Abstract
Focal adhesion kinase (FAK) is an important regulator of integrin signaling in adherent cells and accordingly its activity is significantly modulated during mitosis when cells detach from the extracellular matrix. During mitosis, FAK becomes heavily phosphorylated on serine residues concomitant with its inactivation and dephosphorylation on tyrosine. Little is known about the regulation of FAK activity by serine phosphorylation. In this report, we characterize two novel sites of serine phosphorylation within the C-terminal domain of FAK. Phosphorylation-specific antibodies directed to these sites and against two previously characterized sites of serine phosphorylation were used to study the regulated phosphorylation of FAK in unsynchronized and mitotic cells. Among the four major phosphorylation sites, designated pS1-pS4, phosphorylation of pS1 (Ser722) is unchanged in unsynchronized and mitotic cells. In contrast, pS3 and pS4 (Ser843 and Ser910) exhibit increased phosphorylation during mitosis. In vitro peptide binding experiments provide evidence that phosphorylation of pS1 (Ser722) may play a role in modulating FAK binding to the SH3 domain of the adapter protein p130Cas.
INTRODUCTION
Focal adhesion kinase (FAK) was originally identified as a tyrosine phosphorylated protein targeted to focal adhesions, organized regions of cell-extracellular matrix (ECM) contact (Schaller et al., 1992). FAK signaling from the integrin family of adhesion receptors has been studied extensively and involves primarily tyrosine phosphorylation of FAK and subsequent phosphotyrosine-dependent interactions with other signaling proteins such as c-Src (Schaller et al., 1994), phosphatidylinositol 3′-kinase (Chen and Guan, 1994), and Grb2 (Schlaepfer et al., 1994). In addition to nucleating signaling complexes in response to integrin engagement, FAK also associates with structural proteins, including p130Cas (Polte and Hanks, 1995) and paxillin (Hildebrand et al., 1995). Like FAK, tyrosine phosphorylation of both Cas and paxillin is induced in response to cell adhesion to ECM (Burridge et al., 1992; Petch et al., 1995), and the formation of complexes containing FAK, Cas, and paxillin appears to be central to the activation of signaling pathways involving c-Src, phosphatidylinositol 3′-kinase-Akt, Ras-MAPK (mitogen-activated protein kinase), and Crk-C3G. The regulated disassembly of these signaling complexes is likely to be critical during processes such as mitosis, when cells physically remodel their cytoskeletons, detach from the extracellular matrix, and disengage various metabolic processes until the completion of cell division.
During mitosis, focal adhesion complexes dissociate as cells round up from their ECM substrates, remaining attached through actin-based retraction fibers (Wetzel et al., 1978). FAK, paxillin, and Cas are dephosphorylated on tyrosine during this stage of the cell cycle, concomitant with an increase in the serine phosphorylation of all three of these proteins (Yamaguchi et al., 1997; Yamakita et al., 1999). Tyrosine dephosphorylation of FAK eliminates the c-Src binding site, thereby uncoupling this signaling complex from downstream effectors. Serine phosphorylation of FAK correlates temporally with the dissociation of its binding to Cas (Yamakita et al., 1999), which interacts through a Src homology 3 (SH3) domain with proline-rich sequences in the FAK C terminus (Harte et al., 1996; Polte and Hanks, 1997).
Type II SH3 domain ligands, such as those present in the FAK C terminus, adopt a helical conformation formed by a core PXXPXR consensus sequence (Yu et al., 1994) and appear to interact constitutively with their targets. To date, a single example has been documented where serine phosphorylation negatively regulates the binding of SH3 domains to their ligands, namely, in the interaction of the Grb2 SH3 domains with proline-rich sequences in Sos (Corbalan-Garcia et al., 1996). Tyrosine phosphorylation of the epidermal growth factor receptor results in inducible binding of the SH3-SH2-SH3 adapter protein Grb2. The SH3 domains of Grb2 associate with polyproline ligands in Sos, a guanine nucleotide exchange factor that activates the Ras-MAP kinase signaling cascade (Egan et al., 1993; Gale et al., 1993; Li et al., 1993; Rozakis-Adcock et al., 1993). Activated MAPK phosphorylates Sos on serine residues in the Sos C terminus, proximal to the proline-rich sequences that interact with the Grb2 SH3 domains (Li et al., 1993; Rozakis-Adcock et al., 1993, 1995; Corbalan-Garcia et al., 1996). Sos serine phosphorylation correlates with the dissociation of Grb2-Sos complexes in vivo, suggesting that Sos serine phosphorylation destabilizes its binding to Grb2; accordingly, mutation of the Sos phosphorylation sites leads to the recovery of Grb2–Sos complexes (Corbalan-Garcia et al., 1996). These data suggest that serine phosphorylation of Sos proximal to its SH3 domain-binding ligands negatively influences its binding to Grb2, thus providing a negative feedback mechanism for uncoupling signals from the receptor once MAP kinase has been activated (Rozakis-Adcock et al., 1995).
Because virtually nothing is known about the role of serine phosphorylation in regulating the activities of FAK, we undertook to identify the sites of serine phosphorylation in vivo, to develop antibodies to these phosphorylation sites, and to begin to analyze the biological significance of serine phosphorylation. In this report, we identify two sites of serine phosphorylation in the C terminus of FAK (Ser722 and Ser910), which together with two previously identified sites (Ser840 and Ser843) represent four major sites of FAK serine phosphorylation. Using phosphorylation-specific antibodies directed against each site, we show that in HeLa cells each of these sites is phosphorylated in unsynchronized cells, whereas Ser843 and Ser910 are inducibly phosphorylated in mitotic cells. Ser722 is positioned proximal to a polyproline ligand mediating FAK binding to the Cas SH3 domain, within the sequence PPKPSRPGYPpS. Using a sensitive in vitro peptide competition assay, we provide evidence that phosphorylation of Ser722 in FAK may be important in negatively regulating the ability of this polyproline ligand to bind to the Cas SH3 domain.
MATERIALS AND METHODS
Cell Culture and Protein Expression
Chicken embryo (CE) fibroblasts were prepared from 11-d embryos, cultured as previously described (Reynolds et al., 1989), and transfected with the replication competent retrovirus vector RCAS A encoding FAK-related nonkinase (FRNK) by using calcium phosphate (Hughes et al., 1987; Richardson et al., 1997). HeLa cells were maintained in Iscove's modified Dulbecco's medium supplemented with 10% fetal bovine serum, transfected with various DNA expression constructs by using Lipofectamine (Life Technologies, Gaithersburg, MD), and harvested 48 h after transfection. For studies on mitotic proteins, cells were arrested with 0.5 μg/ml nocodazole (Sigma, St. Louis, MO) in growth medium for 14 to 18 h. Myc- and FLAG-tagged FAK have been described elsewhere (Xiong and Parsons, 1997; Burnham et al., 2000).
