implications of Ca2+ Signaling in Aging
6.1. Aging: A Process of Disorganization
All biological processes involved in the transformation of a fertilized egg into a mature individual capable of reproduction are driven by a purposeful genetic program. Through evolution, natural selection has favored individuals that are reproductively successful [104, 105]. Biological systems, like everything else in the universe, change as a result of entropic changes. Entropy is the tendency for concentrated energy to disperse when unhindered. Natural selection has resulted in sufficient relative strengths of the chemical bonds in our molecules to prevent entropic changes and also installed repair and replacement mechanisms. Evolution has therefore kept the biomolecules in a functional state until reproductive maturation.
After sexual maturation, there is no longer a species-survival benefit for indefinitely maintaining these energy states and, hence, the fidelity in most molecules. As we grow older, stochastic or random events not driven by a genetic program cause energy loss resulting in biologically inactive or malfunctioning molecules. Aging is therefore characterized by increasing entropy. The intrinsic thermodynamic instability of the molecules whose precise three-dimensional structures are no longer maintained leads to covalent modifications such as glycation, conformational changes, aggregation and precipitation, amyloid formation, altered protein degradation, synthesis rates, and nuclear and mitochondrial DNA damage and alterations. When the loss of structure and, hence, function ultimately exceeds repair and turnover capacity, vulnerability to pathology and age-associated diseases increases. Because of the randomness of the molecular disorder underlying aging, the loss of molecular fidelity varies within the body. The weakest links in this system will be the first that lead to disease, like in the vascular system and in cells with a high tendency for cancer development. The very heterogeneous aging process contrasts with the virtually identical stages of development until adulthood [106]. In this respect, we will here focus on the age-related disorganization in the Ca2+ signaling machinery, ROS production, and autophagy.
6.2. Mechanism Involved in Aging: ROS, Mitochondria, and Autophagy
The role of ROS accumulation and subsequent macromolecular damage in age-related degeneration has been supported by a plethora of cellular and biological data from various model systems and organisms [107]. Antioxidants act as ROS scavengers and protect against the detrimental effects of cellular ROS exposure. Genetically, genes that extend lifespan were clustered in the IGF-1/insulin-like signaling pathway in a variety of model systems [108]. Nongenetic mechanisms to extend lifespan in different organisms are achieved by caloric restriction and/or by physical activity [109–113]. The composition of the diet during caloric restriction is important; addition of antioxidants (like vitamins, flavonoids), minerals (like Zn and Se), and other compounds such as caffeine, omega 3, and fatty acids has been shown to enhance lifespan [114]. It should be noted, however, that most studies concerning these mechanisms were performed in yeast and animal models, but not yet in humans [115].
Here, we will discuss the molecular mechanisms of ROS underlying aging. First, we will discuss the remodeling of Ca2+ signaling during aging. This is important since the OMM permeabilization is critically controlled by the elevation of the mitochondrial Ca2+ concentration, thereby serving as a coincidence detector with ROS [116]. Next, we will focus on the signaling cascade involving sirtuins, p66Shc, and autophagy in the regulation of mitochondrial function. A schematic overview of the interaction between the different molecular key players in aging is provided in Figure 3.
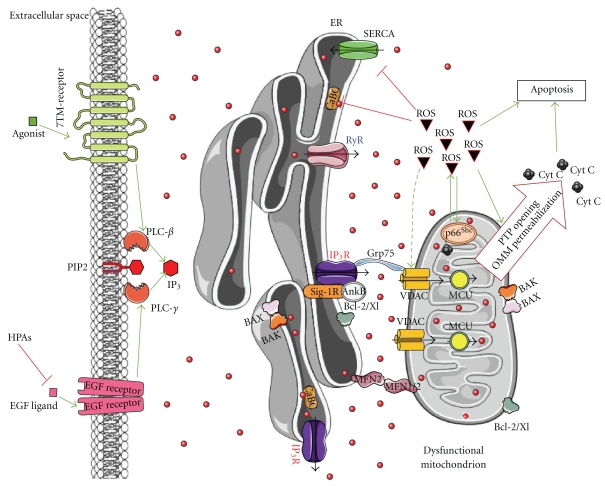
Figure 3.
Ca2+ signalling and key events involved in aging. Aging cells display decreased function or expression of ER proteins (IP3Rs, RyRs, SERCAs, Ca2+-binding chaperones (CaBC)), increased cytosolic [Ca2+], suppressed agonist-mediated signaling, and accumulation of damaged mitochondria due to declined autophagic activity. The simultaneous increase in disorganization and dysfunction of the Ca2+-handling proteins and the decline in autophagy will result in the exaggerated production and excessive accumulation of ROS. These events may lead to both ER stress and mitochondrial dysfunction, like PTP opening and OMM permeabilization with the consequent release of apoptogenic factors and cell death. p66Shc and sirtuins take part in this scenario. P66Shc translocates to mitochondria upon oxidative-stress-induced PKCβ phosphorylation and peptidylprolyl isomerization by Pin1, thereby supporting ROS production. Sirtuins are downregulated and unable to exert its antiaging effect. It is important to note that while p66Shc ablation leads to lifespan extension, high levels of p66Shc have been observed in centenarians. While in normal cells, ROS help to detect and remove altered mitochondria through autophagy, thereby maintaining cellular health, the excessive release of ROS in combination with the decline in autophagy observed during aging may underpin the age-related cell-death processes. In this respect, the recently identified inhibitors of EGF-receptor signaling, the high-performance advanced age phenotype proteins (HPA-1 and HPA-2), whose knockdown promotes locomotory health span of C. elegans, may point towards an important role of proper agonist-induced Ca2+ signaling via the IP3R axis. The relevance of these ligands or of attenuated agonist-induced signaling in humans needs to be established. However, recent evidence indicates that dysfunction of IP3Rs during ER stress promotes cell death and underlies a neurodegenerative disease, like Huntington's disease. Given the central role of proper IP3R function for mitochondrial bioenergetics and ATP production, the decline of IP3R activity observed during ER stress or attenuated upstream signaling linked to IP3 may be very relevant for age-related apoptosis but require further investigation. Green arrows: stimulation; red lines: inhibition; black arrows: Ca2+ flux; dashed-green arrow: stimulation/damage.
6.2.1. Ca2+ Signaling in Aging
Altered intracellular Ca2+ signaling is a hallmark of neurodegeneration, like in Alzheimer's and Huntington's disease [117–120]. Different models have been proposed for familial Alzheimer's-disease-linked presenilin mutations, including the function of presenilins as Ca2+-leak channels [121], an increase in the expression level of IP3Rs [122], or the direct activation of IP3Rs or RyRs [123–125]. In any case, it is clear that exaggerated Ca2+ signaling is an upstream event in the pathophysiology of Alzheimer's disease and contributes to the ROS-mediated cell toxicity [126]. However, the changes in Ca2+ signaling that occur in neurodegenerative diseases may be dependent on the type of disease. For instance, a mouse model for Huntington's disease revealed dysfunctional IP3R Ca2+-release channel activity in the cerebrum and striatum, which was caused by a prominent decline in the association of Grp78, a positive regulator of the IP3R1-channel formation, with the IP3R1 [86].
Other age-related diseases also display altered Ca2+ signaling. Cardiac hypertrophy, for example, is characterized by enhanced IP3 signaling, leading to spontaneous Ca2+-release events that underlie arrhythmias [127]. Also chronic heart failure can be a consequence of excessive phosphorylation of RyR, leading to an increased Ca2+ leak [128] (Figure 2).
However, the role and mechanism of ER Ca2+ signaling in aging is less clear [129], although most studies suggest altered Ca2+ signaling during aging (Figure 2). In most cell types, ER Ca2+ dyshomeostasis was caused by a decreased ER Ca2+ content and a decreased Ca2+ release from the ER, while the cytosolic [Ca2+] was increased. These effects were the result of a decline in SERCA and/or IP3R and/or RyR activity, caused by changes in mRNA or protein levels, phosphorylation events, or oxidative damage to SERCA [7]. In addition, intraluminal Ca2+-buffering protein levels often decline during age, in part also through oxidative damage [130] (Figure 2). Also VDAC undergoes posttranslational modifications in aged cells, possibly through oxidative break-up of tryptophan residues, thereby increasing the susceptibility to apoptosis [131]. This is in line with evidence showing that superoxide can lead to mitochondrial permeabilization in a VDAC-dependent manner [132]. In yeast, this phenomenon can be protected by Cu/Zn-superoxide dismutase, a protein known for its protective role against aging [133].