Cell Lysis
Lysis of adherent unsynchronized cells was performed as previously described (Richardson et al., 1997). Mitotic cells arrested with nocodazole were collected by washing the cells into the culture medium and recovered by centrifugation. Cells were washed gently in phosphate-buffered saline and lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM HEPES, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.5% deoxycholate [pH 7.2]) supplemented with 100 μM leupeptin, 0.05 trypsin inhibitor units (TIU) per milliter aprotinin, 10 μM pepstatin, 40 mM p-nitrophenylphosphate, 20 μg/ml soybean trypsin inhibitor, 1 mM Na3VO4, 40 mM NaF, 10 mM NaPPi, and 1 mM phenylmethylsulfonyl fluoride. The lysate was clarified by centrifugation and the soluble fraction collected in the supernatant.
Metabolic Labeling with 32P and Phosphopeptide Mapping
Labeling of CE cells in vivo and phosphopeptide mapping was performed using methods identical to those previously described (Richardson et al., 1997). Briefly, CE cells overexpressing FRNK or FRNK variants were labeled in vivo with 50-100 mCi [32P]orthophosphate (NEN, Boston, MA) for 12 h at 37°C. CE cells were lysed in ice-cold RIPA buffer supplemented with protease and phosphatase inhibitors. FRNK was immunoprecipitated with the monoclonal antibody 2A7. Immune complexes were resolved by SDS-PAGE and transferred to nitrocellulose. The bands containing FRNK were excised and treated with trypsin for 6 h at 37°C. Phosphopeptides were spotted onto thin layer chromatography (TLC) plates and subjected to high-voltage electrophoresis at 1000 V for 45 min in pH 8.9 buffer followed by chromatography in isobutyric acid buffer.
Phosphorylation-specific Anti-FAK Antibodies
Phosphorylation site-specific antibodies against FAK peptides encompassing the site of interest were generated by BioSource International (Hopkinton, MA). Rabbit polyclonal anti-pS1 (raised against the peptide sequence CPSRPGYP[pS]PRSSEGF-NH2), anti-pS2 (Ac-DVRL[pS]RGSIDRE[Ahx]KC-NH2), anti-pS3 (Ac-DVRLSRG[pS]IDRE[Ahx]KC-NH2), and anti-pS4 (LQPQEI[pS]PPPTANLC-NH2) were isolated by both negative and positive affinity purification. ‘Ac‘ represents acetylation and ‘Ahx‘ represents amino-hexonoic acid, a six-carbon spacer. These antibodies were used at a concentration of 2-3 μg/ml for Western blots. Endogenous FAK was detected in HeLa cells by using monoclonal antibody (mAb) 2A7. Myc- and FLAG-tagged proteins were detected using 9E10 (Santa Cruz Biotechnology, Santa Cruz, CA) and M5 (Sigma) antibodies, respectively.
For peptide preincubation, each phospho-specific antibody (2-3 μg/ml) was incubated for 1 h at room temperature in blocking buffer (Tris-buffered saline with 0.1% Tween-20 and 5% nonfat dry milk) with a 20- to 100-fold molar excess of the appropriate phosphorylated or nonphosphorylated synthetic peptide, also produced by BioSource International. After preincubation, antibody solutions were directly applied to nitrocellulose strips previously blocked in Tris-buffered saline with 0.1% Tween-20 with 5% milk for Western blotting.
Expression and Purification of Recombinant Proteins
Glutathione-S-transferase (GST) fusion proteins were expressed in DH5 bacteria and affinity purified using glutathione Sepharose (Pharmacia, Piscataway, NJ). Hexahistidine-tagged FRNK (His-FRNK) was provided by Peter Sheffield in the bacterial expression vector pHIS.Parallel1 (Sheffield et al., 1999). His-tagged wild-type and mutant FRNK were expressed in BL-21 bacteria and purified using TALON affinity resin (Clontech, Palo Alto, CA). Beads with bound His-tagged proteins were washed twice in lysis buffer (20 mM Tris-HCl, 100 mM NaCl, 0.5% NP-40 [pH 8.0]) and twice in wash buffer (50 mM Tris-HCl, 100 mM NaCl [pH 8.0]). His-FRNK was eluted in two to three bed volumes of elution buffer (50 mM Tris-HCl, 100 mM NaCl, 250 mM imidazole [pH 8.0]) for 20 min at 4°C. The elution step was performed three times. The pooled eluate was dialyzed against storage buffer (50 mM HEPES [pH 7.5], 100 mM NaCl, 1 mM dithiothreitol, 50% glycerol [vol/vol]) overnight at 4°C and stored at −20°C.
Peptide Competition Studies
Peptides for the in vitro competition studies were synthesized by the BioMolecular Research Facility, University of Virginia. The peptides used for the competition assays were designated wild type (EAPPKPSRPGYPSPRSS), phospho (EAPPKPSRPGYPpSPRSS), and P→A (EAPPKASRPGYPSPRSS). For peptide preincubation studies, 400 nM glutathione-S-transferase (GST) fusion protein was incubated with 0-100 μM peptide in RIPA buffer at 4°C for 30 min with constant rocking. After this incubation, the mixture was diluted twofold with 500 μg of CE cell lysate and incubated for an additional 2 h at 4°C with constant rocking. The reactions were washed twice in RIPA and once in Tris-buffered saline. Proteins were eluted by boiling in Laemmli sample buffer, resolved by SDS-PAGE, and transferred to nitrocellulose for Western blotting.
For peptide competition assays, 200 nM GST fusion protein coupled to glutathione Sepharose was mixed with 400 nM purified His-FRNK and varying concentrations of peptide competitor in binding buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 0.5% Triton X-100, 0.25% bovine serum albumin). Reactions were incubated for 90 min at 4°C with constant rotation, and then diluted 1.75-fold with binding buffer containing 25% Sepharose CL-6B (Sigma) as a carrier. Complexes were washed twice with ice cold 0.05% SDS-RIPA (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.05% SDS, 0.5% sodium deoxycholate) and once with ice cold Tris-buffered saline over a vacuum manifold. Proteins were eluted by boiling in Laemmli sample buffer, resolved by 10% SDS-PAGE, and transferred to nitrocellulose for Western blotting.
Endogenous FAK and FRNK in CE cell lysates were detected in Western blots by using the polyclonal antiserum BC3. Recombinant FRNK was detected using the monoclonal antibody 2A7. GST was detected using the monoclonal antibody 9D9. For densitometric quantitation of recombinant FRNK, bands were visualized with anti-mouse IgG labeled with 125I (NEN) followed by autoradiography.
RESULTS
The Carboxyl Terminus of FAK Is Phosphorylated In Vivo at Sites Corresponding to Ser722 and Ser910
In certain cell types, the carboxyl terminal domain of FAK is expressed as a separate protein called FRNK (Schaller et al., 1993; Nolan et al., 1999). Previous work from our laboratory showed that endogenous FRNK expressed in cultured CE cells is phosphorylated on serine residues in vivo (Schaller et al., 1993). Because the amino acid sequences of FRNK and the carboxyl terminus of FAK are identical, we used FRNK as a tool to identify phosphorylation sites in the FAK C terminus. Figure 1D shows a typical tryptic phosphopeptide map of FRNK immunoprecipitated from CE cells overexpressing FRNK and labeled with 32P in vivo. FRNK contains four major tryptic phosphopeptides, two of which were previously shown to result from phosphorylation of Ser148 and Ser151 (denoted peptides A and B, Figure 1D) (Richardson et al., 1997).
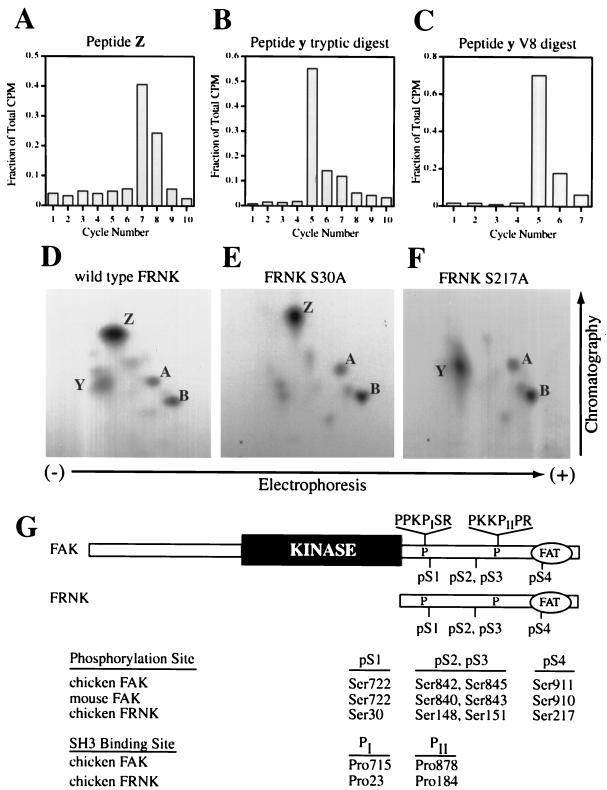
Figure 1.
FRNK, the autonomously expressed carboxyl terminus of FAK, is phosphorylated in vivo on Ser30 (pS1) and Ser217 (pS4). (A–C) Sequential Edman degradation of phosphopeptides collected from in vivo labeling of FRNK. Phosphopeptides were isolated from TLC plates and subjected to sequential Edman degradation as described in MATERIALS AND METHODS. The amount of radioactivity released was determined after each cycle of degradation. Tryptic peptide Z was collected from CE cells overexpressing wild-type FRNK and labeled in vivo with [32P]orthophosphate (A). For peptide y, GST-FRNK was phosphorylated in vitro with protein kinase A and unlabeled ATP, followed by labeling with casein kinase I and [γ-32P]ATP. GST-FRNK was digested with trypsin (B) or V8 (C) before TLC analysis and Edman degradation. Values are expressed as a fraction of total radioactivity recovered after the indicated number of cycles. (D–F) Two-dimensional phosphopeptide mapping of wild-type FRNK (D) or FRNK point mutants at pS1 (E) or pS4 (F) expressed in CE cells labeled in vivo with [32P]orthophosphate. (G) Unified nomenclature for FAK and FRNK serine phosphorylation sites and critical Cas-binding proline residues based on their relative positions in each protein. ‘FAT‘ represents the focal adhesion-targeting sequence at the extreme C termini of FAK and FRNK.
To identify the sites of phosphorylation represented in peptides Y and Z, CE cells overexpressing FRNK were labeled in vivo with 32P and peptides Y and Z were recovered from the tryptic map. Edman degradation analysis showed that the majority of the radioactivity was released from peptide Z after seven cycles of sequencing (Figure 1A). Analysis of the primary sequence of FRNK revealed two serine residues as candidate sites of phosphorylation represented by peptide Z; both Ser217 and Ser247 reside seven residues downstream of a trypsin cleavage site (e.g., Arg or Lys).
Similar Edman degradation studies on peptide Y were inconclusive (our unpublished results). However, previous studies had shown that peptide Y could be labeled in vitro by phosphorylation of a GST-FRNK fusion protein with protein kinase A and unlabeled ATP followed by incubation with casein kinase I and [γ-32P]ATP (Richardson et al., 1997). Because peptide y generated by labeling in vitro comigrated with peptide Y derived from labeling in vivo, we used peptide y for Edman degradation studies. Analysis of peptide y clearly showed that the majority of the radioactivity was released after five cycles of sequencing (Figure 1B). Examination of the FRNK sequence showed six candidate serine residues, each of which was positioned five residues C-terminal to a trypsin cleavage site: Ser10, Ser30, Ser90, Ser151, Ser217, and Ser356.
Mutation of Ser151 to Ala did not affect the appearance of phosphopeptide Y in maps derived from CE cells (Richardson et al., 1997). To help distinguish among the five remaining candidate sites, peptide y derived from in vitro labeling was further digested with V8 protease and mapped. One major phosphopeptide was resolved, and sequential Edman sequencing revealed that the majority of the radioactivity was still released after five cycles (Figure 1C). Cleavage by V8 was confirmed by mixing samples of peptide y before and after digestion, demonstrating a mobility shift attributable to protease treatment (our unpublished results). Because V8 cleaves after glutamate residues, these data suggested that a Glu residue was positioned C-terminal to the phosphorylated serine. Because the phosphorylated serine must be five residues downstream of Arg or Lys with no Glu residues in the intervening amino acid sequence, serines 90, 217, and 356 could be eliminated. Thus, the remaining candidate sites of phosphorylation were Ser10 and Ser30 for peptide Y and Ser217 and Ser247 for peptide Z.
Each of the candidate Ser residues in FRNK was individually mutated to Ala and the mutant FRNK constructs were subcloned into the retroviral vector RCAS A for expression in CE cells. In vivo labeling and tryptic mapping revealed that expression of the S30A mutant resulted in loss of peptide Y (Figure 1E), whereas expression of the S217A mutant resulted in loss of peptide Z (Figure 1F). Expression of either the S10A or the S247A mutants did not affect the appearance of the phosphopeptide map (our unpublished results). Thus, we were able to assign a single serine residue that was phosphorylated in each of the four peptides in the tryptic map: Ser148 in peptide A, Ser151 in peptide B, Ser30 in peptide Y, and Ser217 in peptide Z.These sites correspond to Ser842, Ser845, Ser722, and Ser911, respectively, in the amino acid sequence of chicken FAK.
Although the phosphorylation sites are conserved between FAK and FRNK and among FAK orthologues in different species, the numbering of such sites is different due to slight differences in amino acid sequence of the FAK proteins and the alternative start of the FRNK protein. We refer to the phosphorylation sites by their relative positions in the FAK/FRNK protein sequence (Figure 1G). Thus, Ser722, Ser842, Ser845, and Ser911 of chicken FAK correspond to pS1, pS2, pS3, and pS4, respectively.