Some cell types, however, display Ca2+ dyshomeostasis in a different way (Figure 2). Studies in aged rat hearts, for example, showed increased IP3R levels [134]. Also aged neuronal cells displayed reduced sensitivity towards caffeine, which may be caused by a decline in the steady-state ER Ca2+ levels [135–137]. The latter may be due to a decreased SERCA Ca2+-pump activity, a limited supply of ATP or an increased Ca2+ leak from the ER. Other studies pointed to a prolonged Ca2+-induced Ca2+ release, resulting in an inhibition of synaptic strength and long-term potentiation [138, 139].
Interestingly, IP3R characteristics also appear to be altered in aged brain tissues [140], as IP3R density and IP3 binding to the IP3R were decreased in aged rat cerebellum. The same observation of decreased IP3 binding was made in aged mice cerebellum [141]. However, the cellular IP3 content increased with age [142]. These findings suggest a role for the phosphoinositide/Ca2+ signaling in the impaired neuronal responsiveness during aging. In this respect, more recent work revealed that stimulation of IP3Rs in old astrocytes increased protection against ROS and subsequently neuroprotection [143].
Moreover, in aged MII-stage eggs, it was found that the IP3R1 was proteolytically cleaved by caspase-3, resulting in a leaky 95-kDa C-terminal IP3R1 fragment containing the channel pore [144, 145]. In contrast, when the C-terminal channel domain was recombinantly expressed in the mouse oocytes, the sperm-factor-induced Ca2+ oscillations were abolished and the eggs displayed an apoptotic and fragmented phenotype. Previously, we had shown that caspase-3-dependent cleavage of the IP3R augmented the late phase of apoptosis by providing a prolonged ER Ca2+ leak [146]. However, in healthy cells, the Ca2+ leak through a recombinantly expressed C-terminal channel domain was very small. Hence, the caspase-3-dependent cleavage of the IP3R may participate in cellular Ca2+ overload via a second-hit mechanism. In the case of aged oocytes, accumulated ROS may be the second hit. Currently, it is not clear whether IP3R cleavage contributes to the aging process by overloading the mitochondria with Ca2+ and sensitizing them towards ROS accumulation. In addition, ROS may also directly regulate IP3R activity, since it is known that oxidizing agents like thimerosal sensitize IP3Rs by stimulating intramolecular interactions between the suppressor and ligand-binding domain [147]. Taken together, IP3R/Ca2+ signaling appears to be affected in aged cells. Abnormal Ca2+ signals may then affect many processes (ROS production/protection, autophagy, apoptosis, synaptic transmission, etc.) that are altered during aging (summarized in Figure 5). Nevertheless, the overall changes in ER Ca2+ handling observed during aging seem relatively small compared to the changes found in Alzheimer's disease [129].
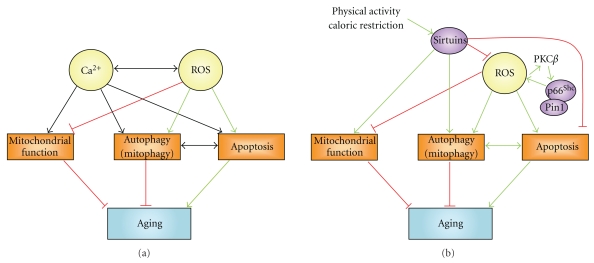
Figure 5.
Network of interactions between sirtuins, p66Shc, Ca2+, and ROS, which affect mitochondrial function, autophagy, and apoptosis, thereby controlling aging-dependent processes. (a) Ca2+ signals may increase or prevent aging. Ca2+ signals are characterized by different spatiotemporal characteristics and subsequently different outcomes on mitochondrial function, autophagy, and apoptosis. For example, a constitutive Ca2+ transfer from ER to mitochondria would stimulate mitochondrial function and inhibit autophagy and apoptosis, while a mitochondrial Ca2+ overload would be proapoptotic. The interplay between mitochondrial Ca2+ elevations and ROS production is a critical determinant in the apoptotic outcome at the level of the mitochondria, which function as co-incidence detectors. Therefore, high mitochondrial Ca2+ concentrations and ROS act as a double-hit mechanism, triggering mitochondrial-dependent apoptosis. (b) Sirtuins are mainly antiaging genes via the promotion of mitochondrial function and autophagy and inhibition of apoptosis. They also act inhibitingly on ROS. Sirtuin function may be enhanced by restricting caloric intake or increasing physical activity, thereby extending lifespan. Increased ROS activate the Pin1- p66Shc complex, which, in turn, promotes the production of ROS and subsequently mitochondrial damage. Therefore, p66Shc may help to target damaged mitochondria and activate cellular processes that deal with dysfunctional mitochondria and oxidative stress. The outcome, however, can be dual: aging may be enhanced via a complete removal of the cell through apoptosis, while the selective removal of the damaged mitochondria through mitophagy, leaving the cell with predominantly healthy mitochondria, may slow down the aging process. Green arrows: stimulation; red lines: inhibition; black arrows: stimulation or inhibition.
Recently, an elegant study on Caenorhabditis elegans re-enforced the paradigm that the activation of IP3R pathways may be considered in therapeutic applications for treating age-related decline in skeletal muscle function (sarcopenia) [148]. Indeed, using an RNAi screen, the authors identified two critical factors that delayed the age-associated decline in locomotory health span of C. elegans in a high-performance advanced age phenotype (HPA-1 and HPA-2). The concept underpinning this study was that locomotory decline in humans contributes to frailty and loss of independence. Although the exact mechanism is not yet known, it is clear that HPA-1 and HPA-2 attenuate epidermal-growth-factor-(EGF-) dependent signaling via the EGF receptor [148]. When HPA-1 and HPA-2 are disrupted, EGF signaling via the EGF receptor will increase. The activation of the EGF-signaling pathway normally leads to cell proliferation, survival, integrity, and differentiation. Importantly, phospholipase C-γ (PLC-γ) and IP3Rs were demonstrated to act downstream of EGF-receptor signaling, thereby contributing to prolonged health span in these animals. This is the very first report considering the role of EGF signaling in aging. Therefore, the exact mechanism of how these signaling pathways affect human aging remains to be further clarified, but restoring the attenuated IP3R-mediated Ca2+ signaling and reestablishing normal mitochondrial function may be an attractive hypothesis in combination with chemical induction of autophagy (Figure 4). Nevertheless, a decline in G-protein-coupled receptor-dependent signaling has been observed in the skeletal muscle and intestine of aged rats [149]. The underlying mechanism involved a prominent decrease in the levels of Gq/11 and Gi protein levels.
Figure 4.
A speculative antiaging strategy based on restoring IP3R-mediated Ca2+ signaling and chemical induction of autophagy. Provided the concept that aging cells are characterized by suppressed IP3 signaling or attenuated IP3R, Ca2+-release activity is relevant in humans, and elevating IP3 levels may compensate for the decline in the IP3/IP3R-signaling axis. This may contribute to a decline in the p66Shc-mediated ROS production, an activation of sirtuin-dependent mitochondrial biogenesis, and the lowering of ROS production. The final step of this compensatory response consists in the autophagic removal of the damaged mitochondria. Hence, chemical induction of autophagy (e.g., by rapamycin or spermidine) is likely critical for successful and healthy aging in human beings. It is important to note that this concept is based on a recent report on C. elegans, in which ablations of inhibitors of EGF signaling enhance IP3R signaling and promote healthy lifespan extension. Green arrows: stimulation; red lines: inhibition; black arrows: Ca2+ flux.