Antibodies Raised against Phosphorylated Peptide Antigens Mimicking the Serine Phosphorylation Sites in FAK Specifically Recognize Phosphorylated Epitopes
To facilitate the analysis of FAK serine phosphorylation, we developed antibodies specific to each site of serine phosphorylation (see MATERIALS AND METHODS). To confirm that the antibodies were specific to individual phosphorylated FAK epitopes, we tested each antibody in Western blots to assess binding to FAK proteins in which individual phosphorylation sites were mutated from Ser to Ala. Expression constructs encoding either wild-type or mutant FAK tagged with either the myc or the FLAG epitope were transfected into HeLa cells and lysates of the transfected cells were immunoblotted with the phospho-specific antibodies. Blotting of a portion of the lysates with the anti-myc or anti-FLAG antibodies yielded a single band, indicating that all of the constructs were expressed to a similar degree (Figure 2, A and B, lanes 1-3). However, immunoblotting of the lysates with an antibody that recognizes both endogenously and ectopically expressed FAK showed that myc-tagged FAK (but not FLAG-tagged FAK) could be resolved from endogenous FAK, migrating as a separate band slightly above the endogenous protein (illustrated in Figure 2A, lanes 4-9).
Figure 2.
Site-specific recognition of serine-phosphorylated residues in the C terminus of FAK by phospho-specific antibodies raised against each site of phosphorylation. (A) Myc-tagged wild-type FAK or FAK variants harboring mutations at pS2 or pS3 were transfected into HeLa cells. Lysates of transfected cells were immunoblotted with anti-myc (lanes 1-3), anti-pS2 (lanes 4-6), or anti-pS3 (lanes 7-9) to demonstrate that mutation of the immunogenic phosphorylated serine residue abolished epitope recognition by the phospho-specific antibodies. Similarly, in B, FLAG-tagged FAK or FAK variants harboring mutations at pS1 or pS4 were tested with the anti-FLAG (lanes 1-3), anti-pS1 (lanes 4-6), or anti-pS4 (lanes 7-9) antibodies. Endogenous FAK is indicated by the open arrows, ectopically expressed FAK by the closed arrows.
Immunblotting the lysates with phosphorylation-specific antibodies showed that anti-pS1 reacted strongly with lysates of cells transfected with wild-type FLAG-FAK, indicating recognition of both endogenous and exogenous proteins (Figure 2B, lane 4). The reactivity of anti-pS1 was appreciably reduced when tested on cells expressing the pS1A mutant (lane 5), consistent with reduced binding of anti-pS1 to the FAK pS1A mutant. Reactivity of anti-pS1 with FLAG-FAK pS4A was indistinguishable from reactivity with wild-type FLAG-FAK (lanes 4 and 6), consistent with this antibody being specific for pS1.
AntipS2 recognized two bands in cells expressing wild-type myc-FAK or the myc-FAK pS3A mutant (Figure 2A, lanes 4 and 6). In contrast, anti-pS2 recognized only a single band, comigrating with endogenous FAK, in cells expressing myc-FAK pS2A (lane 5). These data indicate that anti-pS2 is selective for phospho-Ser840. Anti-pS3 reacted strongly with wild-type myc-FAK (Figure 2A, lane 7) but poorly with either the pS2A or pS3A FAK proteins (lanes 8 and 9). Mutation of pS3 (Ser843) to Ala reduced the binding of anti-pS3, consistent with this antibody recognizing pS3. However, as we have previously shown, pS3 is optimally phosphorylated only when pS2 (Ser840) is prephosphorylated (Richardson et al., 1997). Thus, the failure to observe binding of anti-pS3 to FAK pS2A likely reflects the fact that preventing phosphorylation of pS2 through mutagenesis prevented the efficient phosphorylation of pS3, resulting in a weak signal in the immunoblot of FAK pS2A with the anti-pS3.
Anti-pS4 reacted with both wild-type FLAG-FAK and FAK pS1A (Figure 2B, lanes 7 and 8) but not appreciably with FAK pS4A (lane 9). Reactivity of anti-pS4 with FAK pS1A (lane 8) showed that phosphorylation of pS1 was not required for efficient phosphorylation of pS4. Similarly, the reactivity of anti-pS1 with FAK pS4A showed that phosphorylation of pS4 was not required for efficient phosphorylation of pS1 (Figure 2B, lane 6). We also observed that anti-pS4 reacted with a FAK construct with the three other sites of phosphorylation mutated (FAK pS1/pS2/pS3A; our unpublished results), suggesting that phosphorylation of pS4 was not dependent on phosphorylation of pS1, pS2, or pS3.
Serine Phosphorylation of the Carboxyl Terminus of FAK Is Induced during Mitosis
FAK is highly phosphorylated on serine residues during mitosis and the increase in serine phosphorylation is concomitant with a decrease in tyrosine phosphorylation (Yamakita et al., 1999; Ma and Parsons, unpublished observations). Therefore, we used the phosphorylation-specific antibodies to investigate the serine phosphorylation of individual sites in unsynchronized and mitotic cell extracts. Anti-pS1 reacted equally well with FAK present in lysates collected from unsynchronized and mitotic cells (Figure 3A, lanes 5 and 6). Interestingly, anti-pS1 also reacted with several other proteins present in the mitotic extracts (indicated by dots in lane 6). Because the reactivity of anti-pS1 was blocked with phosphopeptides that mimic the pS1 site (see below), we conclude that anti-pS1 cross reacts with cellular proteins that are presumably phosphorylated within similar, perhaps identical sequences during mitosis. Anti-pS2, anti-pS3, and anti-pS4 reacted weakly with proteins in lysates of unsynchronized cells but strongly with proteins in the mitotic samples (Figure 3A, lanes 9 and 10, 13 and 14, and 17 and 18). Detection of FAK with anti-pS2 indicated that FAK appeared to be phosphorylated to a similar degree in both unsynchronized and mitotic cells (Figure 3B, lanes 9 and 10), although anti-pS2 also reacted with at least nine other bands in the mitotic cells (indicated by dots in Figure 3A, lane 10). Anti-pS3 was highly specific, reacting with a single polypeptide in both unsynchronized and mitotic cells (Figure 3A, lanes 13 and 14). Anti-pS4 also reacted strongly with FAK in the mitotic cells (lane 18) but like anti-pS2, this antibody also reacted with additional proteins in mitotic cell lysates (indicated by dots). As with anti-pS1, reactivity of anti-pS2 and anti-pS4 with the additional bands was blocked by preincubation with the phosphopeptide mimic, but not by the unphosphorylated version (see below).
Figure 3.