6.2.2. Sirtuins
Sirtuins are a conserved family of proteins that are linked to longevity and stress tolerance in Saccharomyces cerevisiae [150]. Sirtuins have been identified as antiaging genes, since increasing their activity prolonged lifespan not only in yeast, but also in C. elegans and Drosophila melanogaster and is thought to act similarly in mammals [151–153]. In this respect, age is often associated with reduced sirtuin levels. In aged mouse embryonic fibroblasts, progressive loss of the sirtuin-1 protein, but not mRNA, was observed [154]. However, other studies show that this is at least tissue specific; sirtuin-1 activity was reduced in rat hearts, but not in adipose tissue [155], and reduced sirtuin-1 expression was found only in distinctive parts of the mouse brain [156]. Sirtuins, which retard aging as a function of their gene dosage, display unique biochemical activities, that is, NAD-dependent protein deacetylase [157, 158]. The subsequent deacetylation of sirtuin substrates alters their activity (activation or inhibition). In mammals, sirtuin-1 deacetylates a variety of key transcription factors and cofactors, like p53 [159], FOXO proteins [160, 161], peroxisome proliferation activating receptor (PPAR)-γ co-activator-1α (PGC-1α) [162], and nuclear factor-κB [163]. The effects of sirtuin-1 on these factors elicit stress tolerance and metabolic changes reminiscent of caloric restriction, while caloric restriction upregulates sirtuin-1 levels, and mice lacking sirtuin-1 did not display phenotypic responses upon caloric restriction [160, 164–166]. Since sirtuins are regulated by NAD+, their activity will be influenced by the NAD+/NADH ratio and thus by the metabolic state of the cell [167]. Hence, sirtuins may be influenced not only by caloric restriction but also by physical activity, both associated with longevity and increased insulin sensitivity [168, 169].
Importantly, sirtuin-1 also regulates mitochondrial biology [150, 167], another key aspect in aging, since the number of functional mitochondria is known to decline during aging. This has been proposed to underlie aging in diseases like type-2 diabetes [170, 171]. In contrast, increasing mitochondrial activity will increase the metabolic rate, enhance glucose metabolism, and improve insulin sensitivity. Even without an increase in the metabolic rate, caloric restriction might be beneficial by inducing mitochondrial biogenesis via sirtuin-1 [165, 172, 173]. Activation of sirtuin-1 has been shown to be involved in mitochondrial biogenesis and improved mitochondrial function by deacetylation of PGC-1α, thereby lowering ROS production [162].
Sirtuin-1 also suppressed stress-induced apoptosis, while the lack of sirtuin-1 inhibited autophagy in vivo [174]. In addition, the extension of lifespan upon caloric restriction was proposed to be dependent on the induction of autophagy by sirtuin-1 [175]. The underlying mechanism probably involves the deacetylation of certain autophagy proteins, such as Atg5, Atg7, and Atg8 [174, 175]. A schematic overview of the role of sirtuins in aging is depicted in Figure 5.
6.2.3. p66Shc
Recent research revealed the role of p66Shc, the 66 kDa isoform of the Shc (Src homolog and collagen homolog) family [176]. Although p66Shc forms stable complexes with Grb2, an adaptor protein for the Ras-exchange factor SOS, it has little effect on Ras-mediated signaling [177].
Nevertheless, p66Shc is activated by oxidative stress via phosphorylation on Ser36, and this mechanism is indispensable for p66Shc's lifespan regulation [178, 179]. Mice in which p66Shc has been deleted displayed a prolonged lifespan with a decreased mitochondrial metabolism and ROS production, while lacking pathophysiological characteristics or effects on body size. MEF cells from p66Shc-/- animals displayed resistance towards oxidative-stress-induced apoptosis in a p53-dependent manner [176].
ROS arise from the mitochondrial electron-transfer chain or from exogenous sources, like UV and ionizing radiations. p66Shc is involved in mitochondrial ROS production. In basal conditions, about one fifth of p66Shc is localized to the intermembrane space of the mitochondria, while oxidative stress dramatically increases the mitochondria-associated p66Shc due to its mitochondrial translocation from the cytosol [180]. In the mitochondria, p66Shc interacts with CytC, promoting the shuttling of electrons from CytC to molecular oxygen [181]. The latter may underlie the increased ROS production upon p66Shc overexpression and the decreased ROS production in p66Shc knockout cells. In addition, p66Shc knockout cells displayed decreased oxidative capacity, thereby redirecting metabolic energy conversion from oxidative toward glycolytic pathways. Therefore, p66Shc may provide a molecular switch to oxidative-stress-induced apoptosis by controlling mitochondrial ROS production. It should be noted, however, that studies in yeast correlated higher respiration rates combined with decreased oxidative stress and increased lifespan [182]. This suggests that the respiration rate per se is not the important factor for ROS production, but more likely the electron transmit time and the availability of oxygen [183].
In normal cells, oxidative stress leads to compromised mitochondrial Ca2+ homeostasis, which is an early event of mitochondrial damage [107, 176]. This is observed as a decreased mitochondrial Ca2+ signal upon agonist stimulation in cells challenged with H2O2 despite a normal cytosolic Ca2+ signal. Importantly, cells lacking p66Shc seemed to be protected against oxidative challenge, since their mitochondrial Ca2+ signaling upon agonist stimulation was not impaired in the presence of H2O2 [176]. Similar results were found in MEF cells lacking Pin-1, a peptidylprolyl isomerase catalyzing cis/trans isomerization of phosphorylated Ser-Pro bonds, where the reduction of agonist-induced Ca2+ signals in mitochondria upon oxidative stress was significantly smaller. These findings suggest a phosphorylation-dependent conformational change in Pin-1 targets, like p66Shc.
Recent work provided important mechanistic insights into the role of p66Shc in the early mitochondrial response to oxidative stress [178, 179]. ROS are known to activate a variety of kinases, including protein kinase C (PKC) β. The activation of PKCβ will cause the phosphorylation of p66Shc on Ser36, although other kinases may also participate in this process. Indeed, the mitochondrial fraction of p66Shc during oxidative challenge was severely reduced after treatment with PKCβ inhibitors. As a result, Ser36-phosphorylated p66Shc will interact with Pin-1. The catalytic activity of Pin-1 may result in cis/trans isomerization of Ser36-Pro37, thereby triggering the exposure of a mitochondrial targeting sequence or an interaction with mtHsp70, a mitochondrial heat-shock protein. This process may underlie selective targeting of p66Shc to mitochondria undergoing oxidative challenge. The mitochondrial targeting of p66Shc involves its protein-phosphatase-(PP-) 2A-mediated dephosphorylation and dissociation from mtHsp70, although the mechanism of their contribution is not fully elucidated. In the intermembrane space, p66Shc will interact with reduced CytC and enhance intramitochondrial H2O2 production. The latter and its more damaging reaction products, the hydroxyl radicals, have been shown to trigger the opening of the PTP [184]. This will perturb mitochondrial structure and function, resulting in mitochondrial permeabilization, CytC release, and apoptosis induction, and subsequently lead to a coordinated cell-death response and the removal of the cell containing damaged mitochondria. However, in addition to apoptosis, autophagy may be involved in removing the subpopulation of compromised mitochondria suffering from oxidative challenge. Interestingly, this autophagy-mediated removal of damaged mitochondria can be triggered through PTP opening [185]. This will result in the removal of the organelles that are damaged by the oxidative stress (a process termed mitophagy), while maintaining the healthy mitochondria. According to these findings, it is interesting to note that aging has been associated with declined autophagy activity [186], while autophagy activity is a requisite for lifespan extension in C. elegans [187]. In this way, p66Shc may be important for mitochondrial quality control through the autophagy-mediated removal of damaged mitochondria. However, during aging, the number of mitochondria suffering from oxidative stress may increase, while their cleanup by the autophagic system may become limiting, leading to the accumulation of unprocessed oxidation-damaged mitochondria. Importantly, in mouse models for aging, the levels of p66Shc seemed to decline, while its phosphorylation at Ser36 was enhanced [188]. This correlated with higher free-radical production and accumulation of damage caused by ROS.
Strikingly, fibroblasts obtained from centenarians displayed elevated levels of p66Shc [189], indicating that basal mitochondrial p66Shc plays an important role in normal cell-damage management of stress and in damage repair. Indeed, the selective removal of damaged mitochondria may contribute to lifespan extension. In addition, it is interesting to note that increased physical activity has been associated with lifespan extension and lower mortality, although this is associated with increased mitochondrial ROS production due to an increased metabolic rate. Therefore, it is conceivable that exercise may promote adaptation to ROS by upregulating ROS scavengers, causing a natural resistance against ROS or against cellular damage in general [167]. Hence, it may be worth investigating whether p66Shc levels are affected by exercise and whether this may contribute to increased cleanup of damaged mitochondria or resistance against ROS. A schematic overview of the role of p66Shc in aging is depicted in Figure 5.
6.2.4. Autophagy
It has become increasingly clear that autophagy plays a central role in the aging process, in which it is involved in the removal of damaged organelles or of protein aggregates by engulfment in autophagosomes followed by lysosomal degradation. First of all, autophagy was demonstrated to decrease with increasing life time [186]. Caloric restriction slowed down the age-related impairment of autophagy in skeletal muscle of rats [190]. In addition, chemical induction of autophagy by spermidine or by rapamycin prolonged lifespan [191, 192]. In contrast, animals with compromised capacity to perform autophagy were short living and displayed neurodegenerative phenotypes, probably due to the accumulation of deleterious accumulation of protein aggregates [193–195]. Moreover, it is clear that damaged mitochondria ought to be removed, while harboring the healthy mitochondria, which are needed for cell survival. In any case, the accumulation of damaged mitochondria and their impaired removal is a hallmark of aging and will contribute to decreased cell viability. Therefore, mitochondrial quality control is essential for proper cell survival.
The “selective” recognition of damaged mitochondria by autophagosomes without affecting healthy mitochondria remains very poorly understood. However, the first components essential for “selective” mitophagy have been identified in yeast: Uth1, an OMM protein, and Aup1, a mitochondrial phosphatase [196–198]. Additional components of organelle-specific autophagy have been revealed in a systematic screen, including Atg11, Atg20, Atg24, Atg32, and Atg33 [199, 200]. Atg32 is proposed as the receptor for mitophagy via the local recruitment of Atg8, an essential component of the autophagosome formation. NIX/BNIP3L [201, 202], BNIP3 [203], PARKIN [204], and PINK-1 [205–210] were proposed to be involved in mitochondrial degradation in mammalian cells. PARKIN is selectively recruited by dysfunctional mitochondria, thereby mediating the engulfment of these mitochondria by the autophagosomes [204]. A recent study provided clear insights into the underlying mechanism, which required the accumulation of the kinase PINK-1 on damaged mitochondria. In healthy mitochondria, PINK-1 is maintained at a low level by voltage-dependent proteolysis [210]. In mitochondria with sustained damage, PINK-1 levels rapidly accumulated. The latter was required and sufficient to recruit PARKIN to the mitochondria providing a mechanism for the selective removal of damaged mitochondria by autophagy. Importantly, mutations in PINK-1 or PARKIN associated with Parkinson's disease abolished the recruitment of PARKIN by PINK-1 to the mitochondria, allowing the accumulation of damaged mitochondria. Another recent study revealed the mitochondrial protein NIX as the selective mitophagy receptor for the removal of damaged mitochondria by binding and recruiting LC3/GABARAP proteins [211]. The latter are ubiquitin-like modifiers required for the elongation of autophagosomal membranes.
Besides these mitophagy receptors, mitochondrial proteases and chaperones were needed to prevent the accumulation of misfolded and aggregated proteins within the mitochondria [167].
Finally, various studies point towards a role of ROS upstream of autophagy [212]. Accumulation of ROS directly affects different key players essential for the induction of autophagy, including the activation of the protein kinases AMPK and JNK, the inhibition of other kinases (Akt and TOR), and the inhibition of LC3 delipidation. These processes will stimulate autophagy, thereby alleviating the oxidative stress by removing the ROS-generating mitochondria.
7. Conclusions
Upstream Ca2+ and ROS signaling tightly control cellular homeostasis by regulating fundamental cell-death and cell-survival processes like apoptosis and autophagy. It is clear that many proteins that mediate apoptosis and autophagy directly affect Ca2+ signaling through interaction with the ER and mitochondrial Ca2+-release and/or Ca2+-uptake mechanisms. Furthermore, these Ca2+-signaling proteins contribute to the functional and physical linking between ER and mitochondria. Importantly, the interplay between ER and mitochondrial Ca2+ signaling and ROS signaling mediates the detection, the efficient targeting, and removal of mitochondria with sustained damage. This is the key for cellular homeostasis as well as for homeostasis at the level of the whole organism. In this respect, the efficient and selective removal of damaged mitochondria by autophagy is a crucial element in the maintenance of cellular health, whereby the poisonous accumulation of ROS from dysfunctional mitochondria and eventual cell death via apoptosis are avoided. Recent studies point towards a central role for impaired autophagy and inadequate removal of damaged mitochondria during aging. At the level of the organism, apoptosis will be the ultimate resort to remove seriously damaged cells. This will particularly affect the lifespan of nondividing cells, like neurons, thereby affecting the lifespan of the whole organism.
Acknowledgments
Work performed in the laboratory of the authors in this area was supported by the Research Council of the K.U.Leuven (Concerted Action GOA 04/07 and 09/012 and OT-START research funding STRT1/10/044) and by the Research Foundation Flanders (FWO-Vlaanderen) (Grants G.0604.07, G073109N, and G072409N). J. P. Decuypere and G. Monaco are, respectively, recipients of a Ph.D. fellowship from the Agency for Innovation by Science and Technology (IWT) and the Research Foundation Flanders (FWO-Vlaanderen).
References
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature Reviews Molecular Cell Biology. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Görlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxidants and Redox Signaling. 2006;8(9-10):1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 3.Corbett EF, Michalak M. Calcium, a signaling molecule in the endoplasmic reticulum? Trends in Biochemical Sciences. 2000;25(7):307–311. doi: 10.1016/s0968-0004(00)01588-7. [DOI] [PubMed] [Google Scholar]
- 4.Foskett JK, White C, Cheung KH, Mak DOD. Inositol trisphosphate receptor Ca2+ release channels. Physiological Reviews. 2007;87(2):593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutko JL, Airey JA. Ryanodine receptor Ca2+ release channels: does diversity in form equal diversity in function? Physiological Reviews. 1996;76(4):1027–1071. doi: 10.1152/physrev.1996.76.4.1027. [DOI] [PubMed] [Google Scholar]
- 6.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40(5-6):405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Puzianowska-Kuznicka M, Kuznicki J. The ER and ageing II: calcium homeostasis. Ageing Research Reviews. 2009;8(3):160–172. doi: 10.1016/j.arr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Sammels E, Parys JB, Missiaen L, de Smedt H, Bultynck G. Intracellular Ca2+ storage in health and disease: a dynamic equilibrium. Cell Calcium. 2010;47(4):297–314. doi: 10.1016/j.ceca.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Giorgi C, Romagnoli A, Pinton P, Rizzuto R. Ca2+ signaling, mitochondria and cell death. Current Molecular Medicine. 2008;8(2):119–130. doi: 10.2174/156652408783769571. [DOI] [PubMed] [Google Scholar]
- 10.Harr MW, Distelhorst CW. Apoptosis and autophagy: decoding calcium signals that mediate life or death. Cold Spring Harbor Perspectives in Biology. 2010;2(10) doi: 10.1101/cshperspect.a005579. Article ID a005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262(5134):744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 12.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiological Reviews. 1990;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 13.Carafoli E. The fateful encounter of mitochondria with calcium: how did it happen? Biochimica et Biophysica Acta. 2010;1797(6-7):595–606. doi: 10.1016/j.bbabio.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends in Cell Biology. 2009;19(2):81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoshan-Barmatz V, de Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Molecular Aspects of Medicine. 2010;31(3):227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Walsh C, Barrow S, Voronina S, Chvanov M, Petersen OH, Tepikin A. Modulation of calcium signalling by mitochondria. Biochimica et Biophysica Acta. 2009;1787(11):1374–1382. doi: 10.1016/j.bbabio.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. Journal of Physiology. 1999;516(1):1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. Journal of Physiology. 2000;529(1):57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Science’s STKE . 2004;2004(215):p. re1. doi: 10.1126/stke.2152004re1. [DOI] [PubMed] [Google Scholar]