Recognition of FAK by phospho-specific antibodies against serine phosphorylated antigens and specific competition by phosphorylated peptides. (A) Reactivity of the phospho-specific antibodies with immune complexes of endogenous FAK (lanes 3 and 4, 7 and 8, 11 and 12, and 15 and 16) and proteins in lysates (lanes 5 and 6, 9 and 10, 13 and 14, and 17 and 18) of unsynchronized (U) and mitotic (M) HeLa cells. FAK is indicated by the arrow; additional cross-reactive bands are indicated by dots (●). (B) Reactivity of the phospho-specific antibodies in Western blots can be specifically blocked by preincubation with phosphorylated synthetic peptide antigen. Each phospho-specific antibody was preincubated at a working concentration of 2-3 μg/ml in the absence of peptide or with a 20- to 100-fold molar excess of nonphosphorylated or phosphorylated peptide. Nitrocellulose strips containing U and M HeLa cell proteins resolved by SDS-PAGE were blocked and then incubated with the pretreated antibody solutions. Total FAK was detected by immunoblotting with the monoclonal antibody 2A7 (lanes 1 and 2). The band corresponding to FAK is indicated by the open arrow; in the anti-pS2 blot, FAK appears as a weak signal migrating above the strong band at ∼120 kDa.
To confirm that the signals generated by the phospho-specific antibodies were due to reactivity with FAK, we also immunoprecipitated FAK from the same extracts that were used for direct immunoblotting. FAK was immunoprecipitated from the unsynchronized and mitotic HeLa cell lysates with the monoclonal antibody 2A7, directed against the extreme C terminus of the FAK protein (Hildebrand et al., 1995). Proteins collected in the immune complex were divided into five equal portions and subjected to Western blot analysis with anti-FAK (Figure 3A, lanes 1 and 2) or with each of the phospho-specific antibodies (lanes 3 and 4, 7 and 8, 11 and 12, and 15 and 16). As shown in Figure 3A, the major band in the FAK immune complexes reacting with anti-pS1, anti-pS3, and anti-pS4 comigrated with FAK as detected by immunoblotting the immune complexes with anti-FAK (lanes 1 and 2). Interestingly, the reactivity of anti-pS2 and of anti-pS4 was greatly reduced toward FAK collected in immune complexes (lanes 7 and 8 and 15 and 16), suggesting that pS2 and pS4 were dephosphorylated during immune complex preparation, or that phosphorylation of pS2 and pS4 prevented efficient immunoprecipitation of FAK.
Because the phospho-specific antibodies reacted with proteins other than FAK in Western blots of mitotic cells, each antibody was tested in peptide preabsorption experiments with phosphorylated and nonphosphorylated peptide antigens as an additional measure of antibody specificity. Preincubation of the antibodies with the appropriate nonphosphorylated peptides did not affect their reactivity toward these proteins in Western blots compared with antibodies incubated without peptide (Figure 3B, lanes 5 and 6, 11 and 12, 17 and 18, and 23 and 24). However, incubation of the antibodies with the phosphorylated peptides abolished their reactivity, confirming that the antibodies were in fact specific to phosphorylated epitopes (lanes 7 and 8, 13 and 14, 19 and 20, and 25 and 26). These experiments thus confirmed that the antibodies reacted only with serine-phosphorylated antigens and not with unphosphorylated proteins. Notably, reactivity of anti-pS2 with the nine major bands in mitotic cells was specifically competed away with the phosphorylated peptide, indicating that a phosphorylated epitope recognized by this antibody is induced in mitosis. The band corresponding to FAK is marked by the open arrow; reactivity of anti-pS2 appears to be equivalent in unsynchronized and mitotic cells.
A Possible Role for Serine Phosphorylation of the FAK Carboxyl Terminus in Regulating Binding to the SH3 Domain of p130Cas
Previous work demonstrated that induction of FAK serine phosphorylation during mitosis correlates with its dissociation from binding to p130Cas (Yamakita et al., 1999). FAK associates with the SH3 domain of Cas primarily through a type II polyproline consensus sequence in the FAK C terminus (P712PKP715SR; Figure 1G) (Polte and Hanks, 1995; Harte et al., 1996). Serine phosphorylation has previously been implicated in modulating the interaction of polyproline ligands with their cognate SH3 domains (Corbalan-Garcia et al., 1996). Because pS1 (Ser722) is positioned five residues C-terminal to the Cas-binding polyproline sequence, we sought to determine whether phosphorylation of pS1 affected the interaction of FAK with the Cas SH3 domain. For these studies we again used FRNK as a tool because the amino acid sequences of FRNK and the C terminus of FAK are identical, and because FRNK is more amenable to manipulation in vitro than FAK.
FRNK was expressed as a GST fusion protein (GST-FRNK) and tested for its ability to associate with Cas in extracts of CE cells in vitro. To study the effect of phosphorylation, FRNK pS1 (Ser30) was mutated either to Ala (to block phosphorylation) or to Glu (to mimic phosphorylation). Neither mutation significantly affected the ability of GST-FRNK to interact with Cas as detected by GST pulldown and Cas immunoblotting (unpublished observations). These data parallel previously published experiments that showed that a GST fusion protein of Grb2 failed to distinguish between phosphorylated and unphosphorylated forms of Sos (Rozakis-Adcock et al., 1995), suggesting that GST coprecipitation assays may not be sufficiently sensitive to detect subtle changes in ligand binding to SH3 domains.
To detect changes in FRNK binding to the Cas SH3 domain, we devised a more sensitive method to test whether the extent of this interaction was affected by serine phosphorylation. A synthetic peptide corresponding to the SH3-binding region in FRNK encompassing the phosphorylation site at pS1 was generated containing the sequence EAPPKPSRPGYPSPRSS (designated wild-type peptide). To study the effect of phosphorylation, a second peptide was synthesized with phosphoserine incorporated at the position corresponding to pS1 (EAPPKPSRPGYPpSPRSS, designated phosphopeptide). A third peptide, denoted peptide P→A (EAPPKASRPGYPSPRSS), was synthesized with a Pro-to-Ala point mutation at the position corresponding to Pro23 of FRNK. In the context of full-length FAK, this Pro residue (FAK Pro715) has been shown to be critical for FAK binding to the Cas SH3 domain (Harte et al., 1996; Polte and Hanks, 1997). For clarity, this residue is referred to as PI within the site I sequence (Figure 1G). Although site I is implicated as a preferred ligand for the Cas SH3 domain, Cas also can bind independently to a second polyproline sequence in FAK, P875KKP878PR, where FAK Pro878 is the critical residue (Harte et al., 1996; Polte and Hanks, 1997). This site is designated PII, denoting the site specified by Pro878 in site II (Figure 1G).
To show that the synthetic peptides were capable of blocking the binding of the Cas SH3 domain to FAK and FRNK, binding of a GST-Cas SH3 domain fusion protein to endogenous FAK and FRNK present in CE cell lysates was assessed by GST-pulldown and immunoblotting. As shown in Figure 4, no binding of FAK or FRNK was observed using GST alone or GST fused to the SH3 domain of cortactin, an actin-binding protein, or to the SH3 domain of Src in the absence or presence of 100 μM wild-type peptide. A GST fusion protein containing the Cas SH3 domain bound both FAK and FRNK efficiently (lane 3), and preincubation of this fusion protein with increasing amounts of wild-type peptide (derived from the site I sequence) resulted in a dose-dependent decrease in Cas SH3 domain association with both FAK and FRNK (lanes 4-8). Under these assay conditions, preincubation with the phosphopeptide yielded results similar to those observed with the wild-type peptide (unpublished observations), confirming that the GST coprecipitation assay was not sufficiently sensitive to detect changes in binding attributable to serine phosphorylation. Preincubation with 100 μM P→A peptide did not affect the ability of the Cas SH3 fusion protein to associate with FAK or FRNK. As an additional control, a GST fusion protein containing the N terminus of paxillin (which binds to the extreme C termini of FAK and FRNK at regions discrete from the SH3 binding sites; Hildebrand et al., 1995) efficiently bound to both FAK and FRNK, and this interaction was not perturbed by preincubation of the fusion protein with 100 μM wild-type peptide (lanes 13 and 14). These results demonstrate that the blocking effects of the peptides were specific to the Cas SH3 domain and validate their use in a peptide competition binding assay.
Figure 4.
A synthetic peptide derived from the site I sequence of FAK specifically interacts with the SH3 domain of Cas. GST alone (lanes 1 and 2) or fused to the SH3 domain of Cas (lanes 3-8), cortactin (lanes 9 and 10), or Src (lanes 11 and 12), or the N terminus of paxillin (lanes 13 and 14) was preincubated with increasing amounts of wild-type peptide (EAPPKPSRPGYPSPRSS, 0 to 100 μM), followed by incubation with 500 μg of CE cell lysate. Protein complexes were captured, resolved by SDS-PAGE, and blotted for the presence of FAK (top) and FRNK (bottom) with the polyclonal antiserum BC3.
The ability of the wild-type and phosphorylated peptides to inhibit the binding of the Cas SH3 domain to FRNK was assessed using an in vitro competition assay with purified recombinant FRNK labeled with a hexahistidine tag (His-FRNK). Purified His-FRNK was incubated with purified GST-Cas SH3 and increasing concentrations of each peptide. GST-Cas SH3 complexes were collected using glutathione Sepharose, and the amount of FRNK associating with the Cas SH3 domain was determined by immunoblotting with the monoclonal antibody 2A7 and detection with 125I anti-mouse IgG. As shown in Figure 5A, both the wild-type peptide and the phosphopeptide were able to compete with wild-type His-FRNK for Cas SH3 binding, whereas the P→A peptide failed to compete. Quantitation of these data (Figure 5B) showed that in this assay the ability of wild type and phosphopeptides to compete for Cas SH3 binding was not significantly different from each other at low peptide concentrations (25 μM), but that the wild-type peptide appeared to be a better competitor at higher peptide concentrations (100 μM). Although this effect was small and required large amounts of peptide, these data nevertheless implied that phosphorylation negatively influenced the ability of the phosphopeptide to compete with full-length His-FRNK compared with the wild-type peptide.
Figure 5.
Analysis of the binding of phosphorylated peptides derived from the site I sequence to the Cas SH3 domain. (A) Representative Western blot of peptide competition assays by using GST-CasSH3 and wild-type His-tagged FRNK. Components were incubated in the presence of increasing amounts (1, 10, 25, 50, or 100 μM) of each peptide indicated. His-FRNK was detected by immunoblotting with the mAb 2A7 followed by detection with 125I anti-mouse IgG and densitometric analysis. (B) Quantitation and graphical representation of PhosphorImager data with wild-type His-FRNK with 25 or 100 μM peptide competitor. Values are expressed as a fraction of a control reaction performed in the absence of peptide (open bar, normalized to 1). P values were calculated using a paired t test. Statistical differences (P < 0.05) between either the wild-type peptide or the phosphopeptide and the control P→A peptide are indicated by an asterisk (∗) above the appropriate bar. Statistical differences between the wild type and phosphopeptides themselves are indicated by a double asterisk (∗∗) above the bar representing the wild-type peptide. Data points represent mean values from three independent experiments; error bars represent SD from the mean.
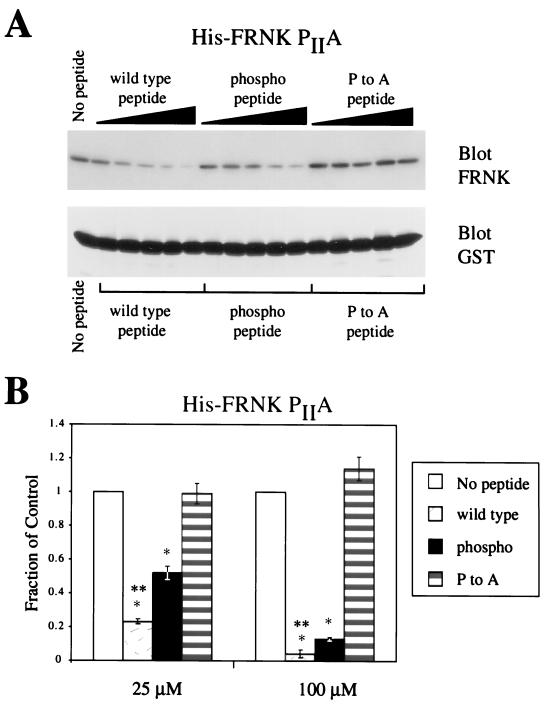
Because the Cas SH3 domain binds to polyproline sequences surrounding both PI and PII in FRNK (Figure 1G), the effect of serine phosphorylation on Cas SH3 binding to site I may be masked by Cas binding to site II. To investigate the effect of pS1 phosphorylation on Cas binding to site I only, we used a His-FRNK construct in which site II was disrupted by a Pro-to-Ala point mutation at PII. When the competition assay was performed with His-FRNK PIIA, where GST-Cas SH3 bound only to site I of His-FRNK, the wild-type peptide was clearly a more effective competitor than was the phosphopeptide (Figure 6, A and B). These data indicate that in vitro, phosphorylation on serine inhibits the ability of peptides containing the site I sequence to interact with the SH3 domain of Cas, and that the inhibitory effects are most evident when studied in the context of Cas SH3 domain binding to a single ligand-binding site.
Figure 6.
Effects of serine phosphorylation on FAK peptide binding to the Cas SH3 domain when Cas binding is restricted to site I of His-FRNK. (A) Representative Western blot of peptide competition assays by using GST-Cas SH3 and His-FRNK PIIA. As in Figure 5, components were incubated in the presence of increasing amounts (1, 10, 25, 50, or 100 μM) of each peptide and FRNK was detected by immunoblotting with the mAb 2A7 and 125I anti-mouse IgG. (B) Quantitation and graphical representation of PhosphorImager data by using His-FRNK PIIA with 25 or 100 μM peptide competitor. Data are presented using the same methods detailed in Figure 5.