- 20.Goetz JG, Genty H, St.-Pierre P. P, et al. Reversible interactions between smooth domains of the endoplasmic reticulum and mitochondria are regulated by physiological cytosolic Ca2+ levels. Journal of Cell Science. 2007;120(20):3553–3564. doi: 10.1242/jcs.03486. [DOI] [PubMed] [Google Scholar]
- 21.Taylor CW, Genazzani AA, Morris SA. Expression of inositol trisphosphate receptors. Cell Calcium. 1999;26(6):237–251. doi: 10.1054/ceca.1999.0090. [DOI] [PubMed] [Google Scholar]
- 22.Bezprozvanny I. The inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2005;38(3-4):261–272. doi: 10.1016/j.ceca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. The EMBO Journal. 1999;18(5):1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in smooth muscle cells of the guinea pig taenia caeci. Journal of General Physiology. 1990;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- 26.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,S)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 27.Parys JB, Sernett SW, DeLisle S, Snyder PM, Welsh MJ, Campbell KP. Isolation, characterization, and localization of the inositol 1,4,5-trisphosphate receptor protein in Xenopus laevis oocytes. Journal of Biological Chemistry. 1992;267(26):18776–18782. [PubMed] [Google Scholar]
- 28.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annual Review of Biochemistry. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 29.Vanderheyden V, Devogelaere B, Missiaen L, de Smedt H, Bultynck G, Parys JB. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochimica et Biophysica Acta. 2009;1793(6):959–970. doi: 10.1016/j.bbamcr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yule DI, Betzenhauser MJ, Joseph SK. Linking structure to function: recent lessons from inositol 1,4,5-trisphosphate receptor mutagenesis. Cell Calcium. 2010;47(6):469–479. doi: 10.1016/j.ceca.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizzuto R, Marchi S, Bonora M, et al. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochimica et Biophysica Acta. 2009;1787(11):1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes DA, Thompson M, Souto NC, et al. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. Journal of Biological Chemistry. 2005;280(49):40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- 33.Simpson PB, Mehotra S, Langley D, Sheppard CA, Russell JT. Specialized distributions of mitochondria and endoplasmic reticulum proteins define Ca2+ wave amplification sites in cultured astrocytes. Journal of Neuroscience Research. 1998;52(6):672–683. doi: 10.1002/(SICI)1097-4547(19980615)52:6<672::AID-JNR6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 34.Vermassen E, Parys JB, Mauger JP. Subcellular distribution of the inositol 1,4,5-trisphosphate receptors: functional relevance and molecular determinants. Biology of the Cell. 2004;96(1):3–17. doi: 10.1016/j.biolcel.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Vermassen E, van Acker K, Annaert WG, et al. Microtubule-dependent redistribution of the type-1 inositol 1,4,5-trisphosphate receptor in A7r5 smooth muscle cells. Journal of Cell Science. 2003;116(7):1269–1277. doi: 10.1242/jcs.00354. [DOI] [PubMed] [Google Scholar]
- 36.Colosetti P, Tunwell REA, Cruttwell C, Arsanto JP, Mauger JP, Cassio D. The type 3 inositol 1,4,5-trisphosphate receptor is concentrated at the tight junction level in polarized MDCK cells. Journal of Cell Science. 2003;116(13):2791–2803. doi: 10.1242/jcs.00482. [DOI] [PubMed] [Google Scholar]
- 37.Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochemical Journal. 2001;358(1):147–155. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapizzi E, Pinton P, Szabadkai G, et al. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. Journal of Cell Biology. 2002;159(4):613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature Reviews Molecular Cell Biology. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 40.Tait SWG, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature Reviews Molecular Cell Biology. 2010;11(9):621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 41.Tsujimoto Y, Shimizu S. VDAC regulation by the Bcl-2 family of proteins. Cell Death and Differentiation. 2000;7(12):1174–1181. doi: 10.1038/sj.cdd.4400780. [DOI] [PubMed] [Google Scholar]
- 42.Tsujimoto Y, Shimizu S. The voltage-dependent anion channel: an essential player in apoptosis. Biochimie. 2002;84(2-3):187–193. doi: 10.1016/s0300-9084(02)01370-6. [DOI] [PubMed] [Google Scholar]
- 43.Malli R, Graier WF. Mitochondrial Ca2+ channels: great unknowns with important functions. FEBS Letters. 2010;584(10):1942–1947. doi: 10.1016/j.febslet.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427(6972):360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 45.Michels G, Khan IF, Endres-Becker J, et al. Regulation of the human cardiac mitochondrial Ca2+ uptake by 2 different voltage-gated Ca2+ channels. Circulation. 2009;119(18):2435–2443. doi: 10.1161/CIRCULATIONAHA.108.835389. [DOI] [PubMed] [Google Scholar]
- 46.Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. Journal of Biological Chemistry. 2001;276(24):21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- 47.Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nature Cell Biology. 2007;9(4):445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perocchi F, Gohil VM, Girgis HS, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature. 2010;467(7313):291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rimessi A, Marchi S, Fotino C, et al. Intramitochondrial calcium regulation by the FHIT gene product sensitizes to apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12753–12758. doi: 10.1073/pnas.0906484106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baines CP. The molecular composition of the mitochondrial permeability transition pore. Journal of Molecular and Cellular Cardiology. 2009;46(6):850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12(5):815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 52.Rasola A, Sciacovelli M, Pantic B, Bernardi P. Signal transduction to the permeability transition pore. FEBS Letters. 2010;584(10):1989–1996. doi: 10.1016/j.febslet.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rusinol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. Journal of Biological Chemistry. 1994;269(44):27494–27502. [PubMed] [Google Scholar]
- 54.Wadhwa R, Taira K, Kaul SC. An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress and Chaperones. 2002;7(3):309–316. doi: 10.1379/1466-1268(2002)007<0309:ahfcmm>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaul SC, Deocaris CC, Wadhwa R. Three faces of mortalin: a housekeeper, guardian and killer. Experimental Gerontology. 2007;42(4):263–274. doi: 10.1016/j.exger.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 56.Szabadkai G, Bianchi K, Várnai P, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. Journal of Cell Biology. 2006;175(6):901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monnet FP, Maurice T. The sigma protein as a target for the non-genomic effects of neuro(active)steroids: molecular, physiological, and behavioral aspects. Journal of Pharmacological Sciences. 2006;100(2):93–118. doi: 10.1254/jphs.cr0050032. [DOI] [PubMed] [Google Scholar]
- 58.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacology and Therapeutics. 2009;124(2):195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131(3):596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi T, Maurice T, Su TP. Ca2+ signaling via σ1-receptors: novel regulatory mechanism affecting intracellular Ca2+ concentration. Journal of Pharmacology and Experimental Therapeutics. 2000;293(3):788–798. [PubMed] [Google Scholar]
- 61.Hayashi T, Su TP. Regulating ankyrin dynamics: roles of sigma-1 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456(7222):605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 63.Guo X, Chen KH, Guo Y, Liao H, Tang J, Xiao RP. Mitofusin 2 triggers vascular smooth muscle cell apoptosis via mitochondrial death pathway. Circulation Research. 2007;101(11):1113–1122. doi: 10.1161/CIRCRESAHA.107.157644. [DOI] [PubMed] [Google Scholar]
- 64.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Molecular Cell. 2010;37(3):299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baffy G, Miyashita T, Williamson JR, Reed JC. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. Journal of Biological Chemistry. 1993;268(9):6511–6519. [PubMed] [Google Scholar]
- 66.Lam M, Dubyak G, Chen L, Nunez G, Miesfeld RL, Distelhorst CW. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(14):6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joseph SK, Hajnóczky G. IP3 receptors in cell survival and apoptosis: Ca2+ release and beyond. Apoptosis. 2007;12(5):951–968. doi: 10.1007/s10495-007-0719-7. [DOI] [PubMed] [Google Scholar]