DISCUSSION
In this report, we attempt to delineate the role of serine phosphorylation in regulating the interactions of FAK and FRNK with downstream effectors. To this end, we identify four sites of serine phosphorylation in focal adhesion kinase, two of which were previously characterized only in the context of FRNK. We use phosphorylation-specific antibodies targeted against each site of phosphorylation to confirm that the sites of phosphorylation mapped using in vitro and in vivo labeling approaches are phosphorylated in unsynchronized and mitotic cells in vivo. We show that phosphorylation of two sites, pS3 and pS4, is increased in mitotic cells, whereas the phosphorylation of the remaining sites, pS1 and pS2, remains unchanged in unsynchronized and mitotic cells. In addition, antibodies to pS1, pS2, and pS4 identify additional polypeptide bands in extracts from mitotic cells, indicating the efficacy of using phosphorylation-specific antibodies to characterize other proteins whose phosphorylation is increased during mitosis. Finally, analysis of the binding properties of peptides mimicking the site I binding site in FAK/FRNK indicate that phosphorylation of pS1 may play a role in regulating binding of FAK/FRNK to p130Cas.
The analysis of serine phosphorylation in modulating the activity of receptor- and nonreceptor tyrosine kinases as well as serine/threonine kinases has been markedly enhanced by the availability of antibodies to defined phosphorylation sites. Using phosphorylation-specific antibodies directed against four major sites of serine phosphorylation within the FAK carboxyl terminus, we show that the phosphorylation of two sites is significantly increased in extracts of mitotic cells. We also observed that phosphorylated epitopes recognized by anti-pS1, anti-pS2, and anti-pS4 are induced in other proteins during mitosis, suggesting that a kinase(s) activated during mitosis directs the phosphorylation of FAK and other proteins within similar, if not identical, sequences. Recognition of multiple bands by the phospho-specific antibodies was particularly striking in immunoblots of mitotic extracts with anti-pS2, which recognized at least nine bands. PS2 in FRNK has previously been characterized as a target of protein kinase A (PKA) (Richardson et al., 1997), and it is well established that PKA is critical for proper transit through the cell cycle. PKA activity is elevated during mitosis (Costa et al., 1976; Grieco et al., 1996), and its function has been implicated in exit from mitosis (Kotani et al., 1998), chromatin condensation (Collas et al., 1999), and transcriptional silencing (Segil et al., 1991), although its mitotic substrates have not been fully defined. Because PKA clearly plays an important role in mitotic progression and anti-pS2 recognizes phosphorylated epitopes presumably related to PKA substrates, anti-pS2 could be a useful reagent to identify other PKA targets phosphorylated during mitosis.
Two of the four antibodies (anti-pS2 and anti-pS4) that reacted strongly with FAK in mitotic cell lysates reacted poorly with FAK purified from the same lysates by immunoprecipitation. This result was unexpected and suggests either that FAK was dephosphorylated on these residues during immune complex preparation (see below), or that FAK proteins phosphorylated on these residues in vivo were not efficiently captured in the immune complex. The antibody used for immunoprecipitation in these experiments (monoclonal antibody 2A7) recognizes an epitope within the C-terminal 150 amino acids of FAK (Hildebrand et al., 1995). Serine phosphorylation of FAK may directly interfere with antibody binding, or phosphorylated FAK may be sequestered by the binding of other cellular proteins that in turn block antibody 2A7 binding.
Work from other groups has documented the localization of the delta isoform of protein phosphatase 1 (PP1δ) to focal adhesions and the coprecipitation of PP1δ with FAK (Murata et al., 1997; Villa-Moruzzi et al., 1998). The proximity of PP1δ to FAK in adherent cells may account for the lack of FAK serine phosphorylation in unsynchronized cells, particularly in light of the inability of FAK to be inducibly serine phosphorylated in response to growth factor stimulation, integrin engagement, and treatment with pharmacological agonists (Ma and Parsons, unpublished data). Interestingly, the targeting subunit of PP1δ is specifically phosphorylated during mitosis and phosphorylation appears both to increase its affinity for myosin and its associated myosin phosphatase activity (Totsukawa et al., 1999). It would be of interest to determine whether PP1δ specifically dissociated from FAK in mitosis and whether its activity toward nonmyosin substrates was specifically decreased because this could be a possible mechanism for the maintenance of serine phosphorylation on focal adhesion proteins during M phase.
Yamakita et al. (1999) reported that FAK and Cas serine phosphorylation are induced during mitosis and that increased serine phosphorylation of these proteins correlates with the dissociation of FAK–Cas complexes. Using a synthetic peptide to represent the FAK ligand in an in vitro binding assay, we show that serine phosphorylation of the FAK peptide reduces its ability to compete with recombinant full-length FRNK in binding to the Cas SH3 domain. These results indicate that serine phosphorylation of FAK and/or FRNK proximal to the Cas SH3 binding site may modulate Cas SH3 domain binding interactions. Although we observed only a slight increase in the phosphorylation of FAK at pS1 in mitotic cells, it is possible that the increased phosphorylation of FAK at other sites, e.g., pS3 and pS4, as well as serine phosphorylation of Cas itself, may contribute to the breakdown of FAK–Cas complexes during mitosis. Work is in progress to identify the enzymes that phosphorylate pS1 and pS4. Once these activities have been defined, we will be able to recapitulate FRNK in vivo serine phosphorylation in vitro and to assess the full effects of serine phosphorylation on FRNK binding to Cas.
The data presented here, together with our observations that FAK pS1 is constitutively phosphorylated during the cell cycle, suggest that phosphorylation of pS1 may contribute to regulating FAK–Cas interactions in normal adhesive interactions of cells with the ECM. Cary et al. (1998) demonstrated that FAK–Cas coupling through FAK through FAK Pro715 (PI) and the Cas SH3 domain promotes cell migration toward fibronectin. Further, Cas tyrosine phosphorylation correlates positively with cell migration and requires both Cas coupling to FAK at site I and Src binding to FAK at tyrosine 397. Thus, FAK appears to position Cas in a way that promotes Cas tyrosine phosphorylation by Src in cell migration responses. Our in vitro studies are consistent with a model that implicates serine phosphorylation of the site I peptide ligand as a potential regulator of FAK–Cas interactions. Regulated phosphorylation of this site may be important in the control of focal adhesion turnover and/or the dynamic regulation of focal adhesion structures during cell migration (Horwitz and Parsons, 1999).
ACKNOWLEDGMENTS
We are grateful to John Shannon and the University of Virginia BioMolecular Research Facility for Edman degradation analysis; Andy Catling for help with protein chemistry; Wen Xiong and Peter Sheffield for providing expression constructs; and Jennifer Havens, Shauna Hodge, and Amy Diepold for technical assistance. This work was supported by Grants CA-29243 and CA-40042 from the Department of Health and Human Services-National Cancer Institute to J.T.P. A.M. was supported by training grants in Molecular Medicine and Pharmacological Sciences. The BioMolecular Research Facility is supported by a grant from the University of Virginia Pratt Committee.