- 68.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27(50):6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annual Review of Physiology. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 70.Oakes SA, Scorrano L, Opferman JT, et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(1):105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White C, Li C, Yang J, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nature Cell Biology. 2005;7(10):1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li C, Wang X, Vais H, Thompson CB, Foskett JK, White C. Apoptosis regulation by Bcl-XL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(30):12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eckenrode EF, Yang J, Velmurugan GV, Kevin Foskett J, White C. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. Journal of Biological Chemistry. 2010;285(18):13678–13684. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen R, Valencia I, Zhong F, et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. Journal of Cell Biology. 2004;166(2):193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rong YP, Bultynck G, Aromolaran AS, et al. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rong YP, Aromolaran AS, Bultynck G, et al. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals. Molecular Cell. 2008;31(2):255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szado T, Vanderheyden V, Parys JB, et al. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(7):2427–2432. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khan MT, Wagner L, Yule DI, Bhanumathy C, Joseph SK. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. Journal of Biological Chemistry. 2006;281(6):3731–3737. doi: 10.1074/jbc.M509262200. [DOI] [PubMed] [Google Scholar]
- 79.Giorgi C, Ito K, Lin H-K, et al. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science. 2010;330(6008):1247–1251. doi: 10.1126/science.1189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones AWE, Szabadkai G. Transfer from the ER to mitochondria: channeling cell death by a tumor suppressor. Developmental Cell. 2010;19(6):789–790. doi: 10.1016/j.devcel.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 81.Culjkovic-Kraljacic B, Borden KLB. Puzzled by PML. Science. 2010;330(6008):1183–1184. doi: 10.1126/science.1199405. [DOI] [PubMed] [Google Scholar]
- 82.Hajnóczky G, Csordás G, Yi M. Old players in a new role: mitochondria-associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria. Cell Calcium. 2002;32(5-6):363–377. doi: 10.1016/s0143416002001872. [DOI] [PubMed] [Google Scholar]
- 83.Azzolin L, von Stockum S, Basso E, Petronilli V, Forte MA, Bernardi P. The mitochondrial permeability transition from yeast to mammals. FEBS Letters. 2010;584(12):2504–2509. doi: 10.1016/j.febslet.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiological Reviews. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 85.Szalai G, Krishnamurthy R, Hajnóczky G. Apoptosis driven by IP3-linked mitochondrial calcium signals. The EMBO Journal. 1999;18(22):6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Higo T, Hamada K, Hisatsune C, et al. Mechanism of ER stress-induced brain damage by IP3 receptor. Neuron. 2010;68(5):865–878. doi: 10.1016/j.neuron.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 87.Cárdenas C, Miller RA, Smith I, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142(2):270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Celsi F, Pizzo P, Brini M, et al. Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochimica et Biophysica Acta. 2009;1787(5):335–344. doi: 10.1016/j.bbabio.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rong YP, Barr P, Yee VC, Distelhorst CW. Targeting Bcl-2 based on the interaction of its BH4 domain with the inositol 1,4,5-trisphosphate receptor. Biochimica et Biophysica Acta. 2009;1793(6):971–978. doi: 10.1016/j.bbamcr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nature Reviews Molecular Cell Biology. 2007;8(11):931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 91.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nature Reviews Molecular Cell Biology. 2007;8(9):741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 92.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death and Differentiation. 2009;16(7):966–975. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 93.Vicencio JM, Lavandero S, Szabadkai G. Ca2+, autophagy and protein degradation: thrown off balance in neurodegenerative disease. Cell Calcium. 2010;47(2):112–121. doi: 10.1016/j.ceca.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 94.Høyer-Hansen M, Bastholm L, Szyniarowski P, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-β, and Bcl-2. Molecular Cell. 2007;25(2):193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 95.Wang SH, Shih YL, Ko WC, Wei YH, Shih CM. Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cellular and Molecular Life Sciences. 2008;65(22):3640–3652. doi: 10.1007/s00018-008-8383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grotemeier A, Alers S, Pfisterer SG, et al. AMPK-independent induction of autophagy by cytosolic Ca2+ increase. Cellular Signalling. 2010;22(6):914–925. doi: 10.1016/j.cellsig.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 97.Sarkar S, Floto RA, Berger Z, et al. Lithium induces autophagy by inhibiting inositol monophosphatase. Journal of Cell Biology. 2005;170(7):1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams A, Sarkar S, Cuddon P, et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nature Chemical Biology. 2008;4(5):295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Criollo A, Maiuri MC, Tasdemir E, et al. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death and Differentiation. 2007;14(5):1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- 100.Khan MT, Joseph SK. Role of inositol trisphosphate receptors in autophagy in DT40 cells. Journal of Biological Chemistry. 2010;285(22):16912–16920. doi: 10.1074/jbc.M110.114207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vicencio JM, Ortiz C, Criollo A, et al. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death and Differentiation. 2009;16(7):1006–1017. doi: 10.1038/cdd.2009.34. [DOI] [PubMed] [Google Scholar]
- 102.Gordon PB, Holen I, Fosse M, Rotnes JS, Seglen PO. Dependence of hepatocytic autophagy on intracellularly sequestered calcium. Journal of Biological Chemistry. 1993;268(35):26107–26112. [PubMed] [Google Scholar]
- 103.Brady NR, Hamacher-Brady A, Yuan H, Gottlieb RA. The autophagic response to nutrient deprivation in the hl-1 cardiac myocyte is modulated by Bcl-2 and sarco/endoplasmic reticulum calcium stores. FEBS Journal. 2007;274(12):3184–3197. doi: 10.1111/j.1742-4658.2007.05849.x. [DOI] [PubMed] [Google Scholar]
- 104.Hayflick L. Living forever and dying in the attempt. Experimental Gerontology. 2003;38(11-12):1231–1241. doi: 10.1016/j.exger.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 105.Hayflick L. Biological aging is no longer an unsolved problem. Annals of the New York Academy of Sciences. 2007;1100:1–13. doi: 10.1196/annals.1395.001. [DOI] [PubMed] [Google Scholar]
- 106.Hayflick L. The future of ageing. Nature. 2000;408(6809):267–269. doi: 10.1038/35041709. [DOI] [PubMed] [Google Scholar]
- 107.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radical Biology and Medicine. 2009;47(4):333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 108.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299(5611):1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 109.Weindruch R. Caloric restriction, gene expression, and aging. Alzheimer Disease and Associated Disorders. 2003;17(2):S58–S59. doi: 10.1097/00002093-200304002-00008. [DOI] [PubMed] [Google Scholar]
- 110.Ingram DK, Anson RM, de Cabo R, et al. Development of calorie restriction mimetics as a prolongevity strategy. Annals of the New York Academy of Sciences. 2004;1019:412–423. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 111.Lane MA, Black A, Handy A, Tilmont EM, Ingram DK, Roth GS. Caloric restriction in primates. Annals of the New York Academy of Sciences. 2001;928:287–295. doi: 10.1111/j.1749-6632.2001.tb05658.x. [DOI] [PubMed] [Google Scholar]
- 112.Stewart KJ. Physical activity and aging. Annals of the New York Academy of Sciences. 2005;1055:193–206. doi: 10.1196/annals.1323.029. [DOI] [PubMed] [Google Scholar]
- 113.Rolland Y, Abellan van Kan G, Vellas B. Healthy brain aging: role of exercise and physical activity. Clinics in Geriatric Medicine. 2010;26(1):75–87. doi: 10.1016/j.cger.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 114.Rockenfeller P, Madeo F. Ageing and eating. Biochimica et Biophysica Acta. 2010;1803(4):499–506. doi: 10.1016/j.bbamcr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 115.Smith DL, Nagy TR, Allison DB. Calorie restriction: what recent results suggest for the future of ageing research. European Journal of Clinical Investigation. 2010;40(5):440–450. doi: 10.1111/j.1365-2362.2010.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baumgartner HK, Gerasimenko JV, Thorne C, et al. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. Journal of Biological Chemistry. 2009;284(31):20796–20803. doi: 10.1074/jbc.M109.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bezprozvanny I, Hayden MR. Deranged neuronal calcium signaling and Huntington disease. Biochemical and Biophysical Research Communications. 2004;322(4):1310–1317. doi: 10.1016/j.bbrc.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 118.Bezprozvanny I. Inositol 1,4,5-tripshosphate receptor, calcium signalling and Huntington’s disease. Sub-Cellular Biochemistry. 2007;45:323–335. doi: 10.1007/978-1-4020-6191-2_11. [DOI] [PubMed] [Google Scholar]
- 119.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends in Neurosciences. 2008;31(9):454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends in Molecular Medicine. 2009;15(3):89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, Bezprozvanny I. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. Journal of Clinical Investigation. 2007;117(5):1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kasri NN, Kocks SL, Verbert L, et al. Up-regulation of inositol 1,4,5-trisphosphate receptor type 1 is responsible for a decreased endoplasmic-reticulum Ca2+ content in presenilin double knock-out cells. Cell Calcium. 2006;40(1):41–51. doi: 10.1016/j.ceca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 123.Ferreiro E, Oliveira CR, Pereira CMF. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloid-β peptide. Journal of Neuroscience Research. 2004;76(6):872–880. doi: 10.1002/jnr.20135. [DOI] [PubMed] [Google Scholar]
- 124.Cheung K-H, Shineman D, Müller M, et al. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58(6):871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheung KH, Mei L, Mak DOD, et al. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Science Signaling. 2010;3(114):p. ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muller M, Cheung KH, Foskett JK. Enhanced ROS generation mediated by Alzheimer’s disease presenilin regulation of InsP3R Ca2+ signaling. doi: 10.1089/ars.2010.3421. Antioxidants & Redox Signaling. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harzheim D, Talasila A, Movassagh M, et al. Elevated InsP3R expression underlies enhanced calcium fluxes and spontaneous extra-systolic calcium release events in hypertrophic cardiac myocytes. Channels. 2010;4(1):67–71. doi: 10.4161/chan.4.1.10531. [DOI] [PubMed] [Google Scholar]
- 128.Reiken S, Lacampagne A, Zhou H, et al. PKA phosphorylation activates the calcium release channel (ryanodine receptor)-in skeletal muscle: defective regulation in heart failure. Journal of Cell Biology. 2003;160(6):919–928. doi: 10.1083/jcb.200211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends in Neurosciences. 2004;27(10):614–620. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 130.Naidoo N. ER and aging-protein folding and the ER stress response. Ageing Research Reviews. 2009;8(3):150–159. doi: 10.1016/j.arr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 131.Groebe K, Klemm-Manns M, Schwall GP, et al. Age-dependent posttranslational modifications of voltage-dependent anion channel 1. Experimental Gerontology. 2010;45(7-8):632–637. doi: 10.1016/j.exger.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 132.Madesh M, Hajnóczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. Journal of Cell Biology. 2001;155(6):1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Karachitos A, Galganska H, Wojtkowska M, et al. Cu,Zn-superoxide dismutase is necessary for proper function of VDAC in Saccharomyces cerevisiae cells. FEBS Letters. 2009;583(2):449–455. doi: 10.1016/j.febslet.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 134.Kaplan P, Jurkovicova D, Babusikova E, et al. Effect of aging on the expression of intracellular Ca2+ transport proteins in a rat heart. Molecular and Cellular Biochemistry. 2007;301(1-2):219–226. doi: 10.1007/s11010-007-9414-9. [DOI] [PubMed] [Google Scholar]
- 135.Kirischuk S, Verkhratsky A. Calcium homeostasis in aged neurones. Life Sciences. 1996;59(5-6):451–459. doi: 10.1016/0024-3205(96)00324-4. [DOI] [PubMed] [Google Scholar]
- 136.Verkhratsky A, Shmigol A, Kirischuk S, Pronchuk N, Kostyuk P. Age-dependent changes in calcium currents and calcium homeostasis in mammalian neurons. Annals of the New York Academy of Sciences. 1994;747:365–381. doi: 10.1111/j.1749-6632.1994.tb44423.x. [DOI] [PubMed] [Google Scholar]
- 137.Murchison D, Griffith WH. Age related alterations in caffeine-sensitive calcium stores and mitochondrial buffering in rat basal forebrain. Cell Calcium. 1999;25(6):439–452. doi: 10.1054/ceca.1999.0048. [DOI] [PubMed] [Google Scholar]
- 138.Clodfelter GV, Porter NM, Landfield PW, Thibault O. Sustained Ca2+-induced Ca2+-release underlies the post-glutamate lethal Ca2+ plateau in older cultured hippocampal neurons. European Journal of Pharmacology. 2002;447(2-3):189–200. doi: 10.1016/s0014-2999(02)01843-5. [DOI] [PubMed] [Google Scholar]
- 139.Kumar A, Foster TC. Enhanced long-term potentiation during aging is masked by processes involving intracellular calcium stores. Journal of Neurophysiology. 2004;91(6):2437–2444. doi: 10.1152/jn.01148.2003. [DOI] [PubMed] [Google Scholar]
- 140.Igwe OJ, Filla MB. Aging-related regulation of myo-inositol 1,4,5-trisphosphate signal transduction pathway in the rat striatum. Molecular Brain Research. 1997;46(1-2):39–53. doi: 10.1016/s0169-328x(96)00269-0. [DOI] [PubMed] [Google Scholar]
- 141.Simonyi A, Xia J, Igbavboa U, Wood WG, Sun GY. Age differences in the expression of metabotropic glutamate receptor 1 and inositol 1,4,5-trisphosphate receptor in mouse cerebellum. Neuroscience Letters. 1998;244(1):29–32. doi: 10.1016/s0304-3940(98)00127-x. [DOI] [PubMed] [Google Scholar]
- 142.Igwe OJ, Ning LI. Inositol 1,4,5-trisphosphate arm of the phosphatidylinositide signal transduction pathway in the rat cerebellum during aging. Neuroscience Letters. 1993;164(1-2):167–170. doi: 10.1016/0304-3940(93)90883-m. [DOI] [PubMed] [Google Scholar]
- 143.Wu J, Holstein JD, Upadhyay G, et al. Purinergic receptor-stimulated IP3-mediated Ca2+ release enhances neuroprotection by increasing astrocyte mitochondrial metabolism during aging. Journal of Neuroscience. 2007;27(24):6510–6520. doi: 10.1523/JNEUROSCI.1256-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Verbert L, Devogelaere B, Parys JB, Missiaen L, Bultynck G, de Smedt H. Proteolytic mechanisms leading to disturbed Ca2+ signaling in apoptotic cell death. Calcium Binding Proteins. 2007;2(1):21–29. [Google Scholar]
- 145.Verbert L, Lee B, Kocks SL, et al. Caspase-3-truncated type 1 inositol 1,4,5-trisphosphate receptor enhances intracellular Ca2+ leak and disturbs Ca2+ signalling. Biology of the Cell. 2008;100(1):39–49. doi: 10.1042/BC20070086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Assefa Z, Bultynck G, Szlufcik K, et al. Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate-independent calcium release during apoptosis. Journal of Biological Chemistry. 2004;279(41):43227–43236. doi: 10.1074/jbc.M403872200. [DOI] [PubMed] [Google Scholar]
- 147.Bultynck G, Szlufcik K, Kasri NN, et al. Thimerosal stimulates Ca2+ flux through inositol 1,4,5-trisphosphate receptor type 1, but not type 3, via modulation of an isoform-specific Ca2+-dependent intramolecular interaction. Biochemical Journal. 2004;381(1):87–96. doi: 10.1042/BJ20040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iwasa H, Yu S, Xue J, Driscoll M. Novel EGF pathway regulators modulate C. elegans healthspan and lifespan via EGF receptor, PLC-gamma, and IP3R activation. Aging cell. 2010;9(4):490–505. doi: 10.1111/j.1474-9726.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Facchinetti MM, de Boland AR. Aging and calcitriol regulation of IP3 production in rat skeletal muscle and intestine. Hormone and Metabolic Research. 2001;33(1):10–15. doi: 10.1055/s-2001-12619. [DOI] [PubMed] [Google Scholar]