Abbreviations used:
- CE
chicken embryo
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- FRNK
FAK-related nonkinase
- GST
glutathione-S-transferase
- SH3
Src homology 3
- PKA
protein kinase A
- pS
phosphoserine
REFERENCES
- Burnham MR, Bruce-Staskal P, Harte MT, Weidow CL, Ma A, Weed SA, Bouton AH. Regulation of c-SRC by the adapter protein CAS. Mol Cell Biol. 2000;20:5865–5878. doi: 10.1128/mcb.20.16.5865-5878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary LA, Han DC, Polte TR, Hanks SK, Guan J-L. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Guan JL. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collas P, Le Guellec K, Tasken K. The A-kinase anchoring protein AKAP95 is a multivalent protein with a key role in chromatin condensation at mitosis. J Cell Biol. 1999;147:1167–1179. doi: 10.1083/jcb.147.6.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbalan-Garcia S, Yang SS, Degenhardt KR, Bar-Sagi D. Identification of the mitogen-activated protein kinase phosphorylation sites on human Sos1 that regulate interaction with Grb2. Mol Cell Biol. 1996;16:5674–5682. doi: 10.1128/mcb.16.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Gerner EW, Russell DH. Cell cycle-specific activity of type I and type II cyclic adenosine 3′:5′-monophosphate-dependent protein kinases in Chinese hamster ovary cells. J Biol Chem. 1976;251:3313–3318. [PubMed] [Google Scholar]
- Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AM, Weinberg RA. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993;363:45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Gale NW, Kaplan S, Lowenstein EJ, Schlessinger J, Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993;363:88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- Grieco D, Porcellini A, Avvedimento EV, Gottesman ME. Requirement for cAMP-PKA pathway activation by M phase-promoting factor in the transition from mitosis to interphase. Science. 1996;271:1718–1723. doi: 10.1126/science.271.5256.1718. [DOI] [PubMed] [Google Scholar]
- Harte MT, Hildebrand JD, Burnham MR, Bouton AH, Parsons JT. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Schaller MD, Parsons JT. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz AR, Parsons JT. Cell migration - movin’on. Science. 1999;286:1102–1103. doi: 10.1126/science.286.5442.1102. [DOI] [PubMed] [Google Scholar]
- Hughes SH, Greenhouse JJ, Petropoulos CJ, Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S, Tugendreich S, Fujii M, Jorgensen P-M, Watanabe N, Hoog C, Hieter P, Todokoro K. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol Cell. 1998;1:371–380. doi: 10.1016/s1097-2765(00)80037-4. [DOI] [PubMed] [Google Scholar]
- Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar-Sagi D, Margolis B, Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signaling. Nature. 1993;363:85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Murata K, Hirano K, Villa-Moruzzi E, Hartshorne DJ, Brautigan DL. Differential localization of myosin and myosin phosphatase subunits in smooth muscle cells and migrating fibroblasts. Mol Biol Cell. 1997;8:663–673. doi: 10.1091/mbc.8.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan K, Lacoste J, Parsons JT. Regulated expression of focal adhesion kinase-related nonkinase, the autonomously expressed C-terminal domain of focal adhesion kinase. Mol Cell Biol. 1999;19:6120–6129. doi: 10.1128/mcb.19.9.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petch LA, Bockholt SM, Bouton A, Parsons JT, Burridge K. Adhesion-induced tyrosine phosphorylation of the p130 Src substrate. J Cell Sci. 1995;108:1371–1379. doi: 10.1242/jcs.108.4.1371. [DOI] [PubMed] [Google Scholar]
- Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polte TR, Hanks SK. Complexes of focal adhesion kinase (FAK) and Crk-associated substrate (p130(Cas)) are elevated in cytoskeleton-associated fractions following adhesion and Src transformation. Requirements for Src kinase activity and FAK proline-rich motifs. J Biol Chem. 1997;272:5501–5509. doi: 10.1074/jbc.272.9.5501. [DOI] [PubMed] [Google Scholar]
- Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular Src gene. Mol Cell Biol. 1989;9:629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Shannon JD, Adams RB, Schaller MD, Parsons JT. Identification of integrin-stimulated sites of serine phosphorylation in FRNK, the separately expressed C-terminal domain of focal adhesion kinase - a potential role for protein kinase A. Biochem J. 1997;324:141–149. doi: 10.1042/bj3240141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M, Fernley R, Wade J, Pawson T, Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993;363:83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M, van der Geer P, Mbamalu G, Pawson T. MAP kinase phosphorylation of mSos1 promotes dissociation of mSos1-Shc and mSos1-EGF receptor complexes. Oncogene. 1995;11:1417–1426. [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK, a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Parsons JT. Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK. Mol Cell Biol. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by Grb2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Segil N, Roberts SB, Heintz N. Mitotic phosphorylation of the Oct-1 homeodomain and regulation of Oct-1 DNA binding activity. Science. 1991;254:1814–1816. doi: 10.1126/science.1684878. [DOI] [PubMed] [Google Scholar]
- Sheffield PJ, Garrard SM, Derewenda ZS. Overcoming expression and purification problems of RhoGDI using a family of ‘parallel‘ expression vectors. Protein Exp Purif. 1999;15:34–39. doi: 10.1006/prep.1998.1003. [DOI] [PubMed] [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hosoya H, Hartshorne DJ, Matsumura F. Activation of myosin phosphatase targeting subunit by mitosis-specific phosphorylation. J Cell Biol. 1999;144:735–744. doi: 10.1083/jcb.144.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Moruzzi E, Tognarini M, Cecchini G, Marchisio PC. Protein phosphatase 1 delta is associated with focal adhesions. Cell Adhes Commun. 1998;5:297–305. doi: 10.3109/15419069809040299. [DOI] [PubMed] [Google Scholar]
- Wetzel B, Kendig EM, Jones GM, Sanford KK. A systematic scanning electron microscope (SEM) analysis of mitotic cell populations in monolayer culture. Scanning Electron Microsc. 1978;2:1–10. [Google Scholar]
- Xiong W-C, Parsons JT. Induction of apoptosis after expression of PYK2, a tyrosine kinase structurally related to focal adhesion kinase. J Cell Biol. 1997;139:529–539. doi: 10.1083/jcb.139.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R, Mazaki Y, Hirota K, Hashimoto S, Sabe H. Mitosis specific serine phosphorylation and downregulation of one of the focal adhesion protein, paxillin. Oncogene. 1997;15:1753–1761. doi: 10.1038/sj.onc.1201345. [DOI] [PubMed] [Google Scholar]
- Yamakita Y, Totsukawa G, Yamashiro S, Fry D, Zhang X, Hanks SK, Matsumura F. Dissociation of FAK/p130CAS/c-Src complexes during mitosis: role of mitosis-specific serine phosphorylation of FAK. J Cell Biol. 1999;144:315–324. doi: 10.1083/jcb.144.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Chen JK, Feng S, Dalgarno DC, Brauer AW, Schreiber SL. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994;76:933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]