- 150.Westphal CH, Dipp MA, Guarente L. A therapeutic role for sirtuins in diseases of aging? Trends in Biochemical Sciences. 2007;32(12):555–560. doi: 10.1016/j.tibs.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 151.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes and Development. 1999;13(19):2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410(6825):227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 153.Wood JG, Regina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 154.Sasaki T, Maier B, Bartke A, Scrable H. Progressive loss of SIRT1 with cell cycle withdrawal. Aging Cell. 2006;5(5):413–422. doi: 10.1111/j.1474-9726.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- 155.Ferrara N, Rinaldi B, Corbi G, et al. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Research. 2008;11(1):139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 156.Lafontaine-Lacasse M, Richard D, Picard F. Effects of age and gender on Sirt 1 mRNA expressions in the hypothalamus of the mouse. Neuroscience Letters. 2010;480(1):1–3. doi: 10.1016/j.neulet.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 157.Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annual Review of Biochemistry. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- 158.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annual Review of Biochemistry. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 159.Vaziri H, Dessain SK, Eaton EN, et al. hSIR2 functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 160.Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 161.Motta MC, Divecha N, Lemieux M, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116(4):551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 162.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 163.Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. The EMBO Journal. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305(5682):390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 165.Civitarese AE, Carling S, Heilbronn LK, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Medicine. 2007;4(3, article e76) doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen D, Steele AD, Lindquist S, Guarente L. Medicine: increase in activity during calorie restriction requires Sirt1. Science. 2005;310(5754):p. 1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 167.Weber TA, Reichert AS. Impaired quality control of mitochondria: aging from a new perspective. Experimental Gerontology. 2010;45(7-8):503–511. doi: 10.1016/j.exger.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 168.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. Canadian Medical Association Journal. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Teramoto M, Bungum TJ. Mortality and longevity of elite athletes. Journal of Science and Medicine in Sport. 2010;13(4):410–416. doi: 10.1016/j.jsams.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 170.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. The New England Journal of Medicine. 2004;350(7):664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283(5407):1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 173.Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Journal of the American Medical Association. 2006;295(13):1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee IH, Cao L, Mostoslavsky R, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(9):3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Morselli E, Maiuri MC, Markaki M, et al. The life span-prolonging effect of sirtuin-1 is mediated by autophagy. Autophagy. 2010;6(1):186–188. doi: 10.4161/auto.6.1.10817. [DOI] [PubMed] [Google Scholar]
- 176.Pinton P, Rizzuto R. p66Shc, oxidative stress and aging: importing a lifespan determinant into mitochondria. Cell Cycle. 2008;7(3):304–308. doi: 10.4161/cc.7.3.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bonfini L, Migliaccio E, Pelicci G, Lanfrancone L, Pelicci PG. Not all Shc’s roads lead to Ras. Trends in Biochemical Sciences. 1996;21(7):257–261. [PubMed] [Google Scholar]
- 178.Pinton P, Rimessi A, Marchi S, et al. Protein kinase C β and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66. Science. 2007;315(5812):659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 179.Hajnóczky G, Hoek JB. Cell signaling: mitochondrial longevity pathways. Science. 2007;315(5812):607–609. doi: 10.1126/science.1138825. [DOI] [PubMed] [Google Scholar]
- 180.Orsini F, Migliaccio E, Moroni M, et al. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. Journal of Biological Chemistry. 2004;279(24):25689–25695. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- 181.Giorgio M, Migliaccio E, Orsini F, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122(2):221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 182.Barros MH, Bandy B, Tahara EB, Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. Journal of Biological Chemistry. 2004;279(48):49883–49888. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- 183.Bonawitz ND, Shadel GS. Rethinking the mitochondrial theory of aging: the role of mitochondrial gene expression in lifespan determination. Cell Cycle. 2007;6(13):1574–1578. doi: 10.4161/cc.6.13.4457. [DOI] [PubMed] [Google Scholar]
- 184.Costantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. Journal of Biological Chemistry. 1996;271(12):6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 185.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. The FASEB Journal. 2001;15(12):2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 186.Meijer AJ, Codogno P. Signalling and autophagy regulation in health, aging and disease. Molecular Aspects of Medicine. 2006;27(5-6):411–425. doi: 10.1016/j.mam.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 187.Meléndez A, Tallóczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301(5638):1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 188.Lebiedzinska M, Duszynski J, Rizzuto R, Pinton P, Wieckowski MR. Age-related changes in levels of p66Shc and serine 36-phosphorylated p66Shc in organs and mouse tissues. Archives of Biochemistry and Biophysics. 2009;486(1):73–80. doi: 10.1016/j.abb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 189.Pandolfi S, Bonafè M, Di Tella L, et al. p66shc is highly expressed in fibroblasts from centenarians. Mechanisms of Ageing and Development. 2005;126(8):839–844. doi: 10.1016/j.mad.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 190.Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Experimental Gerontology. 2010;45(2):138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Eisenberg T, Knauer H, Schauer A, et al. Induction of autophagy by spermidine promotes longevity. Nature Cell Biology. 2009;11(11):1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 192.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 194.Komatsu M, Kominami E, Tanaka K. Autophagy and neurodegeneration. Autophagy. 2006;2(4):315–317. doi: 10.4161/auto.2974. [DOI] [PubMed] [Google Scholar]
- 195.Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 196.Kiššová I, Deffieu M, Manon S, Camougrand N. Uth1p is involved in the autophagic degradation of mitochondria. Journal of Biological Chemistry. 2004;279(37):39068–39074. doi: 10.1074/jbc.M406960200. [DOI] [PubMed] [Google Scholar]
- 197.Tal R, Winter G, Ecker N, Klionsky DJ, Abeliovich H. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. Journal of Biological Chemistry. 2007;282(8):5617–5624. doi: 10.1074/jbc.M605940200. [DOI] [PubMed] [Google Scholar]
- 198.Kanki T, Klionsky DJ. Mitophagy in yeast occurs through a selective mechanism. Journal of Biological Chemistry. 2008;283(47):32386–32393. doi: 10.1074/jbc.M802403200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kanki T, Wang KE, Baba M, et al. A genomic screen for yeast mutants defective in selective mitochondria autophagy. Molecular Biology of the Cell. 2009;20(22):4730–4738. doi: 10.1091/mbc.E09-03-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Okamoto K, Kondo-Okamoto N, Ohsumi Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Developmental Cell. 2009;17(1):87–97. doi: 10.1016/j.devcel.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 201.Sandoval H, Thiagarajan P, Dasgupta SK, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454(7201):232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schweers RL, Zhang J, Randall MS, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shaw J, Yurkova N, Zhang T, et al. Antagonism of E2F-1 regulated Bnip3 transcription by NF-κB is essential for basal cell survival. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(52):20734–20739. doi: 10.1073/pnas.0807735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. Journal of Cell Biology. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dagda RK, Chu CT. Mitochondrial quality control: insights on how Parkinson’s disease related genes PINK1, parkin, and Omi/HtrA2 interact to maintain mitochondrial homeostasis. Journal of Bioenergetics and Biomembranes. 2009;41(6):473–479. doi: 10.1007/s10863-009-9255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cherra SJ, Dagda RK, Tandon A, Chu CT. Mitochondrial autophagy as a compensatory response to PINK1 deficiency. Autophagy. 2009;5(8):1213–1214. doi: 10.4161/auto.5.8.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dagda RK, Cherra SJ, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. Journal of Biological Chemistry. 2009;284(20):13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Geisler S, Holmström KM, Treis A, et al. The PINK1/Parkin-mediated mitophagy is compromised by PD-associated mutations. Autophagy. 2010;6(7):871–878. doi: 10.4161/auto.6.7.13286. [DOI] [PubMed] [Google Scholar]
- 209.Geisler S, Holmström KM, Skujat D, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nature Cell Biology. 2010;12(2):119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 210.Narendra DP, Jin SM, Tanaka A, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biology. 2010;8(1) doi: 10.1371/journal.pbio.1000298. Article ID e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Novak I, Kirkin V, McEwan DG, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Reports. 2010;11(1):45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dewaele M, Maes H, Agostinis P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy. 2010;6(7):838–854. doi: 10.4161/auto.6.7.12113. [DOI] [PubMed] [Google Scholar]